Abstract
An intravenous solution is a dosage forms intended for administration into the bloodstream. This route is the most rapid and the most bioavailable method of getting drugs into systemic circulation, and therefore it is also the most liable to cause adverse effects. In order to reduce the possibility of side effects and to ensure adequate clinical dosage of the formulation, the primarily formulated composition should be optimized. It is also important that the composition should retain its therapeutic effectiveness and safety throughout the shelf-life of the product. This paper focuses on the optimization and stability testing of a parenteral solution containing miconazole and ketoconazole solubilized with a ternary solvent system as model drugs. Optimization of the solvent system was performed based on assessing the risk/benefit ratio of the composition and its properties upon dilution. Stability tests were conducted based on the EMEA (European Medicines Agency) “guideline on stability testing: stability testing of existing active substances and related finished products”. Experiments show that both the amount of co-solvent and surface active agent of the solvent system could substantially be reduced, while still maintaining adequate solubilizing power. It is also shown that the choice of various containers affects the stability of the compositions. It was concluded that by assessing the risk/benefit ratio of solubilizing power versus toxicity, the concentration of excipients could be considerably decreased while still showing a powerful solubilizing effect. It was also shown that a pharmaceutically acceptable shelf-life could be assigned to the composition, indicating good long-term stability.
Key words: azole, composition optimization, parenteral, solubility, stability testing
INTRODUCTION
The intravenous solution is a dosage form intended for administration into the blood stream. This route allows 100% bioavailability and is the most rapid method of getting a drug into systemic circulation (1,2). On the other hand, one must also consider that the undesirable effects of parenteral administration are as prompt as the desirable ones. Therefore, the primary task is to formulate a dosage form that contains the therapeutically effective dose of the active ingredient in a system that exhibits the least possible toxicity. In the case of liquid formulations comprising one or more active ingredients which show poor water solubility, solubility enhancers are often used to ensure the therapeutically effective dose of the compounds (3–9). The effect of such excipients on the pharmacokinetics of the drugs and their impact on the blood have been widely studied (10–13) and although the toxicity of a formulation can by no means be explained solely by the concentration of the applied excipients, it is widely accepted that the lower the level of an excipient in a formulation, the less likely it is to induce toxicity. An inappropriate amount or composition of solvents can cause hemolysis, precipitation of the drug, phlebitis, and pain (14–17). On the other hand, if the composition does not contain suitable amounts of excipients, stability upon administration and dilution or sorption to infusion sets may become an issue (18,19).
Consequently, reducing the amount of solvents in a parenteral composition will probably lead to fewer side effects, which is essential in pharmaceutical development. The stability of the final composition is also of utmost importance, especially in liquid formulations where the possibility of active ingredient degradation is usually much more acute than in other dosage forms.
An intravenous solution (concentrate for infusions) for azole type antifungal drugs was developed and published by our research team (20). It was the aim of the development to formulate a solution of miconazole (≤1.03 μg/mL water solubility (21)) or ketoconazole (0.026 mg/mL water solubility (22)), without Cremophor EL as a possible solubilizer. Cremophor EL was previously used in a marketed formulation of miconazole, but was withdrawn from the market, probably due to the severe side effects induced by the solubilizer (21,23). The developed solution contains one of the azoles and a solvent system comprising a pH adjuster, a co-solvent, and a surfactant. The significant solubility enhancing effect exerted by the novel combination of ammonium acetate solution (pH 3.1), 5% polysorbate 80, and 25% ethanol can be attributed to the synergistic solubilizing impact of the components. In this case, the synergistic effect means that the solubility enhancing effect of each solubilizer taken individually and then added up, results in a weaker solubilizing effect than that obtained in the experiments. Optimization of the solvent system was performed using miconazole as the model drug and the optimization process was then repeated with ketoconazole.
The aim of the present work was to optimize the composition of the above solvent system from the aspect of the amount of excipients, that is, to reduce the amount of both polysorbate 80 and ethanol and still be able to solubilize therapeutically effective amounts of miconazole (at least 30 mg/mL). Since the composition is intended for use after dilution with an appropriate large-volume parenteral (LVP), another purpose of the investigation was to perform precipitation tests (6,24). Furthermore, it was the purpose of the study to determine whether the same steps of optimization could be used for the optimization of the same solvent system but with another azole type drug, ketoconazole. It was also the aim of present work to test the stability of the final composition containing miconazole.
MATERIALS AND METHODS
Materials
Miconazole (MW, 416.1) and ketoconazole (MW, 531.44) both of minimum 98% purity were obtained from Bosche Scientific, New Brunswick, NJ, USA. Acetic acid, ammonium acetate, ethanol 96%, and polysorbate 80 of pharmacopoeial grade were used for the preparation of the samples (Molar Chemicals Ltd., Budapest, Hungary). Ammonium acetate, acetic acid, methanol, and water of HPLC gradient grade were used for the analytical tests (Sigma-Aldrich Ltd., Budapest, Hungary). Glucose, sodium chloride, hydrochloric acid, and water for injections were used for the production of large-volume parenterals and were all Ph.Eur.5. grade (Reanal Ltd., Budapest, Hungary).
Methods
The Analytical Method for the Quantitative Determination of Miconazole and Ketoconazole
An HPLC method was chosen for the determination of the active ingredients. The HPLC system consisted of an L-6200 A Merck-Hitachi pump connected to an L-4250 Merck-Hitachi UV detector set at 230 nm for miconazole and 225 nm for ketoconazole. Ten microliters samples were injected by an AS-4000 automatic sampler on an octylsilane (XDB-C8) phase Agilent-Zorbax column (4.6 mm × 150 mm, 5 µm). The mobile phase consisted of a 70:30 (v:v) mixture of methanol and 0.05 M acetate buffer, pH 3.5; the flow rate was 1.0 mL/min. Peak area integration was performed using a Merck-Hitachi Model D-7000 Chromatography Data Station Software Version 4.1 (Merck KGaA, Darmstadt, Germany and Higuchi Instruments Inc., San Jose, USA). This method has been previously validated and showed good linearity, reproducibility, and accuracy (25).
