Abstract
Background and purpose:
TRPC5 is a Ca2+-permeable channel with multiple modes of activation. We have explored the effects of genistein, a plant-derived isoflavone, on TRPC5 activity, and the mechanism(s) involved.
Experimental approach:
Effects of genistein on TRPC5 channels were investigated in TRPC5-over-expressing human embryonic kidney 293 (HEK) cells and bovine aortic endothelial cells (BAECs) using fluorescent Ca2+ imaging and electrophysiological techniques.
Key results:
In TRPC5-over-expressing HEK cells, genistein stimulated TRPC5-mediated Ca2+ influx, concentration dependently (EC50= 93 µM). Genistein and lanthanum activated TRPC5 channels synergistically. Effects of genistein on TRPC5 channels were mimicked by daidzein (100 µM), a genistein analogue inactive as a tyrosine kinase inhibitor, but not by known tyrosine kinase inhibitors herbimycin (2 µM), PP2 (20 µM) and lavendustin A (10 µM). Action of genistein on TRPC5 channels was not affected by an oestrogen receptor inhibitor ICI-182780 (50 µM) or a phospholipase C inhibitor U73122 (10 µM), suggesting genistein did not act through oestrogen receptors or phospholipase C. In BAECs, genistein (100 µM) stimulated TRPC5-mediated Ca2+ influx. In patch clamp studies, both genistein (50 µM) and daidzein (50 µM) augmented TRPC5-mediated whole-cell cation current in TRPC5 over-expressing HEK cells. Genistein stimulated TRPC5 channel activity in excised inside-out membrane patch, suggesting that its action was relatively direct and did not require cytosolic factors.
Conclusions and implications:
The present study is the first to demonstrate stimulation of a TRP channel by isoflavones. Genistein is a lipophilic compound able to stimulate TRPC5 activity in TRPC5-over-expressing HEK cells and in native vascular endothelial cells.
Keywords: TRPC5, genistein, daidzein, isoflavones, calcium channel, transient receptor potential
Introduction
TRPC5 is a Ca2+-permeable non-selective cation channel (nomenclature follows Alexander et al., 2009) that is expressed in many different tissues/cells (Beech, 2007b). Functionally, the channel plays an important role in a variety of physiological processes including neuronal growth cone extension (Greka et al., 2003) and smooth muscle motility (Xu et al., 2006b). Excessive expression of TRPC5 channels is associated with cardiovascular disorders including hypertension (Liu et al., 2006) and heart failure (Bush et al., 2006).
There are diverse modes of activation for TRPC5 channels (Zeng et al., 2004). The channel can be activated by Ca2+ store depletion, lanthanides, lysophosphatidylcholine, extracellular thioredoxin and vesicular trafficking (Beech, 2007a,b; Xu et al., 2008). The channel activity is also stimulated following the activation of G-protein-coupled receptors and/or receptor tyrosine kinases (Schaefer et al., 2000). Although TRPC5 can be activated by multiple factors, there is still insufficient knowledge on the endogenous agonist(s) that directly opens the channel. In this regard, a recent study suggests that reduced thioredoxin may directly activate TRPC5 by breaking a disulphide bridge in the predicted extracellular pore loop adjacent to the ion selectivity filter of the channel (Xu et al., 2008). There is also evidence that lysophospholipids can activate TRPC5 through a relatively direct action, probably by causing a physical distortion of lipid bilayers (Xu et al., 2006a).
Plant-derived isoflavones have received considerable attention for their potential beneficial properties in protecting against cancer, heart diseases, rheumatoid arthritis, Alzheimer's disease and other pathological conditions (Si and Liu, 2007). Genistein is one of the most widely studied isoflavones and is a phytoestrogen that can act on oestrogen receptors at nanomolar level (Escande et al., 2006). At the concentration range of 10–100 µM, it also inhibits tyrosine kinases (Barnes et al., 2000). In addition, genistein may exert a direct, non-catalytic blocking effect on several ion channels, including L-type Ca2+ channels (Chiang et al., 1996; Belevych et al., 2002), voltage-gated Na+ channels (Paillart et al., 1997), voltage-gated K+ channels (Smirnov and Aaronson, 1995) and inwardly rectifying K+ channels (Chiang et al., 2002), in a tyrosine kinase-independent manner.
In the present study, we used the live-cell fluorescent calcium imaging and patch clamp technique to study the effect of genistein on TRPC5 channels. We found that genistein was capable of stimulating TRPC5 activity in human embryonic kidney (HEK) cells that heterologously expressed TRPC5 channels. This stimulatory effect was independent of tyrosine kinase activity and oestrogen receptors. Genistein also increased TRPC5 channel open probability as recorded in excised membrane patches, suggesting a relatively direct action. Furthermore, genistein also potentiated the stimulating action of La3+ on TRPC5 activity. To our knowledge, this is the first report showing an action of genistein on a TRP cation channel.
Methods
Cell culture and stable transfection
Mouse TRPC5 channel clone was a generous gift from Dr L Birnbaumer, NIH. The full-length cDNA was subcloned into pcDNA3 and transfected into HEK cells (ATCC). Transfection was done as described elsewhere (Kwan et al., 2004). Briefly, 4 µg of DNA plasmid was transfected into HEK cells using 6 µL of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in 200 µL Opti-MEM reduced serum medium (Invitrogen) in six-well plates, which contained ∼6 × 104 cells per well. HEK cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS). Then, 1 mg·mL−1 G-418 (Invitrogen) was added during culture to establish a stable TRPC5-expressing cell line. The primary cultures of bovine aortic endothelial cells (BAECs) were prepared from bovine aorta as described previously (Cheng et al., 2008). Briefly, bovine aortic segments were cut open longitudinally. The intima layer was peeled off and then digested with 0.1% collagenase in phosphate-buffered saline (PBS; in mM: 140, NaCl; 3, KCl; 25, Tris, pH 7.4) for 15 min at 37°C under vigorous shaking. Dissociated cells were centrifuged, resuspended and then grown in a culture medium RPMI-1640 (Invitrogen) supplemented with 10% FBS. Ca2+ imaging experiments with BAECs were carried out 2 days after seeding the cells on glass coverslips.
Immunoblots
The procedures were as described elsewhere (Kwan et al., 2004). Briefly, whole-cell lysates were extracted with detergent extraction buffer, which contained 1% (v/v) Nonidet P-40, 150 mM NaCl, 20 mM Tris–HCl, pH 8.0, with addition of protease inhibitor cocktail tablets. Extracted proteins (80 µg) were applied to each lane and separated on 7.5% sodium dodecyl sulphate/polyacrylamide gel electrophoresis. The proteins were then blotted onto a PVDF membrane. The membrane was incubated at 4°C overnight with anti-TRPC5 antibody (dilution 1:200; Alomone Laboratory, Jerusalem, Israel) in PBS buffer containing 0.1% Tween 20 and 5% non-fat dry milk. Immunodetection was accomplished with horseradish peroxidase-conjugated secondary antibodies, followed by the ECL detection system. Immunoblots with anti-β-tubulin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used to confirm that an equal amount of proteins was loaded onto each lane. The intensity of the bands was analysed by FluorChem 8000 imaging system (Alpha Innotech, San Leandro, CA, USA).
