Abstract
At present, the ease of subdivision of scored tablets is estimated in vivo. In order to replace such in vivo testing and to develop a surrogate test which uses in vitro techniques, the association between physical parameters of scored tablets and their ease of subdivision was studied. The physical properties of 23 brands of scored tablets of which their ease of subdivision in vivo was known were established. Statistical modeling using a logistic regression model was used to fit the data and estimate the contribution of each physical parameter to the goodness of the fit. For scored oblong tablets, the critical parameters for their ease of subdivision are: diameter; diameter/width ratio; depth of score line and resistance to crushing. Criteria for each of these parameters were derived. All criteria need to be complied with to guarantee sufficient ease of subdivision of scored oblong tablets. For scored round tablets the critical parameters, in decreasing order of importance, for their ease of subdivision, are: resistance to crushing, diameter, score mark (one- or two-sided), and shape (flat or biconvex). A five-parameter predictive model was developed, showing excellent discrimination. For development, the proposed surrogate tests are sufficiently reliable. For release testing and stability studies, resistance to crushing of a scored tablet is a reliable predictor of its ease of subdivision.
Key words: break-mark, score line, statistical modeling, subdivision, tablet
INTRODUCTION
Tablets for oral administration are the most common pharmaceutical dosage form in the USA and many of these tablets bear score mark(s) (1). Patients split tablets for a variety of reasons. The most important advantage of score lines is that they provide for dose flexibility: the patient can easily adjust the dose in response to medication effects or to comply with the labeled dosage and administration instructions. Dose flexibility can be especially important for medications that show strongly patient-dependant effect levels, or have a narrow therapeutic index, such as warfarin or levothyroxin (2).
But tablet splitting has also become an important method to reduce health care costs, at least in the USA. Many manufacturers charge the same or similar prices for different strengths of tablets of the same medication, and hence it is possible to purchase high-strength tablets, split them and use them as relatively cheap low-strength tablets (3); this has led many healthcare plans to establish mandatory tablet splitting policies.
These developments gave rise to a growing interest in the performance of score lines. Many studies show large variations in the mass of the subdivided parts (2,4).
In April 2002, the European Pharmacopoeia (Ph.Eur.) changed the monograph “Tablets” and requirements were introduced on the mass distribution of the parts obtained from subdivided scored tablets (5). The adoption of this requirement was a milestone: for the first time a pharmacopoeial requirement on scored tablets was set. The US Pharmacopoeia (USP) is also invited to define requirements on scored tablets (6).
Uniformity of mass of subdivided scored tablets is an important quality attribute of tablet score lines. But another quality attribute is sufficient ease of subdivision along the score line. Patients may perceive bad functioning score lines as a quality defect, and it was reported that 39% of patients were in some way dissatisfied with the subdivision characteristics of their scored tablets (7). Subdivision performance of scored tablets cannot be interpreted as a purely technical quality attribute, as badly performing score lines may result in patients taking a double dose at once, or not taking their medication at all (8).
Previously, we proposed an in vivo test method for ease of subdivision,1 using a panel of healthy elderly with a mean age of ≥75 years and none of them younger than 65 years (9). As a criterion, we proposed that there should be at least 90% probability that not less than 80% of the elderly would be able to subdivide the scored tablet by hand. This test is non-invasive, mimics closely the real-life situation and takes less than a minute per volunteer. However, there are also drawbacks. Volunteers need to be recruited and the test requires endorsement by a medical-ethical committee. In addition, large panels are needed to achieve sufficient statistical power; panels of 25–50 volunteers may be needed, and it may not be known beforehand how many volunteers are needed. Therefore a mechanical test, correlating well with the in vivo test, is needed in tablet development (9). In this paper, we describe the possibility to predict the ease of subdivision of scored tablets from their physical parameters.
EXPERIMENTAL
Tablets
In the study, 28 different tablets were included: three oblong-scored tablets, used as the training set labeled OBLONG, 20 round-scored tablets, used as training set ROUND and five round-scored tablets used for the external validation of the ROUND model. All tablets had been obtained during a market surveillance study on scored tablets in which their in vivo ease of subdivision had been measured. The procedure and results of this market surveillance study have been described previously (10). The tablets, coded (A–Ħ) are shown in Table I.
