Abstract
Membrane technology is broadly applied in the medical field. The ability of membranous systems to effectively control the movement of chemical entities is pivotal to their significant potential for use in both drug delivery and surgical/medical applications. An alteration in the physical properties of a polymer in response to a change in environmental conditions is a behavior that can be utilized to prepare ‘smart’ drug delivery systems. Stimuli-responsive or ‘smart’ polymers are polymers that upon exposure to small changes in the environment undergo rapid changes in their microstructure. A stimulus, such as a change in pH or temperature, thus serves as a trigger for the release of drug from membranous drug delivery systems that are formulated from stimuli-responsive polymers. This article has sought to review the use of stimuli-responsive polymers that have found application in membranous drug delivery systems. Polymers responsive to pH and temperature have been extensively addressed in this review since they are considered the most important stimuli that may be exploited for use in drug delivery, and biomedical applications such as in tissue engineering. In addition, dual-responsive and glucose-responsive membranes have been also addressed as membranes responsive to diverse stimuli.
Key words: Dual-responsive membranes, Glucose-responsive membranes, Membranous drug delivery systems, “On–off” gating mechanisms, pH, Stimuli-responsive polymers, Temperature
Introduction
The membrane industry is a rapidly growing field of research that has found extensive application in industry and medical fields (1,2). Industrially, membranes have found application in ultra-filtration, microfiltration, reverse osmosis, gas separation, pre-evaporation, and electro-dialysis, while medically, applications include incorporation into artificial organs, hemodialysis, drug delivery systems, diagnostics, coatings for medical devices, tissue regeneration, and biosensors among others (1,2). Membrane technology stemmed from use as laboratory tools in the early twentieth century to microporous collodion membranes in the early 1930s. Eventually, ultra-filtration and microfiltration membranes where developed, which are extensively being used to date in the process of water purification (1). Following this development was the advancement of membranes for medical applications and drug delivery.
The earliest application of membrane systems in drug delivery was reflected in the work of Australian physiologists Rose and Nelson (3), who developed an oral osmotic drug delivery device that was used to deliver medication to the gut of sheep and cattle. This drug delivery device ensured a constant or ‘zero-order’ drug release rate over a prolonged period. This technology was further improved and simplified by the Alza Corporation (Mountain View, California, USA) to produce the Higuchi-Theeuwes pumps. These pumps overcame many of the challenges such as the short shelf-life and the need for immediate administration to the body associated with the Rose-Nelson device (3). Eventually, the Alza Corporation successfully managed to deliver drugs transdermally by developing an adhesive patch containing scopolamine, with a zero-order release rate, for the treatment of motion sickness (4). Adalat CR® (controlled release nifedipine) is the most successful product developed by the Alza Corporation based on the OROS® (osmotic-controlled release oral delivery system) technology. This later led to the development of various prolonged release delivery systems for many other drugs such as Acutrim® (phenylpropanololamine), Minipress XL® (prazosin), and Volmax® (salbutamol) (3). The potential for membrane technology in the medical industry supersedes all its other applications due to its versatility in medical applications (2).
Membranes have been vastly utilized in the medical and pharmaceutical fields, in particular drug delivery, due to their ability to control the permeation rate of chemical substances (2). Synthetic functional polymers have witnessed much advancement in the last two decades, and said polymers are capable of responding in a desirable manner to a change in temperature, pH, and electric or magnetic fields (5). These polymers are often referred to as stimuli-responsive materials. Other names include smart, intelligent, or environmentally sensitive/responsive polymers. Recent studies have aimed at formulating ‘smart’ or stimuli-responsive materials that are able to offer altered physicomechanical properties under differing environmental conditions in order to influence or control the polymeric properties (6). Polymers with physical and chemical properties that can be manipulated by the surrounding environment, such as hydrogels, have potential applications in surgical implants, scaffolds for tissue engineering, supports for in vitro cell culture and biotechnological screening, as well as in drug delivery systems (2). In drug delivery, they may be used to target drugs to specific sites in the body or as a ‘smart’ surface which can be switched from an adhesive to a non-adhesive state or from a hydrophilic to a hydrophobic state and thus can swell or shrink. The changes are reversible which implies that the polymer is capable of returning to its initial state as soon as the trigger is removed (7).
This review therefore addresses the usefulness of stimuli-responsive polymers in the development of membranous drug delivery systems and the application of the latter in the medical, biomedical, and pharmaceutical fields. Membranous drug delivery systems engineered from polymers that exhibit a desired response to external signals such as pH, temperature, electric fields, magnetic fields, ionic strength, ultrasonic, and light radiation are discussed. In addition, dual-responsive and glucose-responsive membranes, block copolymers, and various geometrical and chemical architectures, such as polymer brushes and nanoparticles, are also concisely described.
METHODS EMPLOYED FOR THE FABRICATION OF POLYMERIC MEMBRANES
Various methods have been employed in the preparation of polymeric membranes. The type of the polymer and method employed, as well as the conditions during formulation determines the final morphology of the membranes. Phase separation, electrospinning, foaming, particle leaching, emulsion freeze-drying, and sintering are some of the common approaches that have been used for the production of membranes (8).
Phase Separation
Phase separation is a technique of membrane fabrication that has been widely applied in the production of scaffold membranes typically used in tissue engineering (9). This fabrication method may be applied to pure polymers as well as polymeric composites (2,9). The technique involves the formation of an initial homogenous polymeric solution that becomes thermodynamically unstable upon exposure to an external stimulus. Upon reaching thermodynamic instability, the polymeric solution divides into two phases, a high and low polymer concentration phase. The concentrated phase then solidifies after phase separation, resulting in the formation of a polymeric membrane (2). The resultant membrane often has pores that become occupied by the less concentrated polymer phase. Studies have indicated that phase separation can be induced by a variety of external effects. These effects have led to the development of four techniques based on the concept of phase separation. The key difference between each technique is the desolvation ability (8).
Vapor-Induced Phase Separation
Vapor-induction is a phase-separation technique that involves the penetration of non-solvent vapor into the polymeric solution. It is a dry–wet casting process where the vapor of the non-solvent (usually water) allows for the slow transfer of non-solvent molecules into the polymeric solution. The polymeric membrane then forms upon exposure of the polymeric solution to the non-solvent vapor due to thermodynamic instability (9). Vapor-induced phase separation (VIPS) is frequently used in the production of asymmetric membranes, comprising of a dense skin layer and a porous sub-layer. These membranes are typically applied in the separation of gaseous and liquid mixtures. Membranes produced via VIPS include polycarbonate/polyacrylonitrile composite membranes (10), cellulose nitrate membranes commonly used in immunological and biochemical assays (9) and poly(vinylidene fluoride) (PVDF) dimethylformamide (DMF) porous membranes (11).
Thermal-Induced Phase Separation
Thermal-induced phase separation (TIPS) is applicable to polymers that cannot form membranes through other phase separation methods due to inadequate solubility. The effects of temperature changes on the solvent, used to initially dissolve the polymer, leads to the formation of the polymeric membrane (12). A polymer is initially mixed at high temperatures to form a homogenous solution. The hot polymeric solution is then cast onto a cold surface of desired dimensions. A decrease in the surrounding temperature causes a decrease in the quality of the solvent used. As a result, phase separation of the polymeric solution occurs, ultimately forming a microporous membrane (12,13). Porous films of isotropic, anisotropic, or asymmetric membrane structures can be formed, with high porosity percentages, using the TIPS method. Other methods of phase separation involve a multi-component mass transfer reaction for phase separation to occur. In the TIPS method, heat transfer primarily induces phase separation, and therefore this method can be adapted to a wider range of membrane applications (13). Examples of membranes produced via the TIPS method include crystalline poly(ethylene-co-vinyl alcohol) membranes applied in blood purification methods such as hemodialysis and high-density polyethylene hollow fiber membranes (14).
Immersion Precipitation
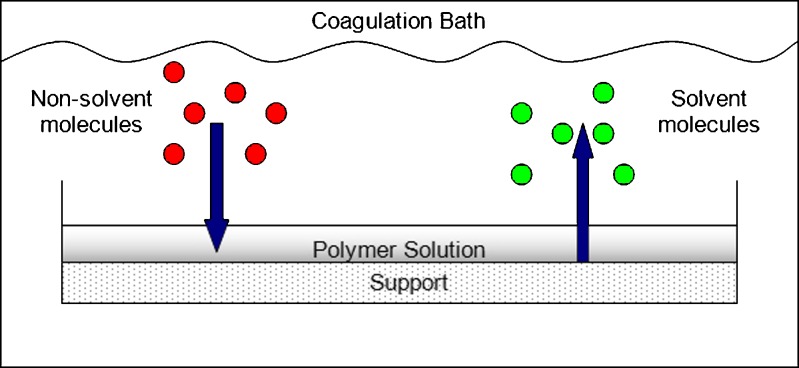
This method involves the casting of a polymeric solution into a mold or support as a thin film. The thin film-like polymeric solution is then immersed into a non-solvent-containing bath that eventually leads to the precipitation of the polymeric solution. This occurs through a series of liquid–solid and/or liquid–liquid phase separation events. Solvent molecules from the polymeric solution are replaced by the non-solvent molecules, as illustrated in Fig. 1, resulting in the formation of a porous polymeric membrane that is subsequently dried to remove the liquid phase (15,16). Membranes produced via this method are widely applied in microfiltration/ultra-filtration and include symmetric and asymmetric poly(vinylidene fluoride) microporous membranes (16) and membranes composed of self-organizing blends of poly(vinylidene fluoride) and an amphiphilic ‘comb-like’ polymer having a PVDF backbone and poly(methacrylic acid) side chains (15).
Fig. 1.
Schematic illustration of the mass transfer of solvent and non-solvent occurring during immersion precipitation (adapted from: Pereira et al. (17))
Film/Dry Casting Technique
Film or dry casting essentially involves dissolving of the polymer in a volatile solvent and a less volatile non-solvent. The polymer becomes less soluble in the non-solvent as the solvent evaporates, which eventually culminates into phase separation that results in the formation of a polymeric membrane (8). Poly(ethylene-co-vinyl alcohol) (EVAL) and poly(acrylic acid) (PAA) blends were dry cast to produce a pH-responsive membrane for the colonic delivery of 5-fluorouracil (18) and ammonio methacrylate copolymer membranes were dry cast and used as pH-responsive coatings to control the release of drug molecules (19). The primary disadvantage of phase separation is that it involves the use of organic solvents in the formulation and development of membrane systems. Incomplete removal of these solvents may result in toxicity when these membrane systems are used in biomedical and pharmaceutical applications (19). The removal of these organic solvents from these membranes is thus necessary; however, it is a costly exercise. Additionally, the formation of polymeric membranes using phase separation is a lengthy process with the solvents often being environmentally harmful. Furthermore, slight changes in the membrane fabrication process may significantly affect the final membrane morphology and hence drug release performance (8,19,20).
Electrospinning
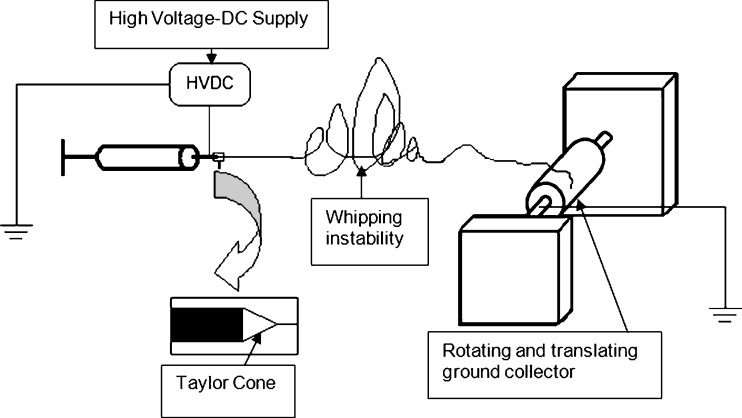
Electrospinning is based on charging a polymeric solution followed by ejecting the solution through a fine capillary tip or needle. The syringe pump forces the polymeric solution through a small-diameter capillary, thereby forming a pendant drop at the tip. A voltage is applied to the tip of the syringe in order to create a charge of certain polarity in the solution. The collecting surface is usually of opposite polarity. As a result of the applied electric field, a leading edge or Taylor cone that eventually forms a fiber jet is formed. The fiber jet then travels towards the collecting surface where solid fibers are deposited as the solvent evaporates. This ultimately leads to the formation of a membranous structure through the continuous deposition of polymeric fibers on the collecting surface. Figure 2 illustrates the polymer electrospinning process. For successful determination of the optimal parameters for the preparation of the membranous matrices, the distance of the needle from the collecting surface, the applied voltage as well as the solution flow rate must be carefully manipulated (21,22), and thus electrospun membranous matrices can be customized to achieve desired drug release patterns (21).
Fig. 2.
An overview of the electrospinning process (adapted from: Sill and von Recum (22))
Electrospun nanofibrous membranes have many promising applications in the pharmaceutical industry, including tissue engineering, drug delivery, and filtration. Thus far, several studies examined the potential of electrospun matrices for use as controlled drug delivery systems for the delivery of drugs such as antibiotics and anticancer drugs as well as for tissue engineering applications (22). Approximately 100 polymers have successfully been manipulated into electrospun nanofibres. The various polymers that are used to form membranes currently used in the medical industry are listed in Table I. The electrospun nanofibrous membranes are able to modulate drug delivery due to their three-dimensional porous structure that renders upon them a relatively large surface area thus making them valuable scaffolds in tissue engineering. A key advantage of utilizing electrospinning in the formation of membrane systems is that the obtained membrane has high flexibility and good mechanical properties. Furthermore, the electrospun fibers can be aligned and functionalized to induce tissue alignment. However, a drawback associated with this technique arises during fabrication of electrospun membranes, there is a possibility that fibers may break, leading to the formation of poor quality nanofibrous membranes (2,22).
