Abstract
In the present study, an attempt was made to prepare immediate-release enteric-coated pellets of aceclofenac, a poorly soluble nonsteroidal anti-inflammatory drug that has a gastrointestinal intolerance as its serious side effect. Formulation of enteric-coated pellets with improved solubility of aceclofenac could address both of these problems. To achieve these goals, pellets were prepared by extrusion–spheronization method using pelletizing agents that can contribute to the faster disintegration and thereby improve the solubility of the drug. Different disintegrants like β-cyclodextrin, kollidon CL, Ac-Di-Sol, and sodium starch glycolate were tried in order to further improve disintegration time. The pellets were characterized for drug content, particle size distribution, flow properties, infrared spectroscopy, surface morphology, disintegration rate, and dissolution profile. The formulations, which showed best disintegration and dissolution profiles, were coated with Eudragit L100-55, an enteric-coated polymer which does not dissolve at gastric pH but dissolves at intestinal pH, releasing the drug immediately in the dissolution medium. The optimized enteric-coated formulation containing 20% κ-carrageenan, lactose, and sodium starch glycolate as a disintegrant did inhibit the release of the drug for 2 h in 0.1 N HCl, whereas 87% of the drug was released within 45 min. The improvement was substantial when it was compared with solubility of pure drug under the same conditions. Thus, dissolution profiles suggested that combination of κ-carrageenan and sodium starch glycolate resulted into fast-disintegrating, immediate-release pellets, overcoming the bioavailability problem of the poorly soluble drug, aceclofenac, and enteric coating of these pellets avoids the exposure of aceclofenac to ulcer-prone areas of the gastrointestinal tract.
Key words: aceclofenac, enteric-coated, κ-carrageenan, pellets
INTRODUCTION
Aceclofenac is chemically [[[2-[(2, 6-Dichlorophenyl)amino]phenyl]acetyl]oxy] acetic acid, a potent nonsteroidal anti-inflammatory drug (NSAID) mainly used in osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis (1). As with most NSAIDs, irritation of the gastrointestinal (GI) tract is one of the major side effects reported after oral administration of aceclofenac (2). The GI intolerance of aceclofenac is not only related to the inhibition of the prostaglandin synthesis but also to acute local contact of the drug with the gastric mucosa when it is given orally. This GI intolerance can be reduced significantly by avoiding contact of the drug with the gastric mucosa. According to the Biopharmaceutical Classification System, aceclofenac is regarded as a class II compound characterized by low water solubility and high permeability. Thus, low water solubility is another major problem with this potentially useful drug candidate. For class II drugs, bioavailability is limited by their dissolution rate. Bioavailability can be increased by improving dissolution rate, and dissolution rate can be increased by achieving faster disintegration.
Literature reports various ways to overcome both these problems of aceclofenac. The acute local contact of aceclofenac has been avoided by formulating a combination of extended-release NSAID and immediate-release prostaglandins (3), dual-release compositions of Cox-2 inhibitors (4). Improvement in the water solubility and hence bioavailability of aceclofenac has been achieved by formulation of soft capsules containing drug and solubilizers (5), solid dispersions with mixed surfactants (6), complexation with HP-β-cyclodextrin (HP-β-CD) (7), spherical agglomerates using PVP and sodium alginate (8), chitosan–aceclofenac cocrystals(9), aceclofenac-loaded agarose beads (10), and fast-dissolving tablets (11,12). All these approaches seem to be pretty attractive as far as improvement in dissolution of aceclofenac is concerned, but none of these addresses the problem of aceclofenac causing GI side effects. It is very likely that GI toxicity may aggravate when more amount of soluble drug is available in the stomach at a given time.
Thus, there is a need for a formulation that is not only providing improvement in solubility but at the same time reducing the GI adverse effects of aceclofenac. It is also important that such a formulation should involve simple technique so that it can be easily employed at a commercial level.
These objectives can be achieved by formulating enteric-coated aceclofenac-loaded pellets which are fast disintegrating in nature. Pellets being smaller in size offer larger surface area for dissolution than any other solid dosage form (13). The surface area can further be increased by making these pellets disintegrate immediately. Decreasing the particle size of poorly soluble drugs is an established approach for improving solubility, thus improving bioavailability (14). Enteric coating of these pellets will prevent the drug to come into contact with ulcer-prone area of GI lining and hence will avoid GI toxicity. Besides, pellets are easy to coat and have good flow properties.
Aceclofenac pellets were prepared by using extrusion–spheronization method. Microcrystalline cellulose (MCC) is the most widely used pelletization aid. But MCC is known to interfere with the release of poorly soluble drugs (15). Because of this reason, various pelletization aids are available nowadays, which may be used to formulate fast-disintegrating pellets. Recently, κ-carrageenan, extracted from cell walls of red seaweeds, has been used as a pelletization aid (16,17). In the present work, κ-carrageenan was also explored as pelletization agent along with MCC for the formulation of aceclofenac pellets. In addition to that, the role of lactose and maize starch as fillers and the role of β-cyclodextrin, sodium starch glycolate, croscarmellose sodium (Ac-Di-Sol), and crospovidone (Kollidon® CL) as disintegrants were evaluated on the disintegration and other characterization parameters of these pellets.
