Abstract
Bovine serum albumin-loaded beads were prepared by ionotropic gelation of alginate with calcium chloride and chitosan. The effect of sodium alginate concentration and chitosan concentration on the particle size and loading efficacy was studied. The diameter of the beads formed is dependent on the size of the needle used. The optimum condition for preparation alginate–chitosan beads was alginate concentration of 3% and chitosan concentration of 0.25% at pH 5. The resulting bead formulation had a loading efficacy of 98.5% and average size of 1,501 μm, and scanning electron microscopy images showed spherical and smooth particles. Chitosan concentration significantly influenced particle size and encapsulation efficiency of chitosan–alginate beads (p < 0.05). Decreasing the alginate concentration resulted in an increased release of albumin in acidic media. The rapid dissolution of chitosan–alginate matrices in the higher pH resulted in burst release of protein drug.
Key words: alginate, chitosan, drug release, encapsulation efficiency, particle size
INTRODUCTION
In recent years, there has been a significant increase in the number of targeting mechanisms available to the pharmaceutical scientist to provide site-specific delivery in the gastrointestinal (GI) tract (1). Research on site-specific and temporal control of drug delivery systems is receiving a major impetus toward the development of new and/or improved drug therapies. Generally, it is highly beneficial to target a drug to a particular site within the GI tract either to maximize a therapeutic response or to reduce side effects caused by drug delivery to an inopportune region of the gut (1,2). The development of delivery formulations for bioactive materials like proteins, peptides, and enzymes has been recognized as one of the most important fundamentals for a successful therapeutic effect in clinical medicine because the oral administration of peptide and protein drugs requires their protection from degradation in the gastric environment and the improvement of their absorption in the intestinal tract (1,3).
In the design of oral delivery of peptide or protein drugs, pH-sensitive hydrogels like alginate and chitosan (CS) have attracted increasing attention since most of the synthetic polymers are immunogenic and the incorporation of proteins into these polymers require a harsh environment which may denature and inactive the desired protein (4). Alginate and chitosan are natural polymers that they are biocompatible, biodegradable, and produce no systemic toxicity on administration. Alginate is a family of polysaccharides composed of α-l-guluronic acid (G) and β-d-mannuronic acid (M) residues, arranged in homopolymeric blocks of each type (MM, GG) and heteropolymeric blocks (MG). Alginates form strong complexes with polycations that are more resistant in the presence of calcium chelators and can be used to both stabilize the gel and reduce its porosity (4–7). Among those polycations, CS has received considerable attention for its safety and its mucoadhesive properties (8,9). Due to its hydrophilic and cationic characteristics, chitosan has the ability to gel on contact with counter anions. It has been reported that the amino groups of chitosan are capable of interacting with an anionic polymer that has carboxylic groups, such as carboxymethylcellulose or alginate by ionic binding (10–13). Alginate–chitosan complexes can be of important use in oral protein delivery systems. Alginate has the property of shrinking in low pH and getting dissolved in higher pH, whereas chitosan dissolves in low pH and is insoluble in higher pH ranges (4).
In this regard, the use of alginate gelled by the addition of calcium ions has been proposed to prepare beads. A mild encapsulation method can enhance the protein stability and retain the biological activity of the encapsulated materials. In addition, the beads can protect the protein as it passes through the acidic and enzymatic environment of the stomach and can release the protein via diffusion and capsule degradation once they reach the small intestinal region, at which the protein is effectively absorbed into the blood stream (10,13). In the encapsulation method, the particle size of beads was shown to be predominantly affected by the ratio of alginate to chitosan, the molecular weight of the biopolymers, and the solution pH (11).
On the basis of these considerations, the aim of this study was to evaluate the influence of the incorporation of two oppositely charged hydrophilic natural polymers, chitosan and sodium alginate, on the retention of a model protein, bovine serum albumin (BSA), and the particle size of the beads during manufacture. The achievement of a controlled release at small intestinal pH was also an objective, in order to reduce the potential degradation of BSA before absorption. Although there are different colloidal devices for protein delivery using alginate and chitosan polymers (4,10,12,14,15), to date, only a few attempts have been made to correlate statistically the formulation variables with the final bead properties. Therefore, in this work, central composite design was used to simultaneously study the effect of the two formulation variables of the chitosan–alginate delivery system on two response variables. Central composite design (CCD) and analysis of response surfaces were used because they are systematic and efficient methods to study the effect of multiple variables and to find an optimum formulation (16).
MATERIALS AND METHODS
Materials
Sodium alginate (Protonal LF 120 M) was a gift from FMC Biopolymer (USA). The viscosity of the 1% (w/w) aqueous sodium alginate solution at 20°C was measured with a rotational viscometer (Brookfield, USA), as 720 cps.
Chitosan H was a gift from Dainichiseika Color & Chem. Mfg. (Japan). BSA was from Amresco Chemicals Co. (USA). Calcium chloride, sodium hydroxide, and acetic acid were obtained from Sigma-Aldrich (Germany). All chemicals were of analytical grade.