Table I.
Polymers Employed in Electrospun0020drug Delivery Applications (Adapted from Sill and von Recum (22))
| Polymer | Solvents | Applications |
|---|---|---|
| PLGA, PEG-b-PLA, and PLA | DMF | Electrospun fibers for sustained drug delivery (Mefoxin® and Cefoxitin) with successful retention of the structure and bioactivity of the drug molecules |
| PCL | TFE | Drug delivery of BSA, core/shell nanofibres were prepared via coaxial spinning capable of higher protein loading |
| PEG | Water | |
| PCL+PEG | CHF+DMF | Drug delivery of BSA, core/shell nanofibres were prepared via coaxial spinning capable of higher protein loading and improved control over protein release with the addition of PEG |
| Dextran | Water | |
| (b) PCL-co-ethyl ethylene phosphate | DCM+PBS | Core/shell nanofibers were prepared via coaxial spinning for delivery of β-nerve growth factor and BSA, PEG addition improved control over protein release |
DCM dichloromethane, PBS phosphate buffered saline, DMF dimethyl formamide, PCL poly(ε-caprolactone), PLA poly(lactic acid), BSA bovine serum albumin, TFE 2,2,2-trifluoroethanol, PLGA poly(d,l-lactic-co-glycolic acid), PEG poly(ethylene glycol), CHF chloroform
Foaming
This method requires a soluble inert gas such as CO2 and N2. The process involves the saturation of the polymer with the gas at a high pressure. The polymer and gas mixture is subsequently quenched into a supersaturated state either by a reduction of the pressure or an increase of the temperature. The properties of the formed membranes may be varied by changing the foaming process conditions. This technique is applicable to pure polymers as well as polymer composites and ceramics. It does not require solvent use and thereby eliminates the risk of residues (9). However, the technique requires very high temperatures, which could lead to the degradation of the polymers employed and these membranes are not suitable for the application in tissue engineering due to their small pore size range (2,9,20). Porous membranes composed of cellulose acetate, polystyrene (11,14), and other polymers with high Tg values (20) have been formulated employing this approach.
Particle Leaching
Particle leaching is generally performed in combination with other methods of membrane formation methods such as foaming and film-casting. The process involves the incorporation of salts, sugars, or other specifically prepared spheres into a polymer sample. The polymer is then processed into a membrane using an appropriate method (e.g., foaming, film-casting etc.). The particles within the membrane are then dissolved and washed out to create additional pores in the membrane. This method ensures that membranes with highly controlled porosity and pore sizes are produced (23). However, a drawback associated with this method is that it may not be applicable to all materials (e.g., soluble protein scaffolds). Furthermore, the washing out post-process is time-consuming and there is a risk of residues remaining from the method of processing (i.e., organic solvents) (2). Thus, this method is preferred for polymers that are not readily soluble in common organic solvents (2,23). Porous membranes produced via this method include polyethylene membranes using tapioca starch as the leachable component (23) and 2,3-dialdehydecellulose membranes with sodium chloride (NaCl) used as the leachable component (24).
Emulsion Freeze-Drying
This method involves an emulsification process that is attained through homogenization of a polymer-solvent and water system in which the polymer-rich phase is the continuous phase while water is the dispersed phase. The produced emulsion is cooled down rapidly and frozen, a process that results in the direct solidification of the polymer from the liquid phase, thus creating a porous polymeric structure. Ultimately, the structure is freeze-dried in order to remove water and solvent. Studies indicated that this technique produces membranous scaffolds that are ideal for use in tissue engineering since the pores produced are large, and the membranes are relatively thicker than membranes formed through other methods (25). Furthermore, incorporating proteins during fabrication is possible. However, there is a possibility of pores forming which are not highly interconnected. This creates a challenge when using certain ceramics as the resultant membranous scaffold becomes brittle (2,25). This method is applied to polymers such as konjac gllucomannan combined with carboxymethylcellulose to prepare films (25) and poly(l-lactic acid) (PLLA), poly(d,l-lactic-co-glycolic acid) (PLGA), chitosan, and alginate porous scaffolds (26).
Sintering
This method involves heating powdered polymeric material, and thus allowing the particles to adhere to one another, thereby forming a membranous scaffold. These kinds of membranous scaffolds show potential in tissue engineering applications, and separation processes. This technique is applied to certain polymers and their composites, and ceramic powders. The principal advantage of this method is that sintering allows for the production of polymeric membranes with a controlled and graded porosity (2). Sintering ceramic powders produces membranes with greater properties including higher thermal and mechanical stabilities and a better chemical and microbial resistance. In addition to their potential use in harsh environments (higher temperatures and exposure to various chemicals), ceramic membranes have a relatively long life of use, and are typically applied to separation processes due to their permeation characteristics. Pore size, porosity, and pore tortuosity can be carefully controlled by adjusting the conditions of the sintering procedure such as the temperature, pressure, particle size, green density, and the addition of sintering additives (27). Liu and Li (28) investigated the effects of sintering temperature and time on the final membrane morphology. The study concluded that higher sintering temperatures result in membranes with lower porosities, whereas lower sintering temperatures produces highly porous membranes (28). Sintering, combined with phase inversion, can be used to prepare hollow fiber membranes (28) and multilayer structures can be produced via the co-sintering method (29). Wang and co-workers (30) studied the effects of sintering on the changes in pore size of alumina microfiltration membranes, and concluded that an increase in sintering temperature caused a decrease in the number of pores and pore length of the alumina membranes (30). In order to improve the porosity of sintered membranes, a macroporous membrane structure was prepared by Wang and co-workers (30) using a sintering process of sol-coated alumina, and an organic pore-forming substance (2% w/v corn starch). As the temperature rises during sintering, the pore-forming substance is ‘burnt out’ thus leaving behind a highly uniform and porous structure (30). Medically, sintering can be conducted to form 3D porous scaffolds by sintering polymeric microspheres onto existing molds. Such porous scaffolds produced are widely applied in bone tissue engineering, with Bioglass® composite microspheres as a key component (31).
APPLICATIONS OF MEMBRANES IN DRUG DELIVERY
Membranes in the medical field have been used extensively in drug delivery, hemodialysis artificial organs (oxygenators, pancreas etc.), and tissue engineering. The development and formulation of these membranous systems requires biocompatible and sometimes biodegradable materials as the systems would ultimately be in contact with biological fluids and tissues. In addition, these materials require other imperative properties such as blood compatibility, size, shape, and porosity to be carefully controlled. Controlled drug permeability and good release properties are the characteristics required for a membranous system to function as an efficient drug delivery system. Drug release from biodegradable matrices is directly related to the degradation rate of the materials used, i.e., the faster the material degrades, the higher the blood levels of the drug, resulting in overdoses which may often be fatal. Therefore, for implantable drug delivery systems that are biodegradable, erosion and degradability are key parameters. In addition, the swelling dynamics of certain polymers are also crucial for the mass transport of drug (diffusion) and also needs to be considered. This is also true for biocompatible polymeric membranes utilized in tissue engineering (2). An ideal drug delivery system should ensure a sustained drug release pattern for a specific period of time at a specific site. A key advantage of site-specific drug delivery is that molecules that have problematic toxicology profiles, localized delivery can often mitigate such issues and is therefore a key driver for the development of biocompatible and/or biodegradable sustained release drug delivery systems. Membrane-based drug delivery systems have the potential to ensure sustained drug release based on mechanisms of osmosis or diffusion (2). Diffusion-controlled membrane drug delivery systems depend on drug diffusion across the membrane, operating according to Fick’s law, and on membrane thickness (2). These systems have been applied in pills, implants, and patches. Smart polymers are extensively used in membrane systems as permeation switches or “gates” (32). The pores within the membrane become blocked when swelling is initiated in response to a stimulus, thereby preventing the release of the drug. Conversely, they may open and release the drug when the polymeric surface collapses.
“SMART”/STIMULI-RESPONSIVE MEMBRANOUS SYSTEMS
Biopolymers are basic components found in all living organisms. Biopolymers, such as proteins, polysaccharides, and nucleic acids, respond to changes in stimuli by undergoing a change in their properties. ‘Smart’ synthetic polymers are able to mimic these biopolymers. Fundamentally, stimuli-responsive polymers are those polymers that when exposed to a small change in the surrounding environment, undergo a relatively significant and immediate change in its properties. This change occurs as due to the polymer recognizing a specific stimulus as a signal, judging the magnitude of this signal, and then changing its chain conformation in direct response (7,33). The driving force behind these changes include neutralization of charged groups by either a pH shift or addition of an oppositely charged polymer, changes in the efficiency of hydrogen bonding with an increase in temperature or ionic strength and collapse of hydrogels and interpenetrating polymeric networks. Electric, magnetic, light, and radiation stimuli are capable of inducing reversible phase transitions and are currently being considered as possible driving forces (5).
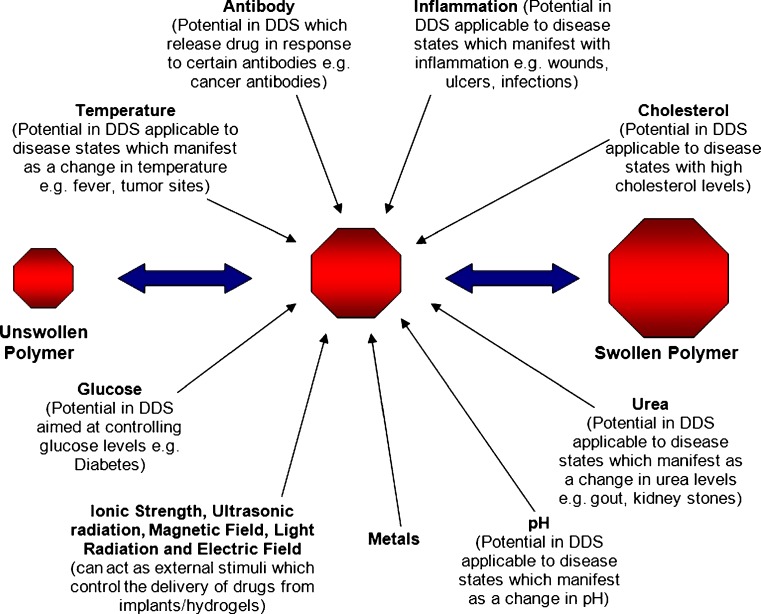
Among the most important stimuli that are applicable in drug delivery systems are temperature and pH. Responses to stimuli may present as a change in physical conformation (size, shape, formation of an intricate molecular assembly), solubility, mucoadhesion and surface characteristics, and hydration state of the polymer or release of a bioactive molecule (e.g., drug molecule) (7). Changes in response to stimuli in stimuli-responsive polymers are typically reversible. The response may also occur as a combination of several responses occurring simultaneously in response to one or more stimuli (i.e., dual responsiveness). Figure 3 illustrates the potential stimuli that may cause changes in the physical properties (such as swelling) of stimuli-responsive polymers (5,7,33). Some polymeric systems have been developed to combine two or more responsive mechanisms into one polymer (e.g., a polymer that responds to temperature may also be responsive to a change in pH). Simultaneous exposure to the stimuli is not a pre-requisite for the required response in these polymers (29). These smart polymers in drug delivery have been applied in the delivery of proteins such as insulin (34), and coenzyme A (35); anticancer drugs such as doxorubicin (36) and paclitexal (37); and levonorgestrel (38) among others.
Fig. 3.
Factors that can transform unswollen polymer into swollen polymer (adapted: Dispenza (39))
Cellular absorption of polymers involves fluid-phase pinocytosis or receptor-mediated endocytosis that leads into the formation of endosomes. Endosomes eventually fuse with lysozomes which contain enzymes. The pH drops from 6.2 to 5.0 within these endosomes during cellular absorption. This change in pH has been utilized in effecting the release molecules into the cellular cytoplasm. Studies indicate that stimuli-responsive polymers that are cationic in nature can be used for the intracellular delivery of nucleic acids. The cationic polymer binds to the negatively charged nucleic acid, and as this complex enters the endosomes, the cationic polymers are deprotonated. This deprotonation eventually leads to the disruption of the endosomal membrane, prior to the fusion with the lysozome, thereby allowing the release of drug molecules into the cytoplasm. Intracellular delivery of drugs using pH-responsive polymers has vast potential in drug delivery applications and may eventually lead to safer and more efficient drug delivery systems (7).
Stimuli-responsive polymers can be used to functionalize polymers, silica, and metal surfaces to produce highly responsive interfaces between two phases, usually a solid and a liquid. The modified interface produces a dynamic on–off system, through changing either the hydrophilic and/or hydrophobic balance of the surface (33). Polymers may be conjugated to form conjugates with a modulated stimuli-responsive ability. The conjugation can be effected by either a covalent bond or a secondary bond such as electrostatic forces or hydrophobic interactions. The responsive nature of these conjugates depends on the hydrophilic and hydrophobic changes of the conjugated polymer. Conjugation may also help in improving desirable properties such as mechanical strength and biodegradability of polymeric systems (33).
It should be acknowledged that the use of stimuli-responsive polymers have existed for a while. In their most elemental form, polymeric micelles for example can be considered stimuli responsive. Granted, they are not fabricated as a film or membrane, but the conformal changes that polymers in solution undergo is well-known. Such transitions in polymer structure have also been explored for the formation of film-like or membranous drug delivery systems.