These pellets were then coated with Eudragit® L 100-55, an anionic copolymer based on methacrylic acid and ethyl acrylate. The carboxylic groups begin to ionize in aqueous media at pH 5.5 and above, rendering the polymer resistant to acidic media but soluble in intestinal fluid, i.e., the pellets were coated using enteric coating polymer.
MATERIAL AND METHODS
Aceclofenac, maize starch, MCC, lactose, talc, and β-cyclodextrin were obtained as gift samples from Zim Laboratories Ltd., Nagpur, India, and κ-carrageenan (Gelcarin GP-911, NF) was a gift sample from Signet chemical corporation, Mumbai, India. Triethyl citrate and isopropyl alcohol were purchased from Sigma Aldrich Ltd. Eudragit L 100-55 was donated by Röhm GmbH (Darmstadt, Germany). Sodium starch glycolate and croscarmellose sodium (Ac-Di-Sol) were gift samples from Signet chemical corporation, Mumbai, India; crospovidone (Ph. Eur. Type A, US Pharmacopeia (USP)/NF, JPE; Kollidon®-CL) was obtained from BASF, The Chemical Company, Germany.
Preparation of Pellets
Pellet formulations were prepared by varying the quantities of MCC, κ-carrageenan, maize starch, and lactose using extrusion–spheronization method (Table I). MCC and κ-carrageenan were used as pelletizing agents. Maize starch and lactose were used as fillers. Out of all the prepared formulations, formulation A6 was found to behave best in the characterization tests. Therefore, formulation A6 was selected for the further evaluation of β-cyclodextrin, sodium starch glycolate, Ac-Di-Sol, and Kollidon® CL as disintegrants. A fixed quantity of all these disintegrants was added to the formulation A6 (Table II).
Table I.
Composition of Pellet Batches
| Formulation code | Aceclofenac | MCC | κ-Carrageenan | M. starch | Lactose |
|---|---|---|---|---|---|
| A1 | 40% | 30% | – | 30% | – |
| A2 | 40% | 20% | 10% | 30% | – |
| A3 | 40% | 10% | 20% | 30% | – |
| A4 | 40% | – | 30% | 30% | – |
| A5 | 40% | – | 10% | – | 50% |
| A6 | 40% | – | 20% | – | 40% |
| A7 | 40% | – | 30% | – | 30% |
Table II.
Composition of Pellet Batches Containing Disintegrants
| Formulation code | A6 | A6-a | A6-b | A6-c | A6-d |
|---|---|---|---|---|---|
| Aceclofenac | 37.03% | 37.03% | 37.03% | 37.03% | 37.03% |
| MCC | – | – | – | – | – |
| κ-Carrageenan | 18.51% | 18.51% | 18.51% | 18.51% | 18.51% |
| Lactose | 37.03% | 37.03% | 37.03% | 37.03% | 37.03% |
| β-CD | – | 7.40% | – | – | – |
| Sodium starch glycolate | – | – | 7.40% | – | – |
| Ac-Di-sol | – | – | – | 7.40% | – |
| Kollidon® CL | – | – | – | – | 7.40% |
For the preparation of pellets, all the ingredients were mixed intimately, and the dry powder mixture was blended for 10 min in laboratory-scale blender. Binder solution (deionized water) was then poured and mixed until wet mass was obtained. The wet mass was extruded using twin screw extruder (EXP-65/10/2000, Umang Pharma Tech. Ltd. Thane, India) and spheronized for 10 min at 750 rpm in a spheronizer (SPH-150/05, Umang Pharma Tech. Ltd. Thane, India) fitted with a cross-hatched rotor plate of 150-mm diameter and 3-mm thickness. Resulting pellets were dried in hot air oven at 40°C until constant weight.
Coating of Pellets
As the goal was to prevent the release of drug in the stomach, the pellets were enteric-coated using Eudragit L100-55 polymer. The coating solution was prepared by dissolving Eudragit-L100-55 (5% w/v) in isopropyl alcohol. Triethyl citrate (10% w/w of dry polymer) and talc (50% w/w of dry weight of polymer) were dispersed into the Eudragit solution. The suspension was then filtered through 200 # sieve and agitated continuously throughout the coating process. The weighed quantity of pellets was charged into the glass coating pan. The pan kept rotating, and coating dispersion was sprayed on the surface of pellet bed under the processing conditions mentioned below (Table III). A spray gun was used to spray the coating solution. To avoid the formation of agglomerates, purified talc was used as antisticking agent whenever required.
Table III.
Conditions for Coating
| Batch size | 100 g |
| Inlet air temperature | 40-45°C |
| Spray rate | 0.5 ml/min |
| Spray nozzle diameter | 0.029 mm |
| Spray pressure | 4 kg/cm2 |
| Pan rotation speed | 15 rpm |
| Pan angle | 45° |
Characterization of Pellets
Drug Loading and Content Uniformity
The aceclofenac content of the pellet formulations was determined in accurately weighed 100-mg pellets. The pellets were powdered in a mortar, and the powder was dissolved in methanol using ultrasonication. After filtration, the UV absorbance of the suitably diluted filtrate was measured at λ = 276 nm to determine the drug content. The content uniformity test was carried out three times for each of the formulation, and the results were expressed with the standard deviations (Table IV).