Methods
Experimental Design
A response surface method central composite experimental design was applied to evaluate the relationship between the independent variables and their responses as well as their interactions in an effective model. According to the model, it contains four full factorial design points, four axial points, and three center points. Two variables and two responses were involved in the experimental design. The dependent response factor variables measured were encapsulation efficiency and particle size. The independent variables are the concentration of alginate (X1) and the concentration of chitosan (X2; Table I). The formulation variables and the high and low levels of each variable were defined based on preliminary experiments. Center points were repeated three times to estimate the experimental error. The design matrix in coded form was shown in Table II.
Table I.
Factors and Their Corresponding Levels Implemented for the Construction of CCD
| Factor | Factor level X | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| X 1, alginate (%) | 1.19 | 1.5 | 2.25 | 3 | 3.31 |
| X 2, chitosan (%) | 0.15 | 0.25 | 0.5 | 0.75 | 0.85 |
Table II.
Central Composite Design in Various Runs and the Correspondent Response
| Formulation | Coded levels | Normal levels | |||
|---|---|---|---|---|---|
| Alginate concentration (%) | Chitosan concentration (%) | Alginate concentration (%) | Chitosan concentration (%) | ||
| K1 | Factorial points | 1 | 1 | 3 | 0.75 |
| K2 | 1 | −1 | 3 | 0.25 | |
| K3 | −1 | 1 | 1.5 | 0.75 | |
| K4 | −1 | −1 | 1.5 | 0.25 | |
| K5 | Axial points | 1.414 | 0 | 3.31 | 0.5 |
| K6 | −1.414 | 0 | 1.19 | 0.5 | |
| K7 | 0 | 1.414 | 2.25 | 0.85 | |
| K8 | 0 | −1.414 | 2.25 | 0.15 | |
| K9 | Center points | 0 | 0 | 2.25 | 0.5 |
| K10 | 0 | 0 | 2.25 | 0.5 | |
| K11 | 0 | 0 | 2.25 | 0.5 | |
Preparation of Beads
BSA was dissolved in water and added to the sodium alginate solution. Chitosan and calcium chloride (CaCl2) were dissolved in 5% acetic acid solution. The pH of solution was adjusted to 5 using 0.1 N NaOH solution and was stirred for further 30 min. Then the mixture solution (sodium alginate–BSA) added dropwise into chitosan–CaCl2 solution at room temperature using a peristaltic pump with a polyethylene tubing nozzle (21 gauge) at a pumping rate of 10 ml/min. Beads were removed from the encapsulation medium via filtration, rinsed twice with 0.1 N HCl, and placed in an oven overnight to dry (37°C) and the final dried mass carefully recorded.
Encapsulation Efficiency
The beads were initially broken in pH 6.8 phosphate buffer. The BSA content was determined by measuring the absorbance at 740 nm with an UV spectrophotometer. Lowry method was used for protein estimation (17). The encapsulation efficiency of BSA in the chitosan–alginate beads was calculated by the formula shown in Eq. 1.
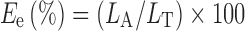 |
1 |
where LA is the actual loading and LT is the theoretical loading of BSA (weight–weight percentage) in the beads. The encapsulation efficiency results are listed in Table III.
Table III.
Results of Particle Size (Y 1) and Encapsulation Efficiency (Y 2) Obtained for Formulations by CCD
| Formulation code | Particle size (μm) | Encapsulation efficiency (%) |
|---|---|---|
| K1 (1, 1) | 1,546 | 66.7 |
| K2 (1, −1) | 1,501 | 98.5 |
| K3 (−1, 1) | 1,588 | 71.5 |
| K4 (−1, −1) | 1,514 | 99 |
| K5 (1.41, 0) | 1,549 | 96 |
| K6 (−1.41, 0) | 1,532 | 70 |
| K7 (0, 1.41) | 1,541 | 68 |
| K8 (0, −1.41) | 1,478 | 91 |
| K9 (0, 0) | 1,530 | 92.9 |
| K10 (0, 0) | 1,572 | 96.4 |
| K11 (0, 0) | 1,563 | 95.6 |
Particle Size Analysis
Particle size analysis was determined using the laser scattering particle size distribution analyzer (Sympatec GmbH, Germany). The particle size results are listed in Table III.
Statistical Analysis
Data evaluation is done using stepwise multivariate linear regression analysis. The model predictor equations are estimated for each dependent variable separately. The general type of predictor equation resulting from a three-level experimental design is a second-order polynomial shown in Eq. 2.
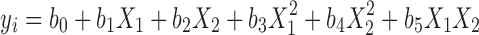 |
2 |
where y is the responsible variable, b0 is a constant, and b1,...,b5 are regression coefficient. X1 and X2 are the independent variables and X1X2 is the interaction term, showing how response changes when two factors are simultaneously changed. 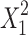 and
and 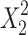 are quadratic terms of the independent variables to evaluate the nonlinearity. In the final model equations, only the significant factors were included.
are quadratic terms of the independent variables to evaluate the nonlinearity. In the final model equations, only the significant factors were included.