Over the past two decades, there has been a great focus on research directed towards synthetic environmentally responsive membranes (15,40–42). These membranes are able to reversibly vary their own permeation characteristics in response to various environmental stimuli. They have potential applications in the treatment of wastewater streams, water softening, fractionation of macromolecules, drug delivery and cell encapsulation, electronic devices, and sensors (15). Typically, drug release from membrane systems depends on various parameters such as initial drug loading, membrane thickness, pH of the surrounding medium, and the mechanism involved in membrane fabrication (43). Several of the membranous drug delivery systems that have been developed respond to various stimuli including glucose concentration (40,44), temperature (42,45), and pH (15,41,46). These systems are further elaborated in this review.
Thermo-Responsive Polymers
Of all the studied stimuli-responsive systems, temperature-responsive drug delivery systems have attracted much attention since certain disease states manifest themselves by a change in temperature (45). Thermo-responsive polymers have been applied in drug and gene delivery, tissue adhesion prevention, wound covering, and recently, as cell carriers in tissue regeneration and in situ forming systems such as the “molecular condom” used for the prevention of STI’s and HIV transmission (47,48). Hydrogel polymers that exhibit sol–gel behavior in response to temperature changes have been classified as thermo-responsive polymers. These polymers generally exhibit lower critical solution temperature (LCST) behavior, where if a certain temperature threshold is surpassed the polymer undergoes a reversible phase separation. Hydrogel solutions are typically liquids and are usually soluble in a solvent (usually water) at lower temperatures, however, once the LCST has been reached; they gel and become insoluble at temperatures above the LCST (7).
Drug delivery through these hydrogels usually occurs at the soluble stage of the polymer, i.e., at temperatures lower than the LCST, demonstrating a negative thermo-responsive behavior (6,42,49). In principle, the LCST of a given polymer can be ‘‘tuned’’ to create the desired property by varying the hydrophilic or hydrophobic co-monomer content (6). Thermo-responsive polymers that are widely employed include poly(N-isopropylacrylamide) [p(NIPAAm)] and its copolymers, poly(N-vinylalkylamides), poly(l-lactic acid)-co-poly(ethylene glycol) (PEG)–poly(l-lactic acid) triblock copolymers and Pluronic® (poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) copolymer). Other commonly used thermo-responsive polymers and their uses are listed in Table II. The majority of the applications of thermo-responsive polymers utilize the change from room temperature to body temperature as a trigger for conformational change to a gel phase (7). Recent studies have been aimed at developing controlled release systems where a temperature variation triggers the alteration of the polymeric configuration (6,7,42). The change in polymeric configuration results in an alteration in the release rate of compounds incorporated in the system, e.g., a pulsatile release behavior resulting from thermal “on–off” responses. Thermo-responsive polymers can be incorporated into nanoparticle or microparticle drug delivery systems that offer a significant advantage over the macrogel type structure as they have a wider variety of applications (50).
Table II.
Thermo-Responsive Polymers and Their Applications
| Polymer | Drug delivery and other biomedical applications | References |
|---|---|---|
| P(NIPAAm) copolymers | ||
| Poly(NIPAAm-co-DMAAm-co-AMA)-b-PUA | Doxorubicin and folic acid | (55) |
| Poly(HEMA-g-NIPAAm) | Theophylline and insulin | (56) |
| Poly(NIPAAm)-g-HA | Post-surgical tissue adhesions | (57) |
| Poly(NIPAAm)-g-gelatin | Hemostatic aid during surgery | (57) |
| PEG/PLGA block copolymers | ||
| PLGA-b-PEG-b-PLGA triblock copolymers | Delivery of proteins (lysozymes) | (34) |
| PLGA–PEG–PLGA triblock copolymers | Testosterone | (58) |
| PEG-b-PLGA-b-PEG triblock copolymers | In situ generated implants | (59) |
| Poly(organophosphazenes) | ||
| Poly(organophosphazene) hydrogel | Doxorubicin | (60) |
| Poly(organophosphazene) hydrogel | Dextran and albumin | (61) |
| PEG/biodegradable polyester copolymers | ||
| Poly(N-HPMAmDL-b-PEG) | Paclitaxel | (62) |
| PEG and PPG | In situ generated implants | (63) |
| PLLA/PEG/PLLA | Doxorubicin | (64) |
| PEO/PPO block copolymers | ||
| Pluronic® F127 (PEO99–PPO67–PEO99) | Metronidazole and methylene blue | (65) |
| Crosslinked PEO–PPO | In situ generated implants | (63) |
| Pluronic® F127 | In situ implant (hGH) | (66) |
DMAAm dimethylacrylamide, PCL poly(caprolactone), PLLA poly(l-lactic acid), P(NIPAAm) poly(N-isopropylacrylamide), PPO poly(propylene oxide), PLGA poly(d,l-lactide-co-glycolic acid), HPMAmDL 2-hydroxypropyl methacrylamide lactate, HEMA hydroxyethyl methacrylate, PEO poly(ethylene oxide), PEG poly(ethylene glycol), PPG poly(propylene glycol), HA hyaluronan, hGH human growth hormone
Studies have indicated that previously developed thermo-responsive drug delivery systems showing negative thermo-responsive behavior were not able to effectively deliver drug to the body since the LCST of the polymer used to be reached at body temperature causing the surface of the gel to form a dense surface layer that often resulted into poor drug permeation (51). However, this property can be used to develop controlled release systems. Kitano and co-workers (51) then achieved the development of hydrogels composed of poly(acrylamide-co-butyl methacrylate) and poly(acrylic acid) that exhibited positive thermo-responsive behavior whereby swelling increased as the temperature increased. Positive thermo-responsive drug release may also be achieved by modification of thermo-responsive properties such as the combination of an inert and rigid housing system comprised of pores that are filled with hydrogels exhibiting negative thermo-responsive swelling (52) or utilization of an inert membrane containing channels or holes composed of a thermo-responsive polymer (53,54).
Applications of Thermo-Responsive Membranes in Drug Delivery
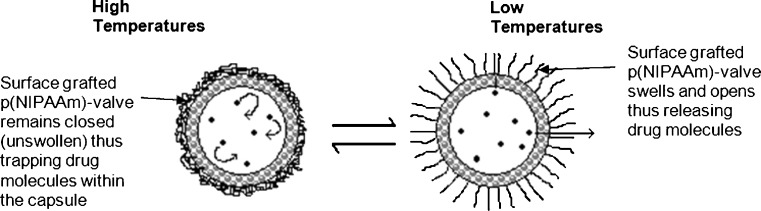
The technology of thermo-responsive polymeric membranes began as early as 1986 (67). In this pioneering study, the permeation of NaCl and dyes from a large capsule membrane composed of nylon, with a surface grafted p(NIPAAm), was reversibly regulated by ambient temperature changes, where the grafted polymer acted as a thermo-responsive permeation valve on the membrane (67). Figure 4 illustrates the release of drug from the capsule at low temperatures.
Fig. 4.
Schematic illustration of a capsule membrane that has been grafted with polymers functioning as a permeation valve (adapted from: Okahata et al. (67))
The study showed that permeability of the polymer-grafted capsule was greatly reduced above 35°C that was in contrast to the non-grafted capsule where an increase in temperature resulted in an increase in the permeability of the membrane (67). Conversely, Ichikawa and Fukumori (50), aimed to develop a positively thermo-responsive controlled release microcapsule (MC), utilizing a thermo-responsive membrane produced from p(NIPAAm) dispersed in an ethylcellulose matrix. The MC showed higher release of carbazochrome sodium sulfonate at elevated temperatures (45–50°C), compared to the release observed at lower temperatures of approximately 20°C (50).
In membranous drug delivery systems, positively behaving thermo-responsive drug delivery systems are more practical in the delivery of drugs to the body, because the LCST of the polymer is usually reached at body temperature, hence allowing drug release. Chun and Kim (68), aimed at developing a novel composite membrane system which could effectively deliver a drug above its LCST. Fabrication of this membrane system involved the crosslinking of gelatin with p(NIPAAm) and then incorporating 4-acetoaminophen as a drug. The composite membrane was prepared by in situ polymerization of entrapped monomers in vacant spaces of the gelatin matrix. Diffusion of 4-acetoaminophen was three- to fourfold greater at 40°C than at 25°C from the crosslinked p(NIPAAm), compared to the pure p(NIPAAm) membrane where drug permeation above the LCST was lower than that below the LCST. The mechanism of drug release in this study was based on a valve mechanism where the swelling state of the thermo-responsive polymer determined the ‘on’ or ‘off’ stage of drug delivery (68).
Membranous Stimuli-Responsive Polymeric Brushes
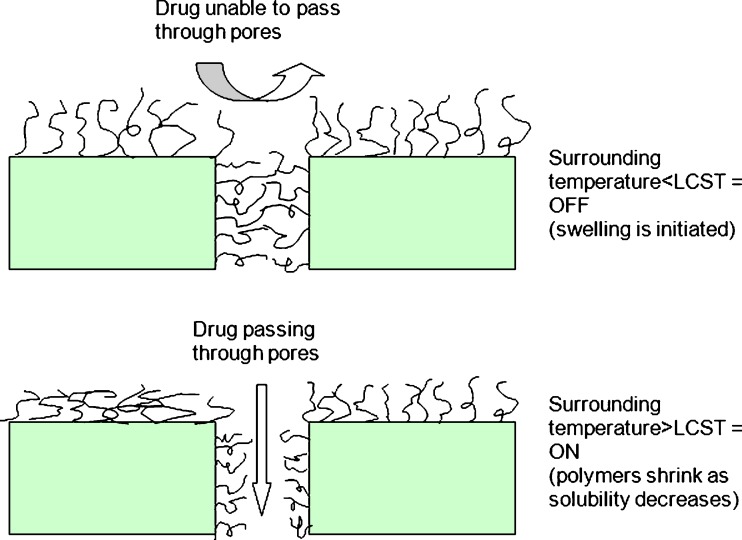
Porous membranes with ‘smart polymeric brushes’ are of particular interest due to their excellent mechanical strength and quick responses to external stimuli, and can thus be used to develop ‘on–off’ drug delivery systems (69). ‘On–off’ valve membrane systems can be prepared by grafting a thermo-responsive polymer onto the surface of microporous polymeric membranes (42,69). This is achieved when a responsive polymer is covalently attached to the surface of pores or physically placed in the pores. Porous substrates are usually inert and provide physical strength and support, whereby the drug diffusion is controlled by the stimuli-responsive polymers present on the surfaces of the pores (69). The ‘on’ or ‘off’ state of the valve may be manipulated by the swelling or shrinkage of the thermo-responsive polymers grafted onto the surfaces, or brush surfaces of such membranes (6,42). The drug permeation through such ‘on–off’ membrane systems is illustrated in Fig. 5. The ‘off’ stage results when the polymeric brushes become soluble in water at temperatures below the LCST which leads to swelling that ultimately leads to closing of the membrane system pores. In contrast, the ‘on’ stage results when the polymer becomes insoluble in water at temperatures above the LCST and shrinks, thus allowing for the opening of the pores within the membrane system. In this case, drug delivery increases with an increase in temperature (6,42,49). Stimuli-responsive drug delivery systems that release drug only above certain temperatures may be usefully applied as drug reservoir systems. One of the areas where they find great potential for use is in chemotherapeutics delivery under local hyperthermia (70).
Fig. 5.
‘On–off’ valve mechanism of polymeric brushes grafted onto porous support (adapted from: Lue et al. (6))
Surface grafting of existing membranes changes the membrane pore size distribution, leading to a change in permeability (42). The use of p(NIPAAm) as polymeric brush surfaces in the formation of ‘on–off’ valve systems has been thoroughly investigated (Table III). Iwata and co-workers (71) prepared thermo-responsive polymeric membranes by grafting p(NIPAAm) and its copolymers onto a porous poly(vinylidene fluoride) membrane. Permeability of these membranes varied more than tenfold between temperatures above and below the LCST. Sensitivity was found to be both reproducible and reversible. Temperature-responsive p(NIPAAm) polymeric brushes of known molecular weight were grafted onto microporous polycarbonate (PC) films using argon plasma treatment by Lue and co-workers (6). In vitro drug release studies showed that the release of 4-acetamidophenol and ranitidine HCl occurred at temperatures above the LCST (the ‘on’ stage) whereas permeability of these drugs decreased at temperatures lower than that of the LCST (the ‘off’ stage) (6). Grafting can be carried out by irradiation (72,73), UV photo-grafting (49,74), ion tracking (75), and plasma modification (76,77).
Table III.
Application of the Thermo-Responsive Polymer p(NIPAAm) in ‘On–Off’ Valve Mechanisms
| Grafting mechanism | Porous membranes | Drug delivery and other biomedical applications | References |
|---|---|---|---|
| Ultraviolet photo-polymerization | Hydrophilic PP microfiltration membranes | Solute separations | (49) |
| Photo-immobilization via photo-irradiation | PC membranes | Permeation of tryptophan | (72) |
| Irradiation | PVDF membranes | Polynucleotide, peptide, and protein delivery | (78) |
| Photo-grafting using UV light | PET track membranes | None used | (74) |
| Plasma-induced graft polymerization | PE membranes | Separation and filtration | (77) |
| Plasma-induced graft polymerization | Track-etched polycarbonate films | 4-acetamidophenol and ranitidine HCl | (53) |
| Plasma-induced graft polymerization | PA microcapsules | NaCl and Vitamin B12 | (76) |
| Radiation-induced graft polymerization | PET and PP membranes | Separation and filtration | (73) |
| Surface-initiated polymerization by FRP and ATRP | PET membranes | Separation and filtration | (79) |
| In situ polymerization of hydrogels within pores of sintered glass | Disk-shaped sintered glass filter | Permeation of salicylic acid and bovine albumin | (80) |
| Surface-initiated ATRP | PS substrates | Cell-based therapy for severe disorders | (54) |
FRP free radical polymerization, PET polyethylene terephtalate, PC polycarbonate, PE polyethylene, PS polystyrene, ATRP atom transfer radical polymerization, PP polypropylene, PVDF poly(vinylidene fluoride), PA polyamide
Aminated Thermo-Responsive Polymers
A study conducted by Zhang and co-workers (45) suggested that the sensitivity of thermo-responsive membranes can be promoted by amination. Charged (aminated) and uncharged bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPO)-g-p(NIPAAm) membranes were investigated for differences in thermal responsiveness by assessing the release characteristics of sodium salicylate. The charged and uncharged grafted membranes were exposed to two media, sodium chloride and deionized water, and drug release was assessed at a range of temperatures including 25, 37, and 43°C. Permeability changes, and hence drug release, of sodium salicylate were greater in charged membranes than that of the uncharged membranes at the same temperature changes. This study deduced that the permeability coefficient of the drug markedly increased after grafted membranes were aminated (45).