Table I.
Properties of the Tablets Included in the Study. Definition of the Parameters: See “EXPERIMENTAL”, Figs. 1 and 2
| Code | Round or oblong | Scoreform | In vivo ease of subdivision | Score mark (one-sided = 0; two-sided = 1) | Shape (biconvex = 0; flat = 1) | Diameter (mm) | Thickness (mm) | Width of score line (mm) | Depth of score line (mm) | Width (mm) | Curvature (rad) | Resistance to crushing (N) | Mass (mg) | Use of tablet |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Round | Line | 0.652 | 0 | 1 | 9.0 | 3.9 | 0.7 | 0.4 | 43.3 | 297 | ROUND | ||
| B | Round | Line | 0.043 | 0 | 0 | 5.9 | 3.9 | 0.5 | 0.2 | 0.567 | 83.0 | 90 | ROUND | |
| C | Round | Line | 0.565 | 0 | 0 | 9.1 | 3.3 | 1.0 | 0.3 | 0.281 | 94.2 | 264 | ROUND | |
| D | Round | Line | 0.391 | 0 | 0 | 8.5 | 4.2 | 1.3 | 0.8 | 0.491 | 81.0 | 251 | ROUND | |
| E | Round | Line | 1.000 | 0 | 1 | 8.1 | 3.2 | 0.6 | 0.2 | 34.1 | 202 | ROUND | ||
| F | Round | Line | 0.696 | 0 | 1 | 8.0 | 3.0 | 0.8 | 0.3 | 41.4 | 199 | ROUND | ||
| H | Round | Line | 0.912 | 0 | 1 | 8.0 | 2.6 | 1.0 | 0.4 | 49 | 175 | EXT ROUND | ||
| I | Round | Cross | 0.478 | 0 | 1 | 7.1 | 2.8 | 0.5 | 0.2 | 45.4 | 142 | ROUND | ||
| J | Round | Line | 0.909 | 0 | 0 | 8.0 | 3.4 | 0.8 | 0.4 | 0.487 | 30.0 | 184 | ROUND | |
| K | Round | Line | 0.909 | 0 | 0 | 8.0 | 3.4 | 0.9 | 0.4 | 0.444 | 50 | 184 | EXT ROUND | |
| L | Oblong | Line | 1.000 | 1 | 0 | 16.1 | 4.1 | 1.8 | 1.4 | 5.1 | 110 | 301 | OBLONG | |
| M | Oblong | Line | 1.000 | 1 | 1 | 10.1 | 2.9 | 1.2 | 0.5 | 5.1 | 75 | 171 | OBLONG | |
| N | Oblong | Line | 1.000 | 0 | 0 | 11.7 | 3.9 | 1.0 | 0.6 | 6.3 | 300 | OBLONG | ||
| O | Round | Line | 1.000 | 0 | 1 | 10.0 | 2.8 | 0.8 | 0.3 | 61.3 | 261 | ROUND | ||
| P | Round | Cross | 0.955 | 0 | 0 | 6.8 | 2.5 | 0.5 | 0.4 | 0.361 | 26.7 | 100 | ROUND | |
| Q | Round | Line | 0.909 | 0 | 0 | 8.0 | 2.3 | 0.6 | 0.3 | 0.189 | 38.8 | 149 | ROUND | |
| R | Round | Line | 0.500 | 0 | 1 | 7.9 | 2.4 | 0.8 | 0.4 | 72.5 | 178 | ROUND | ||
| S | Round | Line | 0.955 | 0 | 0 | 9.8 | 3.9 | 1.4 | 0.6 | 0.344 | 48.1 | 302 | ROUND | |
| T | Round | Line | 0.571 | 0 | 1 | 6.0 | 2.6 | 1.0 | 0.2 | 32 | 100 | EXT ROUND | ||
| U | Round | Line | 1.000 | 0 | 1 | 9.1 | 3.0 | 0.8 | 0.4 | 32.4 | 256 | ROUND | ||