Table IV.
Drug Content in Pellet Formulations (±SD, n = 3)
| S. no. | Formulation | % Drug content (average) ± SD |
|---|---|---|
| 1 | A1 | 93.37 ± 0.09 |
| 2 | A2 | 95.32 ± 0.10 |
| 3 | A3 | 98.34 ± 0.32 |
| 4 | A4 | 98.66 ± 0.22 |
| 5 | A5 | 96.98 ± 0.03 |
| 6 | A6 | 99.98 ± 0.01 |
| 7 | A7 | 99.99 ± 0.01 |
| 8 | A6-a | 99.98 ± 0.80 |
| 9 | A6-b | 99.99 ± 0.05 |
| 10 | A6-c | 98.67 ± 0.08 |
| 11 | A6-d | 99.01 ± 0.10 |
FTIR
Fourier transform infrared (FTIR) studies were carried out to check for any interaction between κ-carrageenan and aceclofenac. Aceclofenac powder and κ-carrageenan powder and pellets of formulation A6 were powdered finely, and infrared spectrums of these powders were carried out using FTIR (Shimadzu FTIR-8101) according to the KBr disk method. Chemical integrity of the drug was determined by comparing the infrared (IR) spectra of pellets with that of the pure drug and κ-carrageenan.
Flow Properties
The flow properties of all the batches were investigated by measuring the angle of repose, bulk density, tapped density, and Hausner ratio in triplicate by standard methods mentioned in US Pharmacopeia 30-NF25 (Table V).
Table V.
Flow Properties and Friability Values of All the Batches
| Formulation code | Parameters | ||||
|---|---|---|---|---|---|
| Angle of repose (°) | Bulk density (g/ml) | Tapped density (g/ml) | Hausner ratio | Friability (%) | |
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | |||
| A1 | 36.15 ± 0.4 | 0.558 ± 0.003 | 0.856 ± 0.004 | 1.53 | 0.01 |
| A2 | 37.30 ± 0.2 | 0.728 ± 0.002 | 0.999 ± 0.003 | 1.37 | 0.03 |
| A3 | 33.58 ± 0.3 | 0.588 ± 0.003 | 0.787 ± 0.004 | 1.33 | 0.03 |
| A4 | 31.39 ± 0.4 | 0.698 ± 0.004 | 0.898 ± 0.003 | 1.28 | 0.04 |
| A5 | 30.80 ± 0.5 | 0.587 ± 0.008 | 0.783 ± 0.004 | 1.33 | 0.04 |
| A6 | 30.00 ± 0.4 | 0.559 ± 0.003 | 0.658 ± 0.005 | 1.17 | 0.03 |
| A7 | 34.70 ± 0.7 | 0.543 ± 0.005 | 0.692 ± 0.005 | 1.27 | 0.04 |
| A6-a | 30.20 ± 0.3 | 0.554 ± 0.003 | 0.632 ± 0.003 | 1.14 | 0.02 |
| A6-b | 27.00 ± 0.2 | 0.578 ± 0.002 | 0.689 ± 0.005 | 1.19 | 0.02 |
| A6-c | 26.30 ± 0.5 | 0.532 ± 0.005 | 0.635 ± 0.003 | 1.19 | 0.015 |
| A6-d | 25.70 ± 0.2 | 0.541 ± 0.007 | 0.622 ± 0.001 | 1.14 | 0.018 |
Particle Size Distribution
In order to determine pellet size distributions of the prepared aceclofenac pellets, standard sieve method was used (18). Standard sieves between apertures 420–2,000 µm were used. The weighed sample of pellet batch was kept on the top of sieve shaker individually. The shaker was operated for 20 min to separate the pellets into various size fractions. The fraction collected on each sieve was weighed and expressed as percent weight (Table VI).
Table VI.
Particle Size Distribution Data of All the Batches
| Formulation code | Percent weight retained | |||
|---|---|---|---|---|
| # 10 | # 14 | # 20 | # 40 | |
| A1 | 2.0 | 23 | 72.3 | 2.7 |
| A2 | 1.8 | 29.2 | 69.0 | – |
| A3 | 2.0 | 31.2 | 65.0 | 1.8 |
| A4 | 1.2 | 33.5 | 63.9 | 1.4 |
| A5 | 1.6 | 35.9 | 62.5 | – |
| A6 | 2.0 | 43.1 | 54.9 | – |
| A7 | 2.3 | 45.9 | 51.8 | – |
| A6-a | 1.9 | 38.2 | 59.90 | – |
| A6-b | 1.77 | 33.7 | 64.53 | – |
| A6-c | 1.1 | 36.1 | 62.8 | – |
| A6-d | 1.68 | 41.2 | 57.12 | – |
Friability
Resistance to abrasion was determined using USP method for the measurement of tablet friability. A sample of accurately weighed uncoated pellets (10 g) was placed in the Roche TAR 10 friabilator. Drum was rotated 100 times, and pellets were removed. After dedusting, weight loss from the pellets was measured by sieving the pellets through #20 sieve. The percent of weight lost was calculated and reported (Table V).