Liquid Crystals as Potential ‘Smart’ Materials for Membranous Systems
Studies have demonstrated that the efficacy of liquid crystals (LC) as potential ‘smart’ materials is due to their sharp, positive, reversible, and multi-stimuli responsiveness. However, the thermotropic use of LCs as stimuli-responsive materials in responsive drug delivery systems is relatively new. Atyabi and co-workers (70) demonstrated that the use of a monolayer composite membrane may be prepared from n-heptyl cyanobiphenyl. In this study, LC’s were embedded on cellulose acetate and cellulose nitrate membranes. These thermo-responsive LC-embedded membranes showed higher permeability to drug molecules (methimazole and paracetamol) at temperatures above 41.5°C. The permeability of the membranes to drug molecules increased with an increase in temperature, and was both reproducible and reversible (70).
Membranous Thermo-Responsive Polymeric Implants
Ng and co-workers (69) developed a thermo-responsive polymeric membrane that can be used to encapsulate an implant for on demand drug delivery. Pores within a porous polyethylene membrane were filled with alkanes (docosane and eicosane) with a melting point of 42°C. The melting point of alkane corresponded to a diffusional switch, and thus functioned as a trigger for drug release form the membranes. The permeation of propranolol HCl through the membrane was increased 200- to 400-fold when the temperature was increased to 42.5–45°C. This occurred as the alkanes blocking the pores became liquid thus allowing drug to permeate through. The implant developed by Ng and co-workers (69) may be applied as a sub-dermal implant that can be activated by external sources of heat such as deep-heating ultrasonic devices (69). The potential to apply p(NIPAAm)-grafted hyaluronan [p(NIPAAm–HA)] and p(NIPAAm)-grafted gelatin [p(NIPAAm)–gelatin] as thermo-responsive polymers for preventing tissue adhesion, or as a hemostatic aid in bleeding blood vessels was investigated by Ohya and co-workers (57). Results showed that an in situ swollen precipitate of p(NIPAAm)–HA formed spontaneously and effectively functioned in preventing tissue adhesion in the rat cecum. Hemostasis of bleeding tissues within blood vessels was effectively controlled by p(NIPAAm)–gelatin membranes. Due to the high elasticity of the p(NIPAAm)–gelatin membranes, there was no interference with the periodic pulsatile action of the high-pressured circulatory system (57).
Membranous Thermo-Responsive Transdermal Patches
Thermo-responsive systems have been successfully utilized in the development of transdermal patches for drug delivery. Csóka and co-workers (81) formulated a thermo-responsive drug delivery system for possible application in transdermal drug delivery, where drug release was modulated by temperature. Metolose SM® (methylcellulose) and Metolose 60 SH-4000® (hydroxypropyl methylcellulose) was used as the thermo-responsive component and was modified by different salts (NaCl, NaHCO3, and KCl) of various concentrations to alter the transition of the polymer (to a low viscosity gel phase) closer to physiological temperatures. A rise in the body temperature (fever) led to the decrease in viscosity of the system, thereby enhancing drug release (81). Hydrogel membranes composed of three kinds of latex particles within carboxymethylcellulose matrices were prepared by Don and co-workers (82) for the purpose of transdermal drug release. Caffeine was the model drug loaded into the hydrogel membrane. The membranes were produced by a crosslinked p(NIPAAm) copolymer synthesized from p(NIPAAm) with an acrylic acid monomer. The addition of acrylic acid not only led to the ionic interaction with two other latex particles via an aluminum ion but also produced a shift in the LCST toward a higher temperature. Other latex particles composed of poly(acrylic acid-co-sodium acrylate) were used to increase the swelling ability of the hydrogel while poly(acrylic acid-co-2-ethylhexyl acrylate) was used to increase the adhesive ability of the hydrogel membrane. This resulted in the formulation of a hydrogel membrane for transdermal application that could successfully control the release of drugs in response to changes in temperature (82).
pH-Responsive Polymers
The use of pH-responsive hydrogels as stimuli-responsive membranous drug delivery systems is based on the variability of the physiological pH is at different sites of the body, including the gastrointestinal tract, vagina, and blood vessels (83,84). pH-responsive polymers are used to target drug delivery to inflamed tissues, certain cancer cells, and damaged or wounded tissues. Table IV lists commonly targeted drug delivery sites in the body and their respective pH ranges. For a polymer to possess pH-responsive properties, they should be ionizable and preferably have a pKa value of 3–10 (7,83). A change in pH results in a deviation in the ionization state of these polymers and therefore a conformational change, which culminates in the changing of the solubility and swelling behavior of the polymer. This pH-responsive swelling ability has been exploited to induce the controlled release of drugs and proteins (7,83).
Table IV.
pH Variation in Different Tissue and Cellular Compartments (Adapted from Schmaljohann (7))
| Tissue/cellular compartment | pH |
|---|---|
| Blood | 7.35–7.45 |
| Stomach | 1.00–3.00 |
| Duodenum | 4.80–8.20 |
| Colon | 7.00–7.50 |
| Early endosome | 6.00–6.50 |
| Late endosome | 5.00–6.00 |
| Lysosome | 4.50–5.00 |
| Golgi | 6.40 |
| Tumor, extracellular | 7.20–6.50 |
| Vagina80 | 3.50–5.00 |
The pH sensitivity of a pH-responsive polymer is as a result of the presence of weakly acidic (e.g., carboxylic and sulfonic acids) and/or weakly basic (e.g., ammonium salts) functional groups on the polymeric backbone, which allow for reversible swelling/de-swelling behavior in acidic or basic media. These functional groups undergo protonation or deprotonation with changes in pH as small as 0.2–0.3 U, thus resulting in water uptake by the polymer, thereby increasing the solubility of solutes within the polymeric matrix (usually with drug particles), and releasing these solutes through the polymeric matrix (85,86). The ionization of these polymers (which depends on the pH and ionic strength of the external medium), renders them pH-responsive thus making them useful in drug delivery systems known as pH-dependent ‘switch-on and switch-off’ systems (83). Thus far, these pH-responsive hydrogels have shown vast potential in the delivery of drugs and therapeutic agents into various areas of the body (46,83,85,86). Table V summarizes certain pH-responsive polymers that have been employed in smart drug delivery systems.
Table V.
pH-Responsive Polymers and Their Application in Drug Delivery
| Polymer | Drug delivery and other biomedical applications | References |
|---|---|---|
| Methacrylic acid copolymers | ||
| Eudragit® L100-55 (pH 5.5) | Mesalazine | (87) |
| Eudragit® P-4135F | Ellagic acid | (88) |
| Poly(GMD-PAA) | 5-aminosalicylic acid | (89) |
| Poly(HEMA–MAA) copolymer | Indomethacin and ephedrine HCl | (90) |
| Poly(MAA–EA) | Procaine HCl and imipramine HCl | (91) |
| Poly(MMA–DMA) | Aminopyrine, caffeine, and theobromine | (46) |
| PEG-b-poly(AlA-co-MA) | Fenofibrate and progesterone | (92) |
| Poly(HEMA) gels | Salicylic acid | (93) |
| Poly(HEMA) | Oral insulin, theophylline, proxyphylline oxprenolol, insulin, and protamine | (94) |
| (95) | ||
| Poly(EA–MAA–BDDA) and Poly(MMA–MAA–EGDMA) | Biomaterials for structural support of soft connective tissues | (96) |
| PEG-b-P(AlA-co-tBMA) block copolymers | Poorly water-soluble drugs: indomethacin, fenofibrate, progesterone | (97) |
| (98) | ||
| Poly(HEMA-co-MAA) hydrogels | Phenylpropanolamine | (99) |
| PEG-b-poly(AlMA-co-methacrylic acid) | Candesartan, cilexetil | (100) |
| Poly(acrylate) derivatives | ||
| PA-g-guar gum | Diltiazem HCl and nifedipine | (86) |
| NPA | Folic acid | (101) |
| Alginate derivatives | ||
| Alginate-guar gum hydrogel | Delivery of proteins and peptides (BSA) | (102) |
| Chitosan | ||
| CHT-TEOS | Lidocaine HCl, sodium salicylate, and 4-acetamidophenol | (41) |
| Polyphosphazenes | ||
| Polyphosphazene-PS polyphosphazene-polysiloxane polyphosphazene-ROMP of norbornene copolymers | No drug delivery application (a dye) Biebrich Scarlet was used to test release from these copolymers | (103) |
| (104) | ||
GMD glycidyl methacrylate dextran, HEMA hydroxyethyl methacrylate, MMA methyl methacrylate, DMA dimethylaminoethyl methacrylate, ATRP atom transfer radical polymerization, AlA alkyl acrylate, HEMA 2-hydroxyethyl methacrylate, EGDMA ethylene glycol dimethacrylate, PA polyacrylamide, NPA p-nitrophenyl acrylate, TEOS tetra ethyl ortho silicate, tBMA t-butyl methacrylate, PAA poly(acrylic acid), MAA methacrylic acid, EA ethyl acrylate, PEG poly(ethylene glycol), BDDA butanediol diacrylate, EG ethylene glycol, MA methacrylamide, CL caprolactone, PVP poly(N-vinylpyrrolidone), CHT chitosan, PS polystyrene, AlMA alkyl (meth)acrylate
Application of pH-responsive Membranes in Drug Delivery
Poly(vinyl alcohol) and poly(acrylic acid) have been used to prepare pH-responsive interpenetrating polymeric network membranes by employing varying crosslinking ratios for application in drug and protein separation. The outcomes of this study may be used to design gel membranes with accurate separation and selectivity characteristics (105). pH-responsive membranes were developed that possess separation characteristics based on self-organizing blends of poly(vinylidene fluoride) and an amphiphilic comb polymer having a PVDF backbone and poly(methacrylic acid) side chains. These membranes were prepared using the immersion precipitation method. The advantage was that these membranes required no post-coagulation processing steps (15).
Ammonio Methacrylate Copolymer Drug Delivery Membranes
Sun and co-workers (19) developed ammonio methacrylate copolymer membranes employing a film-casting process, which have since been used as coatings to control the release of drug molecules. The study investigated the membrane swelling and permeation of ionic drugs through the membranes in media with various pH and ionic strengths. Results indicated that membranes or coatings prepared from the ammonio methacrylate copolymers or their pseudo-latex solutions were insoluble in the aqueous media over the physiological pH range. However, the membranes were swellable and permeable to drugs due to the presence of ionizable quaternary ammonio groups. This study also revealed that anionic and cationic drugs behave differently in transient permeation through membranes. The permeability of the aspirin was found to be higher at a lower pH and lower at higher pH ranges, whereas the permeability of ambroxol was found to be lower at low pH’s and higher at high pH ranges (19).
Organic–Inorganic Hybrid Membrane Delivery Systems
The formulation of organic–inorganic hybrids through the sol–gel process allows for tailoring the solid-state properties of the composite hybrid in relation to the nature and relative content of the constitutive components (106). Park and co-workers (41), reported the use of chitosan as an organic component of a composite membrane and tetra ethyl ortho silicate (TEOS) as an inorganic component. In vitro drug release studies using lidocaine HCl, sodium salicylate, and 4-acetamidophenol showed that drug permeation through the membrane was strongly affected by ionic interactions within the system and the environmental pH range, thus showing potential as a novel pH-responsive drug delivery system. These inorganic–organic hybrid systems have the capability to produce systems that are responsive to various other stimuli by alternating the constitutive components within the system (41).
Reversible pH-Responsive Porous Films
Kai and Min (107) grafted acrylic acid onto porous polycarbonate films via UV irradiation to produce reversible pH-responsive porous films. These devices were found to successfully control the release of 5-fluorouracil (an anticancer drug) and methylene blue in in vitro drug release studies (107). This pH-responsive system demonstrated good drug release properties in acidic media and very poor drug release properties in alkaline conditions (107). The effects of variations in grafting conditions, thickness of membranes, and variation in the pH of the surrounding medium, on the release of glucose from modified chitosan membranes was investigated in a later study. Chitosan membranes were modified by graft copolymerization of 2-hydroxyethylmethacrylate (HEMA) onto chitosan films using γ-ray irradiation. Drug release was reported to increase with a drop in pH. In addition, HEMA influenced the hydrophilicity of the membranes, and hence drug release increased with increasing HEMA levels (43). A similar study investigated poly(methyl methacrylate/dimethylaminoethyl methacrylate) (MMA/DMA) hydrogel membranes crosslinked with divinylbenzene. This study examined the effects of pH and hydration of MMA/DMA (0.1% DVB) as a pH-responsive hydrogel on drug diffusivity, the diffusion characteristics of drugs with different water solubilities and the mechanism of drug release for aminopyrine, caffeine, and theobromine. The membranes were hydrated in an acidic pH range and glassy at neutral pH. Results indicated that drug diffusion through these hydrogels was faster in acidic environments and slower in neutral and alkaline pH ranges. These results confirmed that the transport of highly water-soluble drugs is largely dependent on the degree of hydration and ionization of the hydrogel and that the drug diffusion is controlled by the pH sensitivity of the hydrogel. Thus, at low pH’s the hydrogel became significantly hydrated and ionized which induced extensive opening of pores and more rapid diffusion of the highly water-soluble drugs (46).