Methods for Optimization of the Composition
Optimization tests were divided into three steps and were first performed with miconazole. After completing the test, the same test was performed with ketoconazole. The first step consisted of reducing the concentration of polysorbate, followed by reducing the concentration of ethanol. Finally, based on mixing with LVPs, the concentration of polysorbate was again altered to achieve a composition suitable for dilution. The primary optimization tests were carried out using ternary compositions containing ammonium acetate solution (pH 3.1), 25% ethanol and decreasing amounts of polysorbate 80 (5%–0.1%). In the second phase of optimization, ternary compositions containing ammonium acetate solution (pH 3.1), 0.1% polysorbate 80 and decreasing amounts of ethanol (25%–5%) were analyzed. All samples were prepared in triplicate and the active ingredient concentration was measured in each composition by an HPLC method described above.
Stability upon Dilution
To finalize the optimization of the composition containing miconazole and ensure the applicability of the formulation, dilution tests similar to the ones performed with the originally marketed miconazole solution were performed (26). Two different LVPs were used as diluting solutions: 5% dextrose infusion and 0.9% sodium chloride infusion. Aliquot amounts of the following compositions was diluted with either of the LVPs (tenfold dilution): 10, 20, and 30 mg/mL miconazole in a solvent system comprising 0.1%, 1.0%, 2.0%, and 3.0% of polysorbate 80 + 20% ethanol adjusted to pH 3.1 with ammonium acetate solution. Stability on dilution was observed over a period of 24 h. It was observed in the case of miconazole that the diluted solutions which were agitated with the immersion of a pipette or by measuring pH showed active ingredient precipitation much earlier than the ones which were not disturbed by the immersion of the pipette. To exclude the possibility of precipitation due to the immersion of either of them, aliquot amounts of samples were poured out of the diluted solutions. The miconazole content of each sample was determined with the HPLC method described above. This method was coupled with visual observation for the evaluation of precipitation. A different set of samples was prepared for the evaluation of the pH. The pH of the solutions was measured at regular intervals: t = 0, 1, 2, 4, 8 and 24 h (6,27). Samples were prepared in triplicate and were vigorously shaken at regular intervals. The increase of the polysorbate concentration was performed until the diluted solution proved to be stable for 24 h, irrespective of the miconazole concentration.
In case of ketoconazole, 0.9% sodium chloride infusion was used as diluting solution. The following composition was diluted with the LVP (tenfold dilution): 50, 75, and 100 mg/mL ketoconazole in a solvent system comprising 0.1% of polysorbate 80 + 20% ethanol adjusted to pH 3.1 with ammonium acetate solution. In this case, the immersion of the pipette did not trigger precipitation, and therefore visual evaluation was combined with a quantitative HPLC determination of the active ingredient.
To explain the phenomenon of precipitation upon dilution, the miconazole solubilizing capacity of two compositions comprising polysorbate 80 and ethanol in concentrations equal to the ones after dilution with dextrose or saline infusion was investigated:
Miconazole (20 mg/mL) + 0.1% polysorbate 80 + 20% ethanol + ammonium acetate (pH 3.1), diluted to different extents (0.05, 0.1, 0.25, 0.5) with 5% dextrose or saline infusions and
Miconazole (20 mg/mL) + 3% polysorbate 80 + 20% ethanol + ammonium acetate (pH 3.1), diluted to different extents (0.05, 0.1, 0.25, 0.5) with 5% dextrose or saline infusions.
Accelerated Stability Tests
After finalizing the composition of the solvent system containing miconazole, accelerated stability tests were performed in a pharmaceutical stability chamber (Sanyo type 022, Leicestershire, UK) according to the relevant guideline (28). The guideline states that accelerated stability tests should be performed on an adequate number of samples at 40 ± 2°C and 75 ± 5% RH. Solutions containing 20 mg/mL miconazole and 3% polysorbate, 20% ethanol and ammonium acetate solution (pH 3.1) were prepared. Ten microliters of the solution was filled into either flame-sealed ampoules or into vials sealed with a rubber stopper and cap. An adequate number of ampoules and vials were set upright and an adequate number of vials were set upside down for the duration of the stability tests. At the time of sampling, three samples of each type were analyzed (three vials upright, three vials upside down, and three ampoules). Physical and chemical stability of the formulations were checked for a period of 12 months. Physical stability was confirmed by visually checking for the absence of precipitation, change in color, or turbidity. Chemical stability was determined by measuring pH and miconazole content using the HPLC method described above.
RESULTS
Solvent System for Miconazole
Optimizing the Concentration of Excipients
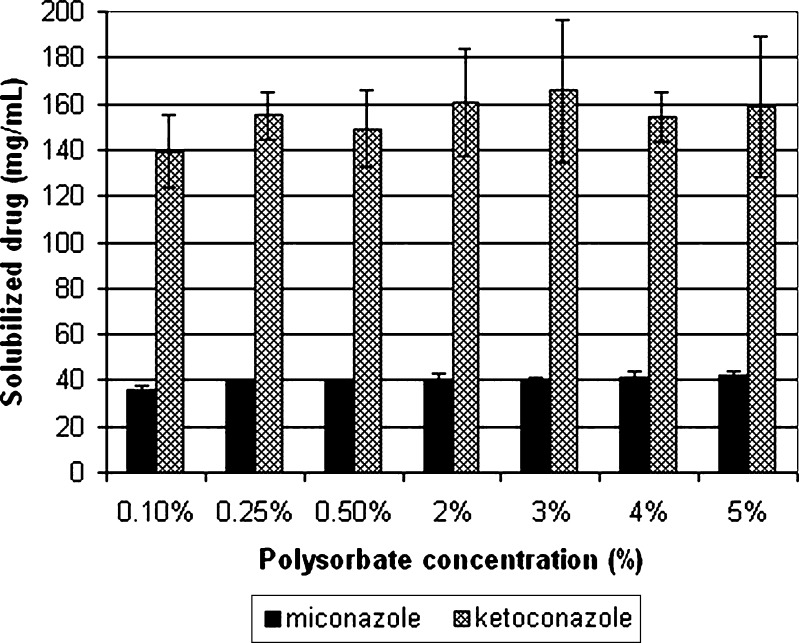
The solvent system developed in an earlier study was able to solubilize 42 mg/mL of miconazole in 5% polysorbate 80, 25% ethanol, and ammonium acetate solution (pH 3.1). Although the developed composition is probably toxicologically adequate, because it contains fewer excipients than the similarly formulated and marketed medications, it was the aim of the present work to further decrease the amount of excipients and still be able to solubilize the therapeutically effective dose of miconazole. It is important to do this because numerous papers deal with the toxicity of the excipients used in this study and all come to a similar conclusion, stating that lower level of excipient results in a lower level of toxicity (1,29–31). Toxic effects of surfactants are associated with their interaction with biological membranes, and as such are proven to exert hemolytic effect (32). Based on such publications and assessing the benefit/risk ratio of each excipient, it was concluded that the amount of excipient to be reduced first and foremost should be polysorbate 80, followed by the reduction of the concentration of ethanol. Therefore, to determine the effect of reducing the concentration of polysorbate, solvent systems containing the surfactant at concentrations from 5% to 0.1% were prepared. Figure 1 shows the solubility of miconazole in solvent systems comprising 25% ethanol, ammonium acetate solution (pH 3.1) and various concentrations of polysorbate 80. The results show that the composition comprising 5% polysorbate solubilized 42.46 ± 1.15 mg/mL miconazole, while the composition comprising 0.1% polysorbate 80 solubilized 35.99 ± 1.50 mg/mL of the drug. There is no statistically significant difference (p = 0.05, n = 6) in the amount of solubilized miconazole between the compositions comprising 0.25%, 0.5%, 2%, 3%, 4%, or 5% of polysorbate. On the other hand, there is a statistically significant difference (p = 0.05, n = 6) in the amount of solubilized miconazole between the compositions comprising 0.25% polysorbate and 0.1% polysorbate. Nevertheless, the latter was chosen for the further optimization because it can dissolve more than 30 mg/mL of miconazole, which was one of the main aims of the study.
