Abstract
Freeze-drying is a relatively expensive process requiring long processing time, and hence one of the key objectives during freeze-drying process development is to minimize the primary drying time, which is the longest of the three steps in freeze-drying. However, increasing the shelf temperature into secondary drying before all of the ice is removed from the product will likely cause collapse or eutectic melt. Thus, from product quality as well as process economics standpoint, it is very critical to detect the end of primary drying. Experiments were conducted with 5% mannitol and 5% sucrose as model systems. The apparent end point of primary drying was determined by comparative pressure measurement (i.e., Pirani vs. MKS Baratron), dew point, Lyotrack (gas plasma spectroscopy), water concentration from tunable diode laser absorption spectroscopy, condenser pressure, pressure rise test (manometric temperature measurement or variations of this method), and product thermocouples. Vials were pulled out from the drying chamber using a sample thief during late primary and early secondary drying to determine percent residual moisture either gravimetrically or by Karl Fischer, and the cake structure was determined visually for melt-back, collapse, and retention of cake structure at the apparent end point of primary drying (i.e., onset, midpoint, and offset). By far, the Pirani is the best choice of the methods tested for evaluation of the end point of primary drying. Also, it is a batch technique, which is cheap, steam sterilizable, and easy to install without requiring any modification to the existing dryer.
Key words: end point, freeze-drying, PAT, primary drying, residual water
INTRODUCTION
Many biopharmaceutical products are not sufficiently stable for long periods of time in aqueous solution, and the low temperature vacuum drying conditions afforded by lyophilization provide a safe and effective mechanism for removing the solvent and converting the material to a solid state thereby promoting long-term stability.
Freeze-drying is carried out in three steps: (1) freezing, (2) primary drying, and (3) secondary drying. In the freezing step, the shelf temperature is reduced to around −40°C, usually in several steps, converting most of the water into ice. The freeze concentrate thus formed usually contains 15–20% unfrozen water. Next, the system is evacuated, and the shelf temperature is increased (such that the product temperature is 2–3°C below the collapse temperature) to carry out primary drying/sublimation drying. Secondary drying is carried out at elevated temperatures to remove most of the unfrozen water from the solute phase by desorption (1). Increasing the shelf temperature for secondary drying before all of the ice is removed from the product will likely cause collapse or eutectic melt, which may have further consequence for product stability. The consequence of having product temperature during primary drying above the collapse temperature is loss of cake structure in the region adjacent to the ice–vapor interface due to glass transition in the amorphous product. An increase in product temperature above the collapse temperature results in viscous flow and, consequently, loss of the pore structure. Such a vial is normally rejected due to lack of elegance. Hence, the economic effect of excessive collapse may be rejection of the entire product batch. Also, collapsed product has lower specific surface area and hence slower secondary drying, which further results in high residual water and an increase in reconstitution time (2). Additionally, collapse can affect product stability due to higher residual water.
The focus of this research was to evaluate the various techniques that have been suggested for determination of the end point of primary drying, with emphasis on applicability to manufacturing processes where practicalities of implementation and concerns over sterility become critical. Freeze-drying is an expensive process with relatively long processing times. Of the three steps in freeze-drying, primary drying is the longest step, and hence, optimization of this step is the focus in industry. Frequently, the end point of primary drying is determined from the product thermocouples, but vials containing temperature sensors are not representative of the entire batch. The vials containing thermocouples nucleate at higher temperature (i.e., less degree of supercooling) than vials without thermocouples. Consequently, any vials containing thermocouple dry faster due to lower product resistance. The time at which the product temperature approaches the shelf temperature indeed marks the end of primary drying for the vials containing thermocouples but not necessarily for the rest of the batch. To account for the differences in the freezing and drying behavior of thermocouple and non-thermocouple vials, an additional soak period of 10–30% is often provided. Also, thermocouples are not compatible with automatic loading system and are usually placed in the front row vials facing the dryer door which experience higher heat transfer due to atypical radiation effects (3). Moreover, the thermocouples are manually placed in the vials by an operator and hence potentially compromise the sterility of the product in a manufacturing operation.
With a technique that can determine the end point of primary drying for the entire batch, the traditionally used additional soak time method can be avoided. With such a technique, the process can meet one of the key objectives in freeze-drying process development and scale-up; that is, one may decrease the cycle time and optimize the process without risking product collapse due to premature increase of shelf temperature for secondary drying. The critical questions to ask before selecting a technique to determine the end point of primary drying is: (a) has all ice been removed, and is the water content sufficiently low to avoid collapse if the temperature is increased for secondary drying? and (b) what is the water content at the end of primary drying as determined by the technique? With the launch of process analytical technology (PAT) initiatives by the Food and Drug Administration, many new online monitoring devices have become available for freeze-drying process control. PAT aims to finalize the quality decision process during the manufacturing process through the assessment of critical process parameters rather than assessing quality offline at the end of process. Thus, not only from process control and optimization stand point but also for product quality, it is very important to detect the precise end point of primary drying.
Nevertheless, applying PAT to freeze-drying faces many problems due to lack of availability of compatible monitoring equipment, particularly for production applications. The extreme conditions of the freeze-drying process (low temperature, low pressure, and sterility) render many devices inappropriate. Therefore, the quest for new monitoring devices to control the freeze-drying process with high degree of assurance has become essential. Currently, there are several potential ways to detect the end point of primary drying, and for this paper the following techniques were studied and compared.
Techniques based on gas composition in the product chamber:
Comparative pressure measurement (i.e., Pirani vs. capacitance manometer)
Dew point monitor (electronic moisture sensor)
Process H2O concentration from tunable diode laser absorption spectroscopy (TDLAS)
Lyotrack (gas plasma spectroscopy)
Others:
-
5.
Product thermocouple response
-
6.
Condenser pressure
-
7.
Pressure rise test (manometric temperature measurement (MTM) or variations of this method)
The first four techniques involve various methods of measuring gas composition in the drying chamber or the duct connecting the chamber and the condenser. During primary drying, the gas composition is essentially all water vapor (4). This is because the molar flux of water vapor is much higher than that of nitrogen, which is used to control the chamber pressure. When essentially all of the vials are finished with primary drying, the gas composition changes from mostly water vapor to nitrogen. Hence, a sharp drop in water vapor composition during primary drying can be used as an indication of the end point of primary drying.