The statistical analysis data through regression model and plotting the response surface graphs were achieved by NCSS (Kaysville, UT, USA). The developed models were tested for its significance using analysis of variance (ANOVA). All tests were performed at a 95% level of significance (α = 0.05).
Evaluation of Protein Release from Beads
A weighed quantity of beads was suspended in pH 1.2 gastric media. This medium was stirred at 140 rpm in a laboratory shaker and maintained at 37 ± 0.1°C in a water bath. The calcium alginate gel beads were tested for drug release for 2 h in 0.1 N HCl as the average gastric emptying time is about 2 h. Then the dissolution medium was replaced with pH 6.8 phosphate buffer and tested for drug release for 3 h. Samples were taken at appropriate intervals from both media, and BSA amount was analyzed spectrophotometrically.
Scanning Electron Microscopy
Gel beads were coated with gold for 5 min under vacuum. Morphological examination of the gel beads was performed using a scanning electron microscope (JSM-840A, Joel Ltd., Japan) at 11 kV.
RESULTS AND DISCUSSION
Particle Size
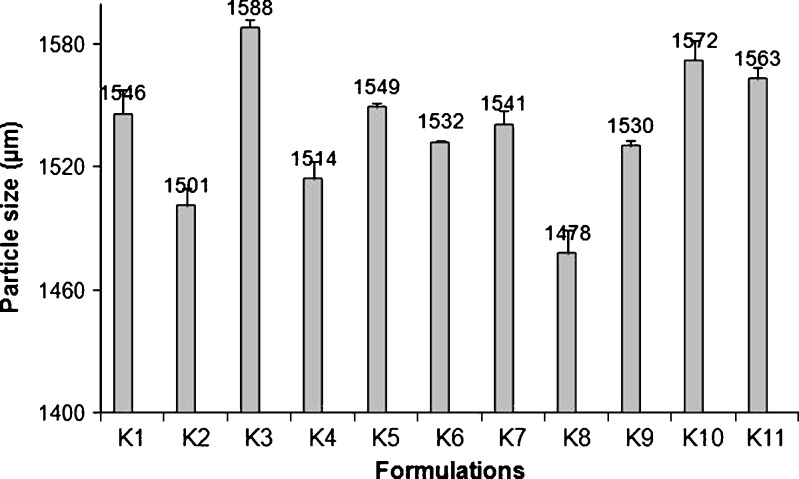
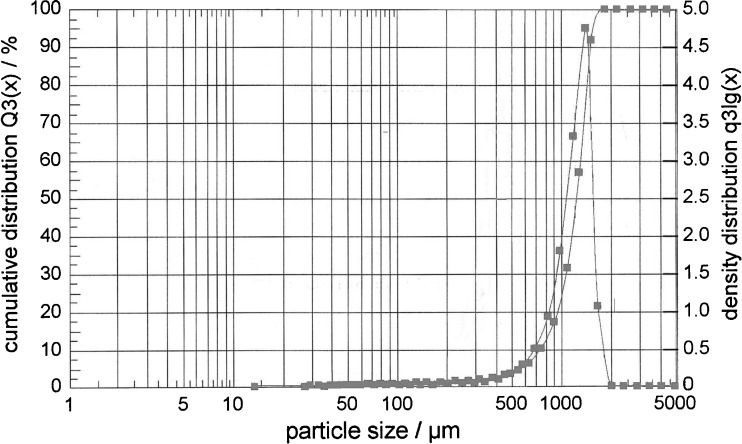
The chitosan/alginate beads showed a spherical shape and a rough polymeric matrix, as can be seen in Fig. 1. The mean particle size of 11 formulations was between 1,478 and 1,588 µm (Fig. 2). It was found that the particle size distribution of each formulation was within a narrow range (Fig. 3), but the mean particle size was slightly different among the formulations. Hence, it can be concluded that the polyethylene tubing nozzle (21 gauge) is an important parameter leading to the formation of larger particle sizes to be produced. In general, beads greater than 1.00 mm in diameter can be prepared by using a syringe with a needle or a pipette (5). Hari et al. (14) also reported that the beads are about 900–1,000 µm in size when sodium alginate solution was dropped through a needle (0.15 mm diameter), from a plastic syringe into a beaker containing CaCl2 solution.
Fig. 1.
Scanning electron microscopy photograph of chitosan–alginate beads (K2 formulation)
Fig. 2.
Particle size of chitosan–alginate beads
Fig. 3.