Lai and co-workers (18) developed a pH-responsive membrane for the colonic delivery of 5-fluorouracil using poly(ethylene-co-vinyl alcohol) and poly(acrylic acid) blends. The pH-responsive membranes were dry cast from different blends of PAA/EVAL solutions at 45 and 60°C and drug release was investigated at a pH range of 2.0 to 7.4. The results portrayed that the membranes prepared at 45°C did not show a significant difference in drug release in media of different pH values (18). However, membranes prepared at 60°C showed that the flux of 5-fluorouracil from the membranes was much higher at pH 7.4 than at 2.0 indicating that PAA/EVAL blended membranes prepared at 60°C have significant potential for use as drug release system for 5-fluorouracil that can be used in the local treatment of colorectal cancer (18). Other attempts aimed at delivering 5-fluorouracil to the colon were prepared by Shieh and co-workers (108). They bonded the EVAL membrane to glycine which resulted in the release of 5-fluorouracil at an appropriate pH of 7.4 as compared to a pH of 2.0 where small quantities of drug was released. There is potential for application of these membranes in the treatment of local ulcerative colitis (108).
pH-Responsive Hydrogel-Based Systems Utilizing ‘On–Off’ Gating Mechanisms
The ‘on–off’ gating mechanism can also be successfully achieved with pH-responsive hydrogels (109,110). Research conducted by Mika and co-workers (109) demonstrated that incorporation of conformationally labile polymeric chains such as poly(4-vinylpyridine) into microporous membranes composed of polypropylene resulted in a specific class of membranes, where permeability was affected by the external chemical and/or physical factors (chemical valves). High pH values (>5) triggered the opening of the valves as a result of unionization of poly(4-vinylpyridine), whereas lower pH values ensured that valves were closed due to the protonation of poly(4-vinylpyridine) (109). In contrast, Hu and Dickson (110) successfully prepared membranes with valves that could open in acidic environments and close within the alkaline pH range. This was achieved by using pore-filled pH-responsive membranes developed by in situ crosslinking of PAA with poly(vinylidene fluoride) microporous membranes (110).
Other Stimuli-Responsive Systems Employed in Drug Delivery
Glucose-Responsive Systems
The next generation of biomaterials is said to focus on hydrogels that demonstrate sensitivity to biomolecules. Thus far, there is no sufficiently effective system for the control of blood glucose levels. In insulin-dependent diabetes, an increase in blood glucose levels requires the administration of insulin to effectively control blood glucose levels. Daily insulin injections cause discomfort and other complications and therefore a conventional oral drug delivery route is preferred. However, this route is not practical for the systemic delivery of proteins and peptides because of their sensitivity to chemical and enzymatic hydrolysis, and poor cellular uptake. Hydrogels that respond to glucose by swelling have potential for a self-regulating insulin delivery system for the treatment of diabetes. Typically, self-regulating glucose-responsive systems should mimic the physiological response of insulin release from the pancreas in response to blood glucose concentrations (107). Thus far, competitive binding, substrate–enzyme reaction, pH-dependent polymer erosion, or drug solubility are employed as glucose-sensing mechanisms (111–114).
Glucose-Oxidase-Containing Membranes
Glucose-responsive hydrogels are commonly known as glucose-oxidase-loaded hydrogels (GOD). These hydrogels requires the presence of glucose oxidase to be rendered pH-responsive. The glucose oxidase converts glucose to gluconic acid and hydrogen peroxide, in the presence of oxygen, thus lowering the pH of the hydrogel (112). This pH change triggers the pH-responsive hydrogel to undergo a conformational change that leads to the release insulin entrapped within the system. The pH of the body drops more rapidly with high glucose concentrations, indicating that the release rate of insulin from the glucose-responsive hydrogel is dependent on the glucose levels in the body (112). Catalase is incorporated into these glucose-responsive systems to eliminate hydrogen peroxide and therefore prevent toxicity, by ensuring that oxygen depletion is prevented (113,114). Various polymers including polyacrylates, polymethacrylates, polyethylene, polypyrroles, silica, and poly(vinyl alcohol) have been successfully used as substrates for the immobilization of GOD (112).
The pioneering study that investigated GOD incorporation in pH-responsive membranes for insulin delivery in response to glucose concentrations was conducted by Albin and co-workers (115,116). Results of their studies were promising and this led to many other studies proposing glucose-responsive systems. Klumb and Horbett (117) successfully developed glucose-responsive hydroxyethyl methacrylate-based copolymer membranes with pendant amine groups containing immobilized enzymes GOD and catalase. However, a problem encountered with early glucose-responsive systems was that pH-responsive polymers required an environmental pH outside of the normal physiological pH range, in order to shrink and allow drug molecules to permeate through the pores (118). Thus, porous poly(vinylidene) filters, grafted with copolymer poly(butyl methacrylate [(BMA)-methacrylic acid (MAAc) co-poly(MAAc-BMA)]) chains and surface immobilized GOD was developed by Cartier and co-workers (118). The study revealed that the use of a copolymer-containing BMA could successfully manipulate the ionization characteristics of the copolymer, thereby ensuring that the copolymer would shrink within physiological pH ranges, therefore delivering drug molecules at physiological pH (118). Further studies by Zhang and Wu (114) attempted to prepare glucose-responsive membranes by film-casting a polymeric solution containing p(NIPAAm/MAA) nanoparticles. Insulin permeation through these membranes was successfully controlled by the surrounding glucose concentration (114).
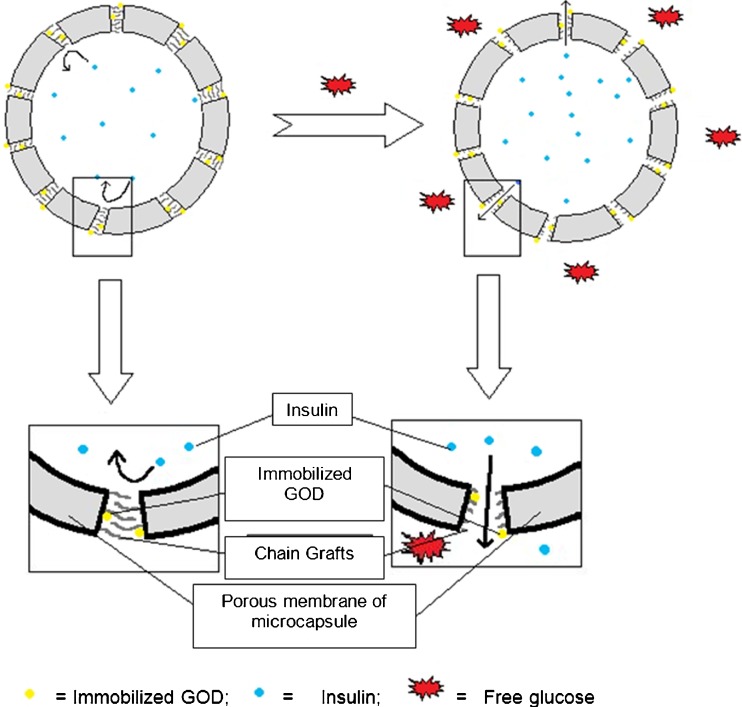
Membranes with good mechanical strength and fast responses to glucose were prepared by Ito and co-workers (119) through a technique that involved grafting of a pH-responsive membrane onto a membrane scaffold. Cellulose films grafted with poly(acrylic acid) were used as pH-responsive membranes. By covalently binding GOD to the surface of these membranes, the membranes were made to respond specifically to changes in glucose concentrations thereby releasing insulin (119). Studies carried out by Chu and co-workers (120) demonstrated the use of a porous polyvinylidene fluoride membrane grafted with poly(acrylic acid) chains for the development of glucose-responsive gating membranes for controlling insulin release (120). Thereafter, the use of porous membranes grafted with linear chains of poly(acrylic acid) in the membrane pores and covalently bound GOD enzymes for the development of microcapsules was reported. The mechanism of insulin release from these microcapsules is as illustrated in Fig. 6 (120).
Fig. 6.
Schematic representation of glucose-responsive microcapsules prepared with a porous membrane containing functional gates. Pores consist of GOD and pH-responsive PAA chains that, upon exposure to glucose, allows for the opening of the pores thus releasing insulin (adapted from: Chu et al. (76))
In order to determine the clinical efficacy of glucose-responsive systems, Tritely and co-workers (121) prepared matrices intended for implantation in rats. They reported that the poly(2-hydroxyethyl methacrylate-co-N,N-dimethylaminoethyl methacrylate) [p(HEMA-co-DMAEMA)] hydrogel entrapped with glucose oxidase, catalase, and insulin were able to effectively immobilize insulin, was biocompatible, and effective in reducing blood glucose levels in rats (121).
Lectin-Loaded Membranes
Lectin-loaded hydrogels have also been employed as glucose-responsive systems (111,113). These systems are based on the glucose-binding properties of concanavalin A (con A), a lectin that possesses four binding sites. Insulin derivatives are able to form complexes with con A, which are released in response to free glucose (111,113). Insulin and lysozyme release from membranes comprised of glucose-containing polymers complexed with con A, and sandwiched between porous poly(hydroxyethyl methacrylate) was investigated by Obaidat and Park (40). The rate of protein release through the membranes depended on the surrounding glucose concentrations. A further attempt to produce glucose-responsive systems was performed by Tang and co-workers (111), where membranes were constructed from crosslinked dextrans, with a con A-glycosylated insulin complex. Results from this study confirmed that the rate of insulin release is dependent on surrounding glucose concentration. Overall, these studies reflect that glucose-responsive phase-reversible membrane systems may be used to regulate insulin release as a function of free glucose concentration in the environment (40,111).
Membranes Containing Phenylboronic Acid Moieties
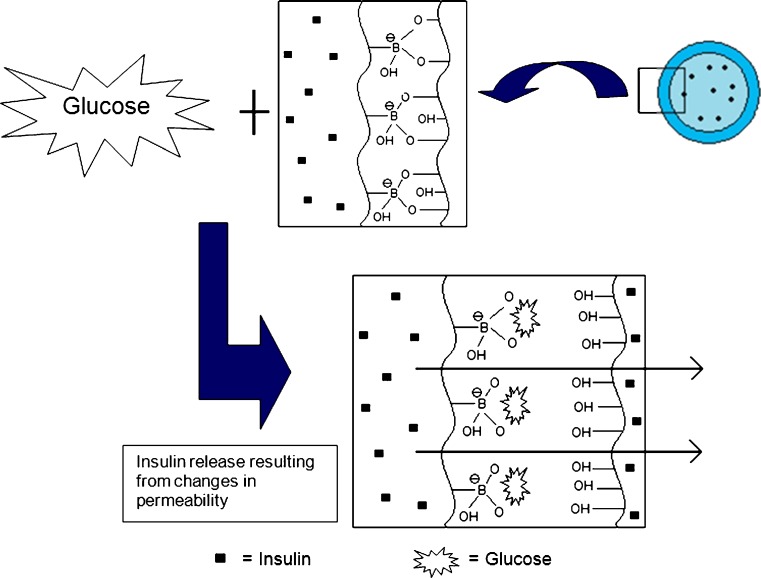
The concept of competitive binding can be utilized to develop glucose-responsive systems. These glucose-responsive systems do not require proteins such as glucose oxidase or lectins in the fabrication of the glucose-responsive hydrogel. Complex formation between phenylboronic acid and glucose is utilized as the insulin-releasing trigger. In aqueous solutions, phenylboronic acid and its derivatives form complexes with polyol compounds like glucose. Initially, phenylboronic acid forms complexes with other polyol groups such as polyvinyl acetate (PVA). When the complex is exposed to glucose, the phenylboronic acid binds more readily to the free glucose molecules (113). This competitive binding of glucose and PVA has been exploited to develop a glucose-responsive system (51). A study conducted by Kitano and co-workers (51) demonstrated the use of copolymers bound with phenylboronic acid moieties and PVA. Poly(N-vinyl-2-pyrollidine) [p(NVP)] copolymerized with 3-(acrylamide) phenylboronic acid (PBA) formed a glucose-responsive poly(NVP-co-PBA). Exposure of the copolymer to glucose resulted in the dissociation of poly(NVP-co-PBA) from PVA with subsequent complex formation with glucose. This complexation resulted in a conformational change of the polymeric system thus allowing the release of insulin as depicted in Fig. 7 (51,113).
Fig. 7.