Immunodetection of TRPC5 channels in BAECs
BAECs were fixed with 3.7% formaldehyde and permeabilized with 0.1% Triton X-100. Non-specific immunostaining was blocked by pre-incubating the cells with 5% BSA. The fixed cells were incubated with or without anti-TRPC5 (dilution 1:100, Alomone Laboratory) at 4°C overnight, followed by incubation with secondary goat anti-rabbit IgG conjugated to Alexa Fluor 546 (1:200). Immunofluorescence was detected by a FV1000 confocal system (Olympus, Tokyo, Japan). Fluorescence signals were acquired at the settings under which the signals from the control cells (without anti-TRPC5) were absent.
Preparation of a TRPC5-blocking antibody T5E3
T5E3 was raised in rabbits using the strategy developed by Xu et al. (2005b). Briefly, a peptide corresponding to TRPC5 channel putative pore region (CYETRAIDEPNNCKG) conjugated to keyhole limpet haemocyanin (Alpha Diagnostic International, San Antonio, TX, USA) was injected subcutaneously in the back of a rabbit followed by two boost doses. T5E3 antiserum was collected 4 weeks after the second boost. IgG was purified from T5E3 antiserum and pre-immune serum using a protein G column. The specificity of T5E3 on TRPC5 channel activity has been previously demonstrated by Dr Beech's lab (Xu et al., 2006a). We also tested the specificity of our batches of T5E3. The results showed that T5E3 inhibited carbachol-induced activation of TRPC5 channels in TRPC5-over-expressing HEK cells, but it had no effect on 1-oleoyl-2-acetyl-sn-glycerol (OAG)-induced rise of intracellular Ca2+ ([Ca2+]i) in TRPC3-expressing HEK cells (data not shown), confirming the specificity of T5E3 to TRPC5.
Measurement of [Ca2+]i
[Ca2+]i was measured as described elsewhere with slight modification (Kwan et al., 2004). Briefly, cells were seeded onto poly-L-lysine-coated glass discs 1 day before [Ca2+]i measurement. The cells were loaded for 1 h in dark with 5 µM Fluo-3/AM and 0.02% Pluronic F-127 in culture media, before mounting onto a microscope chamber containing the bath solution. Normal physiological saline solution (NPSS) contained in mM: 140, NaCl; 5, KCl; 2, MgCl2; 1, CaCl2; 10, glucose; 10, HEPES, pH 7.4. 0Ca2+–PSS contained in mM: 140, NaCl; 5, KCl; 2, MgCl2; 10, glucose; 0.2, EGTA; 10, HEPES, pH 7.4. When needed, cells were pretreated with genistein or LaCl3 for 10 min. In experiments with BAECs, cells were loaded with 5 µM Fura-2/AM for ratiometric Ca2+-fluorescent measurement, and the bath solution was HBS, which contained in mM: 107, NaCl; 6, KCl; 1.2, MgSO4; 2, CaCl2; 11.5, glucose; 20, HEPES, pH 7.4. Ca2+-free (0[Ca2+]o) HBS was equivalent to HBS except that there was no CaCl2. Fluorescence [Ca2+]i signals were recorded by InCyt Basic Fluorescence Imaging System (Intracellular Imaging, Cincinnati, OH). In HEK cell experiments using Fluo-3 indicator, [Ca2+]i response was expressed as a ratio (Ft/F0) of real-time fluorescence (Ft; excitation at 490 nm) relative to the intensity at the beginning of the experiment (F0). In experiments with BAECs using Fura-2 indicator, [Ca2+]i response was expressed as ratio of the fluorescence signal from excitation at 340 nm to that at 380 nm (F340/F380). All experiments were performed at room temperature (∼23°C).
Electrophysiology
TRPC5 channel activity was measured in whole-cell or excised inside-out patch mode as described elsewhere (Shen et al., 2008). Briefly, whole-cell or single-channel activity was recorded using an EPC9 patch clamp amplifier (HEKA, Freiburg, Germany) controlled by Pulse software (HEKA). In time-series recordings of whole-cell current, the currents at +60 and −60 mV were sampled at 5 s intervals after break-in. Whole-cell current–voltage relationship (I/V) was determined from the currents recorded in response to successive voltage pulses of 500 ms duration, increasing in 20 mV increments from −80 to +80 mV. For whole-cell recording, the patch pipette contained in mM: 145, CsCl; 2, MgCl2; 10, glucose; 0.2, ATP; 10, HEPES, pH 7.2 titrated with CsOH. The bath contained NPSS. Free Ca2+ in the deionized water was estimated to be <50 pM. Although deionized water was used to prepare all solutions, there would be some calcium contamination in the major salts used (CsCl, NaCl). For the excised inside-out patches, the pipette solution was NPSS, and the bath solution contained in mM: 135, CsCl; 2, MgCl2; 1, EGTA; 20, HEPES, pH 7.2 titrated with CsOH. All currents were sampled at 50 kHz and filtered at 3 kHz, and the data were analysed with PulseFit and TAC. Electrophysiological experiments were performed at room temperature (∼23°C).
Data analysis and presentation
Data points in Ca2+-imaging traces represent the mean ± SEM from cells in one experiment. Bar charts represent means ± SEM of multiple experiments. Current–voltage curves in electrophysiology were means ± SEM of multiple experiments with number of experiments given as n in parenthesis. Student's t-test was used for statistical comparison, with P < 0.05 (*) or P < 0.01 (**) as a significant difference. Half-maximal response of TRPC5 channels to genistein (EC50) was determined by curve-fitting with Hill's equation using Prism 3.0 (GraphPad software).
Materials
Genistein, PP2, lavaendustin A, carbachol, OAG and 2-aminoethoxydiphenyl borate (2-APB) were from Calbiochem/Merck KGaA (Darmstadt, Germany). Daidzein, herbimycin, LaCl3 and dimethyl sulphoxide (DMSO) were from Sigma (St Louis, MO, USA). ICI-182780 was from Tocris Bioscience (Bristol, UK).