COMPARATIVE PRESSURE MEASUREMENT (i.e., PIRANI vs. CAPACITANCE MANOMETER)
During the drying step, the chamber pressure is controlled using a capacitance manometer, which measures the absolute pressure in the drying chamber. However, the Pirani vacuum gauge works on the principle of measuring the thermal conductivity of the gas in the drying chamber (5). The Pirani gauge reads about 60% higher than the capacitance manometer (i.e., MKS Baratron) during primary drying when essentially all of the gas in the chamber is water vapor. This is because the thermal conductivity of water vapor is ∼1.6 times the thermal conductivity of nitrogen. With this inherent property, the Pirani vacuum gauge can be used to detect the end of primary drying. The point where the Pirani pressure starts to sharply decrease (i.e., onset) indicates that the gas composition is changing from mostly water vapor to nitrogen; i.e., sublimation is “essentially” complete (Fig. 1).
Fig. 1.
Pirani pressure, dew point, TDLAS (process [H2O]), Lyotrack (gas composition), and P ice (vapor pressure of ice from pressure rise test) profile during primary drying
DEW POINT
An electronic moisture sensor can be used to measure the frost point, which is the temperature at which ice has an equilibrium vapor pressure equal to the measured partial pressure of water. The measurement is based on the principle of changes in the capacitance of a thin film of aluminum oxide arising from adsorption of water at a given partial pressure. The sensor further translates the capacitance into a voltage output which is “calibrated” to read the frost point/dew point or the partial pressure of water (6). Similar to the Pirani, the point where “dew point” starts dropping indicates that the sublimation is “essentially” complete, i.e. gas composition is changing from mostly water vapor to nitrogen (Fig. 1).
PROCESS H2O CONCENTRATION VIA TDLAS
Tunable diode laser absorption spectroscopy (TDLAS) directly measures the water vapor concentration (molecules/cm3) in the duct connecting the chamber and the condenser. The TDLAS unit is commonly installed with two laser beams, one directed with and the other directed against the vapor flow. TDLAS works on basic spectroscopic principles measuring absorption of radiation by water vapor to monitor the trace concentration of water vapor in real time. A laser beam is passed through a gas mixture containing a quantity of the target gas, and the beam’s wavelength is tuned to one of the target gas’s absorption lines to accurately measure the absorption of that beam from which one can deduce the average concentration of target gas molecules integrated over the beam’s path length (6). The gas flow velocity can also be measured from Doppler shifted water vapor absorption spectrum (i.e., how much the peak absorption wavelength has shifted between the two beams or shifted relative to a reference zero velocity gas sample). Further, the sublimation rate can be determined from the gas flow velocity and concentration of water vapor. TDLAS is becoming widely popular as it can determine sublimation rate during primary drying in real time (7). However, for the present study, it is the water vapor concentration profiles during primary drying that are used to determine the end point of primary drying. The point where water concentration starts decreasing sharply (i.e., onset) indicates that the gas composition is changing, and hence sublimation is “essentially” complete (Fig. 1).
LYOTRACK (GAS PLASMA SPECTROSCOPY)
This method is the latest addition to the online monitoring devices for freeze-drying and is manufactured by Alcatel Vacuum Technology, France. Lyotrack is based on optical emission spectroscopy and measures water vapor concentration during the drying process. It consists of a plasma generator and an optical spectrometer. The plasma generator creates a radio frequency wave (440 MHz) whose energy is transmitted to the gases present inside the plasma tube via the radio frequency antenna. The gas inside the plasma tube becomes a plasma, which is composed of atoms and molecules (excited or not), ions, electrons, and photons. The excited atom is not stable and upon de-excitation will return instantaneously to its initial energy level. In this phase, it will emit light. The wavelengths of the emitted light are the characteristic signatures for the identification of the atom or molecule. The point where water vapor concentration starts sharply decreasing (i.e., onset) indicates that the gas composition is changing, and hence sublimation is “essentially” complete (Fig. 1).
Mayeresse et al. (8) have shown that Lyotrack can be used to determine the end point of primary drying with good reproducibility and sensitivity. They also studied the effect of positioning of the probe in the freeze-dryer (i.e., product chamber, duct connecting the chamber and the condenser, and the condenser chamber). Lyotrack gas composition signal was sensitive to gas composition in the chamber as well as the duct but not in the condenser.
Since this technique involves ionization of the gas species, there could be potential in-process degradation of drug products via free radical oxidation. To address this issue, in this research, Lyotrack was used to freeze-dry human growth hormone (hGH), and the degradation via methionine oxidation was studied.
PRODUCT TEMPERATURE DURING PRIMARY DRYING
The end point of primary drying can also be determined from the product thermocouple response, assuming the vials containing the thermocouples are representative of the batch as a whole (9). Product temperature approaching the shelf temperature set point (i.e., “offset” in Fig. 2) is commonly taken as an indication of the end of primary drying. Indeed, primary drying is over in the monitored vial(s) even just slightly after the “onset” point, but the monitored vials are not representative of the batch as a whole, particularly in manufacturing operations carried out in a class 100 environment; here, the low particulate environment results in a large difference in degree of supercooling between thermocouple and non-thermocouple containing vials (6). As mentioned earlier, thermocouple vials nucleate at higher temperature, resulting in low product resistance and hence faster drying. Thus, primary drying times from product thermocouples may be seriously biased, and the actual primary drying time may be much longer, particularly in manufacturing. In addition, the edge vials, where thermocouples are commonly placed in a manufacturing process, dry faster than the center vials because of the side radiation effects from the walls and door of the chamber (3). Usually, a soak period of 10–20% is added to primary drying time once the thermocouple vials reach the shelf temperature to compensate for the bias (10), although the actual time required could be less or much more.
Fig. 2.