A glucose-responsive insulin-releasing system based on PVA/poly(NVP-co-BPA) (adapted from: Kitano et al. (51))
Dual-Responsive Systems
The combination of two or more stimuli-responsive mechanisms in one polymeric system results in the development of a dual-responsive system that responds when exposed to two stimuli simultaneously. Injections of thermo-responsive hydrogels into deep anatomical sites within the body are not suitable due to the premature gelling of the hydrogel within the catheter used to deliver the hydrogel (122). This challenge can be overcome by combining a thermo-responsive polymer with a pH-responsive polymer to form a copolymer that is responsive to both pH and temperature changes (36). As a result, the block copolymer undergoes gelation in response to simultaneous exposure to pH and temperature changes, thus preventing premature gelation. Shim et al. (122) reported a novel pH- and thermo-responsive block copolymer prepared by adding pH-responsive sulfamethazine oligomer (SMO) to thermo-responsive poly(caprolactone-co-lactide)-PEG-poly(caprolactone-co-lactide) resulting in a copolymer solution which demonstrated a reversible sol–gel transition triggered by a change in the pH in the range of 7.2–8.0 at a temperature of 37°C (body temperature). It was then anticipated that the SMO–PCLA–PEG–PCLA–SMO block copolymer would be a solution at a pH above 8.0 at room temperature and form a gel in the body at physiological pH (7.4) and body temperature (37°C) (122). Furthermore, Dong and Hoffman (123) crosslinked various ratios of p(NIPAAm) and poly(N,N′-dimethylaminopropylmethacrylamide) to form a positively charged pH-/thermo-responsive hydrogel. Dual-responsive systems in the form of beads have also been developed by Kim et al. (124) for the release of insulin. These beads were composed of poly(N-isopropylacrylamide-co-butylmethacrylate-co-acrylic-acid) and PAA. The study demonstrated that the rate of insulin release at pH 7.4 and 37°C was controlled by the PAA content of the beads. Insulin bioactivity was retained after a period of 5 h in the rat stomach (124).
Applications Dual-Responsive Membranes in Drug Delivery
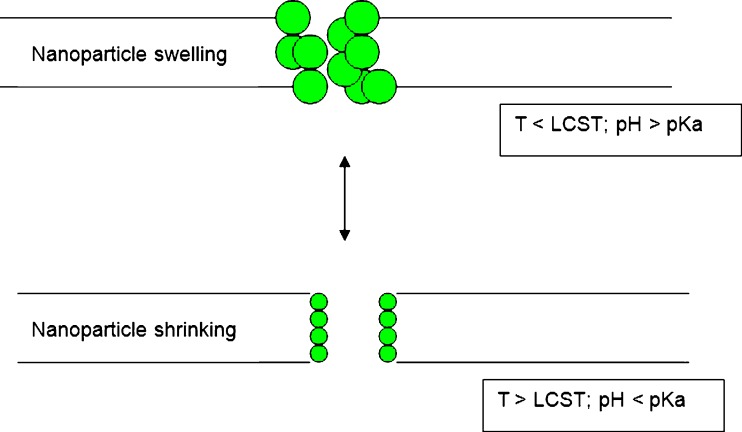
Thus far, thermo- and pH-responsive delivery systems have drawn much attention since a number of disease states manifest as a change in temperature and/or pH (114). Dual-responsive membranes can be prepared by co-grafting thermo- and pH-responsive polymers on porous membrane substrates. Porous polyamide membranes grafted with both PAA and p(NIPAAm) were found to be responsive to both temperature and pH. These were among the first dual-responsive membranes produced (85). Chitosan-based hydrogel films possessing both thermal and pH sensitivity were prepared by blending chitosan with p(NIPAAm) and polyethylene glycol. PEG was added to enhance thermo-mechanical and swelling properties of the film. The data obtained from the study revealed that the blended chitosan/PEG/p(NIPAAm) films had a LCST at around 32°C due to p(NIPAAm) component and showed pH-responsiveness due to the amino groups of chitosan component (125). Dual-responsive membranes consisting of nanoparticles, using the concept of the ‘on–off’ gating mechanism have been prepared by Zhang and Wu (114) as illustrated in Fig. 8. Nanoparticles embedded within the polymeric membrane were prepared using poly(N-isopropylacrylamide-co-methacrylic acid) [p(NIPAAm-co-MAA)]. Upon exposure to environmental stimuli, the nanoparticles shrink or swell, thus controlling the permeability of drug molecules. Permeability of solutes such as peptides, Leuprolide, vitamin B12, insulin, and lysozyme across these membranes increased with increasing temperatures or particle concentration and decreased with increasing pH. To prevent nanoparticles from escaping through the surface of the membranes, Zhang and co-workers (114) improved on this study by coating the nanoparticles with multilayers of polyelectrolytes to promote the stability of the membranes (114).
Fig. 8.
A schematic illustration of the permeation of solutes through a nanoparticulate thermo- and pH-responsive membrane (adapted from: Zhang and Wu (114))
pH- and/or Thermo-Non-dependent Dual-Responsive Systems
Membranes with dual-responsive systems not originating from pH- and/or thermo-responsive properties have also been investigated (47,126). An early attempt by Kontturi and co-workers (126) led to the development of PVDF hydrophobic membranes grafted with PAA via radiation grafting. The resulting PVDF/PAA membranes demonstrated convective permeability that changed significantly with the pH and/or the salt concentration of surrounding fluids, thus representing an appropriate dual-responsive mechanism (126). Subsequently, the effects of a thermo-responsive polymer combined with a magnetic drug-targeting carrier were investigated. The magnetized drug carrier contained doxorubicin and was encapsulated within a thermo-responsive polymeric membrane consisting of dextran-g-poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) [dextran-g-p(NIPAAm-co-DMAAm)]. The drug release was found to be poor at a temperature below the LCST but at temperatures greater than the LCST, there was an initial period rapid drug release followed by controlled drug release (47). Yang and co-workers (127) prepared microcapsules with a dual-responsive behavior using a super paramagnetic porous membrane and thermo-responsive p(NIPAAm) gates. These microcapsules were found to be sensitive to both magnetic fields and environmental temperature. These types of responsive systems offer a high potential for application in the field of drug targeting such as delivering of anticancer drugs to tumor sites, and they are associated with reduced side-effects and controlled drug release in response to temperature changes, as well as magnetic flux (47). The microcapsules are directed to the target and remain there with the aid of the magnetic field (127). The permeability of the microcapsule membrane changed in response to environmental temperature, thus controlling the rate of drug release. A subsequent study investigated the effects of oleic acid modified Fe3O4 nanoparticles embedded in a polyamide microcapsule membrane. p(NIPAAm) chains were grafted onto membrane pores to function as thermo-responsive gates controlling the release of drugs. Other applications of pH- and/or thermo-non-dependent dual-responsive systems include controlled release of chemicals, and the production of biomedical and/or chemical sensors and micro-reactors (127).
OTHER MEDICAL APPLICATIONS OF SMART POLYMERS
Tissue Engineering
Tissue Engineering (TE) is an active and evolving field of scientific research as it is a prominent tool in regenerative medicine. TE scaffolds provide mechanical support, shape, and cell scale architecture for neo-tissue construction as seeded cells expand and organize in vivo (128). For tissues to be successfully regenerated, adequate cell propagation, and appropriate differentiation must be achieved in the three-dimensional cellular composite (129). Three-dimensional porous cell composites allow the attachment of individual cells to the scaffold surface, promoting cell growth, and maintaining the differentiated cell phenotypes. These cell composites may be produced form a range of materials including polymers and ceramics (130). Nonwoven fabrics have been widely used as scaffolds for tissue engineering application, although they have a relatively high porosity and large pore size, and have not been structurally optimized for specific applications. Thus, it has been noted that there is a need for a reliable method that can be easily used to modify the microstructure of nonwoven fibrous matrices so that they can be used as membranes for tissue engineering applications (129). Smart polymers offer promise for innovative improvements in TE scaffolds. Beyond the physical properties of polymers, a major objective is to impart smart biomaterials with the specific properties of signaling proteins such as ECM components and growth factors (128). Chitin membranes prepared from different chitin hydrogels have been investigated as scaffolds for tissue engineering by Nagahama and co-workers (129) for the growth of NIH/3T3 fibroblast cells. The chitin membranes showed good biodegradation, swelling, mechanical, and NIH/3T3 fibroblast cell growth properties. These novel, biodegradable chitin membranes are promising biomaterials in the field of tissue engineering (129).
In situ Forming Hydrogels
In situ forming systems have many biomedical applications including drug delivery, cell encapsulation, and tissue repair. These systems depend on the sol–gel characteristics of polymeric solutions, which are capable of forming a gel drug delivery system upon exposure to physiological conditions. Drug-containing polymeric solutions are injected into a physiological site, and upon exposure to the pH and/or temperature changes, a membrane is formed around the injected solution and gelation occurs. Drug diffusion occurs as the gelled system begins to degrade and dissolve in the surrounding physiological medium (131). Using the thermo-responsive approach in the development of in situ forming systems is beneficial compared to other methods such as solvent exchange (131), photo-polymerization (UV irradiation) (132), ionic crosslinking (133), or as a result of a change in pH (134), as it does not require organic solvents, co-polymerization agents, or an externally applied trigger for gelation to occur. These systems have potential for application in parenteral drug delivery (131), delivery of macromolecules such as glucose using crosslinked dextrans coupled with con A (111), in situ forming implants for prolonged drug delivery (135), orthopedic implants composed of methyl methacrylate polymers (which are thermally or redox cured), and dental fillings composed of dimethacrylate monomers (cured via photo-polymerization) (132).
CONCLUSIONS
‘Smart’ drug delivery systems have shown great potential in combating many disease states due to their targetability and in-built drug release mechanisms. Rather than acting exclusively as a drug carrier, these systems are able to respond to their environments, allowing drugs to be delivered solely to the target site and in response to a disease-related stimulus. This, in turn, allows for safer and more potent therapeutic interventions in many disease states thus improving the quality of life in countless patients. Stimuli-responsive polymers, used in the development of these drug delivery systems, have also been effectively employed in diverse medical fields for the development of implants, tissue engineering scaffolds, and biotechnological screening, in addition to a strong potential in drug delivery. Membranes have widely been utilized in purification techniques, artificial organs such as kidneys and livers, membrane oxygenators, and tissue engineering. Many transdermal, osmotic and diffusion-controlled systems depend on membrane technology. The potential for these systems to act as ‘on–off’ drug delivery systems is high. Patient compliance may be severely compromised in certain disease states requiring complicated therapeutic interventions, which are invasive in nature, require costly treatments, or necessitate long hospital stays. Thus, membranous drug delivery systems developed from the architecture of various drug delivery technologies have a significant potential in solving the problem of uncontrolled and untargeted delivery of drugs to different body sites. Research should therefore be aimed at developing more refined membranous drug delivery systems with enhanced intelligent capabilities using stimuli-responsive polymers for the treatment of various diseases.
References
- 1.Baker RW. Membrane technology and applications. Chichester: Wiley; 2004. [Google Scholar]
- 2.Stamatialis DF, Papenburg BJ, Girones M, Saiful S, Bettahalli SNM, Schmitmeier S, Wessling M. Medical applications of membranes: drug delivery, artificial organs and tissue engineering. J Membr Sci. 2008;308:1–34. [Google Scholar]
- 3.Santus G, Baker RW. Osmotic drug delivery: a review of the patent literature. J Control Release. 1995;35:1–21. [Google Scholar]
- 4.Lonsdale H. The growth of membrane technology. J Membr Sci. 1982;10:81–181. [Google Scholar]
- 5.Kumar A. Smart polymeric materials: Where chemistry and biology can merge. 2009. www.iitk.ac.in/directions/dirnet7/PP~ASHOK~FFF.pdf. Accessed: 29.03.2009.