Results
Effect of genistein on TRPC5 channel current in TRPC5-over-expressing cells
TRPC5 cDNA was stably transfected into HEK cells. Compared to vector-transfected cells, these TRPC5 cDNA-transfected cells had a higher TRPC5 protein level on Western blots (Figure 1A). In whole-cell patch clamp recordings, bath application of 50 µM genistein to TRPC5-over-expressing cells induced an outward current at +60 mV and an inward current at −60 mV, peaking at around 5 min after application (Figure 1B–D). Consistent with the reported characteristics of TRPC5 channels (Jung et al., 2003; Xu et al., 2005a), extracellular LaCl3 (100 µM) enhanced the currents, whereas 2-APB (75 µM) abolished them (Figure 1B and C). Current–voltage relationship of the genistein-stimulated currents displays double rectification (Figure 1F), which is characteristic of TRPC5 channels (Beech, 2007b). In control experiments, genistein (50 µM) failed to increase whole-cell current in wild-type HEK cells (Figure 1H). Because genistein stock was prepared in DMSO, a vehicle control experiment was also performed. Addition of vehicle (DMSO, 0.1%) also had no effect on the whole-cell current (Figure 1G).
Figure 1.
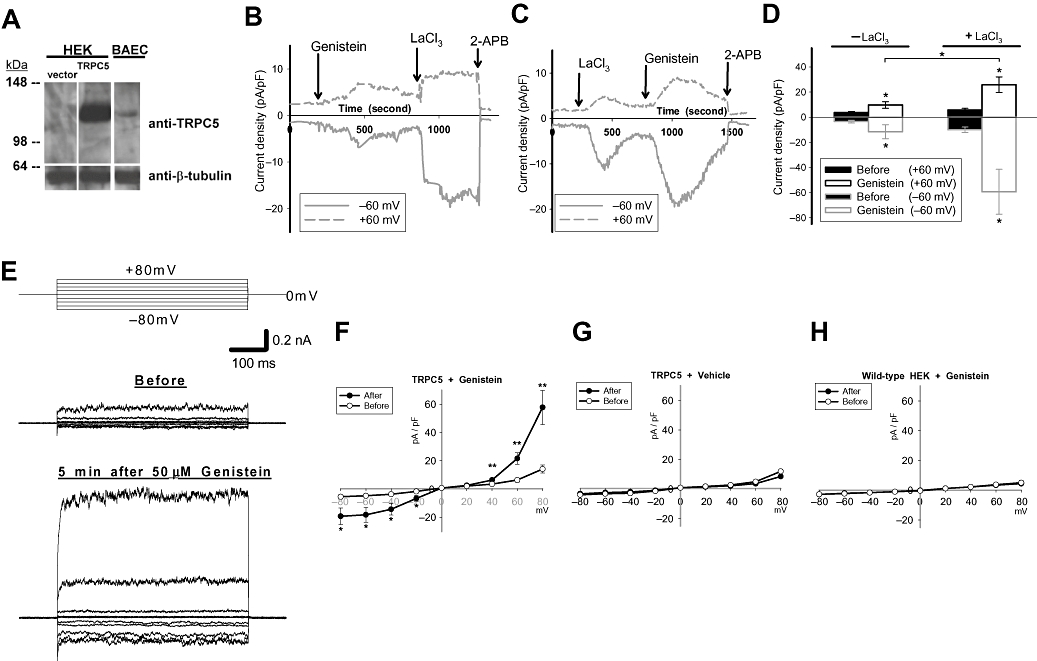
Effect of genistein on TRPC5 channel activity in HEK cells. (A) Representative immunoblot probed with anti-TRPC5 and anti-β-tubulin (n= 3). Left panel, pcDNA3-transfected HEK cells (vector); middle panel, TRPC5-transfected HEK cells; right panel, BAECs. (B and C) Representative time-course (n= 5–6) of whole-cell currents in TRPC5-over-expressing HEK cells. Currents were sampled every 5 s at +60 and −60 mV. Genistein (50 µM), LaCl3 (100 µM) and 2-APB (75 µM) were applied to the bath at the time-points indicated by arrows. (D) Summary of maximal whole-cell currents before and after genistein addition at +60 and −60 mV in TRPC5-over-expressing cells. +LaCl3, with 100 µM LaCl3; −LaCl3, without LaCl3. Mean ± SEM (n= 5–6). (E) Representative traces showing voltage protocol (top) and corresponding whole-cell currents immediately before (middle) and 5 min after (bottom) bath application of 50 µM genistein in TRPC5-over-expressing HEK cells. (F) Current–voltage relationships as in (E). (G and H) Current–voltage relationships in TRPC5-over-expressing (G) or wild-type HEK cells (H) immediately before and 5 min after bath application of 50 µM genistein (H) or 0.1% DMSO (G). Mean ± SEM (n= 3–8). *P < 0.05; **P < 0.01 compared to ‘before’.
Single-channel recordings were made in inside-out membrane patches excised from TRPC5-over-expressing cells. A low level of basal channel activity could be observed before any additions (Figure 2A and B). Application of genistein (50 µM) to the cytoplasmic side caused a significant increase in channel activity (Figure 2B–D), while application of vehicle (DMSO, 0.1%) did not increase the channel activity (Figure 2A). The channel activity was blocked by 75 µM 2-APB (data not shown). Single-channel current–voltage relationship was obtained (Figure 2E), and the single-channel slope conductance was estimated to be ∼46 pS.
Figure 2.
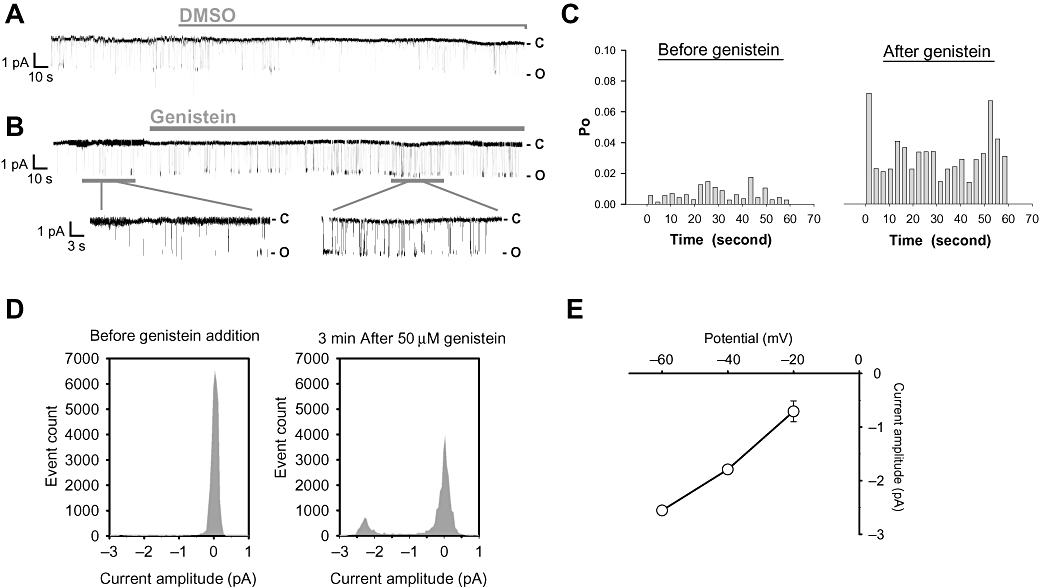
Single-channel recording of genistein-modulated channels in excised inside-out patches from TRPC5-over-expressing HEK cells. (A and B) Single-channel traces of membrane patches excised from TRPC5-over-expressing HEK cells; 0.1% DMSO (A) or 50 µM genistein (B) was applied as indicated by the horizontal bar. The patch potential was held at −60 mV. C, close state; O, open state. (C) Channel open probability (Po) values of the patch (as in A and B) in 3 s intervals. (D) Event histogram of a representative experiment before and after 50 µM genistein application at −60 mV. (E) Single-channel current–voltage relationship of the genistein-modulated channel. Mean ± SEM (n= 3–5).