Pellet Disintegration
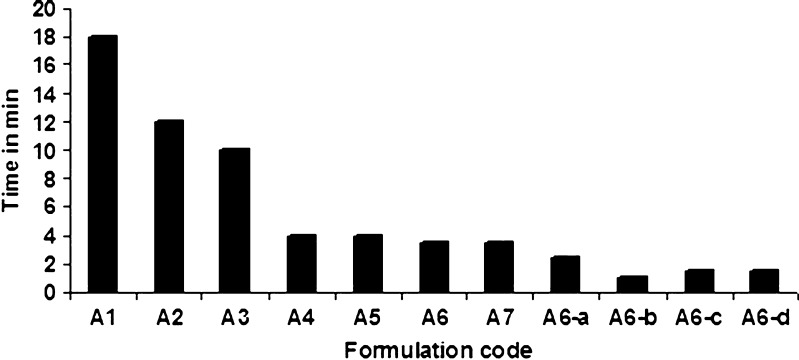
The pellet disintegration in water was evaluated by a tablet disintegration tester DT 2 (ROLEX Tablet disintegration rate test apparatus IP). Special transparent tubes of 10-mm diameter and 15-mm length were used. Sieves of 710-mm mesh size were at the top and the bottom of this tube. After filling 100-mg pellets in each tube, they were inserted in the standard tablet disintegration tester. The disintegration time of six dried samples at 37°C was determined at a speed of 30 dips. Disintegration test was carried out three times for each formulation, and results were expressed with the standard deviations (Fig. 1).
Fig. 1.
Disintegration time of various formulations (±SD; n = 3)
Drug Release from the Pellets
The dissolution studies were carried out according to the USP (XXIV) paddle method, for uncoated pellets (in pH 6.8 phosphate buffer) and enteric-coated pellets (in 0.1 N HCl for 2 h and pH 6.8 phosphate buffer after 2 h), as per the USP general drug release standard for delayed-release dosage form specifications with a paddle speed of 100 rpm at 37 ± 0.5°C in 900 ml of dissolution medium.
Uncoated pellets equivalent to 100 mg aceclofenac were subjected to the dissolution studies in 900-ml phosphate buffer (pH 6.8). The sample of 10 ml was withdrawn from dissolution vessel at 15-min time interval and was assessed spectrophotometrically at a wavelength of 276 nm with a UV spectrophotometer (UV2401PC, SHIMADZU, Kyoto, Japan). Sample amount used for analysis was replaced by fresh phosphate buffer solution (pH 6.8) to maintain the sink conditions.
For the drug release studies of the enteric-coated pellets, 0.1 N HCl (900 ml) was used as a dissolution medium for the first 2 h and then pH 6.8 phosphate buffer (900 ml). Each batch was analyzed in triplicate.
Scanning Electron Microscopy
Scanning electron microscopy is the technique of choice for measuring the shape and surface morphology of the pellets to support visually the other qualitative and quantitative results. Photomicrographs of coated and uncoated pellets were taken using a scanning electron microscope (Jeol-6380A, Japan Electron Optical Laboratory) for visualization of pellet surface morphology and pellet interior. Pellets were coated with platinum by means of sputter coater (Auto Fine Coater Jeol, Tokyo, Japan) to assure conductivity. Pellets were cut in half using steel blade to examine internal structure.
RESULTS AND DISCUSSION
Drug Loading and Content Uniformity
Table IV shows the results of the drug loading and content uniformity of the pellets. It was observed that maximum drug loading was obtained for formulations A6, A7, A6-a, and A6-b. It was also found that, when κ-carrageenan was used as a pelletizing agent, the yield of the pellets was maximum, i.e., 99.99%. This might be because of the good binding property of κ-carrageenan. The results of drug loading and content uniformity also indicate that there is no interaction taking place between the drug and the excipients used for pelletization.