Fig. 1.
Solubility of miconazole and ketoconazole in solvent systems comprising 0.1, 0.25, 0.5, 2.0, 3.0, 4.0, and 5.0% polysorbate 80 + 25% ethanol + 0.05 M ammonium acetate (pH 3.1)
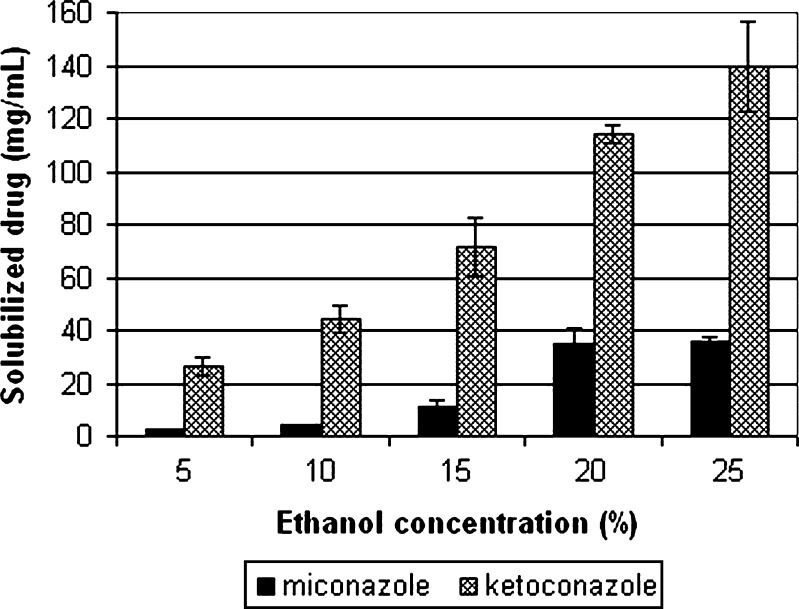
Therefore, after primary solvent system optimization, the second step was initiated with a solvent system containing 0.1% polysorbate 80, 25% ethanol, and ammonium acetate. To determine the effect of reducing the concentration of ethanol on the solvent’s solubilizing power, solutions comprising 0.1% polysorbate 80, ammonium acetate, and ethanol (5%, 10%, 15%, 20%, 25%) were prepared and their solubilizing ability was analyzed. Figure 2 shows the solubility of miconazole in solvent systems comprising 0.1% polysorbate 80, ammonium acetate solution (pH 3.1) and various concentrations of ethanol. The results show that the amount of miconazole solubilized by the compositions containing 25% or 20% was statistically similar (p = 0.05, n = 8). On the other hand, compositions comprising 15%, 10%, and 5% of ethanol only solubilized 10.79 ± 2.84, 4.52 ± 0.03, 2.40 ± 0.13 mg/mL of miconazole, respectively.
Fig. 2.
Solubility of miconazole and ketoconazole in solvent systems comprising 5, 10, 15, 20, and 25% ethanol + 0.1% polysorbate 80 + 0.05 M ammonium acetate (pH 3.1)
Stability upon Dilution
The solution developed in this study was a concentrate for infusions. Such a dosage form should be diluted with an infusion prior to administration for safety reasons. The usual practice is a tenfold dilution performed with 5% dextrose or 0.9% sodium chloride infusions. It is a widely accepted criterion that a diluted solution should be stable for at least 24 h. Stability is determined by the absence of precipitation after a given time. Precipitation of any given active ingredient is most likely when solubilized by modification of pH, co-solvents, or surfactants. Since the formulation of the present work comprises all of the above excipients, it is essential to determine its behavior upon dilution. To achieve this, 20 mL of 0.1%, 1.0%, 2.0%, and 3.0% polysorbate 80, 20% ethanol, and ammonium acetate (pH 3.1) solutions containing 10, 20, or 30 mg/mL of miconazole were prepared. Each solution was diluted separately with the two infusions to 200 mL. Table I shows the tendency of the diluted solutions to precipitate. It can be seen that the solvent system comprising 0.1% polysorbate 80, 20% ethanol, and ammonium acetate (pH 3.1) containing 10 mg/mL of miconazole was stable both in dextrose and sodium chloride infusions during the time of examination. On the other hand, the same solvent system containing 20 or 30 mg/mL showed precipitation within 24 h. The formulation with 3% polysorbate 80 did not precipitate at any given miconazole concentration (Fig. 3). Results of the test also showed that the pH of the diluted solutions remained basically unchanged throughout the 24-h test (Table II.). No difference in pH was observed when diluting with 5% dextrose or 0.9% sodium chloride infusion.
Table I.