Effect of genistein on TRPC5 channel-mediated rise in [Ca2+]i
Fluorescence Ca2+ imaging was used to test the effect of genistein on TRPC5-mediated Ca2+ influx; 100 µM La3+ was included in the bath to block other non-selective cation channels and to select for TRPC5-mediated Ca2+ influx. In one protocol, cells bathed in 0Ca2+–PSS were first treated with a muscarinic agonist carbachol (100 µM), which initiated a [Ca2+]i rise (Figure 3A and B) by releasing Ca2+ from intracellular Ca2+ stores (Schaefer et al., 2000). Subsequent addition of extracellular Ca2+ (1 mM) evoked another [Ca2+]i rise in TRPC5-transfected (Figure 3A), but not in vector-transfected HEK cells (Figure 3B). These results are consistent with a previous report which showed a stimulating action of carbachol on TRPC5 channels via a receptor-operated mechanism (Schaefer et al., 2000). Interestingly, addition of genistein (50 µM) into the bath caused a further rise in [Ca2+]i (Figure 3A). In another protocol, cells bathed in 0Ca2+–PSS were pretreated with genistein (50 µM) for 10 min, followed by the addition of extracellular Ca2+, which elicited a marked rise in [Ca2+]i in TRPC5-over-expressing cells (Figure 3C), but not in cells that were transfected with vector plasmid (Figure 3D). Vehicle control experiments were performed, in which the cells were pretreated with 0.1% DMSO (vehicle) (Figure 3C and D). As expected, the [Ca2+]i rise in response to extracellular Ca2+ addition was much smaller in vehicle-pretreated cells than in genistein-pretreated cells (Figure 3C).
Figure 3.
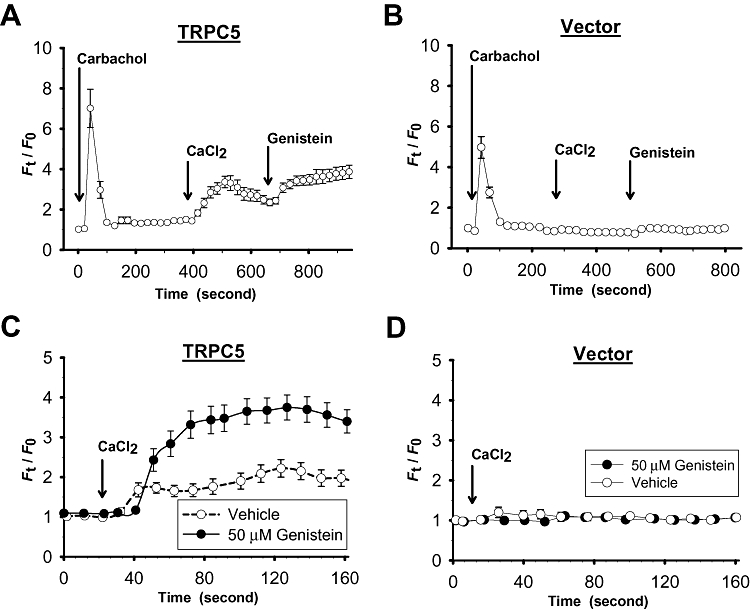
Effect of genistein on TRPC5-mediated [Ca2+]i rise. (A–D) Representative time-course of [Ca2+]i change in vector-transfected (B, D) and TRPC5-transfected HEK cells (A, C). (C and D) Cells were pretreated with genistein (50 µM) or vehicle (0.1% DMSO) for 10 min in 0Ca2+–PSS. In (A–D), 100 µM La3+ was included in the bath to block other non-selective cation channels and to select for TRPC5-mediated Ca2+ influx. Each trace represents mean ± SEM of at least 20 cells in one representative experiment (n= 4–7 experiments).
Synergistic action of genistein and La3+ on TRPC5 channels
We further tested a range of genistein concentrations (10–800 µM) on TRPC5 channel activity. Bath application of genistein elicited a [Ca2+]i rise on TRPC5-over-expressing cells in a concentration-dependent manner (Figure 4A–C). Analysis of the concentration–response curve yielded a half-maximal response (EC50) at ∼93 µM (Figure 4C). Interestingly, when bath solution contained 100 µM LaCl3, application of genistein induced a larger [Ca2+]i rise (Figure 4B and C) and a larger whole-cell current (Figure 1D). Furthermore, La3+ shifted the dose–response curve of genistein to the left, resulting in a lower EC50 at ∼51 µM (Figure 4C). Conversely, the presence of genistein (50 and 100 µM) in bath solution also potentiated the [Ca2+]i response to extracellular La3+ (Figure 4D and E). These experiments indicate that genistein and La3+ act synergistically to stimulate TRPC5 channels.
Figure 4.