Particle size distribution of K2 formulation
In Vitro Drug Release
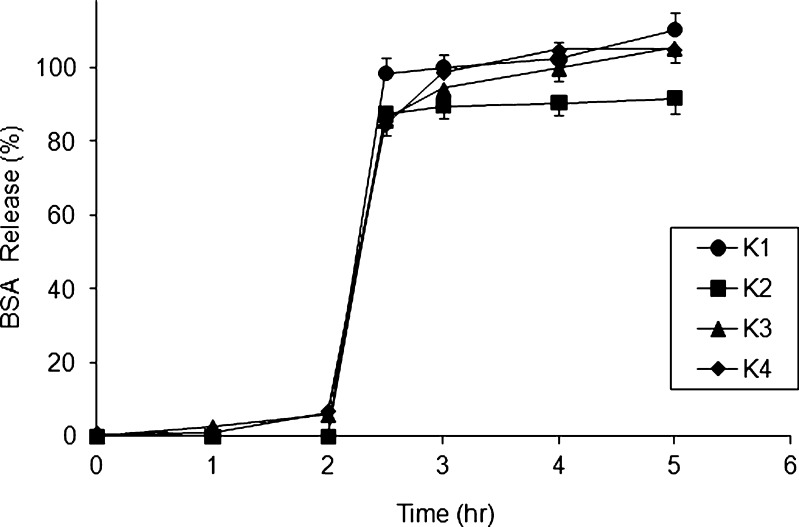
Figures 4, 5, and 6 illustrate the release profiles of the four factorial, four axial, and three central points of the central composite design. The release profiles of BSA from the beads for several of the formulations are shown in gastric media for 2 h and then in pH 6.8 phosphate buffer for 3 h. It is clear from the Fig. 4 that the formulations showed a similar drug release. The drug release was not seen from the K1 and K2 formulations in gastric media for 2 h; however, the cumulative mean percentages BSA released from the formulations K3 and K4 were 5.86 ± 3.2 and 6.73 ± 3.9, respectively, after 2 h of testing in simulated gastric fluids.
Fig. 4.
BSA release profiles for formulations prepared from four factorial points of CCD
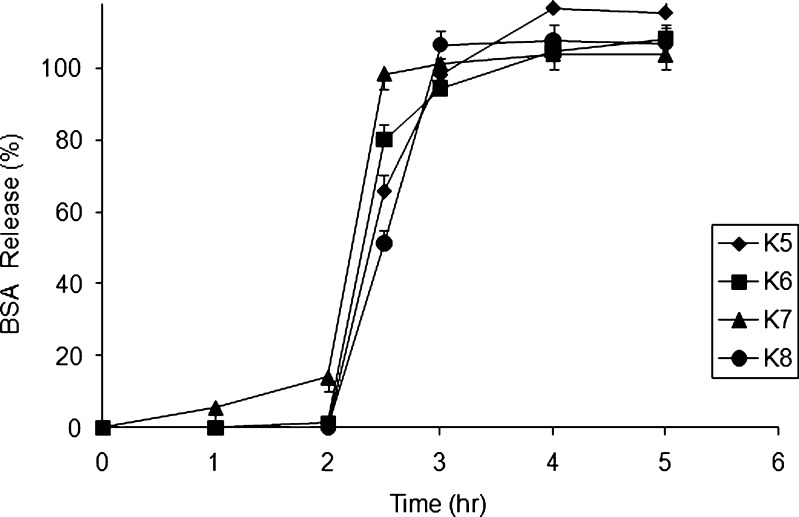
Fig. 5.
BSA release profiles for formulations prepared from four axial points of CCD
Fig. 6.
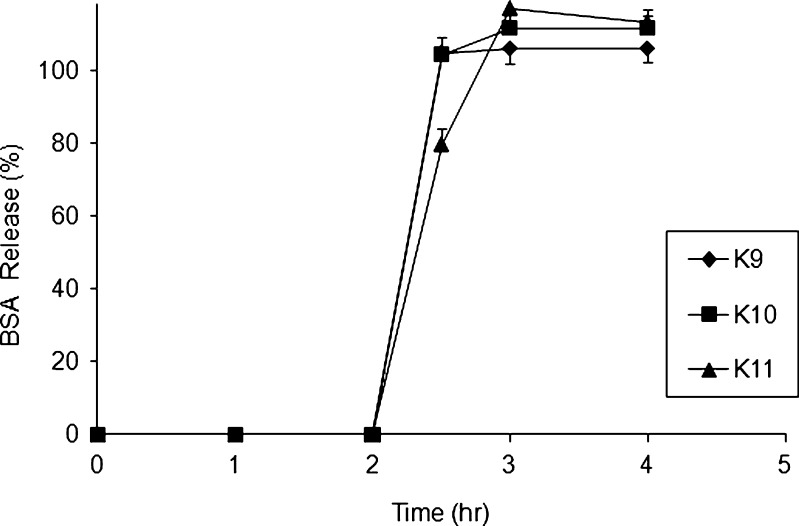
BSA release profiles for formulations prepared from three center points of CCD
The drug release in gastric media in K3 and K4 formulations may be due to the decreased sodium alginate content in the beads. The release of less than 7% of BSA in simulated gastric medium indicates that sodium alginate–chitosan complex is capable of minimizing drug release in the physiological environment of the stomach. At low pH, the alginates do not swell, but a reversal of shrinkage takes place and the contents are not released (4). After the dissolution medium was replaced with pH 6.8 phosphate buffer, all the formulations exhibited burst release and at the end of 1 h of testing in pH 6.8, the cumulative mean percentage drug released from formulations K1, K2, K3, and K4 were found to be 100 ± 4.2, 90.6 ± 3.3, 99.8 ± 4.2, and 104.7 ± 3.8, respectively. In the polymer matrix system, the drug is homogeneously dispersed in a rate-controlling way. When such systems are exposed to the dissolution medium, drug release is modulated by diffusion through matrix swelling and dissolution/erosion at the matrix periphery (6,18).