| V | Round | Line | 0.905 | 0 | 0 | 10.0 | 4.2 | 2.0 | 1.0 | 0.582 | 54 | 360 | EXT ROUND | |
| W | Round | Line | 0.524 | 0 | 1 | 13.1 | 4.5 | 0.9 | 0.4 | 150 | 667 | EXT ROUND | ||
| X | Round | Line | 0.095 | 0 | 0 | 8.1 | 3.8 | 0.7 | 0.2 | 0.703 | 126.3 | 201 | ROUND | |
| Y | Round | Line | 0.810 | 0 | 1 | 9.0 | 3.7 | 0.6 | 0.2 | 63.1 | 305 | ROUND | ||
| Z | Round | Cross | 0.476 | 1 | 1 | 11.0 | 4.6 | 1.5 | 0.5 | 206.0 | 479 | ROUND | ||
| Æ | Round | Line | 0.810 | 0 | 1 | 7.0 | 1.9 | 0.7 | 0.2 | 39.2 | 99 | ROUND | ||
| Ø | Round | Line | 0.143 | 0 | 1 | 7.0 | 3.0 | 0.7 | 0.3 | 83.2 | 151 | ROUND | ||
| Ħ | Round | Cross | 1.000 | 0 | 0 | 7.4 | 3.9 | 0.9 | 0.4 | 0.737 | 8.9 | 226 | ROUND |
In Vivo Ease of Subdivision
Data on the in vivo ease of subdivision were taken from the literature (9,10). In this study, elderly volunteers, both men and women, were recruited from a home for the elderly, their age ranging from 71–95 years and a mean age of 83 years. The tablets were presented to the volunteers in a random order and with the instruction to subdivide the tablet as if it was for their own medical use. Tablets bearing three- and fourfold breakmarks were only subdivided into two parts. Tablets were broken by hand or without the aid of knives or scissors.
The in vivo ease of subdivision of the 28 tablets is shown in Table I as the fraction of the volunteers that had been able to subdivide the tablet.
Physical Parameters
All Tablets
Mass (milligram) was determined with an analytical balance. The presence of a score line or a score cross was described by the parameter scoreform, a score line represented by the value 1 and a score cross by the value 0. The one- or two-sided presence of a score line was described by a score mark, one-sided = 0, two-sided = 1. A biconvex or flat surface of a tablet was described by shape, a biconvex tablet = 0, a flat tablet =1.
Oblong Tablets
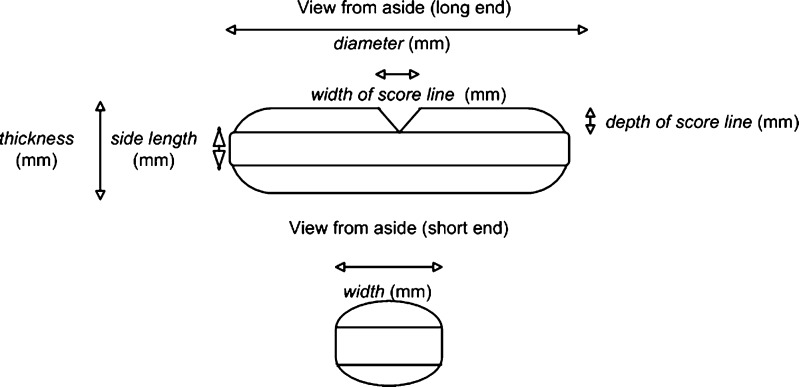
Diameter (millimeter), thickness (millimeter), width of score line (millimeter), depth of score line (millimeter), and width (millimeter) were measured with a sliding caliper, see Fig. 1. Resistance to crushing (N) was determined according to the Ph.Eur. 2.9.8.
Fig. 1.