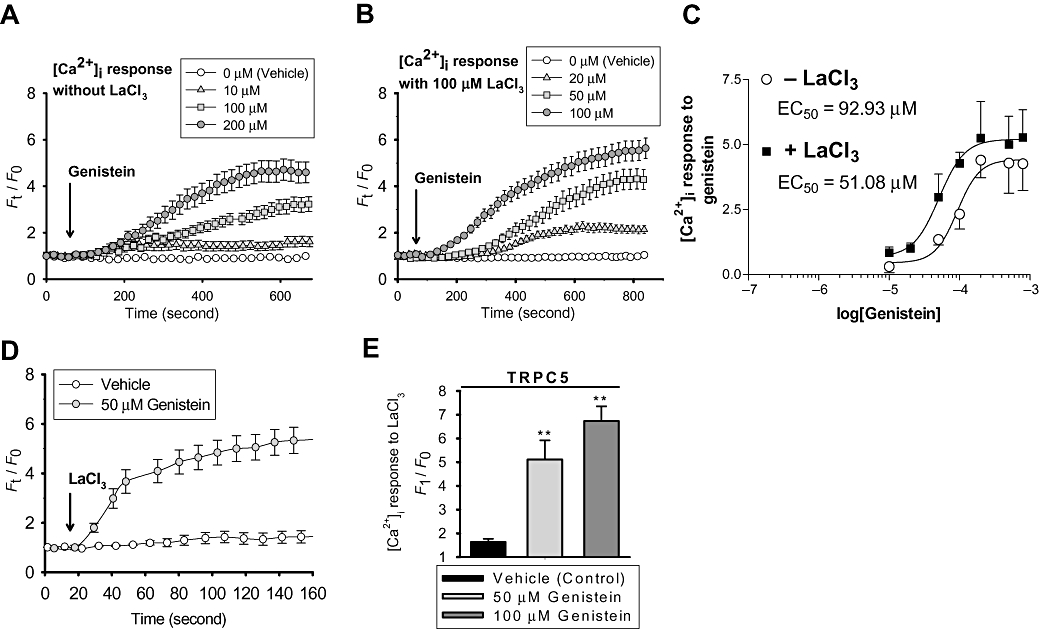
Concentration-dependent stimulation of TRPC5 channels by genistein and synergy between genistein and La3+. (A and B) Representative time-course of [Ca2+]i change in TRPC5-transfected HEK cells in response to different concentrations of genistein. Cells were bathed in NPSS (A) or NPSS + 100 µM LaCl3 (B). (C) Plot of concentration–response with curve fitting to determine the genistein concentration for half-maximal response (EC50). [Ca2+]i response at each concentration was subtracted by the background given by vehicle control; means ± SEM (n= 4–12). (D) Representative time-course of La3+ (100 µM)-induced [Ca2+]i change in TRPC5-expressing HEK cells in the absence or presence of 50 µM genistein. (E) Summary of data showing the maximal [Ca2+]i rise in response to La3+. For A, B and D, each trace represents means ± SEM of at least 20 cells from one representative experiment (n= 4–10 experiments). Vehicle contained 0.1% DMSO. **P < 0.01, compared to vehicle.
Effects of genistein were independent of oestrogen receptors and phospholipase C activity
An oestrogen receptor antagonist ICI-182780 was used to determine if the genistein effect is mediated through the classic oestrogen receptor α or β. As shown in Figure 5, pre-incubation of cells with ICI-182780 (50 µM) had no effect on genistein (100 µM)-induced Ca2+ influx in TRPC5-over-expressing HEK cells (Figure 5A). Other earlier reports have shown that receptor activation of TRPC5 channels relies on phospholipase C activity (Schaefer et al., 2000; Beech, 2007b). However, pretreatment of cells with a phospholipase C inhibitor U73122 (10 µM) did not affect the [Ca2+]i response to genistein (Figure 5B). U73343 (10 µM), an inactive analogue of U73122, also had no effect (Figure 5B). Control experiments were performed to verify the inhibitory effect of U73122 on phospholipase C activity. The results showed that a 30 min pretreatment with U73122 (10 µM) was able to inhibit carbachol-induced [Ca2+]i rise in wild-type HEK cells (F1/F0 of 1.53 ± 0.24 (n= 4) in U73122-treated cells vs. 5.96 ± 0.47 (n= 4) in U73343-treated cells), confirming the effectiveness of U73122 towards phospholipase C. Taken together, these data suggest that the effect of genistein on TRPC5 channels was not mediated through oestrogen receptors or phospholipase C.
Figure 5.
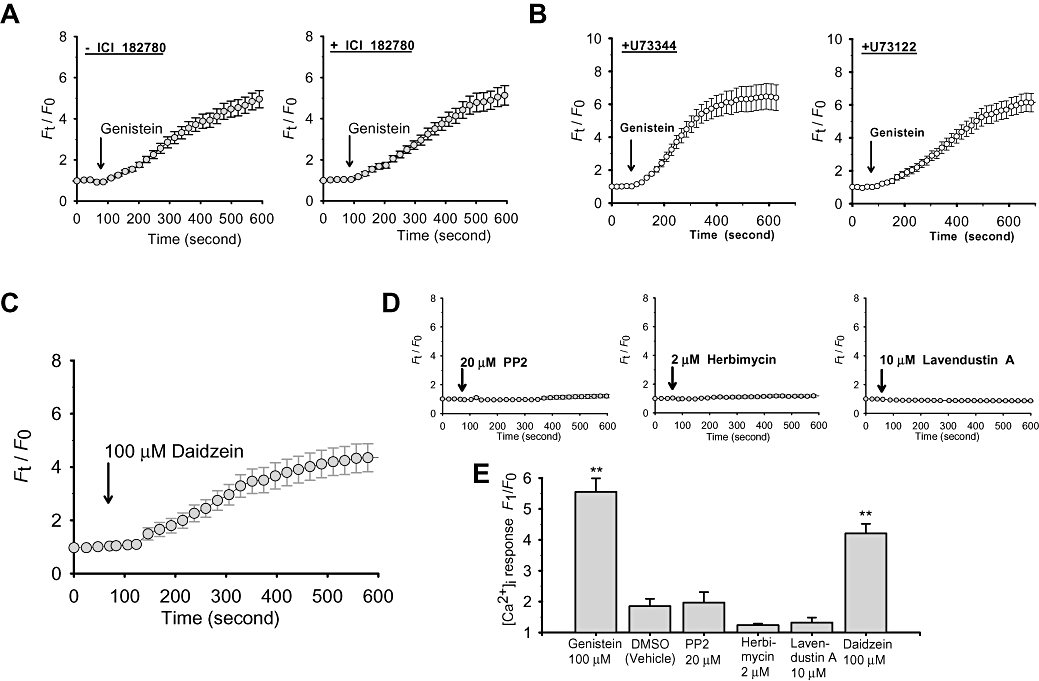
Effect of ICI-182780, U73122, a panel of tyrosine kinase inhibitors and an inactive genistein analogue, daidzein, on TRPC5-mediated [Ca2+]i rise. (A and B) Representative time-course of [Ca2+]i change in response to 100 µM genistein in TRPC5-over-expressing HEK cells in the absence (A, left) or the presence (A, right) of 50 µM ICI-182780, or in the presence of 10 µM U73343 (B, left) or 10 µM U73122 (B, right). Cells were pretreated with ICI-182780 for 5 min, or with U73122 or U73343 for 30 min. (C and D) Representative time-course of [Ca2+]i change in response to 100 µM daidzein (C), 20 µM PP2 (D, left), 2 µM herbimycin (D, middle) or 10 µM lavendustin A (D, right) in TRPC5-over-expressing HEK cells. Each trace in A–D represents mean ± SEM of at least 20 cells from one representative experiment (n= 3–8). Cells were bathed in NPSS in the presence of 100 µM LaCl3. (E) Summary of data showing the maximal [Ca2+]i rise as in A–D. Mean ± SEM (n= 3–8). **P < 0.01, compared to vehicle.