Figure 5 represents the percentage BSA release at 5 h for axial points. As per central composite design, formulation K5 and K8 contain the highest sodium alginate loads (α = 1.14) and exhibited a 65.7 ± 4.5% and 51.4 ± 3.8%, respectively, at 2.5 h. The results from the axial points indicated the significant effect of sodium alginate concentration for a strong complexation. This phenomenon is in agreement with the previous study where it is reported that the release rate was quicker for beads prepared in low concentrations of alginate but slower for beads prepared in high concentrations (19). However, proteins encapsulated in alginate matrices are released at 3 h due to the degradation of the polymeric network.
To calculate the lack of fit for the suggested regression model, center points were included in the design to calculate the pure error due to the experimental procedure. From Fig. 6, we can conclude that the fact that the release of all three center points overlaps each other indicates that the error due to experimental procedure was found to be less in generating a meaningful fitting for the dependent variables.
The formulation and the technological approach in the preparation of BSA loaded beads have to provide a control in the BSA release for a relatively long period or to delay the drug release in the stomach. These expectations were based on the physicochemical properties of the polymers. It has previously been shown that the binding and stability of alginate–chitosan complex depends on both the composition of the alginate gel and the production procedure of the beads (4,15,20). In the one-stage procedure, a complex coacervate membrane is formed at the interface between the alginate and chitosan solutions when the alginate solution is dropped directly into a calcium chloride–chitosan solution. If the beads are made by a one-stage procedure, only low amounts of chitosan are bound. This is probably because the chitosan only binds onto the surface, creating a membrane with such small pores that further diffusion of chitosan into the beads and subsequent binding onto the gel network is restricted (20). In our study, the rapid and complete BSA release was obtained for all the formulations when beads were transferred to phosphate buffer at pH 6.8. This result could be reasonably attributable to the production procedure of the beads which caused low contents of chitosan binding onto the surface and thereby the erosion of the polymer matrix. In respect to achieving a more controlled release of BSA at small intestinal pHs, the prevention of erosion of alginate–chitosan matrix in this pH is considered desirable.
Effect of Formulation Variables on Particle Size
Table IV shows the effect of independent variables on the particle size in terms of a regression model. Along with the model terms, factor X2 was found to be significant and the polynomial equation relating to response Y1 can be written as Eq. 3.
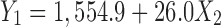 |
3 |
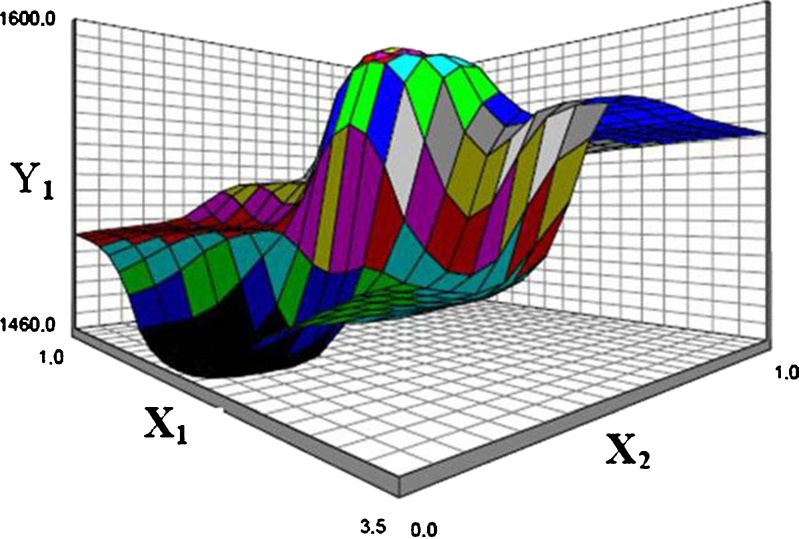
As the chitosan concentration (X2) increases, the corresponding particle size also increases. The probable explanation for this may be due to formation of polyelectrolyte complex between carboxyl groups of sodium alginate and the amino groups of chitosan. The optimal binding ratio of chitosan to alginate was found to be approximately 1:1 (21). Douglas and Tabrizian (11) also reported that smaller particles were formed when the availability of the functional groups are in stoichiometric proportion. Increasing the relative amount of chitosan or alginate causes an increase in particle size.