The Effect of a Tenfold Dilution with 0.9% Sodium Chloride or 5% Dextrose Infusion on the Stability of Compositions Comprising 0.1%, 1.0%, 2.0%, and 3.0% Polysorbate 80 + 20% Ethanol + 0.05 M Ammonium Acetate (pH 3.1)
| Polysorbate 80 concentration (%) | Miconazole concentration (mg/mL) | Large-volume parenteral | Precipitation after 24 h |
|---|---|---|---|
| 0.1 | 10 | NaCl | No |
| 20 | Yes | ||
| 30 | Yes | ||
| 10 | Dextrose | No | |
| 20 | Yes | ||
| 30 | Yes | ||
| 1 | 10 | NaCl | No |
| 20 | Yes | ||
| 30 | Yes | ||
| 10 | Dextrose | No | |
| 20 | Yes | ||
| 30 | Yes | ||
| 2 | 10 | NaCl | No |
| 20 | No | ||
| 30 | No | ||
| 10 | Dextrose | No | |
| 20 | Yes | ||
| 30 | Yes | ||
| 3 | 10 | NaCl | No |
| 20 | No | ||
| 30 | No | ||
| 10 | Dextrose | No | |
| 20 | No | ||
| 30 | No |
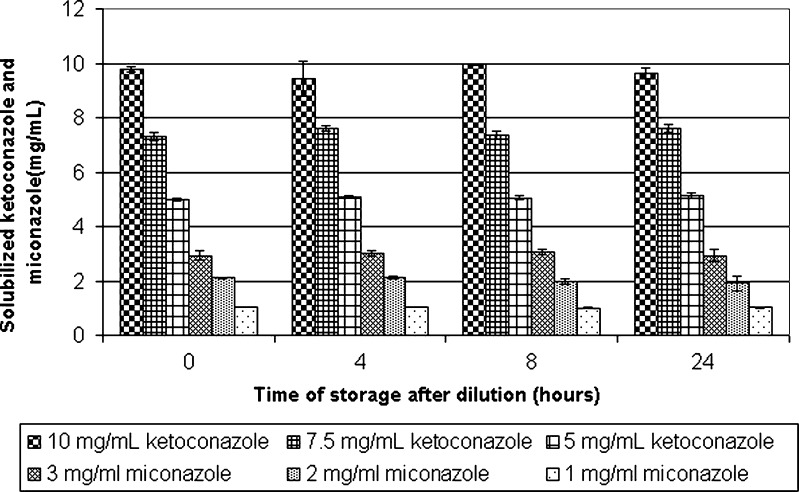
Fig. 3.
The tendency of ketoconazole and miconazole to remain solubilized after dilution. Solvent systems comprising 0.1% polysorbate 80, 20% ethanol, and ammonium acetate (pH 3.1) and 100, 75 and 50 mg/mL ketoconazole and 30, 20, and 10 mg/mL miconazole were diluted tenfold with 0.9% sodium chloride infusion to reach final concentrations of 10 mg/mL, 7.5 mg/mL, and 5 mg/mL for ketoconazole and 3 mg/mL, 2 mg/mL and 1 mg/mL for miconazole
Table II.
The Change in pH Values Over the Time of Storage after Dilution for Solvent Systems Comprising 0.1% Polysorbate 80, 20%, Ethanol and Ammonium Acetate (pH 3.1) and Various Concentrations of Miconazole or Ketoconazole Diluted with 0.9% Sodium Chloride Infusion
| Time of storage after dilution (hours) | 0 | 1 | 2 | 4 | 8 | 24 |
|---|---|---|---|---|---|---|
| Miconazole concentration | ||||||
| 30 mg/mL | 3.38 ± 0.01 | 3.47 ± 0.02 | 3.35 ± 0.02 | 3.39 ± 0.02 | 3.29 ± 0.02 | 3.30 ± 0.01 |
| 20 mg/mL | 3.35 ± 0.01 | 3.47 ± 0.01 | 3.31 ± 0.01 | 3.39 ± 0.01 | 3.31 ± 0.01 | 3.31 ± 0.01 |
| 10 mg/mL | 3.27 ± 0.02 | 3.30 ± 0.01 | 3.19 ± 0.01 | 3.31 ± 0.01 | 3.20 ± 0.01 | 3.28 ± 0.02 |
| Ketoconazole concentration | ||||||
| 100 mg/mL | 3.72 ± 0.01 | 3.75 ± 0.01 | 3.83 ± 0.02 | 3.80 ± 0.01 | 3.77 ± 0.01 | 3.78 ± 0.01 |
| 75 mg/mL | 3.65 ± 0.01 | 3.69 ± 0.01 | 3.73 ± 0.01 | 3.67 ± 0.01 | 3.68 ± 0.02 | 3.69 ± 0.01 |
| 50 mg/mL | 3.51 ± 0.02 | 3.51 ± 0.01 | 3.58 ± 0.02 | 3.56 ± 0.03 | 3.58 ± 0.01 | 3.57 ± 0.01 |
Solvent System for Ketoconazole
Optimizing the Concentration of Excipients
Based on the results of the optimization of the solvent system for miconazole, the same experiments were conducted with the same compositions, but with ketoconazole. The results of the primary optimization are shown in Fig. 1. Reducing the polysorbate concentration of the composition from 5% to 0.1% has a similar effect on the solubility of ketoconazole to that which it has on the solubility of miconazole. In both cases, a slight fall of solubilizing capacity is seen when reducing the polysorbate concentration from 0.25% to 0.1%. A more pronounced difference can be seen in the case of miconazole, where there is a statistically significant difference (p = 0.05, n = 6) in the amount of solubilized miconazole between the composition comprising 0.25% polysorbate and 0.1% polysorbate. In contrast, in the case of ketoconazole, there is no statistically significant difference (p = 0.05, n = 6).
Figure 2 shows that reducing the ethanol concentration of the compositions results in solvent systems that show different solubilizing characteristics for miconazole and ketoconazole. In the case of miconazole, there is no statistically significant difference (p = 0.05, n = 8) in the amount of drug solubilized by the composition containing 25% or 20% ethanol, while in the case of ketoconazole, there is a statistical difference (p = 0.05, n = 6).
Stability upon Dilution
In contrast to the case of miconazole, results showed that no ketoconazole precipitation occurred when a solution containing 0.1% polysorbate 80, 20% ethanol, ammonium acetate (pH 3.1) and 50, 75, or 100 mg/mL ketoconazole was diluted tenfold with a 0.9% sodium chloride infusion. Figure 3 shows that the concentration of ketoconazole in each diluted solution remained constant for 24 h, at 5, 7.5, and 10 mg/mL, respectively.