Dimensions of oblong tablets
Round Tablets
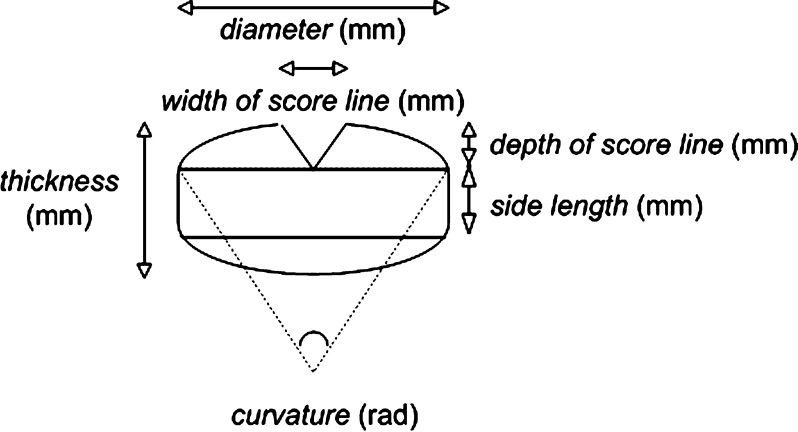
Diameter, thickness, width of score line, depth of score line, and side length (millimeter) were measured with a sliding caliper according to Fig. 2. The curvature of biconvex round tablets (rad) was calculated using computer program CIRCSECT (http://www.1728.com). The program requires segment height, which was calculated from (thickness—side length)/2.
Fig. 2.
Dimensions of round tablets
Resistance to crushing (N) of round tablets was determined according to the Ph.Eur. 2.9.8., but of tablets H, K, L, M, T, V, and W the number of samples available was insufficient. For these tablets, resistance to crushing values were obtained from external sources: of tablets H, T, and V from process validation results; of tablets K and M from stability data and of tablets L and W from their release specification.
The values obtained for the physical parameters of the tablets are also shown in Table I.
Modeling
Oblong Tablets
Tablets L, M, and N of the training set OBLONG all had an in vivo ease of subdivision of 1.000, see Table I. We concluded that the extreme values of the critical parameters of the tablets in OBLONG are the boundaries of a design space defining ease of subdivision = 1.000. We expected these critical parameters to be: diameter, the quotient of diameter and width, depth of score line and resistance to crushing. The extreme values of the critical parameters in OBLONG are: diameter: 10.1 mm (tablet M); diameter/width: 1.86 (tablet N); depth of score line: 0.5 mm (tablet M) and resistance to crushing: 110 N (tablet L), see Table I. Acceptance criteria were set on the conservative side of these extreme values: diameter: not less than (NLT) 10 mm; diameter/width: NLT 2.0; depth of score line: NLT 0.5 mm and resistance to crushing: not more than (NMT) 110 N. These are AND criteria, all these criteria need to be complied with. If all criteria are met, an ease of subdivision of NLT 0.800 is considered to be assured. No internal or external validation was performed.
Round Tablets
20 tablets, in Table I identified as ROUND, were selected as training set for construction of the statistical model for round scored tablets.
Nine parameters were identified as potential predictors for ease of subdivision: scoreform, score mark, shape, curvature, diameter, thickness, width of score line, depth of score line, resistance to crushing, and mass. A logistic regression model was fit to the data incorporating all identified parameters. Then a backward model selection based on the Bayesian Information Criterion (11) was performed in which one parameter at the time was dropped from the model as long as not all remaining parameters had a significant contribution to the ease of subdivision. For this parsimonious model all possible interactions and higher order terms were tested for a possible contribution to the ease of subdivision, resulting in the final model. All statistical analyses were conducted using the free software R (12). The Bayesian information criterion is measure for suitability of a model to the data. The model fit in terms of the log likehood is penalized by a factor based on the number of model parameters and the amount of observed data.
This final model was internally validated using bootstrap techniques. The bootstrap is a resampling technique for obtaining estimates and precisions thereof, without making assumptions about the distribution giving rise to the data (13). The internal validation resulted in a shrinkage factor of 0.912 for the coefficients of the model and an addition of 0.06 for the intercept of the model. These correction factors are close to 1 and 0, respectively, indicating that the model is fairly robust and only small corrections were needed.
The final model for easy of subdivision predicted includes only five parameters: resistance to crushing, diameter, score mark, thickness, and shape:
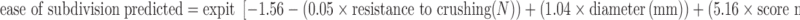 |
The other postulated parameters were dropped during the construction of the model.
A measure to indicate the discriminative properties of the final model is the c-index, or area under the curve. The c-index of the model was 0.861, indicating very good discrimination (14).