Table IV.
Values for Regression Coefficients and Their Levels of Significance for the Factors Used to Model the Best Fit Size Response
| Term | Coeff. | SE Coeff. | T | p |
|---|---|---|---|---|
| Particle size | ||||
| Constant | 1,554.99 | |||
| Chitosan concentration (%, w/v) | 26.014 | 7.367 | 3.53 | 0.0167 |
| Encapsulation efficiency | ||||
| Constant | 94.97 | |||
| Alginate concentration (%, w/v) | 3.93 | 0.648 | 6.07 | 0.0261 |
| Chitosan concentration (%, w/v) | −11.48 | 2.88 | −3.98 | 0.0104 |
| (Alginate concentration)2 | −5.31 | 0.771 | −6.89 | 0.0204 |
| (Chitosan concentration)2 | −7.07 | 0.772 | −9.15 | 0.0117 |
R 2 (encapsulation efficiency) = 0.8217, R 2 (particle size) = 0.7842
Coeff. coefficient, SE Coeff. coefficient of standard error of mean
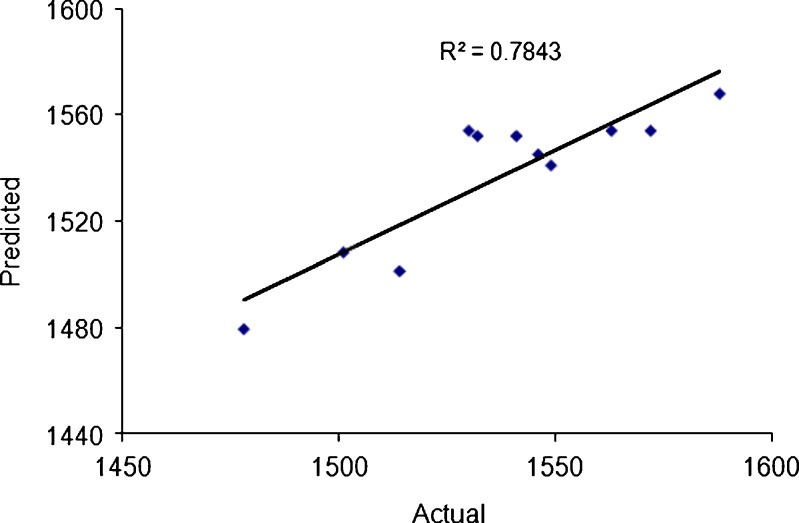
The effect of pH is also an important parameter on particle size. Much of chitosan, which is poorly water soluble likely precipitates upon addition to an alginate solution with pH 7.1 and the majority of amine groups being unprotonated and therefore unable to participate in ionic interactions. The few protonated groups available for interaction would result in weaker electrostatic interactions with the alginate gel, leading to larger particle sizes (11,22). Figure 7 represents the observed response values compared to that of predicted values. The response surface plot in Fig. 8 clearly illustrates the effect of interaction between X1 and X2 on particle size. The effect of the concentration of alginate in the range studied was not statistically significant. This is probably attributed to the size of the needle used in the production method. A larger diameter needle was not affected in the range studied of the alginate concentration. We can conclude that the diameter of the needle was found more effective than the alginate/chitosan ratio to determine the particle size of beads.
Fig. 7.
The observed response values compared to that predicted values for particle size (Y 1)
Fig. 8.
Response surface plot showing the effect of alginate concentration (X 1) and chitosan concentration (X 2) on particle size (Y 1)
Effect of Formulation Variables on Encapsulation Efficiency
The model terms for Y2 (encapsulation efficiency) were found to be significant with an F value of 4.61; high R2 value of 0.8217 indicates the fitting of regression model (Table IV). In this case, all the factors, except X1X2, were found to be significant, and the model describing the encapsulation efficiency can be written as Eq. 4.
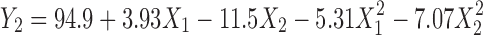 |
4 |
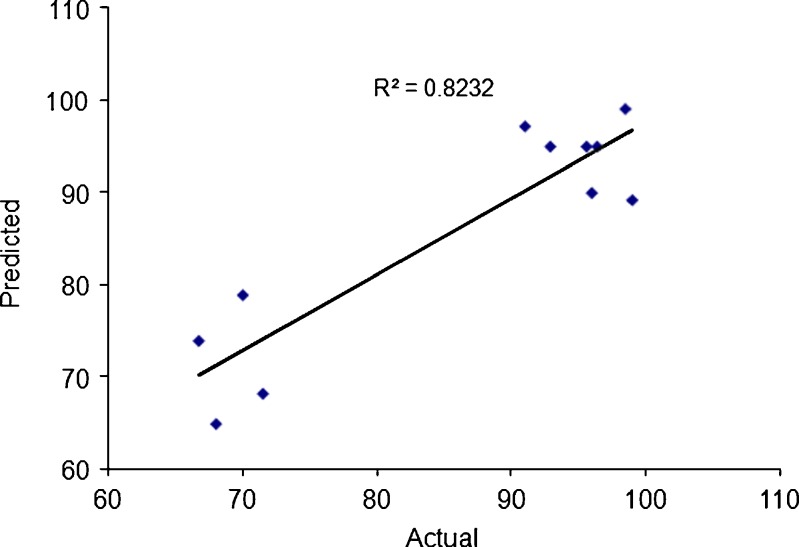
Figure 9 represents the observed response values compared to that of predicted values. The encapsulation efficiency of BSA varied from 66.7% to 99% depending on the alginate and chitosan concentrations.