FTIR
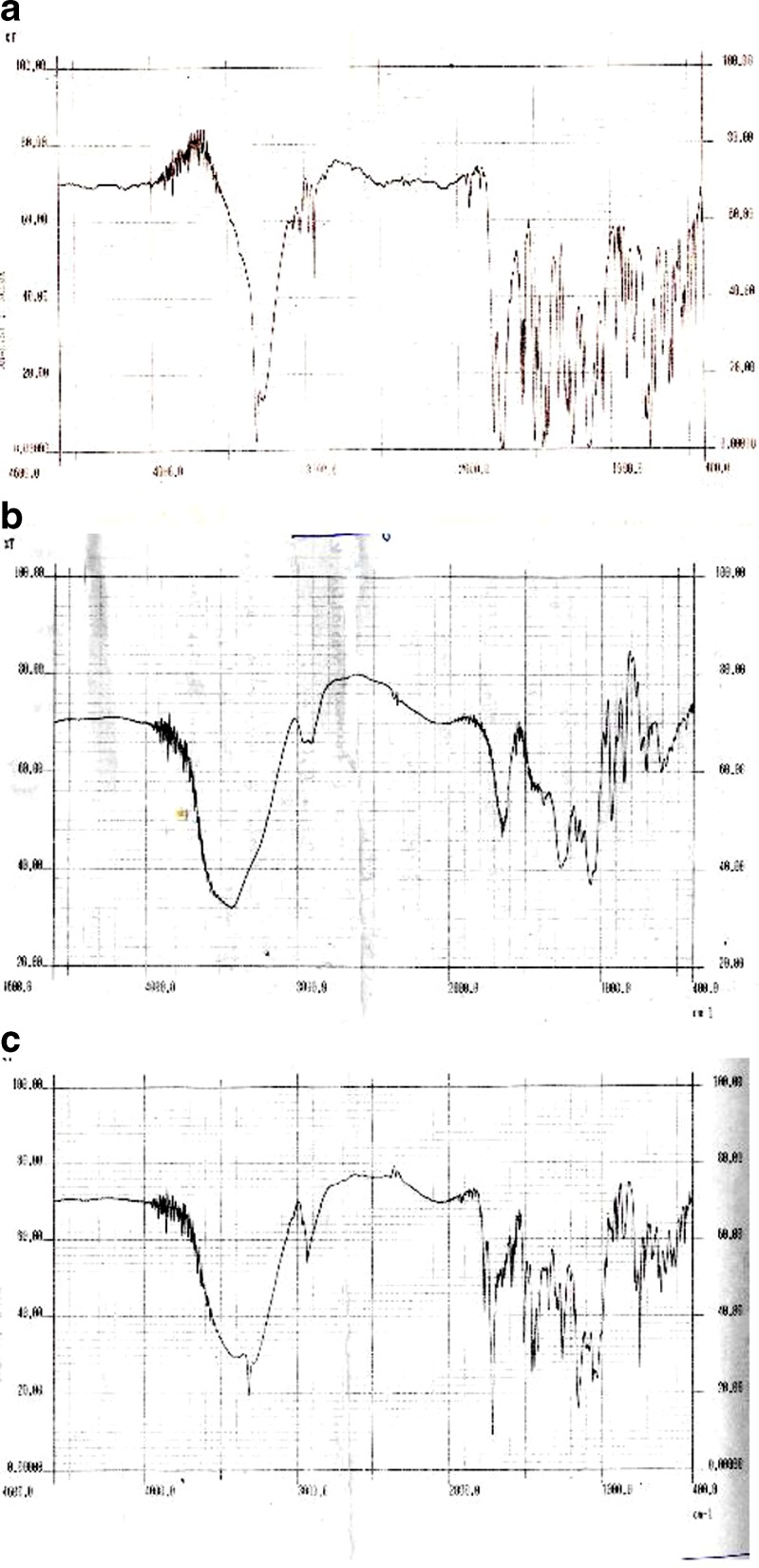
The FTIR spectra of aceclofenac pellets prepared using κ-carrageenan when compared with IR spectra of aceclofenac and κ-carrageenan powder show that there is no interaction of aceclofenac with κ-carrageenan, and hence κ-carrageenan can be retained as an excipient (Fig. 2). Principle peaks of aceclofenac and κ-carrageenan are seen in the IR spectra of the aceclofenac pellet prepared using κ-carrageenan. So it can be concluded that aceclofenac remains unaffected during the extrusion and spheronization process in the presence of κ-carrageenan.
Fig. 2.
FTIR spectra of a aceclofenac, b pellet of aceclofenac and κ-carrageenan, and c κ-carrageenan
Flow Properties and Friability
Results of the investigation of flow properties indicate that all the formulations possess good flow properties (Table V). Addition of κ-carrageenan results in the improvement of flow properties as is revealed by the change in the value of angle of repose from 36.15° for formulation A1 to 30° for formulation A6. Poorly flowable pellets are converted to pellets with good flow properties. Even the discouraging values of Hausner ratio are also improved. The results reveal that the combination of κ-carrageenan and lactose results in the formulation with very good flow properties. Addition of disintegrants retains the flow properties. The pellets from all the batches are not friable at all.
Particle Size Distribution
Study of particle size distribution data (Table VI) reveals that the size distribution becomes narrow with the increased amount of κ-carrageenan. It can also be observed that κ-carrageenan pellets show higher mean pellet size. This might be due to the uniformity in the length of extrudates that were formed when compositions containing κ-carrageenan were extruded. This size distribution was not affected significantly by the addition of disintegrants.
Disintegration of Pellets
As expected, disintegration time decreases as the concentration of MCC was reduced (Fig. 1). Disintegration time for the pellets prepared using MCC (formulation A1) was more than 18 min. Addition of 30% of κ-carrageenan reduces disintegration time from 18 to 4 min (formulation A2). It can be seen that addition of starch did not alter disintegration time considerably, whereas addition of disintegrants further lowered the disintegration time. With sodium starch glycolate, disintegration time was lowest (formulation A6-b), indicating very rapid disintegration of pellets. Pellets prepared using MCC showed slower disintegration. Therefore, it takes longer time for the dissolution medium to penetrate pellets and to help in fragmenting them into small particles. While in the case of other pelletizing agents like κ-carrageenan, its wettability is more, so the rate of diffusion of the dissolution medium into the pellet interior was faster. Disintegration studies become important for coated pellets, where immediate drug release is required after a functional coating has dissolved in gastrointestinal fluids. Faster disintegration will result into more number of smaller particles in the dissolution medium. This will result in a great increase in surface area that contributes largely to the increased water solubility. Rapid disintegration of pellet formulation A6-b must be because of swelling of κ-carrageenan and sodium starch glycolate in the presence of disintegration medium.