Effect of tyrosine kinase inhibitors and daidzein on TRPC5-mediated rise in [Ca2+]i
To determine if the effect of genistein was related to its inhibitory action on tyrosine kinases, a panel of other tyrosine kinase inhibitors was tested. These include a wide-spectrum tyrosine kinase inhibitor (herbimycin), an src family tyrosine kinase inhibitor (PP2) and an inhibitor for epidermal growth factor receptor tyrosine kinase (lavendustin A). Unlike genistein, herbimycin (2 µM), PP2 (20 µM) and lavendustin A (10 µM) all failed to stimulate TRPC5 channels (Figure 5D and E). In contrast, daidzein, which is a genistein analogue not active as an inhibitor of tyrosine kinases, was capable of stimulating TRPC5 channels (Figure 5C and E).
Effect of daidzein on TRPC5 channel whole-cell currents
In agreement with the [Ca2+]i measurement experiments, treatment of TRPC5-over-expressing HEK cells with daidzein (50 µM) increased the whole-cell currents at +60 and −60 mV (Figure 6A–C). Similar to the genistein-stimulated currents (Figure 1B), daidzein-stimulated currents also peaked at around 5 min (Figure 6A). Current–voltage relationship of the daidzein-stimulated currents displays double rectification (Figure 6C), a characteristic of TRPC5 channels. These results are consistent with the notion that genistein potentiates TRPC5 channel activity, independently of tyrosine kinases.
Figure 6.
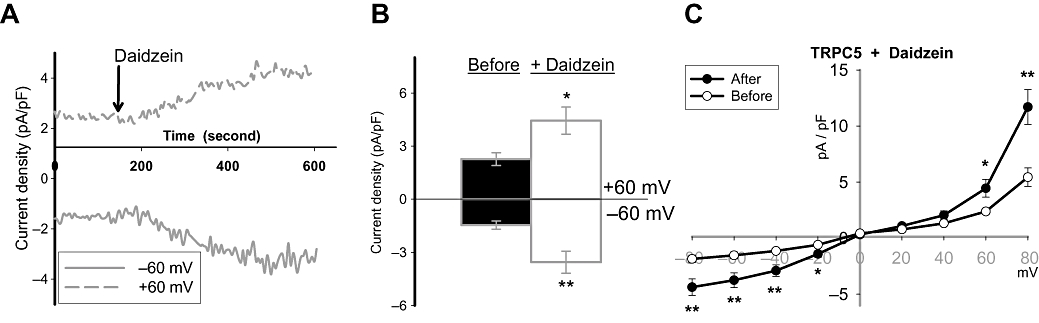
Effect of daidzein on whole-cell currents in TRPC5-over-expressing HEK cells. (A) Representative time-course of whole-cell currents. Currents were sampled every 5 s at +60 and −60 mV. Daidzein (50 µM) was applied to the bath at the time-point indicated by arrow. (B) Summary of maximal whole-cell currents before (before) and after daidzein (+daidzein) addition at +60 and −60 mV. Mean ± SEM (n= 7). (C) Current–voltage relationships of whole-cell currents immediately before and 5 min after bath application of 50 µM daidzein. Voltage protocol is the same as in Figure 1E. Mean ± SEM (n= 7). *P < 0.05; **P < 0.01 compared to ‘before’.
Effect of genistein on TRPC5 channels in native endothelial cells
TRPC5 is known to be expressed in primary cultures of BAECs (Yao and Garland, 2005). This was confirmed in the present study by Western blot and immunochemical staining of cultured BAECs using a TRPC5-specific antibody (Alomone Laboratory, 1:100) (Figures 1A and 7A). In functional studies, bath application of vehicle (DMSO, 0.1%) and 1 µM genistein did not increase [Ca2+]i (Figure 7B and C), but, at higher concentrations (25–200 µM), genistein did elevate [Ca2+]i in these cells (Figure 7B and C). This [Ca2+]i rise was mostly due to Ca2+ influx because it was almost absent in the cells that were bathed in a Ca2+-free medium (Figure 7E). As the [Ca2+]i response was not further enhanced when the genistein concentration was increased from 100 to 200 µM (Figure 7C), 100 µM was chosen as the standard concentration for further study of the effects of genistein on TRPC5 channels in BAECs (Figure 7D and E). As shown, 100 µM genistein induced a [Ca2+]i rise in BAECs pre-incubated with the pre-immune IgG (Figure 7D and E). In contrast, this genistein-induced [Ca2+]i rise was diminished in cells that had been pre-incubated with a TRPC5-specific blocking antibody T5E3 (Figure 7D and E), suggesting an involvement of TRPC5 channels. However, note that the [Ca2+]i response to genistein was rather small, it is probably due to a relatively lower expression of TRPC5 channels in BAECs compared to that in TRPC5-over-expressing HEK cells.
Figure 7.
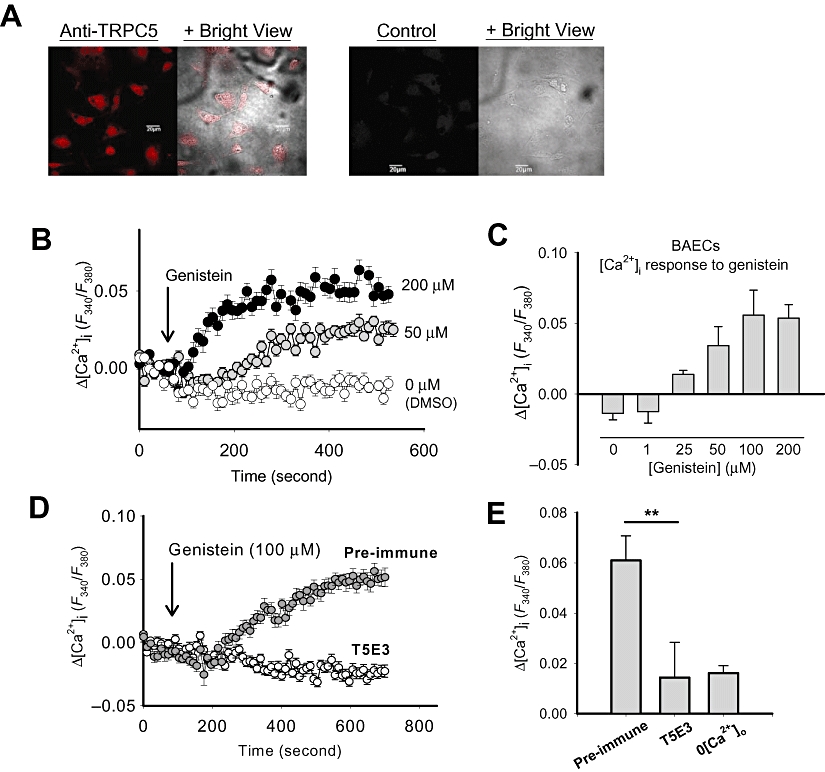
Effect of genistein on TRPC5-mediated [Ca2+]i rise in native vascular endothelial cells. (A) Immunocytochemical detection of TRPC5 proteins in BAECs using confocal microscopy. Shown were the paired images of TRPC5 fluorescent signal (red, left panel) and the BrightView image merged with fluorescent signal. The control images (right panel) were from samples without addition of primary antibodies. Scale bar represents 20 µm. (B and D) Representative time-course of [Ca2+]i change in response to vehicle (0.1% DMSO) or genistein in BAECs. (D) Pre-incubated with pre-immune IgG (4 µg·mL−1) or T5E3 (antibody to TRPC5 channels; 4 µg·mL−1) for 10 min. Each trace represents mean ± SEM of at least 15 cells from one representative experiment (n= 6–12). (C) Summary of data showing the maximal [Ca2+]i changes in response to 0.1% DMSO or various concentrations of genistein. Mean ± SEM (n= 6–12). (E) Summary of data showing [Ca2+]i changes 10 min after genistein application in the presence of pre-immune IgG (4 µg·mL−1) or T5E3 (4 µg·mL−1) or Ca2+-free HBS (0[Ca2+]o). Mean ± SEM (n= 3–8). **P < 0.01.