- 6.Lue SJ, Hsu JJ, Wei TC. Drug permeation modeling through the thermo-sensitive membranes of poly (N-isopropylacrylamide) brushes grafted onto micro-porous films. J Membr Sci. 2008;321:146–154. [Google Scholar]
- 7.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Altinkaya SA, Yenal H, Ozbas B. Membrane formation by dry-cast process: model validation through morphological studies. J Membr Sci. 2005;249:163–172. [Google Scholar]
- 9.Sun G, Zhang XZ, Chu CC. Formulation and characterization of chitosan-based hydrogel films having both temperature and pH sensitivity. J Mater Sci Mater Med. 2007;18:1563–1577. doi: 10.1007/s10856-007-3030-9. [DOI] [PubMed] [Google Scholar]
- 10.Kao ST, Teng MY, Li CL, Kuo CY, Hsieh CY, Tsai HA, Wang DM, Lee KR, Lai JY. Fabricating PC/PAN composite membranes by vapor-induced phase separation. Desalination. 2008;233:96–103. [Google Scholar]
- 11.Matsuyama H, Teramoto M, Nakatani R, Maki T. Membrane formation via phase separation induced by penetration of non-solvent from vapor phase II: membrane morphology. J Appl Polym Sci. 1999;74:171–178. [Google Scholar]
- 12.Hanks PL, Lloyd DR. Deterministic model for matrix solidification in liquid-liquid thermally induced phase separation. J Membr Sci. 2007;306:125–133. [Google Scholar]
- 13.Li D, Krantz WB, Greenberg AR, Sani RL. Membrane formation via thermally induced phase separation (TIPS): model development and validation. J Membr Sci. 2006;279:50–60. [Google Scholar]
- 14.Matsuyama H, Okafuji H, Maki T, Teramoto M, Kubota Preparation of polyethylene hollow fiber membrane via thermally induced phase separation. J Membr Sci. 2003;223:119–126. [Google Scholar]
- 15.Hester JF, Olugebefola SC, Mayes AM. Preparation of pH-responsive polymer membranes by self-organization. J Membr Sci. 2002;208:375–388. [Google Scholar]
- 16.Lin DJ, Chang HH, Chen TC, Lee YC, Cheng LP. Formation of porous poly (vinylidene fluoride) membranes with symmetric or asymmetric morphology by immersion precipitation in the water/TEP/PVDF system. Eur Polym J. 2006;42:1581–1594. [Google Scholar]
- 17.Pereira CC, Nobrega R, Borges CP. Spinning process variables and polymer solution effects in the die-swell phenomenon during hollow fiber membrane formation. Braz J Chem Eng. 2000;17:599–606. [Google Scholar]
- 18.Lai PL, Shieh MJ, Pai CL, Wang CY, Young TY. A pH-sensitive EVAL membrane by blending with PAA. J Membr Sci. 2006;275:89–96. [Google Scholar]
- 19.Sun YM, Hsu SC, Lai JY. Transport properties of ionic drugs in the ammonio methacrylate copolymer membranes. Pharm Res. 2001;18(3):304–310. doi: 10.1023/a:1011098712693. [DOI] [PubMed] [Google Scholar]
- 20.Krause B, van der Vegt NFA, Wessling M. New ways to produce porous polymeric membranes by carbon dioxide foaming. Desalination. 2002;144:5–7. [Google Scholar]
- 21.Kim TG, Lee DS, Park TG. Controlled protein release from electrospun biodegradable fiber mesh composed of poly(caprolactone) and poly(ethylene oxide) Int J Pharm. 2007;338:276–283. doi: 10.1016/j.ijpharm.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomat. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Sanguanruksa J, Rujiravanit R, Supaphol P, Tokura S. Porous polyethylene membranes by template-leaching technique: preparation and characterization. Polym Test. 2004;23:91–99. [Google Scholar]
- 24.Roy-Chowdhury P, Kumar V. Fabrication and evaluation of porous 2, 3-dialdehydecellulose membrane as a potential biodegradable tissue engineering scaffold. J Biomed Mater Res A. 2006;76A(2):300–309. doi: 10.1002/jbm.a.30503. [DOI] [PubMed] [Google Scholar]
- 25.Cheng LH, Karim A, Seow CC. Characterization of composite films made of konjac glucomannan (KGM), carboxymethylcellulose (CMC) and lipid. Food Chem. 2008;107:411–418. [Google Scholar]
- 26.Ho MH, Kuo PY, Hsieh HJ, Hsien TY, Hou LT, Lai JY, Wang DM. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomat. 2004;25:129–138. doi: 10.1016/s0142-9612(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 27.Qiu M, Feng J, Fan Y, Xu N. Pore evolution model of ceramic membrane during constrained sintering. J Mater Sci. 2009;44:689–699. [Google Scholar]
- 28.Liu Y, Li K. Preparation of SrCe0.95Yb0.05O3-α hollow fibre membranes: study on sintering processes. J Membr Sci. 2005;259:47–54. [Google Scholar]
- 29.Feng J, Qiu M, Fan Y, Xu N. The effect of membrane thickness on the co-sintering process of bi-layer ZrO2/Al2O3 membrane. J Membr Sci. 2007;305:20–26. [Google Scholar]
- 30.Wang YH, Cheng JG, Liu XQ, Meng GY, Ding YW. Preparation and sintering of macroporous ceramic membrane support from Titania sol-coated alumina powder. J Am Ceram Soc. 2008;91:825–830. [Google Scholar]
- 31.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomat. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Discher DE, Ahmed F. Polymersomes. Ann Rev Biomed Eng. 2006;8:323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 33.Gil ES, Hudson SM. Stimuli-responsive polymers and their bioconjugates. Prog Polym Sci. 2004;29:1173–1222. [Google Scholar]
- 34.Chen S, Pieper R, Webster DC, Singh J. Triblock copolymers: synthesis, characterization, and delivery of a model protein. Int J Pharm. 2005;288:207–218. doi: 10.1016/j.ijpharm.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Guo BL, Gao QY. Preparation and properties of a pH/temperature-responsive carboxymethyl chitosan/poly(N-isopropylacrylamide) semi-IPN hydrogel for oral delivery of drugs. Carbohydr Res. 2007;342:2416–2422. doi: 10.1016/j.carres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Liu SQ, Wiradharma N, Gao SJ, Tong YW, Yang YY. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomat. 2007;28:1423–1433. doi: 10.1016/j.biomaterials.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Liu SQ, Tong YW, Yang YY. Thermally sensitive micelles self-assembled from poly(N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly(d, l-lactide-co-glycolide) for controlled delivery of paclitaxel. Mol Biosys. 2005;1(2):158–165. doi: 10.1039/b501756b. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Singh J. In vitro release of levonorgestrel from phase sensitive and thermo-sensitive smart polymer delivery systems. Pharm Dev Technol. 2005;10:319–325. doi: 10.1081/pdt-54479. [DOI] [PubMed] [Google Scholar]
- 39.Dispenza A. Radiation process of polymers. www.pa.ibf.cnr.it/nabla/T03.html. Accessed on 29.03.2009. dispenza@dicpm.unipa.it < dispenza@dicpm.unipa.it>
- 40.Obaidat AA, Park K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomat. 1997;18:801–806. doi: 10.1016/s0142-9612(96)00198-6. [DOI] [PubMed] [Google Scholar]
- 41.Park SB, You JO, Park HY, Haam SJ, Kim WS. A novel pH-sensitive membrane from chitosan TEOS IPN; preparation and its drug permeation characteristics. Biomat. 2001;22:323–330. doi: 10.1016/s0142-9612(00)00187-3. [DOI] [PubMed] [Google Scholar]
- 42.Ying L, Kang ET, Neoh KG, Kato K, Iwata H. Drug permeation through temperature-sensitive membranes prepared from poly(vinylidene fluoride) with grafted poly(N-isopropylacrylamide) chains. J Membr Sci. 2004;243:253–262. [Google Scholar]
- 43.Singh DK, Ray AR. Controlled release of glucose through modified chitosan membranes. J Membr Sci. 1999;155:107–112. [Google Scholar]
- 44.Chu LY, Liang YJ, Chen WM, Ju XJ, Wang HD. Preparation of glucose-sensitive microcapsules with a porous membrane and functional gates. Colloid Surf B Biointerfaces. 2004;37:9–14. doi: 10.1016/j.colsurfb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Xu T, Lin Z. Controlled release of ionic drug through the positively charged temperature-responsive membranes. J Membr Sci. 2006;281:491–499. [Google Scholar]
- 46.Varshosaz J, Falamarzian M. Drug diffusion mechanism through pH-sensitive hydrophobic/polyelectrolyte hydrogel membranes. Eur J Pharm Biopharm. 2001;51:235–240. doi: 10.1016/s0939-6411(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Misra RDK. Magnetic drug-targeting carrier encapsulated with thermo-sensitive smart polymer: core-shell nanoparticle carrier and drug release response. Acta Biomat. 2007;3:838–850. doi: 10.1016/j.actbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Ndesendo VMK, Pillay V, Choonara YE, Buchmann E, Bayever DN, Meyer LCR. Current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS PharmSciTech. 2008;9(2):505–520. doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang L, Feng X, Peurrung L, Viswanathan V. Temperature-sensitive membranes prepared by UV photopolymerization of N-isopropylacrylamide on a surface of porous hydrophilic polypropylene membranes. J Membr Sci. 1999;162:235–246. [Google Scholar]
- 50.Ichikawa H, Fukumori Y. A novel positively thermo-sensitive controlled-release microcapsule with membrane of nano-sized poly (N-isopropylacrylamide) gel dispersed in ethylcellulose matrix. J Control Release. 2000;63:107–119. doi: 10.1016/s0168-3659(99)00181-9. [DOI] [PubMed] [Google Scholar]
- 51.Kitano S, Koyama Y, Kataoka K, Okano T, Sakurai Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly(vinyl alcohol) and poly(N vinyl-2-pyrrolidone) with a phenylboronic acid moiety. J Control Release. 1992;19:162–170. [Google Scholar]
- 52.Gutowska A, Bark JS, Kwon IK, Bae YH, Cha Y, Kim SW. Squeezing hydrogels for controlled oral drug delivery. J Control Release. 1997;48:141–148. [Google Scholar]
- 53.Lue SJ, Hsu JJ, Chen CH, Chen BC. Thermally on–off switching membranes of poly (N-isopropylacrylamide) immobilized in track-etched polycarbonate films. J Membr Sci. 2007;301:142–150. [Google Scholar]
- 54.Mizutani A, Kikuchi A, Yamato M, Kanazawa H, Okano T. Preparation of thermo-responsive polymer brush surfaces and their interaction with cells. Biomat. 2008;29:2073–2081. doi: 10.1016/j.biomaterials.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Meng L, Lu X, Zhang L, He Y. Thermo and pH sensitive fluorescent polymer sensor for metal cations in aqueous solution. Poly Adv Tech. 2007;19(2):137–143. [Google Scholar]
- 56.Ankareddi I, Brazel CS. Synthesis and characterization of grafted thermo-sensitive hydrogels for heating activated controlled release. Int J Pharm. 2007;336:241–247. doi: 10.1016/j.ijpharm.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 57.Ohya S, Sonoda H, Nakayama Y, Matsuda T. The potential of poly(N-isopropylacrylamide) (PNIPAM)-grafted hyaluronan and PNIPAM-grafted gelatin in the control of post-surgical tissue adhesions. Biomat. 2005;26:655–659. doi: 10.1016/j.biomaterials.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Chen S, Singh J. Controlled delivery of testosterone from smart polymer solution based systems: in vitro evaluation. Int J Pharm. 2005;295:183–190. doi: 10.1016/j.ijpharm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Jeong BM, Lee DS, Bae YH, Kim SW. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res. 2000;50:171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 60.Kang GD, Cheon SH, Song SC. Controlled release of doxorubicin from thermo-sensitive poly (organophosphazene) hydrogels. Int J Pharm. 2006;319:29–36. doi: 10.1016/j.ijpharm.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 61.Kang GD, Cheon SH, Khang G, Song SC. Thermo-sensitive poly(organophosphazene) hydrogels for a controlled drug delivery. Eur J Pharm Biopharm. 2006;63:340–346. doi: 10.1016/j.ejpb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Soga O, van Nostrum CF, Fens M, Rijcken CJF, Schiffelers RM, Storm G, Hennink WE. Thermo-sensitive and biodegradable polymeric micelles for paclitaxel delivery. J Control Release. 2005;103:341–353. doi: 10.1016/j.jconrel.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Cohn D, Sosnik A, Garty S. Smart hydrogels for in situ generated implants. Biomacromol. 2005;6:1168–1175. doi: 10.1021/bm0495250. [DOI] [PubMed] [Google Scholar]
- 64.Na K, Lee KL, Lee DH, Bae YH. Biodegradable thermo-sensitive nanoparticles from poly(L-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur J Pharm Sci. 2006;27:115–122. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Sosnik A, Cohn D. Ethoxysilane-capped PEO-PPO-PEO triblocks: a new family of reverse thermo-responsive polymers. Biomat. 2004;25:2851–2858. doi: 10.1016/j.biomaterials.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 66.Chung HJ, Lee Y, Park TG. Thermo-sensitive and biodegradable hydrogels based on stereo-complexed Pluronic multi-block copolymers for controlled protein delivery. J Control Release. 2008;127:22–30. doi: 10.1016/j.jconrel.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Okahata Y, Noguchi H, Seki T. Thermo-selective permeation from a polymer-grafted capsule membrane. Macromol. 1986;19:493–494. [Google Scholar]
- 68.Chun SW, Kim JD. A novel hydrogel-dispersed composite membrane of poly(N-isopropylacrylamide) in a gelatin matrix and its thermally actuated permeation of 4-acetamidophen. J Control Release. 1996;38:39–47. [Google Scholar]
- 69.Ng CC, Cheng Y-L, Saville BA. Thermo-responsive polymer membrane for the local delivery of drugs. J Sex Reprod Med. 2001;1:21–27. [Google Scholar]
- 70.Atyabi F, Khodaverdi E, Dinarvand R. Temperature modulated drug permeation through liquid crystal embedded cellulose membranes. Int J Pharm. 2007;339:213–221. doi: 10.1016/j.ijpharm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Iwata H, Oodate M, Uyama Y, Amemiya H, Ikada Y. Preparation of temperature-sensitive membrane by graft polymerization onto a porous membrane. J Membr Sci. 1991;55:119–130. [Google Scholar]
- 72.Park YS, Ito Y, Imanishi Y. Permeation control through porous membranes immobilized with thermo-sensitive polymer. Langmuir. 1998;14:910–914. [Google Scholar]