Product temperature and condenser pressure profile during primary drying
CONDENSER PRESSURE DURING PRIMARY DRYING
Yet another indicator of the end point of primary drying is the condenser pressure. During primary drying, most of the gas in the chamber is water vapor (4), and because the total vapor flux is high, a high ΔP (difference between chamber and condenser pressure) develops to remove the water from the chamber. However, once primary drying is over, ΔP decreases (i.e., condenser pressure (Pcond) increases since chamber pressure (Pc) is held constant). The condenser pressure reflects mostly the partial pressure of nitrogen in the condenser. The purpose of the nitrogen bleed is to control the chamber pressure at the desired set point. The point where condenser pressure starts increasing (i.e., onset) indicates that the sublimation is “essentially” over since the high mass transfer portion of the process (i.e., sublimation) is largely over (Fig. 2). A capacitance manometer installed in the condenser reads the condenser pressure.
PRESSURE RISE TEST
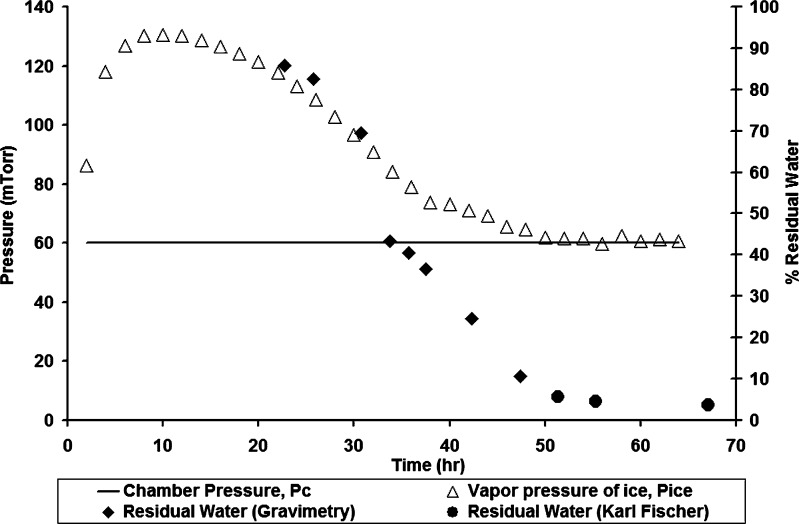
MTM is a procedure to measure the product temperature during primary drying by quickly isolating the chamber from the condenser for a short time (≈25 s) and analyzing the pressure rise during this period. This analysis yields vapor pressure of ice at the sublimation interface, the product temperature, and the mass transfer resistance of the dried product (11). However, the data obtained measure the vapor pressure of ice accurately only as long as the system remains in primary drying. At the end of primary drying, there is little or no pressure rise because all ice is gone, and hence the calculated “vapor pressure of ice” becomes equal to the chamber pressure (Fig. 1). Thus, a close approach of the calculated vapor pressure of ice to the chamber pressure forms the basis of the criterion for end of primary drying.
The objective of this research is to make a comprehensive comparison of the various available techniques for determining the end point of primary drying. Specifically the apparent end point of primary drying is determined at the onset, midpoint, and offset for each technique, and samples are compared for residual water content and cake appearance after primary drying. As mentioned earlier, residual water and the cake structure are the critical product quality attributes. It will be demonstrated that the apparent primary drying time, as determined by the various techniques, can be different as a result of their different working principles. One might be inclined to select a technique that provides a relatively shorter drying time thinking that this technique is quick in response, but this practice could lead to product defects when the samples contain trace ice or high residual water resulting in melt-back or collapse during the secondary drying ramp in temperature. Reports in the literature suggest how these methods can be used to determine the end point of primary drying, but no study compares residual water content and the cake appearance at the apparent end point of primary drying as determined by these various techniques. Such a comparison is the main subject of this paper.
MATERIALS AND METHODS
Crystalline sucrose and d-mannitol USP were obtained from Sigma Aldrich (St. Louis, MO). Vials used for freeze-drying were 5 mL tubing vials from West Pharmaceutical Co (Lionville, PA). Stoppers were 20 mm finish Daikyo Flurotec® stoppers (West Pharmaceutical Co.) designed for lyophilization. Human growth hormone was supplied by Eli Lilly & Co. (Indianapolis, IN). All hGH solutions were buffered to pH 7.4 with 0.277 mg Na2HPO4·7H2O per mg hGH, with final pH adjustment with NaOH or H3PO4. The hGH-sucrose formulation was prepared in the ratio of 1:61.
A dew point monitor (General Eastern, Model# MMY31), capacitance manometer (MKS Baratron, Model# 662), Pirani gauge (Granville Phillips, Helix Technology Corporation, Model# 225), and Lyotrack (Alcatel Vacuum Technologies, France) were mounted on the ports provided on top of the product chamber. Condenser pressure was measured using a capacitance manometer installed in the foreline (OD 2 cm) just above the condenser chamber which connected the condenser chamber with the vacuum pump. TDLAS (Physical Sciences Inc. Model# Lyoflux 100) was installed in the duct connecting the chamber and the condenser above the isolation valve.