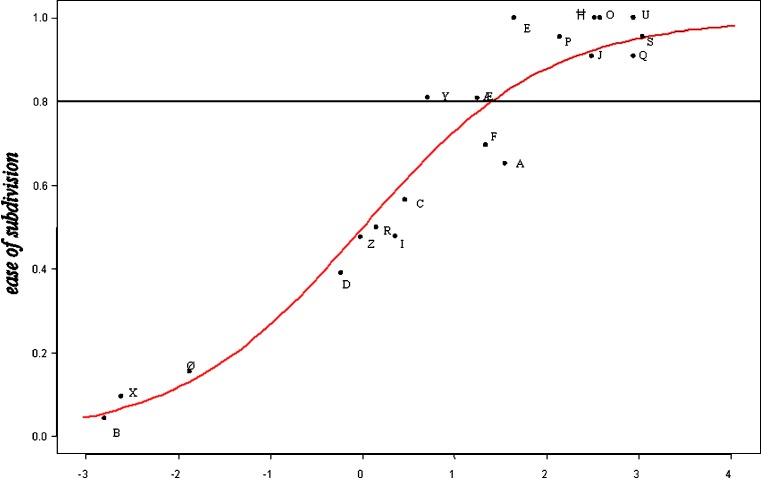
Figure 3 shows the model and the scatter of the training set around the model and Table II shows these differences in a numerical format. The contribution of each of the five parameters in the model was estimated, the result of this sensitivity analysis is shown in Table III.
Fig. 3.
Model and scatter of the tablets of training set ROUND. Horizontal axis: the sum of: −1.56 − (0.05 × resistance to crushing) + (1.04× diameter) + (5.16 × score mark) − (0.82 × thickness) − (0.90 × shape). Also shown: horizontal line ease of subdivision = 0.800, being the cut-off value for an acceptable ease of subdivision
Table II.
Differences between the Statistical Model ROUND and the Tablets Included in the Training Set
| Tablet | In vivo ease of subdivision | Ease of subdivision predicted | Difference |
|---|---|---|---|
| A | 0.652 | 0.824 | −0.172 |
| B | 0.043 | 0.057 | −0.014 |
| C | 0.565 | 0.612 | −0.047 |
| D | 0.391 | 0.441 | −0.050 |
| E | 1.000 | 0.838 | 0.162 |
| F | 0.696 | 0.791 | −0.095 |
| I | 0.478 | 0.587 | −0.109 |
| J | 0.909 | 0.923 | −0.014 |
| O | 1.000 | 0.929 | 0.071 |
| P | 0.955 | 0.894 | 0.060 |
| Q | 0.909 | 0.923 | −0.014 |
| R | 0.500 | 0.535 | −0.035 |
| S | 0.955 | 0.954 | 0.000 |
| U | 1.000 | 0.950 | 0.050 |
| X | 0.095 | 0.068 | 0.027 |
| Y | 0.810 | 0.669 | 0.141 |
| Z | 0.476 | 0.493 | −0.017 |
| Æ | 0.810 | 0.786 | 0.024 |
| Ø | 0.143 | 0.138 | 0.005 |
| Ħ | 1.000 | 0.925 | 0.075 |
Table III.
Sensitivity Analysis of the Parameters of the ROUND Model
| Parameter | Statistical significance to the ROUND model (p value)a |
|---|---|
| Resistance to crushing | 0 |
| Diameter | 1.23e-09 |
| Score mark | 5.90e-07 |
| Thickness | 1.65e-03 |
| Shape | 5.37e-03 |
aThe range of theoretically possible p values is 0–1; the closer the p value of a parameter is to 0, the more important its contribution
External Validation of the Statistical Model for Round Scored Tablets
An external validation of the statistical model for round scored tablets was performed with five tablets not used in the development of the model: tablets H, K, T, V, and W, see Table I.
For these tablets, ease of subdivision predicted was calculated from the formula shown above3 and compared with the in vivo ease of subdivision. The outcomes for ease of subdivision predicted and in vivo ease of subdivision were also interpreted in terms of compliance with the requirement: ease of subdivision NLT 0.800 and the outcome ranked as either correct, i.e. ease of subdivision predicted leading to the same outcome as in vivo ease of subdivision, or false: ease of subdivision predicted leading to a different outcome as in vivo ease of subdivision. The results of the external validation are shown in Table IV.
Table IV.