Fig. 9.
The observed response values compared to that predicted values for encapsulation efficiency (Y 2)
Negative value of X2 indicated that the percent of encapsulation efficiency decreased as the chitosan concentration increased. Motwari et al. (23) also reported that chitosan concentration had a negative effect on the encapsulation efficiency because at higher concentrations, chitosan led to the formation of aggregates upon addition of alginate. Figure 10 shows the effect of sodium alginate concentration and chitosan concentration on encapsulation efficiency.
Fig. 10.
Response surface plot showing the effect of alginate concentration (X 1) and chitosan concentration (X 2) on encapsulation efficiency (Y 2)
As the concentration of alginate increases and concentration of chitosan decreases, the encapsulation efficiency increases. It is observed that only two formulations (K1 and K7) exhibited low encapsulation efficiency. Such a behavior of decreases in encapsulation efficiency may be attributed due to competition between chitosan and positively charged BSA for available acid sites on the alginate chain can occur.
ANOVA, Pure Error, Lack of Fit
Regression analysis was carried out to obtain the regression coefficients (Table IV) and the effects as follows: Only chitosan concentration was found to be significant for Y1. On the other hand, chitosan (X1) and alginate (X2) concentration and the quadratic terms 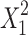 and
and 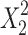 were found to be significant for Y2. The above results conveyed us that both alginate and chitosan concentrations play an important role in the formulation of chitosan–alginate beads containing highly water-soluble protein BSA. The data of pure error and lack of fit are summarized in Table V, which can provide a mean response and an estimate of pure experimental uncertainty (24).
were found to be significant for Y2. The above results conveyed us that both alginate and chitosan concentrations play an important role in the formulation of chitosan–alginate beads containing highly water-soluble protein BSA. The data of pure error and lack of fit are summarized in Table V, which can provide a mean response and an estimate of pure experimental uncertainty (24).
Table V.
Summary of ANOVA Results in Analyzing Lack of Fit and Pure Error
| Source | Sum of squares | df | Mean square | F value | Probability |
|---|---|---|---|---|---|
| Particle size (μm) | |||||
| Model | 7,947.49 | 5 | 1,587.097 | 3.66 | 0.0903 |
| Residual | 2,183.06 | 5 | 436.61 | – | – |
| Total | 10,118.55 | 10 | 2,023.27 | – | – |
| Lack of fit | 1,205.06 | 3 | 401.69 | 0.81 | 0.592 |
| Pure error | 978 | 2 | 489 | – | – |
| Encapsulation efficiency | |||||
| Model | 1,529.01 | 5 | 305.80 | 4.61 | 0.0109 |
| Residual | 331.88 | 5 | 66.38 | – | – |
| Total | 1,860.89 | 10 | 372.18 | – | – |
| Lack of fit | 325.16 | 3 | 108.38 | 32.23 | 0.0302 |
| Pure error | 6.726 | 2 | 3.36 | – | – |
CONCLUSION
The application of a two-factor five-level central composite design resulted in a useful tool for the characterization and optimization of BSA chitosan–alginate beads. The central composite design is used to estimate the effect of the two independent variables, which are sodium alginate and chitosan concentration on the response factors particle size and encapsulation efficiency. The polymer amount is a major factor affecting the release and encapsulation efficiency of the chitosan/alginate beads. According to the studied factors, the selected optimum formulation was that prepared with 3% (w/v) of sodium alginate and 0.25% (w/v) of chitosan corresponding to (1, −1) of the experimental design. No significant release of BSA was observed during treatment with 0.1 N HCl for 2 h; however, the beads released almost all the entrapped protein into phosphate buffer pH 6.8 media within 1 h. Increasing the alginate and/or chitosan concentration in the matrix did not promote a controlled release of BSA from beads at pH 6.8. The preparation of cross-linking reinforced the chitosan–alginate beads to prevent the erosion of the matrix and to achieve a more controlled release of BSA at small intestinal pH that are needed as further studies.
Acknowledgments
This study was supported by a grant from Gazi University (02/2002-03).