Freeze-Drying
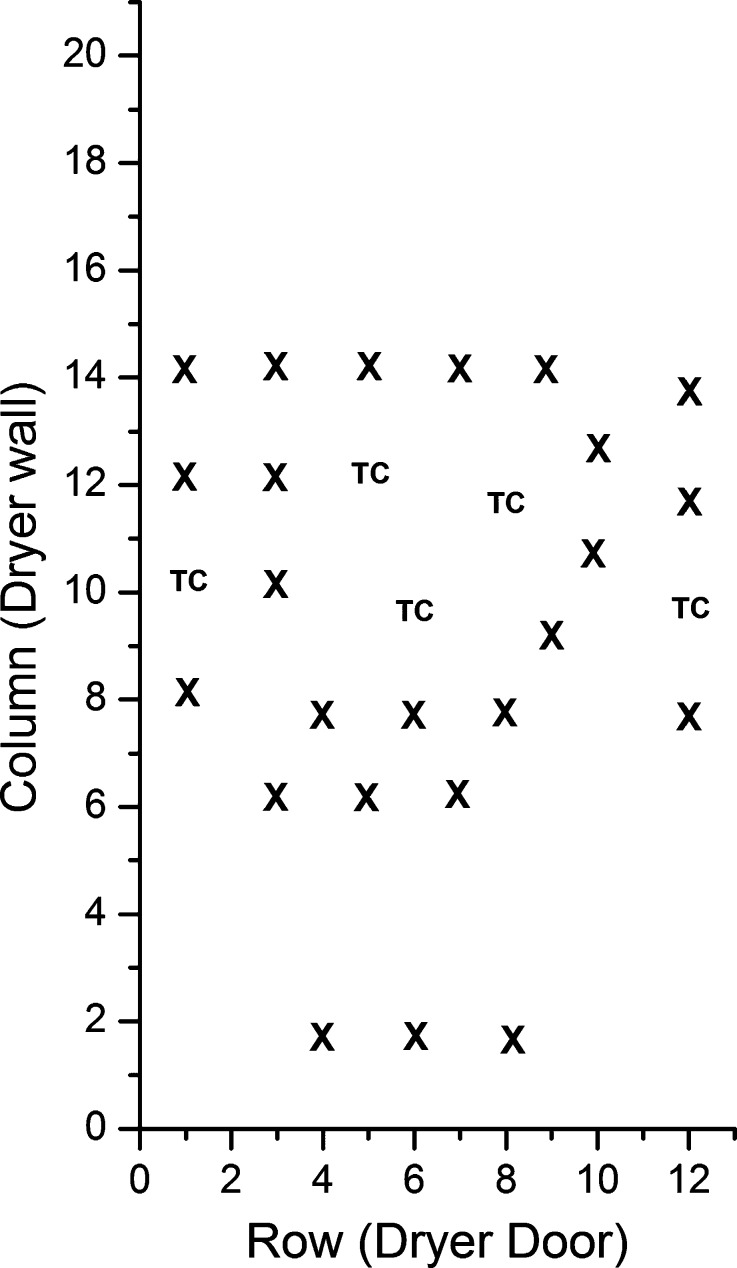
We studied two commonly used excipients as products, 5% (w/v) mannitol and 5% (w/v) sucrose. Freeze-drying was performed in a laboratory-scale Lyostar II freeze-dryer (SP Industries, Stone Ridge, NY) with three shelves and a total shelf surface area of 0.5 m2. A chamber door with a sampling thief was used. Aqueous solutions of the solutes were prepared and filtered through a 0.22-μm membrane filter. A total of 240 vials were filled with 3 mL of solution in each and loaded on to the center shelf of the freeze-dryer. The height of the shelves was adjusted to facilitate easy removal of vials during primary drying using the sample thief. Product temperature was measured using 28-gauge copper-constantan (type T) thermocouples (Omega Engineering, Inc., Stamford, CT) with a resolution of ±0.1°C.Thermocouples were placed in the bottom center of both edge and center vials. The arrangement of sampling vials along with the thermocouple vials is shown in Fig. 3.
Fig. 3.
Arrangement of thermocouple and sampling vials on the shelf (X sampled vials, TC vials containing thermocouple)
A total of four runs were performed, where runs #1 and #2 are for 5% sucrose, while runs #3 and #4 are for 5% mannitol.
The freezing–drying protocol for 5% (w/v) sucrose (runs #1 and #2) was as follows:
Freezing at 1°C/min to −40°C and holding for at least 120 min
Ramping at 0.2°C/min to −25°C at a chamber pressure of 60 mTorr and holding until the desired indicator of primary drying has reached the final plateau value
The freeze-drying protocol for 5% (w/v) mannitol (runs #3 and #4) was as follows:
Freezing at 1°C/min to −40°C and holding for at least 120 min
Ramping at 1°C/min to 5°C at a chamber pressure of 150 mTorr and holding until the desired indicator of primary drying has reached the final plateau value
Freeze-Drying Procedure for hGH Formulations
Vials were filled with 1 mL of 2 mg/mL hGH and loaded onto the center shelf of the freeze-dryer.
The freeze-drying protocol was as follows:
Freezing at 1°C/min to −40°C and holding for at least 120 min
Ramping at 0.3°C/min to −30°C at a chamber pressure of 100 mTorr and holding until the Pirani pressure reached the final plateau value
Ramping 0.2°C/min to +30°C at a chamber pressure of 100 mTorr and holding for 240 min
After secondary drying, the product chamber was vented with dry nitrogen, and the vials were sealed by stoppering in the freeze-dryer.
Reverse-Phase High-Performance Liquid Chromatography Assay
In aqueous solution as well as in solid state, human growth hormone undergoes chemical decomposition via oxidation and deamidation, which was measured by reverse-phase high-performance liquid chromatography (RP-HPLC) using Vydac C4 column with mobile phase of 29% n-propanol and 71% 0.05 M Tris buffer at pH 7.5 (12, 13).
Steam Sterilization of the Pirani Gauge
The Pirani gauge was subjected to three steam sterilization cycles (125°C, 25 psi, 30 min) to test if it can withstand steam sterilization without showing loss in performance.
Determination of Vapor Pressure of Ice
The manometric temperature measurement procedure was executed to determine the vapor pressure of ice (11, 14). The procedure consists of closing the valve between the chamber and the condenser for a brief period of time (25 s) and recording the chamber pressure with time at a frequency of ten data points per second. The MTM equation is fitted to the pressure rise data to obtain vapor pressure of ice at the sublimation interface.
ANALYSIS OF PRODUCT
Determination of Residual Water
Vials were extracted from the drying chamber during primary drying using a sample thief to determine % residual water. When the vials selected had a complete melt-back (i.e, “puddle of solution”) after warming to room temperature, due to presence of residual ice, residual water was calculated gravimetrically. For the vials that retained cake structure, residual water was determined by using Karl Fisher residual moisture analyzer (Metrohm, KF756).
Karl Fisher Residual Water Analysis
The freeze-dried cake was reconstituted with 3 ml of “dry” methanol, and 0.5 mL of the solution was injected into the titration cell. Knowing the weight of the sample and the amount of water in dry methanol, the water content in the sample could be determined.
Visual Characterization
Product was visually characterized and classified as melt-back (due to presence of ice), cake collapse (due to high %H2O), or retention of cake structure.