Stability Test
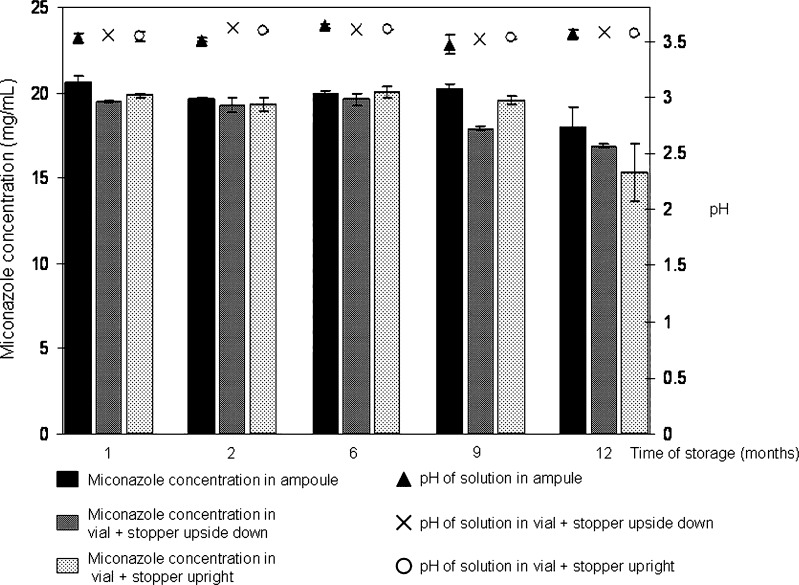
To determine the chemical stability of miconazole and the stability of the solvent system over a long period of time, accelerated stability tests as described in relevant guidelines were performed (28). In addition to testing the shelf-life of the composition, its compatibility with various containers was also examined during the study. The compositions were stored in different containers and thus it was possible to examine the changes occurring in the solution as a result of the choice of container in which it was stored. Figure 4 shows the results of the accelerated stability tests performed over a period of 12 months.
Fig. 4.
Miconazole content and pH of solutions after 12 months of accelerated stability test (40 ± 2°C and 75 ± 5% RH) stored in different containers (miconazole concentration in ampoule ( ), in vial + stopper upside down (
), in vial + stopper upside down ( ), in vial + stopper upright (
), in vial + stopper upright ( ))
))
DISCUSSION
Optimizing the Concentration of Excipients
It can be concluded that reducing the concentration of polysorbate 80 from 5% to 0.1% resulted in a slight decrease of solubilizing capacity for both miconazole and ketoconazole (Fig. 1). To understand the decrease of solubilizing power, components other than the surfactant must also be considered:
pH reduction ionizes any weak base, therefore miconazole and ketoconazole, being weak bases (miconazole pKa 6.7; ketoconazole pKa1, 2.94 and pKa2 6.51) are ionized. Although usually any solute that is in its ionized form is less soluble in the nonpolar surfactant, the solubility of the protonated miconazole or ketoconazole in the ternary solvent system did not decrease drastically on altering the concentration of polysorbate 80 (33). This can be understood by assessing the log D of miconazole and ketoconazole. Based on calculations using Eq. 1 for miconazole and Eq. 2 for ketoconazole:
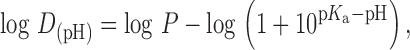 |
1 |
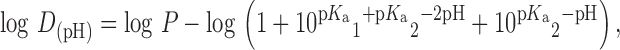 |
2 |
where log D(pH) is the apparent partition coefficient at a given pH, log P is the partitioning coefficient, the log D(pH) value of the azoles can be determined (34). Using Eqs. 1 and 2, log D(pH) of miconazole and ketoconazole was calculated to be between 2.3–2.7, and 0.09–0.61, respectively (in the pH range of the studies). According to the classification of Yalkowsky (33) such solutes are considered to be semipolar, and as such are still solubilized by the surfactant present in the composition, most likely by its polar regions. Besides the effect of pH alteration, the presence of ethanol must also be discussed. Primarily, co-solvents may alter the pKa of the azoles in such a way that they ionize to a lesser extent; secondly ethanol may be incorporated into the micelles of polysorbate and change its characteristics. Alongside these effects, co-solvents compete with the surfactant for the solubilization of the azoles. Therefore, if the decrease of polysorbate concentration results in less azole being incorporated into the micelles, the non-incorporated solute may be solubilized by ethanol. The complex combination of the described effects resulted in only a slight decrease of solubilizing power. Assessing the benefit/risk ratio, it was concluded that a minor decrease of solubilizing capacity is acceptable, especially if compared to the 50-fold decrease of polysorbate concentration reached after optimization.
In case of miconazole, it was observed that reducing the concentration of ethanol from 25% to 20% did not have a major effect on the solubilizing power of the solvent system (Fig. 2). However, further reducing the concentration of ethanol resulted in a negative effect on the solvent system’s solubilizing ability. Since the reduction of co-solvent concentration from 25% to 20% does not dramatically affect the solubilizing power of the miconazole solvent system a composition comprising 0.1% polysorbate 80, 20% ethanol, and ammonium acetate (pH 3.1) was worked out.
Assessing the same optimization process in case of ketoconazole, it was seen that although the deterioration of solubilizing capacity was observed earlier, between 25% and 20% ethanol, a similar tendency was observed.
The possible explanation of the difference is as follows: ionized miconazole has a log D(pH) value of 2.3–2.7, which is semipolar, but is closer to being apolar. Ionized ketoconazole has a log D(pH) value of 0.09–0.61, which is semipolar, but is closer to being polar. As discussed earlier, the reduction of polysorbate concentration led to fewer micelles, which can solubilize the drug. Therefore, most of the miconazole molecules which were primarily solubilized by the micelles were then solubilized by ethanol, but the ketoconazole molecules that were primarily solubilized by the micelles do not dissolve in ethanol as easily as miconazole, due to the difference in their log D(pH) values.
Evaluating the solubilizing power of the solvent systems, it was concluded that the significant decrease in the concentration of excipients is much more beneficial than the solubilizing capacity that would be regained if the concentration of excipients was increased. Based on the tests, the concentration of polysorbate 80 and ethanol was chosen so as to be balanced between physiological tolerability and effectiveness.
Stability upon Dilution
Solvent System for Miconazole
Since the pH of the investigated diluted solutions remained basically unchanged (Table II) it was presumed that precipitation of miconazole occurred due to the lower concentrations of the surfactant/ethanol present in the composition.