References
- 1.Hwang S, Park H, Park K. Gastric retentive drug delivery systems. Crit Rev Ther Drug Carr Syst. 1998;15:243–284. [PubMed] [Google Scholar]
- 2.Lee VH, Grass GM, Dodda-Kashi S, Rubas W. Oral route of peptide and protein drug delivery. In: Lee VH, editor. peptide and protein drug delivery. New York: Marcel & Dekker; 1991. pp. 691–741. [Google Scholar]
- 3.Ingemann M, Frokjaer S, Hovgaard L. Peptide and protein drug delivery systems for non-parenteral routes of administration. In: Frokjaer S, Hovgaard L, editors. Pharmaceutical formulation development of peptides and proteins. Philadelphia: Taylor & Francis; 2000. pp. 189–203. [Google Scholar]
- 4.George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Cont Rel. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–285. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 6.Tonnesen HH, Karlsen J. Alginate in drug delivery. Drug Dev Ind Pharm. 2002;28:621–630. doi: 10.1081/DDC-120003853. [DOI] [PubMed] [Google Scholar]
- 7.Takka S, Acartürk F. Calcium alginate microparticles for oral administration: I. Effect of sodium alginate type on drug release and drug entrapment efficiency. J Microencapsul. 1999;16:275–290. doi: 10.1080/026520499289022. [DOI] [PubMed] [Google Scholar]
- 8.Aral C, Akbuğa J. Alternative approach to the preparation of chitosan beads. Int J Pharm Sci. 1998;168:9–15. doi: 10.1016/S0378-5173(98)00072-6. [DOI] [Google Scholar]
- 9.Şenel S, Hıncal AA. Drug permeation enhencement via buccal route: possibilities and limitations. J Contr Rel. 2001;72:133–144. doi: 10.1016/S0168-3659(01)00269-3. [DOI] [PubMed] [Google Scholar]
- 10.Coppi G, Iannuccelli V, Leo E, Bernabei MT, Cameroni R. Chitosan–alginate microparticles as a protein carrier. Drug Dev Ind Pharm. 2001;27:393–400. doi: 10.1081/DDC-100104314. [DOI] [PubMed] [Google Scholar]
- 11.Douglas KL, Tabriziani M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J Biomater Sci Polym ed. 2005;16:43–56. doi: 10.1163/1568562052843339. [DOI] [PubMed] [Google Scholar]
- 12.Polk A, Amsden B, De Yao K, Peng T, Goosen MFA. Controlled release of albumin from chitosan alginate microcapsules. J Pharm Sci. 1994;83:178–185. doi: 10.1002/jps.2600830213. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Guo J, Peng X, Jin Y. Preparation and release behavior of carboxymethylated chitosan/alginate microspheres encapsulating bovine serum albumin. J Appl Polym Sci. 2004;92:878–882. doi: 10.1002/app.13708. [DOI] [Google Scholar]
- 14.Hari PR, Chandy T, Sharma CP. Chitosan/calcium alginate beads for oral delivery of insulin. J Appl Polym Sci. 1996;59:1795–1801. doi: 10.1002/(SICI)1097-4628(19960314)59:11<1795::AID-APP16>3.0.CO;2-T. [DOI] [Google Scholar]
- 15.Vandenberg GW, De La Noüe L. Evaluation of protein release from chitosan–alginate microcapsules produced using external or internal gelation. J Microencapsul. 2001;18:433–441. doi: 10.1080/02652040010019578. [DOI] [PubMed] [Google Scholar]
- 16.Narendera C, Srinath MS, Prakash Rao B. Development of three layered buccal compact containing metoprolol tartrate by statistical optimization technique. Int J Pharm. 2005;304:102–114. doi: 10.1016/j.ijpharm.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Takka S, Ocak Ö, Acartürk F. Calcium alginate microparticles for oral administration: II. Effect of formulation factors on drug release and drug entrapment efficieny. Eur J Pharm Sci. 1998;6:241–266. doi: 10.1016/S0928-0987(97)10005-7. [DOI] [PubMed] [Google Scholar]
- 19.Mi FL, Sung HW, Shyu SS. Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohyd Polym. 2002;48:61–72. doi: 10.1016/S0144-8617(01)00212-0. [DOI] [Google Scholar]
- 20.Gåserød O, Sannes A, Skjak-Braek G. Microcapsules of alginate–chitosan. II. A study of capsule stability and permeability. Biomaterials. 1999;20:773–783. doi: 10.1016/S0142-9612(98)00230-0. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Takayama K, Machida Y, Nagai T. Characteristics of polyion complexes of chitosan with sodium alginate and sodium polyacrylate. Int J Pharm. 1990;61:35–41. doi: 10.1016/0378-5173(90)90041-2. [DOI] [Google Scholar]
- 22.Gazori T, Khoshayand MR, Azizi E, Yazdizade P. Evaluation of alginate/chitosan nanoparticles as antisense delivery vector: formulation, optimaztion and in vitro characterization. Carbohydr Polym. 2009;77:599–606. doi: 10.1016/j.carbpol.2009.02.019. [DOI] [Google Scholar]
- 23.Motwari SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68:513–525. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Lewis GA, Luu RP, Mathiey D. Pharmaceutical experimental design. New York: Marcel Dekker; 1999. [Google Scholar]