RESULTS AND DISCUSSION
Comparison of Lyotrack and Pirani
The water vapor composition during primary drying as measured using Lyotrack for 10% sucrose is shown in Fig. 4. For comparison, the Pirani pressure is plotted on the same graph, and as one might expect since both measure vapor composition at the same location, Lyotrack and Pirani follow the same trend not only during primary drying but also secondary drying. Similar results were obtained for lysozyme, BSA/sucrose, and hGH (data not shown). Thus, we conclude that the percent residual water, primary drying time, and the cake appearance for Lyotrack would be the same as Pirani at the onset, midpoint, and offset.
Fig. 4.
Comparison of Lyotrack and the Pirani profile during primary drying (10% sucrose, 2 mL fill in 10 cm3, 20 mm vials). Dotted line represents the Lyotrack profile, whereas the solid line is the Pirani pressure profile
Free Radical Oxidation of hGH
Since Lyotrack ionizes the gas present in the chamber to create a plasma, there is potential for free radical oxidation of pharmaceutical drug products. Basically, plasma is partially ionized gas containing atoms, molecules, and ions with free electrons (i.e., free radicals). If free radicals are present in the gas phase, they are likely to be adsorbed on the product and consequently cause degradation of product susceptible to free radical oxidation2. Results for percent decomposition of hGH using RP-HPLC are shown in Fig. 5. Freeze-dried hGH-sucrose formulation showed about the same level of in-process degradation (∼12%) as does freeze-dried hGH without any lyoprotectant. When freeze-dried without using Lyotrack, hGH as well as hGH-sucrose is quite stable, and there is very little in-process decomposition via oxidation (<0.5%) (12). The fact that when Lyotrack was used there was a substantial decomposition, even in the hGH formulation stabilized by sucrose, indicates that Lyotrack should be used with caution when processing a product susceptible to degradation via oxidation.
Fig. 5.
Percent increase in decomposition of hGH after freeze-drying (A = freeze-dried hGH (using Lyotrack), B = freeze-dried hGH+sucrose (using Lyotrack), C = freeze-dried hGH or hGH+sucrose (without using Lyotrack))
A recent publication reported that the Lyotrack signal is not greatly altered when installed in the duct rather than in the chamber, and the sharp drop in signal for both locations occurs about the same time (8). Hence, one possible way to minimize oxidation when using Lyotrack would be to install it in the duct connecting the chamber and the condenser. However, this might require some modifications to the existing dryer.
Comparison of Apparent End Point of Primary Drying and Residual Water
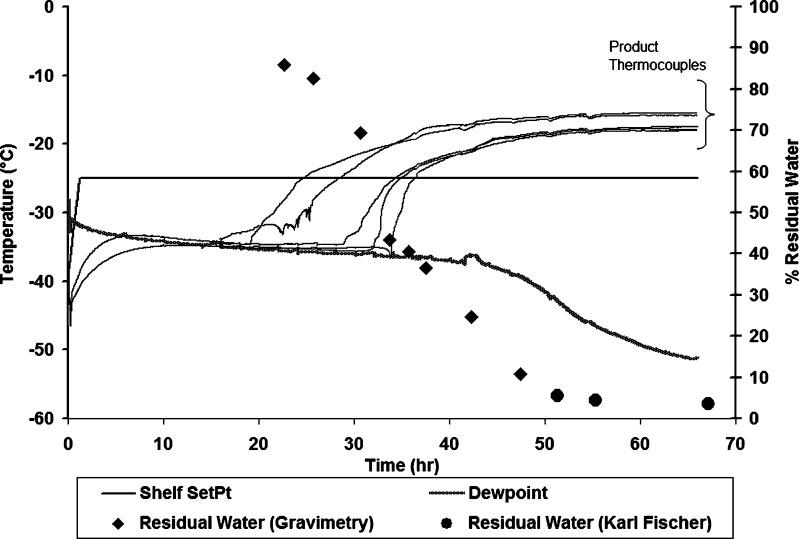
The drying profiles along with the percent residual water for each of the techniques discussed above are shown in Figs. 6, 7, 8, 9, and 10 for 5% sucrose. The residual water data during primary drying are combined from two runs, assuming that the conditions were identical in both the runs. Similar profiles were also obtained for 5% mannitol (data not shown). In Fig. 6, product temperature (both edge and center locations) as measured by thermocouples (TC), dew point (DP), shelf temperature set point (Shelf SetPt), and residual water for center vials is plotted against drying time for 5% sucrose. As primary drying nears completion, there is a sharp rise in product temperature, as well as a sharp drop in condenser temperature (2–3°C) due to the decreased load on the condenser (data not shown). In Fig. 7, chamber pressure as measured by a capacitance manometer, chamber pressure as measured by the Pirani gauge (Pirani), and chamber pressure set point (Vac SetPt) are plotted against drying time. Similarly, the condenser pressure profile during primary drying is shown in Fig. 8. As the sublimation rate decreases, the Pirani pressure decreases and condenser pressure increases, although the increase in condenser pressure is very small (∼4 mTorr). In Fig. 9, process water concentration (i.e., concentration of water vapor in the gas mixture) determined by the TDLAS unit and residual water are plotted against drying time for 5% sucrose. Again, as primary drying nears completion, there is a sharp decline in process water concentration. In Fig. 10, the vapor pressure of ice determined by MTM (Pice) and residual water are plotted against primary drying time. The chamber pressure (Pc) is also shown for comparison. During the portion of primary drying when most vials are still in primary drying, the vapor pressure of ice evaluated by MTM is a valid measure of the sublimation interface temperature, and as product temperature rises during primary drying, the vapor pressure of ice evaluated by MTM increases. However, as more vials finish primary drying, and the amount of vapor being generated by sublimation decreases, the pressure rise decreases as well, and the calculated value of the vapor pressure of ice decreases toward the chamber pressure. When the primary drying is “essentially” complete, the vapor pressure of ice equals the chamber pressure within a few millitorrs.
Fig. 6.
Temperature and percent residual water profile during primary drying for 5% sucrose
Fig. 7.