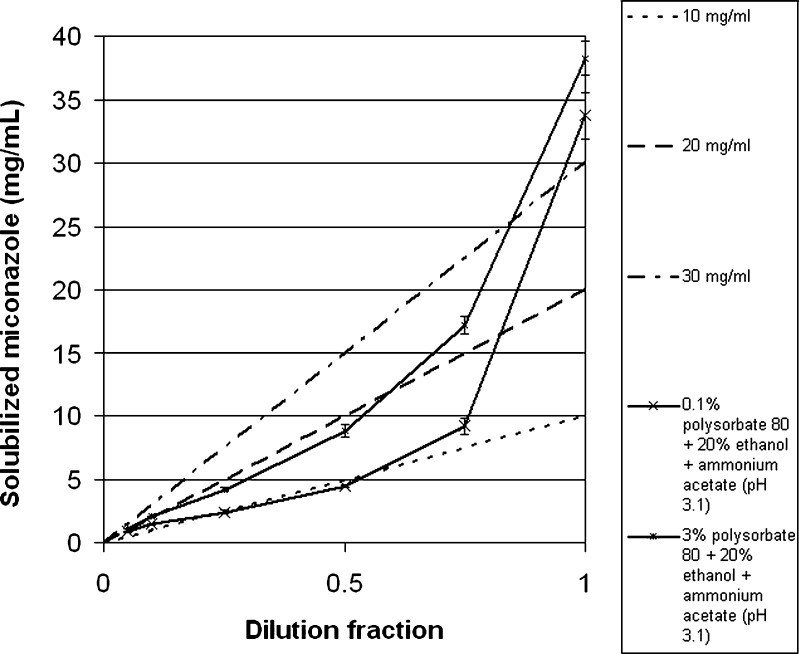
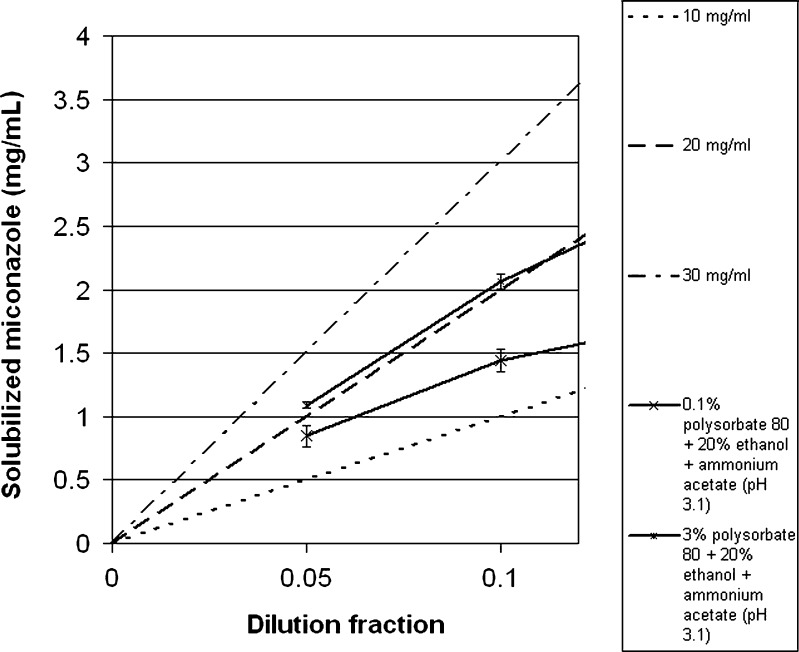
To appreciate this effect, one must understand the phenomena that happen during dilution of the studied compositions. When a surfactant/co-solvent/pH adjusted system solubilizes a solute which is diluted with an LVP, the concentration of solute and the concentration of excipients decrease by the same factor. Therefore, a dilution plot of solute concentration as a function of the composition of the excipients is a straight line that passes from the initial composition to the origin (33). Three such dilution lines (represented by dashed lines), originating from different initial concentrations of miconazole (10, 20, and 30 mg/mL), are illustrated in Figs. 5 and 6. The figures also show the miconazole solubilizing capacity of two compositions comprising polysorbate 80 and ethanol in concentrations equal to the ones after dilution with dextrose or saline infusion (solubility curve): (a) 20 mg/mL miconazole + 0.1% polysorbate 80 + 20% ethanol + ammonium acetate (pH 3.1), diluted to different extents with 5% dextrose or saline infusions [represented by a line of lower-case Xs (-x-)] and (b) 20 mg/mL miconazole + 3% polysorbate 80 + 20% ethanol + ammonium acetate (pH 3.1), diluted to different extents with 5% dextrose or saline infusions [represented by a line of asterisks (-*-)]. The phenomenon of precipitation can be explained by assessing the solubility curve superimposed on the dilution line. In the case in which the solubility curve is above the dilution line, precipitation does not happen. In contrast, where the solubilizing power of the composition is below the theoretical solubility of the diluted composition (dilution line), precipitation may occur. Precipitation does not necessarily occur in these cases, since other factors, such as supersaturation and nucleation determine whether the APIs actually precipitate or not (30). The data show that a composition equivalent to a formulation comprising 0.1% polysorbate, 20% ethanol, and ammonium acetate (pH 3.1) diluted with dextrose or sodium chloride infusions (tenfold dilution) results in a composition exerting lower solubilizing capacity than that which would be needed to stop miconazole from precipitating (since the solubility curve falls below the dilution line). This result confirmed the observed precipitation that was encountered upon dilution (Table I). To achieve stability on dilution the concentration of polysorbate was increased until the absence of precipitation was observed for 24 h. As seen from Table I, the concentration of polysorbate required to achieve stability was 3%. To confirm this result, the solubilizing power of the diluted solutions was further assessed and was compared to the dilution line in Figs. 5 and 6. Although these results show that the solubilizing capacity of the composition equivalent to a tenfold diluted solution falls below 3 mg/mL of miconazole, it was seen that the system was stable for 24 h. This can be explained by the possible supersaturation of the solution, enabling the active ingredient to stay in solution for the tested period of time. Based on these results, the final composition of the solvent system that was chosen to be tested for stability was 3% polysorbate 80, 20% ethanol, and ammonium acetate solution (pH 3.1).
Fig. 5.