Drug Release from Pellets
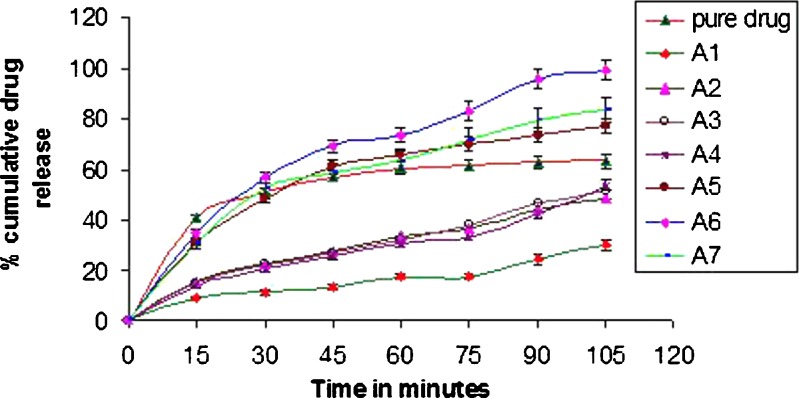
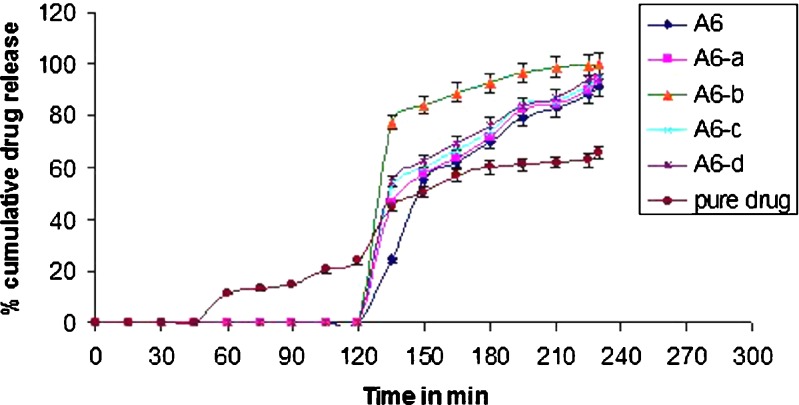
The effect of addition of different pelletizing agents and fillers on the improvement of dissolution rate of aceclofenac is illustrated in Fig. 3.
Fig. 3.
Release profiles of the pellets prepared using various pelletizing agents and fillers in pH 6.8 phosphate buffer (±SD; n = 3)
In vitro drug release profiles of uncoated pellets in pH 6.8 phosphate buffer of all the formulations revealed that formulation A1 did not result in the complete release of aceclofenac. It was even slower than the dissolution rate of the pure drug. As the quantity of MCC was reduced, the total amount of drug released in the first 2 h increased (formulations A2 and A3), as compared to the release from A1, but still the dissolution rate was less than that of the pure drug. Formulation A4 also failed to give acceptable release profile. In 2 h, less than 40% of the drug was released. Formulations A5, A6, and A7 showed significant increase in the dissolution rate of the drug. About 70% of aceclofenac was released within 45 min from A6, whereas A5 and A7 showed 60% release within 45 min.
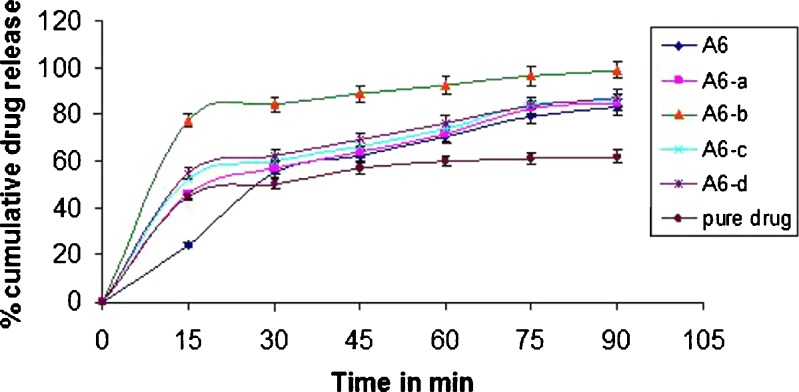
Figure 4 depicts the influence of the addition of disintegrants to the chosen formulation A6 on the dissolution rate of aceclofenac from pellets. Addition of disintegrants in formulation A6 indeed was found to improve the dissolution rates substantially. In the first 15 min, the percent dissolution was improved from 34% (formulation A6) to 77% (formulation A6-b). Fast disintegration of the pellets improved wettability and hence dissolution. The release of the drug increased further up to 90% within 45 min due to the addition of disintegrant sodium starch glycolate (formulation A6-b), which is a water-soluble agent. It absorbs water, rapidly resulting into disintegration of pellets. The resulting particles, because of the presence of wetting agents like κ-carrageenan and sodium starch glycolate, get wetted immediately and hence start dissolving. Porous internal structure of pellets might also be contributing to the rapid dissolution. Because of diffusion of dissolution medium into the pores, the pressure will build up inside the pellet that will fragment or disintegrate the pellet, exposing a large surface area of the wetted drug particles to the dissolution medium.