Pirani vs. differential capacitance manometer and percent residual water profile during primary drying for 5% sucrose
Fig. 8.
Condenser pressure and percent residual water profile during primary drying for 5% sucrose
Fig. 9.
Process [H2O] from TDLAS and percent residual water profile during primary drying for 5% sucrose
Fig. 10.
Vapor pressure of ice and percent residual water during primary drying for 5% sucrose (P c chamber pressure, P ice vapor pressure of ice at sublimation interface)
Residual Water Analysis
Tables I and II show the approximate residual water at the onset, midpoint, and offset of the various indicators of primary drying end point for 5% sucrose and 5% mannitol, respectively. These residual water values were estimated from the residual moisture vs. time data (i.e., as illustrated in Figs. 6, 7, 8, 9, and 10) profile of two runs combined together and by interpolation and extrapolation between data points as necessary to obtain residual moisture values at times corresponding to each of the measures of “end point.”
Table I.
Percent Residual Water at the Onset, Midpoint, and Offset for the Various Potential Indicators of Primary Drying End Point for 5% Sucrose
| Indicator | Approximate % residual water | ||
|---|---|---|---|
| Onset | Midpoint | Offset | |
| Pirani | 25 | 9 | 5 |
| Lyotrack | 25 | 9 | 5 |
| Condenser pressure | 88 | 67 | 25 |
| Dew point | 25 | 6 | 3 |
| Product TC | 43 | 33 | 25 |
| Process [H2O] from TDLAS | 41 | 24 | 10 |
| Pressure rise test | 92 | 69 | 18 |
Table II.
Percent Residual Water at the Onset, Midpoint, and Offset for the Various Potential Indicators of Primary Drying End Point for 5% Mannitol
| Indicator | Approximate % residual water | ||
|---|---|---|---|
| Onset | Midpoint | Offset | |
| Pirani | 9 | 5 | 4 |
| Lyotrack | 9 | 5 | 4 |
| Condenser pressure | 87 | 61 | 9 |
| Dew point | 5 | 4 | 2 |
| Product TC | 63 | 12 | 5 |
| Process [H2O] from TDLAS | 10 | 5 | 4 |
| Pressure rise test | 75 | 29 | 5 |
For 5% sucrose, Pirani, Lyotrack, and dew point all show a similar trend, with residual water around 10% at the midpoint. However, residual water is relatively high at the midpoints of condenser pressure, product TC, and pressure rise test. Indeed, at the offset of condenser pressure change, there is still about 25% residual water in the product. This observation suggests that condenser pressure is not a good indicator of end point of primary drying, at least for amorphous materials like sucrose. In addition, the onset, midpoint, and the offset are not clearly defined with condenser pressure since the condenser pressure rises only slightly, and this small increase is gradual. One would expect a small condenser pressure increase with low sublimation loads, such as products being dried at low temperature, such as sucrose formulations, and particularly with partial loads. The rise in condenser pressure is more clearly defined with a full load (i.e., all shelves loaded fully) compared to partial load (one third of full load used in this study) (16). Also, it is important to note that there was ∼25% residual water at the offset of product thermocouple response. This observation indicates that the thermocouple vials dry slightly faster than the rest of the batch, even in a laboratory run where the drying bias introduced by the thermocouple is generally small. The midpoints of Pirani, Lyotrack, and dew point appear to be good indicators of the end of primary drying, with residual water being <15%. It is significant to point out that the signal from the Pirani gauge remained unaffected even after three steam sterilization cycles (data not shown). That is, the Pirani gauge does withstand steam sterilization in spite of some claims to the contrary3.
For 5% mannitol, Pirani, Lyotrack, dew point, and TDLAS water concentration, all indicate a residual water of around 5% at the midpoint. Values at the onset and offset are also similar for each method. The residual water at the midpoint and the offset for condenser pressure is less than 11%, indicating that the condenser pressure can likely be utilized to monitor the end point of primary drying with mannitol and by analogy, likely for any crystalline solute system. Also, the offset of product thermocouple end point occurs at a residual water of ∼10%.
Analysis of Apparent Primary Drying Time
For 5% sucrose, the condenser pressure measure of apparent primary drying time is the shortest (Table III) among all the indicators. This is expected since mass flux will decrease as the number of vials still in primary drying decreases, even when the gas composition remains at essentially 100% water vapor. Generally, apparent primary drying times from dew point and Pirani measures agree fairly well, but the offset of dew point was not as sharp or obvious. However, the onsets for both the methods are in good agreement with identical drying times and residual water content, being around 25% in both cases. Apparent primary drying time at the offset of product thermocouple is 42.3 h with residual water of around 25%, and hence a soak period of 4–5 h (∼10%) is required before the cycle could be advanced into secondary drying without undue risk of collapse. The increasing order of primary drying time is as follows:
At onset: pressure rise test < condenser pressure < product TC < TDLAS < Pirani = Lyotrack = dew point
At midpoint: condenser pressure < pressure rise test < product TC < TDLAS < Pirani = Lyotrack < dew point
At offset: condenser pressure < product TC < pressure rise test < TDLAS < Pirani = Lyotrack < dew point
Table III.
Approximate Primary Drying Time at the Onset, Midpoint, and Offset for the Various Potential Indicators of Primary Drying End Point for 5% Sucrose
| Indicator | Approximate primary drying time (h) | ||
|---|---|---|---|
| Onset | Midpoint | Offset | |
| Pirani | 42.3 | 48.3 | 54.3 |
| Lyotrack | 42.3 | 48.3 | 54.3 |
| Condenser pressure | 20.2 | 29.9 | 42.2 |
| Dew point | 42.3 | 51.2 | ≈60 |
| Product TC | 33.9 | 38.1 | 42.3 |
| Process [H2O] from TDLAS | 35.7 | 41.5 | 47.3 |
| Pressure rise test | 18 | 31 | 44 |
For 5% mannitol, the onsets of Pirani and dew point primary drying times were 13.9 and 14.5 h, respectively (Table IV), with the residual water being roughly 10% in both cases (Table II). Thus, at onset there is good agreement in drying time between Pirani and dew point, which is expected since both of the techniques are essentially measuring the concentration of water vapor in the drying chamber. Also, similar to 5% sucrose, the offset of dew point was not sharp and obvious. It is interesting to note that the time difference between the Pirani onset and offset for 5% sucrose is 12 h and only 1.6 h for 5% mannitol, a reflection of the much slower secondary drying in an amorphous system (sucrose) than in a crystalline system (mannitol). The increasing order of primary drying time is as follows:
At onset and midpoint: condenser pressure < pressure rise test < product TC < TDLAS < Pirani = Lyotrack < dew point
At offset: condenser pressure < pressure rise test = product TC < TDLAS = Pirani = Lyotrack < dew point
Table IV.