Results of the External Validation of the Statistical Model ROUND to Predict Ease of Subdivision of Round-Scored Tablets
| Tablet | In vivo ease of subdivision | Compliance with requirement | Ease of subdivision predicted | Compliance with requirement | Prediction of compliance |
|---|---|---|---|---|---|
| H | 0.912 | Complies | 0.782 | Out of spec | False |
| K | 0.813 | Complies | 0.878 | Complies | Correct |
| T | 0.571 | Out of spec | 0.512 | Out of spec | Correct |
| V | 0.905 | Complies | 0.937 | Complies | Correct |
| W | 0.524 | Out of spec | 0.494 | Out of spec | Correct |
Also shown: correctness of prediction of the model in terms of compliance with the requirement: ease of subdivision NLT 0.800
RESULTS AND DISCUSSION
Oblong Tablets
The training set OBLONG contained only tablets showing an in vivo ease of subdivision = 1.000, being the highest score possible. This suggests that oblong tablets with values for the critical parameters that are within the design space will always subdivide very easily. Although this is a very small training set, this conclusion is supported by other observations on the ease of breakability of oblong tablets (15).
Crucial parameters for the ease of subdivision of oblong tablets can be expected to be: diameter, depth of score line, diameter/width, and resistance to crushing (2). The first three parameters must not be too small to make an oblong tablet sufficiently breakable (2). In OBLONG, tablet M has the smallest diameter (10.1 mm); tablet M the smallest depth of score line (0.5 mm); tablet N the lowest diameter/width quotient (1.86). The resistance to crushing of a tablet must not be too high to make it sufficiently breakable (2). In OBLONG, tablet L shows the highest resistance to crushing (110 N). These four values mark the boundaries of the design space for an ease of subdivision of 1.000 of oblong scored tablets.
We defined acceptance criteria inside this design space; in this way, these acceptance criteria were set conservatively. Acceptance criteria defined were: diameter NLT 10 mm AND diameter/width NLT 2.0; AND depth of score line NLT 0.5; AND resistance to crushing NMT 100. When these four criteria all are met, we expect, with a probability of more than 90%, that at least 80% of elderly will be able to subdivide the oblong scored tablet by hand (9); note that the boundaries of the space design were derived from situations having an ease of subdivision = 1.000, whereas the acceptance criteria correspond with an ease of subdivision = 0.800, being an easier criterion to comply with.
Round Tablets
The c-index of the final model, i.e., c = 0.861, indicates excellent discrimination, according to the criteria: c-index ≥0.8: excellent discrimination; c-index ≥ 0.9: outstanding discrimination (14).
Also, the results of the external validation are satisfactory, as appears from Table IV. For four out of the five tablets ease of subdivision predicted and in vivo ease of subdivision led to the same outcome. Only tablet H, by its in vivo ease of subdivision being acceptable, would not be accepted on its ease of subdivision predicted. However, this is a false negative, i.e., a producer's risk, not a patient's risk.
Limitations of the Study
Taking a conservative approach, we conclude that the two models yet cannot be considered as sufficiently reliable to replace in vivo testing in all situations, but for product development the models are sufficiently reliable.
In the release testing situation, the formulation and the dimensions of the tablet are known, hence, four of the five parameters present in the formula for ease of subdivision predicted have become invariant: diameter, score mark, thickness, and shape. The only parameter that can change from batch-to-batch and may vary during storage is resistance to crushing. So, for a tablet with known dimensions, limits for resistance to crushing can be calculated that guarantee an acceptable ease of subdivision. For instance, tablet J, having a resistance to crushing of 30.0 N, has an acceptable In vivo ease of subdivision of 0.909, nicely corresponding with an ease of subdivision predicted of 0.923. Substituting different values for the resistance to crushing learns that the ease of subdivision predicted of tablet J will not drop below 0.800 when the resistance to crushing does not exceed 52 N. Taking a conservative approach, setting for tablet J a limit for resistance to crushing of NMT 50 N can replace testing in vivo ease of subdivision at release and in the stability study.
Conclusion
Oblong scored tablets can be regarded as sufficiently breakable when their diameter is NLT 10 mm; AND their diameter/width ratio is NLT 2.0; AND their depth of score line is NLT 0.5 mm; AND their resistance to crushing is NMT 100 N.