Fig. 4.
Release profile of formulation A6 after addition of disintegrants in pH 6.8 phosphate buffer (±SD; n = 3)
The drug release studies of pellets coated 12% w/w with Eudragit L100-55 revealed that no drug was released for 2 h in 0.1 N HCl (Fig. 5). The film formed by Eudragit L100-55 at 12% w/w on the κ-carrageenan-based aceclofenac pellets (as seen from photomicrographs of enteric-coated pellets Fig. 6) had sufficient thickness and uniformity to protect the pellets from releasing the drug in the gastric fluid. Hence, coating of aceclofenac-loaded κ-carrageenan pellets using Eudragit L100-55 showed the drug release at the intestinal pH, enteric-coated pellets with the immediate release of drug at the intestinal pH. For the dissolution studies of enteric-coated pellets, dissolution medium was 0.1 N HCl; for 2 h, the drug did not show any release from the enteric-coated pellets; further faster drug release was observed when the dissolution medium was replaced by pH 6.8 phosphate buffer. Pellets containing 20% κ-carrageenan, 40% lactose, and the disintegrant sodium starch glycolate showed a significantly faster rate of disintegration followed by dissolution.
Fig. 5.
Release profile of pellets in 0.1 N HCl and pH 6.8 phosphate buffer (±SD; n = 3)
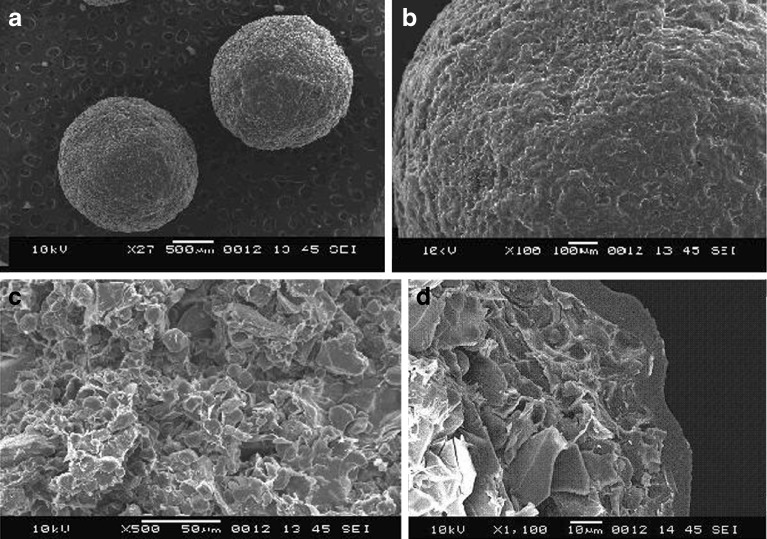
Fig. 6.
Scanning electron micrographs of enteric-coated pellets. a Coated pellets. b Surface morphology of coated pellet formulation A6-b. c Interior of the coated pellet. d Cross section of a coated pellet
Scanning Electron Microscopy
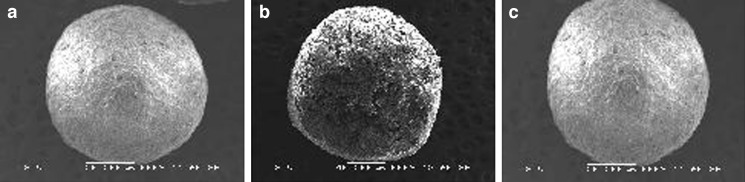
Scanning electron micrographs of the pellet formulations A1, A2, and A6 are shown in Fig. 7. The selected formulations were chosen on the basis of results obtained from the disintegration studies and dissolution studies. The scanning electron micrographs of coated formulations were also taken to study the surface morphology of enteric-coated pellets. Figure 7a represents the pellets prepared using only MCC (A1) showing very slow disintegration; Fig. 7b represents the pellets prepared using a mixture of κ-carrageenan and MCC as a pelletizing agent and maize starch as a diluent (A2), showing moderate disintegration rate, and Fig. 7c represents the pellets prepared using κ-carrageenan as a pelletizing agent and lactose as a diluent (A6), showing fast disintegration rate.
Fig. 7.
Scanning electron micrographs of formulation A1 a, A4 b, and A6 c
Figure 6a represents the enteric-coated pellets prepared using κ-carrageenan as a pelletizing agent, lactose as a diluent, and sodium starch glycolate as a disintegrants, formulation A6-b. Figure 6b represents the surface morphology of the enteric-coated pellets. Figure 6c represents the interior of the coated pellet. Figure 6d represents the cross section of an enteric-coated pellet.
The pellets prepared using only MCC were having smooth surfaces but were not perfectly spherical; the interior of the MCC pellet was not porous, while pellets prepared using a mixture of MCC and κ-carrageenan (starch as a diluent) were almost spherical, but pellet surface was not very smooth and the interior of the pellet was not very porous. The pellets prepared using only κ-carrageenan and lactose as a diluent were perfectly spherical and smooth with fine pores in it, and the interior of the pellet was highly porous. The photomicrographs of the enteric-coated pellets show smoother surface, improved sphericity, whereas coating of the pellets did not affect the internal porous structure of the pellets. The cross section of the pellet shows the coating thickness as well as the porous interior, responsible for the rapid disintegration of the pellet.