Approximate Primary Drying Time at the Onset, Midpoint, and Offset for the Various Potential Indicators of Primary Drying End Point for 5% Mannitol
| Indicator | Approximate primary drying time (h) | ||
|---|---|---|---|
| Onset | Midpoint | Offset | |
| Pirani | 13.9 | 14.7 | 15.5 |
| Lyotrack | 13.9 | 14.7 | 15.5 |
| Condenser pressure | 8.3 | 11.1 | 13.9 |
| Dew point | 14.5 | 16 | ≈17.4 |
| Product TC | 10.8 | 12.6 | 14.4 |
| Process [H2O] from TDLAS | 13.3 | 14.4 | 15.5 |
| Pressure rise test | 10.1 | 12.3 | 14.4 |
Overall, the trends are the same irrespective of product, with dew point giving longest and condenser pressure giving shortest primary drying time.
Cake Appearance
The appearance of product at 25°C when extracted at the onset, midpoint, and offset of the various indicators of the end point of primary drying is shown in Table V for sucrose and Table VI for mannitol. Appearance denoted “cake” indicates no ice and moisture sufficiently low to avoid collapse at 25°C from a glass transition. Thus, observation of “cake” means that particular method would yield an acceptable product if used as the indicator of primary drying for that material. However, observation of “melt-back” means presence of residual ice which melts upon warming at 25°C, whereas “collapse” means sufficiently high residual moisture is present to allow cake collapse when the sample is warmed to ambient. For 5% sucrose, Pirani, Lyotrack, and dew point results in good cake structure at the offset, while other techniques result in either collapse or melt-back. Particularly using a slow (≈0.1°C/min) shelf temperature ramp in the transition between primary and secondary drying, structural collapse could likely be avoided in many if not all of the sucrose samples labeled “collapse” that have residual water less than 20%. For 5% mannitol, all the techniques result in good cake structure at the offset; however, condenser pressure, thermocouples, and pressure rise test result in either collapse or melt-back at both onset and midpoint. Examination of moisture content (Tables I and II) with a requirement of <10% water for a satisfactory “end of primary drying” is perhaps a better criterion. At the end of primary drying, the water content is usually about 15–20% for amorphous materials (17, 18) and likely ∼5–10% for crystalline materials like mannitol.
Table V.
Appearance of Product at the Onset, Midpoint, and Offset for the Various Potential Indicators of Primary Drying End Point for 5% Sucrose
| Indicator | Product appearance | ||
|---|---|---|---|
| Onset | Midpoint | Offset | |
| Pirani | Collapse | Collapse | Cake |
| Lyotrack | Collapse | Collapse | Cake |
| Condenser pressure | Melt-back | Melt-back | Melt-back |
| Dew point | Collapse | Cake | Cake |
| Product TC | Melt-back | Melt-back | Melt-back |
| Process [H2O] from TDLAS | Melt-back | Collapse | Collapse |
| Pressure rise test | Melt-back | Melt-back | Collapse |
Table VI.
Appearance of Product at the Onset, Midpoint, and Offset for the Various Potential Indicators of Primary Drying End Point for 5% Mannitol
| Indicator | Product appearance | ||
|---|---|---|---|
| Onset | Midpoint | Offset | |
| Pirani | Cake | Cake | Cake |
| Lyotrack | Cake | Cake | Cake |
| Condenser pressure | Melt-back | Collapse | Cake |
| Dew point | Cake | Cake | Cake |
| Product TC | Melt-back | Collapse | Cake |
| Process [H2O] from TDLAS | Cake | Cake | Cake |
| Pressure rise test | Melt-back | Melt-back | Cake |
Comparison of the Techniques
Some of the important factors that should be considered when selecting a technique to determine the end point of primary drying are listed in Table VII. Determination of the end point of primary drying from condenser pressure is not satisfactory in many cases as it is very difficult to define the onset, midpoint, and the offset, as discussed earlier. This problem would moderate somewhat, however, with full loads of rapidly freeze-drying products. However, at least with an amorphous material, there is excessive water at the apparent end points, which is expected because mass flux decreases even when a significant number of vials retain ice. The midpoint of Pirani, dew point, and water concentration (from TDLAS) does determine the end point of primary drying in a meaningful and useful way. However, TDLAS is an expensive technique which is not a common accessory with freeze-dryers, and the offset of dew point is sometimes difficult to determine as it reaches the plateau value asymptotically. From a sterility standpoint, it is best to avoid use of thermocouples in the product vials in manufacturing, and furthermore, vials monitored with thermocouples are not representative of the entire batch, with the bias becoming much more serious in manufacturing (16). Thus, thermocouples are not suitable for evaluating the end point of primary drying in manufacturing. For pressure rise test, the valve connecting the chamber and the condenser is closed for a short time (25 s). Thus, the cycle needs to be interrupted briefly, and the dryer needs to be equipped with a quick closing valve. Lyotrack is a costly technique giving information which offers no significant advantage over Pirani. Also, Lyotrack (gas plasma spectroscopy) results in product degradation via oxidation when the probe is mounted on the product chamber, although such degradation could likely be minimized by mounting the probe in the duct connecting the chamber and the condenser.
Table VII.