Discussion and conclusions
In the present study, we found that genistein augmented TRPC5-mediated Ca2+ influx in TRPC5-over-expressing HEK cells and primary cultures of BAECs. Genistein also enhanced TRPC5-mediated cation current. The stimulating effect of genistein synergized with La3+, which is a well-documented potentiator for TRPC5 channels. The effect of genistein could not be mimicked by several other tyrosine kinase inhibitors, but it could be mimicked by daidzein, which is inactive as a tyrosine kinase inhibitor. This action of genistein was not mediated through oestrogen receptors or phospholipase C, because selective inhibitors against these targets had no effect. Importantly, the stimulating action of genistein on TRPC5 channels could be demonstrated in excised inside-out patches. Taken together, these data suggest that the action of genistein on TRPC5 channels is relatively direct and may not require a cytosolic factor.
Several different protocols were used to determine whether genistein could indeed enhance TRPC5 activity in HEK cells that heterologously expressed TRPC5 channels. In the first protocol, the TRPC5-over-expressing cells were treated with carbachol in a Ca2+-free medium, followed by extracellular Ca2+ addition, which initiated a Ca2+ influx. Genistein was then added. It was found that the genistein application elicited a further Ca2+ rise. In the second protocol, cells were pretreated with genistein in a Ca2+-free medium, followed by extracellular Ca2+ addition, which elicited a [Ca2+]i rise. It was found that the magnitude of this [Ca2+]i rise was much larger in genistein-pretreated cells than those without genistein pretreatment. In the third protocol, direct addition of genistein also caused a [Ca2+]i rise in cells bathed in a Ca2+-containing physiological solution. Furthermore, in patch clamp studies, genistein stimulated TRPC5-mediated cation current in whole-cell patches and in excised inside-out patches. These data confirmed a stimulating action of genistein on TRPC5 channel activity. Interestingly, we observed that La3+ and genistein act synergistically on TRPC5 channels. La3+ ions are known to interact with the negatively charged amino acid residues near the extracellular pore region of TRPC5 proteins, thereby potentiating the channel opening (Jung et al., 2003). In the present study, La3+ was able to potentiate the stimulating action of genistein on TRPC5 channels, and genistein was capable of potentiating the action of La3+ on TRPC5. The exact mechanism for the observed synergy is not clear. However, one possibility could be that La3+ (or genistein) elevates [Ca2+]i, which may in turn sensitize TRPC5, resulting in an enhanced response to genistein (or La3+). This interpretation is supported by a recent study which showed a potentiation by high [Ca2+]i on agonist-induced activation of TRPC5 (Blair et al., 2009).
Genistein is a phytoestrogen that can act on oestrogen receptors at nanomolar level (Escande et al., 2006). Activity of oestrogen receptors can alter ion homeostasis in a variety of tissues and cells including the CNS, vascular system and pancreatic endocrine cells (Kelly and Levin, 2001; Nadal et al., 2004; Raz et al., 2008). In the present study, an oestrogen receptor antagonist ICI-182780 had no effect on the stimulating action of genistein on TRPC5, suggesting that the action is independent of classical oestrogen receptors. Genistein is also commonly used as a tyrosine kinase inhibitor, over the concentration range of 10–100 µM (Barnes et al., 2000). Indeed, genistein was found to inhibit ATP-sensitive K+ channels, N-type Ca2+ channels, T-type channels, TRPC3 and TRPM2 channels, via a tyrosine kinase-dependent mechanism (Ogata et al., 1997; Morikawa et al., 1998; Kawasaki et al., 2006; Zhang et al., 2007). In the present study, we found that the genistein action could not be mimicked by other tyrosine kinase inhibitors including herbimycin, PP2 and lavendustin A. In addition, the action of genistein could be mimicked by daidzein, which is inactive as a tyrosine kinase inhibitor. Therefore, the genistein effect on TRPC5 channels was independent of tyrosine kinases. It has been reported that the membrane-anchored phospholipase C is critical to the receptor-operated TRPC5 activation (Schaefer et al., 2000). However, in the present study, inhibition of phospholipase C by U73122 had no effect on the genistein action on TRPC5, suggesting that the genistein action on TRPC5 is not mediated through phospholipase C.
Evidence shows that genistein may inhibit multiple ion channels via a relatively direct mechanism. These include L-type Ca2+ channels (Chiang et al., 1996; Belevych et al., 2002), voltage-gated Na+ channels (Paillart et al., 1997), voltage-gated K+ channels (Smirnov and Aaronson, 1995) and inwardly rectifying K+ channels (Chiang et al., 2002). Unlike most of other studies, we found that genistein stimulates TRPC5 channel activity. Furthermore, the effect of genistein was demonstrated in excised inside-out patches, suggesting that the action is relatively direct and did not require a cytosolic factor. Unfortunately, the present study cannot differentiate whether genistein acts on TRPC5 channels intracellularly or extracellularly. The patch clamp study showed that the genistein applied from either side of the membrane was able to stimulate TRPC5 activity (Figures 1–3), but this was expected because genistein is a membrane-permeable agent (Akiyama et al., 1987). The detailed mechanism of activation of TRPC5 channels by genistein is still unclear, but it could be related to the alteration of lipid bilayer properties. Evidence shows that genistein can be adsorbed and partitioned onto the plasma membrane, thus altering elastic properties of lipid bilayer (Arora et al., 2000; Hwang et al., 2003; Whaley et al., 2006). Such an alteration in lipid environment may cause a change in gating properties of some ion channels. This model has been previously used to explain the genistein-induced activation of gramicidin A channels (Hwang et al., 2003), which are a group of short antibiotic peptides produced by Bacillus brevis. We speculate that a similar mechanism may underlie the stimulating action of genistein on TRPC5 channels. In agreement, daidzein, which only differs from genistein by having one less −OH and is also incorporated into lipid bilayers (Whaley et al., 2006), exerted a similar stimulatory action on TRPC5 channels. Interestingly, several reports have shown that TRPC5 channel activity could be modulated by phosphatidylinositol-4,5-bisphosphate (Trebak et al., 2009), lysophosphatidylcholine (Flemming et al., 2006) and sphingosine 1-phosphate (Beech, 2007a), all of which are capable of inserting into lipid bilayers. Indeed, the TRPC5 channel has been proposed as a sensor for endogenous phospholipids (Flemming et al., 2006; Beech, 2007a,b;). Therefore, it is likely that natural isoflavones, such as genistein and daidzein, may stimulate TRPC5 channels by altering the channel–lipid interactions. The present study is the first to demonstrate the ability of isoflavones to stimulate a TRP channel.