The ease of subdivision of round-scored tablets can be predicted from the developed model, which is sufficiently reliable to be used in product development. Once the dimensions of a tablet have been fixed and its in vivo ease of subdivision established, resistance to crushing is a sufficiently reliable predictor for ease of subdivision and can replace in vivo testing at release and in the stability study.
However, prediction of ease of subdivision of scored tablets from their physical parameters is a surrogate technique and in vivo testing remains the gold standard for that parameter in any case of doubt or dispute.
Acknowledgments
P. Salomons and H. Vogelpoel, both RIVM, are acknowledged for suggesting this study and for suggesting to calculate the specifications for crushing strength from the ease of breaking algorithm, respectively. K. Zuurman, University Groningen, is acknowledged for providing the experimental crushing strengths data.
Footnotes
In this paper, we use the expression: in vivo ease of subdivision, for values actually measured by testing by elderly. Predicted or calculated ease of subdivision is indicated by the expression: ease of subdivision predicted. The expression: ease of subdivision, without an addition, indicates the attribute without specifying the method used to estimate the value of this attribute. Italics indicate attributes of tablets defined in “EXPERIMENTAL”.
Expit is an algebraic expression defined as: 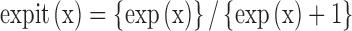 . A demonstration of the use of this formula is given in a footnote in the section External validation of the statistical model for round scored tablets.
. A demonstration of the use of this formula is given in a footnote in the section External validation of the statistical model for round scored tablets.
Example for tablet H: 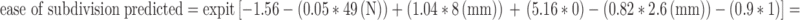 . From this formula is calculated: ease of subdivision predicted = 0.782.
. From this formula is calculated: ease of subdivision predicted = 0.782.
References
- 1.Physicians' Desk Reference. 62nd ed. Montvale, NJ: Thomson PDR; 2008
- 2.van Santen E, Barends DM, Frijlink HW. Breaking of scored tablets: a review. Eur J Pharm Biopharm. 2002;53:139–145. doi: 10.1016/S0939-6411(01)00228-4. [DOI] [PubMed] [Google Scholar]
- 3.Fawell NG, Cookson TL, Scranton SS. Relationship between tablet splitting and compliance, drug acquisition cost, and patient acceptance. Am J Health Syst Pharm. 1999;56(24):2542–2545. doi: 10.1093/ajhp/56.24.2542. [DOI] [PubMed] [Google Scholar]
- 4.Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J Manag Care Pharm. 2009;15(3):253–261. doi: 10.18553/jmcp.2009.15.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Pharmacopoeia Suppl. 4.1. 2002. Monograph 0478: Tablets. Council of Europe; European Directorate for the Quality of Medicine, Strasbourg, France.
- 6.Berg C, Green G, Polli JE, Barends DM. Pharmacopeial standards for the subdivision characteristics of scored tablets. Pharm Forum. 2009;35:1598–1612.
- 7.Rodenhuis N, de Smet PAGM, Barends DM. Patient's experience of the performance of tablet's score lines needed for dosing. Pharm World Sci. 2003;25:173–176. doi: 10.1023/A:1024852529628. [DOI] [PubMed] [Google Scholar]
- 8.Rodenhuis N, de Smet PAGM, Barends DM. The rationale of scored tablets as dosage form. Eur J Pharm Sci. 2004;21:305–308. doi: 10.1016/j.ejps.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Barends DM, Groot DW, Frijlink HW, Rodenhuis N, van der Steen JC. Development of an in vivo test procedure for the ease of breaking of scored tablets. Pharmeur Sci Notes. 2005;1:27–30. [PubMed] [Google Scholar]
- 10.Barends DM, Groot DW, van der Steen JC, de Kaste D, Frijlink HW. Results of a market surveillance study in The Netherlands on break-mark tablets. Pharmeur Sci Notes. 2006;2:1–7. [PubMed] [Google Scholar]
- 11.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 12.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. URL http://www.R-project.org. We used version 2.9.0.
- 13.Harrell FE., Jr . Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- 14.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 2000. [Google Scholar]
- 15.Spang R. Teilbarkeit von tabletten und filmdragees. Pharm Acta Hel. 1982;57:99–111. [PubMed] [Google Scholar]