Solubility curve of miconazole in solutions diluted to different extents (a, 20 mg/mL miconazole + 0.1% polysorbate 80 + 20% ethanol + ammonium acetate (pH 3.1), diluted to different extents with 5% dextrose or saline infusions [represented by a line of lower-case Xs] and b, 20 mg/mL miconazole + 3% polysorbate 80 + 20% ethanol + ammonium acetate (pH 3.1), diluted to different extents with 5% dextrose or saline infusions [represented by a line of asterisks]) superimposed on the dilution lines originating from different initial concentrations (10, 20, and 30 mg/ml [represented by dashed lines])
Fig. 6.
Same data as Fig. 5, but scales are adjusted to dilution ratio of 0.0–0.15 and the solubilized miconazole scale is adjusted to 0.0–4.0
Solvent System for Ketoconazole
As the result showed that no precipitation occurred with the tested compositions (0.1% polysorbate 80, 20% ethanol, ammonium acetate (pH 3.1) and 50, 75, or 100 mg/mL ketoconazole), upon dilution it was concluded that it was not necessary to alter the amount of polysorbate 80 present in the solvent system. The lack of precipitation can be explained by the fact that the dilution lines of ketoconazole solutions containing 50, 75, or 100 mg/mL active ingredient fall below the solubility curve of the tested diluted ketoconazole solutions.
General Trends Guiding Formulation Decisions
Based on the above results and conclusions, a number of general trends were identified that might be of help in other formulation decisions. First and foremost, it can be concluded that any solvent system can be further optimized to ensure better applicability. General trends, which might also apply to other APIs, can be found in the reduction of polysorbate and ethanol concentration. This process shows that the loss of solubilizing capacity experienced due to decreasing the amounts of excipients is more pronounced in the case of alcohol than in the case of the surfactant. This correlates with previously published works that prove the log-linear solubilizing effect of co-solvents (33). This can be said even though in case of combined solvent systems (especially with the alteration of pH) the slope of solubilizing capacity is less steep than in co-solvent–water formulations. Another general observation is that in addition to improving buffer capacity, as suggested by Alvarez-Núñez and Yalkowsky (4), increasing the amount of solubilizing excipients can also be an adequate way to achieve stability upon dilution.
Stability Test
The results of Fig. 4 show that there is no significant difference neither in the quantity of the active ingredient in the solutions nor in the pH of the samples stored in containers with a stopper closure (upright or upside down) or in flame-sealed glass ampoules for the first 6 months. Samples taken after 9 months showed that the amount of active ingredient stored in a vial placed upside down decreased substantially. The probable explanation of this is a widely experienced phenomenon, the sorption of certain ingredients, in this case of miconazole, to stoppers. On the other hand, the active ingredient content of the solutions filled into ampoules or vials placed upright was between the 100 ± 5% limit. Visually checking the solutions revealed no change for 9 months, but after 12 months of storage, opalescence was observed. Therefore, before examining the miconazole content, the solution was filtered on a membrane filter with 0.22 µm pore size. The active ingredient assay performed after filtration indicated that the opalescence was due to the precipitation of the drug, because the amount of drug recovered was markedly lower than the theoretic 20 mg/mL concentration (vials upright, 15.31 ± 1.67; vials upside down, 16.88 ± 0.13; ampoules, 17.95 ± 1.17 mg/mL). The pH of the solutions remained basically unchanged, pH 3.56 ± 0.03 and the gross weight of the containers + compositions also stayed constant, indicating that there was no evaporation or uptake of water. It can be concluded that the composition is adequate for long-term storage, since it was shown that the composition stored in a flame-sealed ampoule stayed stable up to 9 months.
CONCLUSION
Working with a previously developed solvent system, the present study focused on optimizing the solvent system. It was shown that by choosing adequate aims and methods, the composition of the initial solvent system could be advantageously modified. It was proved that both the amount of the surfactant and the co-solvent could be decreased and the composition was still able to exert a very substantial solubilizing effect. Further optimization showed that the composition was stable upon dilution with various LVPs.
The concentration of polysorbate 80 and ethanol in the final composition is balanced between physiological tolerability and solubilizing power and the amount of excipients is as low as possible to display sufficient solubility. The optimization results suggest that due to the decreased amount of excipient, a lower toxicity of the solvent system is expected. The accelerated stability test showed that a pharmaceutically acceptable shelf-life could be assigned to the composition making it readily available for long-term storage.
References
- 1.Yalkowsky SH, Krzyzaniak JF, Ward GH. Formulation-related problems associated with intravenous drug delivery. J Pharm Sci. 1998;87:787–796. doi: 10.1021/js980051i. [DOI] [PubMed] [Google Scholar]
- 2.Egger-Heigold B. The effect of excipients on pharmacokinetic parameters of parenteral drugs (dissertation). Basel; 2005.