In conclusion, genistein stimulated the activity of TRPC5 channels. The action was neither related to tyrosine kinase inhibition nor to oestrogen receptors. Genistein and La3+ ions acted synergistically to stimulate TRPC5 channel activity. The stimulating action of genistein on TRPC5 was mimicked by daidzein, which can also be partitioned into lipid bilayers. Furthermore, the stimulation of TRPC5 channels could be recorded on excised inside-out patches. These data all suggest that the action of genistein was relatively direct. It is possible that genistein enhanced TRPC5 channel activity by modifying the channel–lipid interaction in the lipid bilayer.
Acknowledgments
This study was supported by Hong Kong RGC grants (CUHK477307, CUHK477408 and CUHK479109); Focused Investment Scheme of Chinese University of Hong Kong; and Li Ka Shing Institute of Health Sciences.
Glossary
Abbreviations:
- AM
acetoxymethyl ester
- 2-APB
2-aminoethoxydiphenyl borate
- BAEC
bovine aortic endothelial cell
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulphoxide
- FBS
fetal bovine serum
- G-418
geneticin
- HEK
human embryonic kidney
- IgG
immunoglobulin G
- OAG
1-oleoyl-2-acetyl-sn-glycerol
- pS
picoSiemens
- TRP
transient receptor potential
- TRPC5
canonical transient receptor potential isoform 5
Conflict of interest
None.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102–109. doi: 10.1006/abbi.1999.1525. [DOI] [PubMed] [Google Scholar]
- Barnes S, Boersma B, Patel R, Kirk M, Darley-Usmar VM, Kim H, et al. Isoflavonoids and chronic disease: mechanisms of action. Biofactors. 2000;12:209–215. doi: 10.1002/biof.5520120133. [DOI] [PubMed] [Google Scholar]
- Beech DJ. Bipolar phospholipid sensing by TRPC5 calcium channel. Biochem Soc Trans. 2007a;35(Pt 1):101–104. doi: 10.1042/BST0350101. [DOI] [PubMed] [Google Scholar]
- Beech DJ. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007b:109–123. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- Belevych AE, Warrier S, Harvey RD. Genistein inhibits cardiac L-type Ca(2+) channel activity by a tyrosine kinase-independent mechanism. Mol Pharmacol. 2002;62:554–565. doi: 10.1124/mol.62.3.554. [DOI] [PubMed] [Google Scholar]
- Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009;133:525–546. doi: 10.1085/jgp.200810153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- Cheng KT, Leung YK, Shen B, Kwok YC, Wong CO, Kwan HY, et al. CNGA2 channels mediate adenosine-induced Ca2+ influx in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:913–918. doi: 10.1161/ATVBAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- Chiang CE, Chen SA, Chang MS, Lin CI, Luk HN. Genistein directly inhibits L-type calcium currents but potentiates cAMP-dependent chloride currents in cardiomyocytes. Biochem Biophys Res Commun. 1996;223:598–603. doi: 10.1006/bbrc.1996.0941. [DOI] [PubMed] [Google Scholar]
- Chiang CE, Luk HN, Chen LL, Wang TM, Ding PY. Genistein inhibits the inward rectifying potassium current in guinea pig ventricular myocytes. J Biomed Sci. 2002;9:321–326. doi: 10.1007/BF02256587. [DOI] [PubMed] [Google Scholar]
- Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, et al. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta. Biochem Pharmacol. 2006;71:1459–1469. doi: 10.1016/j.bcp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Koeppe RE, 2nd, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci USA. 2006;103:335–340. doi: 10.1073/pnas.0508030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci USA. 2004;101:2625–2630. doi: 10.1073/pnas.0304471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Scholze A, Zhu Z, Krueger K, Thilo F, Burkert A, et al. Transient receptor potential channels in essential hypertension. J Hypertens. 2006;24:1105–1114. doi: 10.1097/01.hjh.0000226201.73065.14. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Fukuda K, Mima H, Shoda T, Kato S, Mori K. Tyrosine kinase inhibitors suppress N-type and T-type Ca2+ channel currents in NG108-15 cells. Pflugers Arch. 1998;436:127–132. doi: 10.1007/s004240050613. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Fuentes E, Soria B, Ripoll C. Estrogen and xenoestrogen actions on endocrine pancreas: from ion channel modulation to activation of nuclear function. Steroids. 2004;69:531–536. doi: 10.1016/j.steroids.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ogata R, Kitamura K, Ito Y, Nakano H. Inhibitory effects of genistein on ATP-sensitive K+ channels in rabbit portal vein smooth muscle. Br J Pharmacol. 1997;122:1395–1404. doi: 10.1038/sj.bjp.0701532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillart C, Carlier E, Guedin D, Dargent B, Couraud F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J Pharmacol Exp Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Shen B, Cheng KT, Leung YK, Kwok YC, Kwan HY, Wong CO, et al. Epinephrine-induced Ca2+ influx in vascular endothelial cells is mediated by CNGA2 channels. J Mol Cell Cardiol. 2008;45:437–445. doi: 10.1016/j.yjmcc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Si H, Liu D. Phytochemical genistein in the regulation of vascular function: new insights. Curr Med Chem. 2007;14:2581–2589. doi: 10.2174/092986707782023325. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ Res. 1995;76:310–316. doi: 10.1161/01.res.76.2.310. [DOI] [PubMed] [Google Scholar]
- Trebak M, Lemonnier L, Dehaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009;457:757–769. doi: 10.1007/s00424-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley WL, Rummel JD, Kastrapeli N. Interactions of genistein and related isoflavones with lipid micelles. Langmuir. 2006;22:7175–7184. doi: 10.1021/la0606502. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005a;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, et al. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005b;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol. 2006a;291:H2653–H2659. doi: 10.1152/ajpheart.00495.2006. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006b;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, et al. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559(Pt 3):739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Tong Q, Conrad K, Wozney J, Cheung JY, Miller BA. Regulation of TRP channel TRPM2 by the tyrosine phosphatase PTPL1. Am J Physiol Cell Physiol. 2007;292:C1746–C1758. doi: 10.1152/ajpcell.00569.2006. [DOI] [PubMed] [Google Scholar]