Important Factors to be Considered for Application to Industrial-Scale Freeze-Dryer
| Factors | TC | Comparative pressure measurement | DP | TDLAS | Cond. pressure | PRT or MTM | Lyotrack |
|---|---|---|---|---|---|---|---|
| Monitor entire batch | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Auto loading | No | Yes | Yes | Yes | Yes | Yes | Yes |
| CIP | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Aseptic handling | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Steam sterilization | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Leak rate insensitive | Yes | Yes | Yes | Yes | No | No | Yes |
| Implementation | Yes | Yes | Yes | Maybe | Yes | Yes | Yes |
| Calibration | Yes | Yes | Yes | No | Yes | No | No |
| Inexpensive $$$ | Yes | Yes | Yes | No | Yes | Yes | No |
| Robust | Maybe | Yes | No | Yes | Maybe | Yes | Yes |
TC thermocouple, DP dew point monitor, TDLAS tunable diode laser absorption spectroscopy, Cond. condenser, PRT pressure rise test, MTM manometric temperature measurement, CIP Clean in place
CONCLUSION
Comparative pressure measurement (i.e., Pirani) seems, by far, to be the best choice of the methods tested for evaluation of the end point of primary drying. It works very well, is cheap, can withstand steam sterilization, and is easy to install without requiring any modification to the existing dryers. Other techniques also work well and not only determine the end point of primary drying but also provide other useful information for process development and scale-up. Lastly, Lyotrack should not be used for products sensitive to oxidation.
Acknowledgments
The authors would like to acknowledge Pfizer, Inc. for partly funding this project. Also, we thank Alcatel vacuum technologies for providing Lyotrack and Physical Sciences Inc. for providing TDLAS for evaluation in this study.
Footnotes
The starting material used in these studies contained ∼20% degradation, more than normal for fresh material, apparently due to degradation during storage.
Previous results suggest that methionine oxidation in hGH is accelerated by free radical oxidation in the drying chamber (15).
It may be that the deterioration of the gauge depends to some extent on the gauge construction. However, it is important to note that at least one readily available gauge is relatively robust. Also, since it is very easy to “calibrate” the Pirani during each run (i.e., early in primary drying the gauge should read 60% higher than the differential capacitance manometer), one can easily compensate for some loss of performance due to whatever cause.
References
- 1.Pikal MJ. Freeze-drying of proteins: process, formulation, and stability. ACS Symposium Series. 1994;567:120–133. doi: 10.1021/bk-1994-0567.ch008. [DOI] [Google Scholar]
- 2.Adams GDJ, Irons LI. Some implications of structural collapse during freeze-drying using Erwinia caratovora l-asparaginase as a model. J Chem Biotechnol. 1993;58:71–76. doi: 10.1002/jctb.280580110. [DOI] [PubMed] [Google Scholar]
- 3.Rambhatla S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, I: atypical radiation and the edge vial effect. AAPS PharmSciTech. 2003;4(2):111–120. doi: 10.1208/pt040214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pikal MJ, Roy ML, Shah S. Mass and heat transfer in vial freeze-drying of pharmaceuticals: role of the vial. J Pharm Sci. 1984;73(9):1224–1237. doi: 10.1002/jps.2600730910. [DOI] [PubMed] [Google Scholar]
- 5.Nail SL, Johnson W. Methodology for in-process determination of residual water in freeze-dried products. Dev Biol Stand. 1992;74:137–151. [PubMed] [Google Scholar]
- 6.Roy ML, Pikal MJ. Process control in freeze drying: determination of the end point of sublimation drying by an electronic moisture sensor. J Parenter Sci Technol. 1989;43(2):60–66. [PubMed] [Google Scholar]
- 7.Gieseler H, Kessler WJ, Finson M, Davis SJ, Mulhall PA, Bons V, et al. Evaluation of tunable diode laser absorption spectroscopy for in-process water vapor mass flux measurements during freeze drying. J Pharm Sci. 2007;96(7):1776–1793. doi: 10.1002/jps.20827. [DOI] [PubMed] [Google Scholar]
- 8.Mayeresse YVR, Sibille PH, Nomine C. Freeze-drying process monitoring using a cold plasma ionization device. PDA J Pharm Sci Technol. 2007;61(3). [PubMed]
- 9.Bardat A, Biguet J, Chatenet E, Courteille F. Moisture measurement: a new method for monitoring freeze-drying cycles. J Parenter Sci Technol. 1993;47(6):293–299. [PubMed] [Google Scholar]
- 10.Tang X, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21(2):191–200. doi: 10.1023/B:PHAM.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- 11.Tang X, Nail SL, Pikal MJ. Freeze-drying process design by manometric temperature measurement: design of a smart freeze-dryer. Pharm Res. 2005;22(4):685–700. doi: 10.1007/s11095-005-2501-2. [DOI] [PubMed] [Google Scholar]
- 12.Pikal MJ, Dellerman KM, Roy ML, Riggin RM. The effects of formulation variables on the stability of freeze-dried human growth hormone. Pharm Res. 1991;8(4):427–436. doi: 10.1023/A:1015834724528. [DOI] [PubMed] [Google Scholar]
- 13.Riggin RM, Dorulla GK, Miner DJ. A reversed-phase high-performance liquid chromatographic method for characterization of biosynthetic human growth hormone. Anal Biochem. 1987;167(1):199–209. doi: 10.1016/0003-2697(87)90152-7. [DOI] [PubMed] [Google Scholar]
- 14.Milton N, Pikal MJ, Roy ML, Nail SL. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. PDA J Pharm Sci Tech. 1997;51(1):7–16. [PubMed] [Google Scholar]
- 15.Pikal MJ, Roy ML. Unpublished observations.
- 16.Patel SM, Jameel F, Pikal MJ. Effect of dryer load on freeze-drying process design. AAPS Annual Meeting. San Antonio, Texas, USA; 2006.
- 17.Hatley RHM, Mant A. Determination of the unfrozen water content of maximally freeze-concentrated carbohydrate solutions. Int J Biol Macromol. 1993;15(4):227–232. doi: 10.1016/0141-8130(93)90042-K. [DOI] [PubMed] [Google Scholar]
- 18.Zasypkin DV, Lee T-C. Extracellular ice nucleators from pantoea ananas: effects on freezing of model foods. J Food Sci. 1999;64(3):473–478. doi: 10.1111/j.1365-2621.1999.tb15066.x. [DOI] [Google Scholar]