- 3.Torrado S, Torrado S, Cadorniga R, Torrado JJ. Formulation parameters of albendazole solution. Int J Pharm. 1996;140:45–50. doi: 10.1016/0378-5173(96)04545-0. [DOI] [Google Scholar]
- 4.Alvarez-Núñez FA, Yalkowsky SH. Buffer capacity and precipitation control of pH solubilized phention formulations. Int J Pharm. 1999;185:45–49. doi: 10.1016/S0378-5173(99)00129-5. [DOI] [PubMed] [Google Scholar]
- 5.Sweetana S, Akers MJ. Solubility principles and practice for parenteral drug dosage form development. PDA J Pharm Sci Tech. 1999;50(5):330–342. [PubMed] [Google Scholar]
- 6.Ni N, Sanghvi T, Yalkowsky SH. Solubilization and preformulation of carbendazim. Int J Pharm. 2002;244:99–104. doi: 10.1016/S0378-5173(02)00318-6. [DOI] [PubMed] [Google Scholar]
- 7.Rangel-Yagui CO, Pessoa-Jr A, Tavares LC. Micellar solubilization of drugs. J Pharm Pharmaceut Sci. 2005;8(2):147–163. [PubMed] [Google Scholar]
- 8.Lamas MC, Villaggi L, Nocito I, Bassani G, Leonardi D, Pascutti F, et al. Development of parenteral formulations and evaluation of the biological activity of the trypanocide drug benznidazole. Int J Pharm. 2006;307:239–243. doi: 10.1016/j.ijpharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Lin HS, Chean CS, Ng YY, Chan SY, Ho PC. 2-Hydroxypropyl-β-cyclodextrin increases aqueous solubility and photostability of all-trans-retinoic acid. J Clin Pharm Ther. 2000;25:265–269. doi: 10.1046/j.1365-2710.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 10.Brazeau GA, Cooper B, Svetic KA, Smith CL, Gupta P. Current perspectives on pain upon injection of drugs. J Pharm Sci. 1998;87:667–677. doi: 10.1021/js970315l. [DOI] [PubMed] [Google Scholar]
- 11.Kryzaniak JF, Raymond DM, Yalkowsky SH. Lysis of human red blood cells 2: effect of contact time on cosolvent induced hemolysis. Int J Pharm. 1997;52:193–200. doi: 10.1016/S0378-5173(97)00082-3. [DOI] [Google Scholar]
- 12.Amin K, Dannenfelser RM. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95:1173–1176. doi: 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- 13.Krzyzaniak JF, Alvarez Nunez FA, Raymond DM, Yalkowsky SH. Lysis of human red blood cells. 4. Comparison of in vitro and in vivo hemolysis data. J Pharm Sci. 1997;86:1215–1217. doi: 10.1021/js970184o. [DOI] [PubMed] [Google Scholar]
- 14.Lowe K, Furmidge B, Thomas S. Haemolytic properties of pluronic surfactants and effects of purification. Artif Cell Blood Substit Immobil Biotechnol. 1995;23:135–139. doi: 10.3109/10731199509117673. [DOI] [PubMed] [Google Scholar]
- 15.Al-Assadi H, Baillie AJ, Florence AT. The haemolytic activity of non-ionic surfactants. Int J Pharm. 1989;53:161. [Google Scholar]
- 16.Riess JG, Pace S, Zarif L. Highly effective surfactants with low hemolytic activity. Adv Mat. 1991;3:249–251. doi: 10.1002/adma.19910030507. [DOI] [Google Scholar]
- 17.Ohnishi M, Sagitani H. The effect of nonionic surfactant structure on hemolysis. J Am Oil Chem Soc. 1993;70:679–684. doi: 10.1007/BF02641003. [DOI] [Google Scholar]
- 18.Pourroy B, Botta C, Solas C, Lacarelle B, Braguer D. Seventy-two-hour stability of Taxol® in 5% dextrose or 0.9% sodium chloride in Viaflo®, Freeflex®, Ecoflac® and Macoflex N® non-PVC bags. J Clin Pharm Ther. 2005;30:155–458. doi: 10.1111/j.1365-2710.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 19.McGookin AG, Millership JS, Scott EM. Miconazole sorption to intravenous infusion sets. J Clin Pharm Ther. 1989;12:433–437. doi: 10.1111/j.1365-2710.1987.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 20.Kovács K, Stampf Gy, Klebovich I, Antal I, Ludányi K. Aqueous solvent system for the solubilization of azole compounds. Eur J Pharm Sci. 2009;36:352–358. doi: 10.1016/j.ejps.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Piel G, Evrard B, Fillet M, Llabres G, Delattre L. Development of a non-surfactant parenteral formulation of miconazole by the use of cyclodextrins. Int J Pharm. 1998;169:15–22. doi: 10.1016/S0378-5173(98)00103-3. [DOI] [Google Scholar]
- 22.Taneri F, Güneri T, Aigner Z, Kata M. Influence of cyclodextrin complexation on the physicochemical and biopharmaceutical properties of ketoconazole. J Incl Phenom Macro Chem. 2003;47:15–23. doi: 10.1023/B:JIPH.0000003875.46691.f6. [DOI] [Google Scholar]
- 23.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/S0959-8049(01)00171-X. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SL, Patel JP, Jones DL, Partipilo RW. Parenteral formulation development of renin inhibitor Abbott-72517. PDA J Pharm Sci Tech. 1994;48:86–91. [PubMed] [Google Scholar]
- 25.Barillaro V, Bertholet P, de Hassonville SH. Effect of acidic ternary compounds on the formation of miconazole/cyclodextrin inclusion complexes by means of supercritical carbon dioxide. J Pharm Pharmaceut Sci. 2004;7:378–388. [PubMed] [Google Scholar]
- 26.Crane IM, Mulhern MG, Nema S. Stability of reconstituted parecoxib for injection with commonly used diluents. J Clin Pharm Ther. 2003;28:363–369. doi: 10.1046/j.0269-4727.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- 27.Krishna G, Hodnick WF, Lang W, Lin X, Karra S, Mao J, et al. Pharmaceutical development and manufacturing of a parenteral formulation of a novel antitumor agent, VNP40101M. AAPS PharmsciTech. 2001;2(3):article 14. doi: 10.1208/pt020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guideline on stability testing: stability testing of existing active substances and related finished products (CPMP/QWP/122/02)
- 29.Amin K, Dannenfelser RM. In vitro hemolysis: guidance for the pharmaceutical scientist. Wiley InterScience. 2007. doi: 10.1002/jps.20627. [DOI] [PubMed]
- 30.Krzyzaniak JF, Raymond DM, Yalkowsky SH. Lysis of human red blood cells 2 effect of contact time on cosolvent induced hemolysis. Int J Pharm. 1997;152:193–200. doi: 10.1016/S0378-5173(97)00082-3. [DOI] [Google Scholar]
- 31.Krzyzaniak JF, Raymond DM, Yalkowsky SH. Lysis of human red blood cells 3 effect of contact time on surfactant induced hemolysis. J Pharm Sci Technol. 1998;52:66–69. [PubMed] [Google Scholar]
- 32.Ross BP, Braddy AC, McGeary RP, Blanchfield JT, Prokai L, Toth I. Micellar aggregation and membrane partitioning of bile salts, fatty acids, sodium dodecyl sulfate, and sugar-conjugated fatty acids: Correlation with hemolytic potency and implications for drug delivery. Mol Pharm. 2004;1:233–245. doi: 10.1021/mp049964d. [DOI] [PubMed] [Google Scholar]
- 33.Yalkowsky SH. Solubility and solubilization in aqueous media. New York: Oxford University Press; 1999. [Google Scholar]
- 34.Völgyi G, Deák K, Vámos J, Valkó K, Takács-Novák K. RPTLC determination of log P of structurally diverse neutral compounds. J Planar Chrom. 2008;21:143–149. doi: 10.1556/JPC.21.2008.2.12. [DOI] [Google Scholar]