Conclusion
The study has revealed that it is possible to overcome the problem of gastric damage during the use of NSAID aceclofenac, avoiding the exposure of the drug to the ulcer-prone area of the GI tract, by enteric coating of the pellets. It can also be concluded from the study that, in the same formulation, solubility of the drug can also be improved by formulating fast-disintegrating pellets, making the drug available in the intestine for rapid absorption, by using κ-carrageenan as a pelletizing aid and sodium starch glycolate as a disintegrant by extrusion–spheronization technique.
References
- 1.Parfitt K. Analgesic anti inflammatory and antipyretic. In: Reynolds JEF, editor. Martindale: The complete drug reference. 32. Taunton: Pharmaceutical Press; 1999. pp. 2–12. [Google Scholar]
- 2.Yamazaki R, Kawai S, Mastsuzaki T, Kaneda N, Hashimoto S, Yokokura T, Okamoto R, Koshino T, Mizushima Y. Aceclofenac blocks prostaglandin E2 production following its intracellular conversion into cyclooxygenase inhibitors. Eur J Pharmacol. 1997;329:181–187. [PubMed] [Google Scholar]
- 3.Franz M. Stabilized pharmaceutical compositions comprising an extended release nonsteroidal anti-inflammatory agent and immediate release prostaglandin. US Patent 7303761, December 4, 2007.
- 4.Desai S, Nadkarni SR, Wald RJ, Debrincat GA. Dual release compositions of a cyclooxygenase-2 inhibitor. US Patent 7220434, May 22, 2007.
- 5.Yong CS, Oh YK, Lee KH, Park SM, Park YJ, Gil YS. Trials of clear aceclofenac-loaded soft capsules with accelerated oral absorption in human subjects. Int J Pharm. 2005;302:78–83. doi: 10.1016/j.ijpharm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Joshi VY, Sawant MR. Study on dissolution rate enhancement of poorly water soluble drug: contributions of solubility enhancement and relatively low micelle diffusivity. J Disp Sci Technol. 2006;27(8):1141–1150. doi: 10.1080/01932690600859374. [DOI] [Google Scholar]
- 7.Dahiya S, Pathak K. Physicochemical characterization and dissolution enhancement of aceclofenac-hydroxypropyl beta-cyclodextrin binary systems. PDA J Pharm Sci Technol. 2006;60(6):378–388. [PubMed] [Google Scholar]
- 8.Muatlik S, Usha AN, Reddy MS, Ranjith AK, Pandey S. Improved bioavailability of aceclofenac from spherical agglomerates: development, in vitro and preclinical studies. Pak J Pharm Sci. 2007;20(3):218–226. [PubMed] [Google Scholar]
- 9.Mutalik S, Anju P, Manoj K, Usha AN. Enhancement of dissolution rate and bioavailability of aceclofenac: a chitosan-based solvent change approach. Int J Pharm. 2008;350(1–2):279–290. doi: 10.1016/j.ijpharm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Yesmin F, Talukder MMU, Islam MS, Laila S, Haque T. Evaluation of aceclofenac loaded agarose beads prepared by ionotropic gelation method. S J Pharm Sci. 2008;1(1&2):10–17. [Google Scholar]
- 11.Margret Chandira R, Venkataeswarlu BS, Kumudhavalli MV, Bhowmik D, Jayakar B. Formulation and evaluation of the fast dissolving tablets of aceclofenac. The Pharma Review. 2008; 164–67.
- 12.Mallikarjuna Setty C, Prasad DVK, Gupta VRM, SA B. Development of fast dispersible aceclofenac tablets: effect of functionality of superdisintegrants. Ind Pharm Sci. 2008;70(2):180–185. doi: 10.4103/0250-474X.41452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghebre-Sellassie I. Pellets: a general overview. In: Ghebre-Sellassie I, editor. Pharmaceutical pelletization technology. New York: Marcel Dekker; 1989. pp. 1–13. [Google Scholar]
- 14.Nassab PR, Rajkó R, Szabó-Révész P. Physicochemical characterization of meloxicam–mannitol binary systems. J Pharm Biomed Anal. 2006;41:1191–1197. doi: 10.1016/j.jpba.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor RE, Schwartz JB. Spheronization II: drug release from drug-diluent mixtures. Drug Dev Ind Pharm. 1985;11:1837–1157. doi: 10.3109/03639048509057702. [DOI] [Google Scholar]
- 16.Thommes M, Kleinebudde P. Use of κ-carrageenan as alternative pelletization aid to microcrystalline cellulose in extrusion/spheronization, I: influence of type and fraction of filler. Eur J Pharm Biopharm. 2006;63:59–67. doi: 10.1016/j.ejpb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Thommes M, Kleinebudde P. Use of κ-carrageenan as alternative pelletization aid to microcrystalline cellulose in extrusion/spheronization, II: influence of drug and filler type. Eur J Pharm Biopharm. 2006;63:68–75. doi: 10.1016/j.ejpb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.USP . The United States Pharmacopeia 2004 (USP 27) Toronto: Webcom Limited; 2003. [Google Scholar]