- 73.Shtanko NI, Kabanov VY, Apel PY, Yoshida M, Vilenskii AI. Preparation of permeability-controlled track membranes on the basis of ‘smart’ polymers. J Membr Sci. 2000;179:155–161. [Google Scholar]
- 74.Yang B, Yang W. Thermo-sensitive switching membranes regulated by pore covering polymer brushes. J Membr Sci. 2003;218:247–255. [Google Scholar]
- 75.Spohr R, Reber N, Wolf A, Alder GM, Ang V, Bashford CL, Pasternak CA, Omichi H, Yoshida M. Thermal control of drug release by a responsive ion track membrane observed by radio tracer flow dialysis. J Control Release. 1998;50:1–11. doi: 10.1016/s0168-3659(97)00076-x. [DOI] [PubMed] [Google Scholar]
- 76.Chu LY, Park SH, Yamaguchi T, Nakao SI. Preparation of thermo-responsive core-shell microcapsules with a porous membrane and poly(N-isopropylacrylamide) gates. J Membr Sci. 2001;192:27–39. [Google Scholar]
- 77.Huang J, Wang XL, Qi WS, Yu XH. Temperature sensitivity and electrokinetic behavior of a N-isopropylacrylamide grafted microporous polyethylene membrane. Desalination. 2002;146:345–351. [Google Scholar]
- 78.Åkerman S, Viinikka P, Svarfvar B, Putkonen K, Järvinen K, Kontturi K, Näsman J, Urtti A, Paronen P. Drug permeation through a temperature-sensitive poly(N-isopropylacrylamide) grafted poly(vinylidene fluoride) membrane. Int J Pharm. 1998;164:29–36. [Google Scholar]
- 79.Alema H, Duwezb A-S, Lussis P, Lipnik P, Jonas AM, Demoustier-Champagne S. Microstructure and thermo-responsive behavior of poly(N-isopropylacrylamide) brushes grafted in nanopores of track-etched membranes. J Membr Sci. 2008;308:75–86. [Google Scholar]
- 80.Li SK, D’Emanuele A. On-off transport through a thermo-responsive hydrogel composite membrane. J Control Release. 2001;75:55–67. doi: 10.1016/s0168-3659(01)00365-0. [DOI] [PubMed] [Google Scholar]
- 81.Csóka G, Gelencsér A, Makó A, Marton S, Zelkó R, Klebovich I, Antal I. Potential application of Metolose® in a thermo-responsive transdermal therapeutic system. Int J Pharm. 2007;338:15–20. doi: 10.1016/j.ijpharm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 82.Don TM, Huang ML, Chiu AC, Kuo KH, Chiu WY, Chiu LH. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater Chem Phys. 2008;107:266–273. [Google Scholar]
- 83.Lemma F, Spizzirri UG, Puoci F, Muzzalupo R, Trombino S, Cassano R, Leta S, Picci N. pH-Sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int J Pharm. 2006;312:151–157. doi: 10.1016/j.ijpharm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Mania-Pramanik J, Kerkar SC, Mehta PB, Potdar S, Salvi VS. Use of vaginal pH in diagnosis of infections and its association with reproductive manifestations. J Clin Lab Anal. 2008;22(5):375–379. doi: 10.1002/jcla.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee YM, Shim JK. Preparation of pH/temperature responsive polymer membrane by plasma polymerization and its riboflavin permeation. Polymer. 1997;38:1227–1232. [Google Scholar]
- 86.Soppimath KS, Kulkarni AR, Aminabhavi TM. Chemically modified polyacrylamide-g-guar gum-based crosslinked anionic microgels as pH-sensitive drug delivery systems: preparation and characterization. J Control Release. 2001;75:331–345. doi: 10.1016/s0168-3659(01)00404-7. [DOI] [PubMed] [Google Scholar]
- 87.Khan MZ, Prebeg Ž, Kurjakovi N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: manipulation of drug release using Eudragit® L100-55 and Eudragit® S100 combinations. J Control Release. 1999;58:215–222. doi: 10.1016/s0168-3659(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 88.Jeong YI, Prasad YR, Ohno T, Yoshikawa Y, Shibata N, Kato S, Takeuchi K, Takada K. Application of Eudragit® P-4135F for the delivery of ellagic acid to the rat lower small intestine. J Pharm Pharmacol. 2001;53(8):1079–1085. doi: 10.1211/0022357011776469. [DOI] [PubMed] [Google Scholar]
- 89.Kim SY, Cho SM, Lee YM, Kim SJ. Thermo- and pH-responsive behaviors of graft copolymer and blend based on chitosan and N-isopropylacrylamide. J Appl Polym Sci. 2000;78:1381–1391. [Google Scholar]
- 90.Varshosaz J, Hajian M. Characterization of drug release and diffusion mechanism through hydroxyethyl methacrylate/methacrylic acid pH sensitive hydrogel. Drug Deliv. 2004;11(1):53–58. doi: 10.1080/10717540490265298. [DOI] [PubMed] [Google Scholar]
- 91.Tan JPK, Goh CH, Tam KC. Comparative drug release studies of two cationic drugs from pH-responsive nanogels. Eur J Pharm Sci. 2007;32:340–348. doi: 10.1016/j.ejps.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Sant VP, Smith D, Leroux JC. Enhancement of oral bioavailability of poorly water-soluble drugs by poly(ethylene glycol)-block-poly(alkyl acrylate-co-methacrylic acid) self-assemblies. J Control Release. 2005;104:289–300. doi: 10.1016/j.jconrel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 93.Ferreira L, Vidal MM, Gil MH. Evaluation of poly(2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int J Pharm. 2000;194:169–180. doi: 10.1016/s0378-5173(99)00375-0. [DOI] [PubMed] [Google Scholar]
- 94.Mahkam M. Using pH-sensitive hydrogels containing cubane as a crosslinking agent for oral delivery of insulin. Published online. 2005; Wiley InterScience (www.interscience.wiley.com). doi:10.1002/jbm.b.30279. [DOI] [PubMed]
- 95.Khare AR, Peppas NA. Release behavior of bioactive agents from pH-sensitive hydrogels. J Biomater Sci Polym Ed. 1993;4(3):275–289. doi: 10.1163/156856293x00564. [DOI] [PubMed] [Google Scholar]
- 96.Brahim S, Narinesingh D, Guiseppi-Elie A. Release characteristics of novel pH-sensitive p(HEMA-DMAEMA) hydrogels containing 3-(trimethoxy-silyl) propyl methacrylate. Biomacromol. 2003;4:1224–1231. doi: 10.1021/bm034048r. [DOI] [PubMed] [Google Scholar]
- 97.Lally S, Mackenzie P, LeMaitre CL, Freemont TJ, Saunders BR. Microgel particles containing methacrylic acid: pH-triggered swelling behavior and potential for biomaterial application. J Colloid Interface Sci. 2007;316:367–375. doi: 10.1016/j.jcis.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 98.Sant VP, Smith D, Leroux JC. Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: preparation and characterization. J Control Release. 2004;97:301–312. doi: 10.1016/j.jconrel.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Kou JK, Amidon GL, Lee PI. pH-dependent swelling and solute diffusion characteristics of poly(hydroxyethyl methacrylate-co-methacrylic acid) hydrogels. Pharm Res. 1988;5(9):592–597. doi: 10.1023/a:1015998131160. [DOI] [PubMed] [Google Scholar]
- 100.Satturwar P, Eddine MN, Ravenelle F. Jean-Christophe Leroux JC. pH-responsive polymeric micelles of poly(ethylene glycol)-b-poly(alkyl methacrylate-co-methacrylic acid): influence of the copolymer composition on self-assembling properties and release of candesartan cilexetil. Eur J Pharm Biopharm. 2007;65:379–387. doi: 10.1016/j.ejpb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Sáez-Martínez V, Pérez-Álvarez L, Merrero MT, Hernáez E, Katime I. pH-sensitive microgels functionalized with folic acid. Eur Polym J. 2008 [Google Scholar]
- 102.George M, Abraham TE. pH sensitive alginate-guar gum hydrogel for the controlled delivery of protein drugs. Int J Pharm. 2007;335:123–129. doi: 10.1016/j.ijpharm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Allcock HR, Ambrosio AM. Synthesis and characterization of pH-sensitive poly (organophosphazene) hydrogels. Biomat. 1996;17(23):2295–2302. doi: 10.1016/0142-9612(96)00073-7. [DOI] [PubMed] [Google Scholar]
- 104.Garner W. Polyphosphazene Polymers. http://www.ibridgenetwork.org/psu/polyphosphazene-polymers. Accessed on 29.03.2009.
- 105.Gudeman LF, Peppas NA. pH-sensitive membranes from poly(vinyl alcohol)/poly(acrylic acid) interpenetrating networks. J Membr Sci. 1995;107:239–248. [Google Scholar]
- 106.Tian D, Dubois PH, Jerome R. Biodegradable and biocompatible inorganic-organic hybrid materials: effect of acid content and water content on the incorporation of aliphatic polyesters into silica by the sol-gel process. Polymer. 1999;40:951–957. [Google Scholar]
- 107.Kai G, Min Y. AAc photo-grafted porous polycarbonate films and its controlled release system. J Control Release. 2001;71:221–225. doi: 10.1016/s0168-3659(00)00323-0. [DOI] [PubMed] [Google Scholar]
- 108.Shieh MJ, Lai PS, Young TH. 5-Aminosalicylic acid permeability enhancement by a pH-sensitive EVAL membrane. J Membr Sci. 2002;204:237. [Google Scholar]
- 109.Mika AM, Childs RF, Dickson JM. Chemical valves based on poly(4-vinylpyridine)-filled microporous membranes. J Membr Sci. 1999;153:45–56. [Google Scholar]
- 110.Hu K, Dickson JM. Modelling of the pore structure variation with pH for pore-filled pH-sensitive poly(vinylidene fluoride)-poly(acrylic acid) membranes. J Membr Sci. 2008;321:162–171. [Google Scholar]
- 111.Tang M, Zhang R, Bowyer A, Eisenthal R, Hubble J. A reversible hydrogel membrane for controlling the delivery of macromolecules. Biotechnol Bioeng. 2003;82(1):47–53. doi: 10.1002/bit.10539. [DOI] [PubMed] [Google Scholar]
- 112.Podual K, Doyle FJ, III, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J Control Release. 2000;67:9–17. doi: 10.1016/s0168-3659(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 113.Miyata T, Uragami T, Nakamae K. Biomolecule-sensitive hydrogels. Adv Drug Deliv Rev. 2002;54:79–98. doi: 10.1016/s0169-409x(01)00241-1. [DOI] [PubMed] [Google Scholar]
- 114.Zhang K, Wu XY. Modulated insulin permeation across a glucose-sensitive polymeric composite membrane. J Control Release. 2002;80:169–178. doi: 10.1016/s0168-3659(02)00024-x. [DOI] [PubMed] [Google Scholar]
- 115.Albin G, Horbett TA, Buddy Ratner BS. Glucose sensitive membranes for controlled delivery of insulin: insulin transport studies. J Control Release. 1985;2:153–164. [Google Scholar]
- 116.Albin GW, Horbett TA, Miller SR, Ricker NL. Theoretical and experimental studies of glucose-sensitive membranes. J Control Release. 1987;6:267–291. [Google Scholar]
- 117.Klumb LA, Horbett TA. Design of insulin delivery devices based on glucose-sensitive membranes. J Control Release. 1992;18:59–80. [Google Scholar]
- 118.Cartier S, Horbett TA, Ratner BD. Glucose-sensitive membrane coated porous filters for control of hydraulic permeability and insulin delivery from a pressurized reservoir. J Membr Sci. 1995;106:17–24. [Google Scholar]
- 119.Ito Y, Casolaro M, Kono K, Imanishi Y. An insulin releasing system that is responsive to glucose. J Control Release. 1989;10:195–203. [Google Scholar]
- 120.Chu LY, Li Y, Zhu JH, Wang HD, Liang YJ. Control of pore size and permeability of a glucose-responsive gating membrane for insulin delivery. J Control Release. 2004;97:43–53. doi: 10.1016/j.jconrel.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 121.Tritely T, Cohen Y, Cost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomat. 2000;21:1679–1687. doi: 10.1016/s0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 122.Shim WS, Kim JH, Park H, Kwa, Kim K, Kwon IC, Lee DS. Biodegradability and biocompatibility of a pH- and thermo-sensitive hydrogel formed from a sulphonamide-modified poly(e-caprolactone-co-lactide)-poly(ethylene glycol)-poly(e-caprolactone-co-lactide) block copolymer. Biomat. 2006;27:5178–5185. doi: 10.1016/j.biomaterials.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 123.Dong LY, Yan Q, Hoffman AS. Controlled release of amylase from a thermal and pH-sensitive, macroporous hydrogel. J Control Release. 1992;19:171–178. [Google Scholar]
- 124.Kim YH, Bae YH, Kim SW. pH/temperature-sensitive polymers for macromolecular drug loading and release. J Control Release. 1994;28:143–152. [Google Scholar]
- 125.Sun H, Liu S, Ge B, Xing L, Chen H. Cellulose nitrate membrane formation via phase separation induced by penetration of non-solvent from vapor phase. J Membr Sci. 2007;295:2–10. [Google Scholar]
- 126.Kontturi K, Mafé S, Manzanares JA, Svarfvar BL, Viinikka P. Modeling of the salt and pH effects on the permeability of grafted porous membranes. Macromol. 1996;29:5740–5746. [Google Scholar]
- 127.Yang WC, Xie R, Pang XQ, Ju XJ, Chu LY. Preparation and characterization of dual stimuli-responsive microcapsules with a super-paramagnetic porous membrane and thermo-responsive gates. J Membr Sci. 2008;321:324–330. [Google Scholar]
- 128.Furth ME, Atala A, Van Dyke ME. Smart biomaterials design for tissue engineering and regenerative medicine. Biomat. 2007;28:5068–5073. doi: 10.1016/j.biomaterials.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 129.Nagahama H, Nwe N, Jayakumar R, Koiwa S, Furuike T, Tamura H. Novel biodegradable chitin membranes for tissue engineering applications. Carbohydr Polym. 2008;73:295–302. [Google Scholar]
- 130.D’Acunto M, Ciardelli G, Narducci P, Rechichi A, Giusti P. Phospholipid-polyurethane adhesion force observed by atomic force microscopy. Mater Lett. 2005;59(13):1627–1633. [Google Scholar]
- 131.Haglund BO, Joshi R, Himmelstein KJ. An in situ gelling system for parenteral delivery. J Control Release. 1996;41:229–235. [Google Scholar]
- 132.Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Release. 2002;78:199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 133.Kakinoki S, Taguchi T, Saito H, Tanaka J, Tateishi T. Injectable in situ forming drug delivery system for cancer chemotherapy using a novel tissue adhesive: characterization and in vitro evaluation. Eur J Pharm Biopharm. 2007;66:383–390. doi: 10.1016/j.ejpb.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 134.Nanjawade BK, Manvi FV, Manjappa AS. In situ forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 135.Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58:445–455. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]