Abstract
This study aimed to investigate the effects of dry and humid heat curing on the physical and drug release properties of polyvinyl acetate–polyvinyl pyrrolidone matrices. Both conditions resulted in increased tablet hardness; tablets stored under humid conditions showed high plasticity and deformed during hardness testing. Release from the matrices was dependent on the filler's type and level. Release profiles showed significant changes, as a result of exposure to thermal stress, none of the fillers used stabilized matrices against these changes. Density of neat polymeric compacts increased upon exposure to heat; the effect of humid heat was more evident than dry heat. Thermograms of samples cured under dry heat did not show changes, while those of samples stored under high humidity showed significant enlargement of the dehydration endotherm masking the glass transition of polyvinyl acetate. The change of the physical and release properties of matrices could be explained by the hygroscopic nature of polyvinyl pyrrolidone causing water uptake; absorbed water then acts as a plasticizer of polyvinyl acetate promoting plastic flow, deformation, and coalescence of particles, and altering the matrices internal structure. Results suggest that humid heat is more effective as a curing environment than dry heat for polyvinyl acetate–polyvinyl pyrrolidone matrices.
Key words: aging, drug release, physical stability, polyvinyl acetate–polyvinyl pyrrolidone, pycnometric density, thermal curing
INTRODUCTION
The success of the process of development of a drug delivery system (DDS) is highly dependent on the appropriate selection and processing of a carrier material capable of exerting the desired control on the onset and rate of drug delivery (1).
A significant proportion of carriers in oral DDSs are polymeric materials fabricated in matrix-type systems. A matrix device is a simple design consisting of a drug dispersed and/or dissolved homogenously throughout a polymeric matrix (1,2). This simple, but adaptable, design allows a polymer-based matrix DDS to deliver wide variety of drugs at controlled rates. In addition, the ease of manufacture, versatility, cost-effectiveness, and the fact that dose dumping is a highly unlikely event, since the drug is dispersed homogenously in the matrix, contribute to matrix-type devices being amongst the most successful in the field of drug delivery.
In such systems, drug release is preceded by the penetration of the dissolution medium into the porous matrix to dissolve the drug, followed by diffusion of the dissolved molecules out of the matrix.
An erosional component may be present and contributing significantly to the drug release depending on the solubility-swelling properties of the polymer, the degree of agitation and composition of the dissolution medium, and the type and level of filler excipients used (3–5).
The use of polymeric materials in sustained/controlled drug delivery is best exemplified by hydroxypropyl methylcellulose (HPMC) which is probably the most commonly used matrix former in pharmaceutical formulations (6).
Other matrix formers include carbomers (7), ethyl cellulose and related derivatives (8), methacrylate esters copolymers (9), and various natural gums (10).
The co-processed excipient, Kollidon® SR, is probably the latest addition to the limited list of commercially available matrix formers. It is a co-processed polymeric combination consisting of 80% polyvinyl acetate (PVAc) having a molecular weight of about 450,000 and 19% polyvinyl pyrroloidone (PVP) Ph.Eur./USP (Kollidon® 30). About 0.8% of sodium lauryl sulfate and about 0.6% of silica are used as stabilizers (11).
PVAc–PVP-based matrices were found to provide sustained release of model compounds. Certain formulation and processing variables were found to play an important role in controlling release kinetics, such variables include the compression force, drug loading, level of the polymeric material in the matrix, excipient level, as well as the nature of the excipients (water soluble vs. water insoluble) (12,13).
On the other hand, sensitivity of PVAc–PVP-based tablets to temperature and humidity has been pointed out in the pharmaceutical literature. One group (13) reported a decrease in the dissolution rate along with an increase in hardness of diphenhydramine HCl tablets prepared with a high level of PVAc–PVP without diluents or with 15% diluent (lactose, Emcompress®) and subjected to accelerated stability conditions. At higher levels of Emcompress® (25%), no changes occurred (13).
Another group (14) reported that PVAc–PVP-based tablets demonstrated reproducible drug release patterns when stored at 25°C/60% RH and 30°C/70% RH for up to 6 months. On the other hand, drug release from matrix tablets stored at 40°C/75% RH decreased slightly (14). A similar observation was made with PVAc–PVP mini-tablets stored under the same conditions (15).
Since the stability of the performance of a DDS during long storage periods is one of the most important considerations in choosing a carrier material, this physical aging phenomenon constitutes an important limitation to the use of PVAc–PVP as a controlled release matrix former despite it having excellent flow, compression, and release-controlling properties.
Drug release from systems susceptible to such a thermal-aging phenomenon has been stabilized using post-processing thermal treatment or curing (16–18). Curing involves the exposure of the dosage form (matrix system or polymer coated reservoir system) to temperatures above the glass transition temperature (Tg) of the polymeric component for a determined period of time so that adjacent polymer chains and segments can diffuse past each other leading to coalescence of polymeric particles producing a more homogenous, less porous system.
A post-compression curing step was suggested to stabilize drug release from PVAc–PVP matrices (13). However, only dry heat was used for curing and used at one level only (60°C), the inadequacy of such a condition has been pointed out by the same group (19) where they reported that it was found; as a result of a stability chamber malfunction whereby the temperature had temporarily reached 90°C; that tablets were further strengthened into a hardness range beyond the readout capacity of a typical hardness tester.
This gap in our knowledge of optimal curing procedures for PVAc–PVP matrices has prompted us to investigate the effects of exposure to elevated temperature with or without humidity, as candidates for curing conditions, on the drug release and physical properties of PVAc–PVP matrices prepared using different filler excipients.
Our study also aimed to understand the mechanisms mediating such an effect to allow formulation scientists to rationally suggest suitable curing conditions and formulation components necessary to ensure the stability of the quality of PVAc–PVP containing formulations.
MATERIALS AND METHODS
Materials
Chlorpheniramine maleate (CPM), C16H19ClN2, was used as a water-soluble model drug and was generously donated by United Pharmaceutical Manufacturing Co. Ltd., (Amman, Jordan).
PVAc–PVP-based excipient, Kollidon® SR (lot: 69982988Q0), was generously donated by BASF (Germany); microcrystalline cellulose (MCC), Avicel PH-101® (lot: 249557 186), was purchased from FMC (Switzerland); anhydrous dibasic calcium phosphate (DCP; lot: 73218/1), was purchased from Janssen Chimica (Belgium); D (+)-lactose monohydrate (LAC; lot. and filling code: 436197/112303242), was purchased from Fluka BioChemika (Switzerland); colloidal silica powder, Aerosil® (lot: 9888370), was purchased from BDH chemicals Ltd (England); and magnesium stearate was purchased from Unichema International (Malaysia).
Methods
Tablet Preparation
PVAc–PVP matrix tablets with a target weight of 300 mg and CPM content of 20 mg were prepared by direct compression. The formulation composition is shown in Table I. Twelve different formulations were prepared using different weight ratios (8:2, 6:4, 4:6, and 2:8) of PVAc–PVP to specific filler excipients (MCC, DCP, and LAC).
Table I.
Formulation Composition of Polyvinyl Acetate–Polyvinyl Pyrroloidone-Based Tablets
| Component | Milligrams/tablet | Percentage |
|---|---|---|
| Chlorpheniramine maleate | 20 | 6.67 |
| Colloidal silicon dioxide | 3 | 1.00 |
| Magnesium stearate | 3 | 1.00 |
| PVAc–PVP + filler excipient | 274 | 91.33 |
| Total | 300 |
The formulas were labeled using the letters LAC, MCC, or DCP indicating the type of diluent used in combination with the numbers 8:2, 6:4, 4:6, or 2:8 to denote the weight ratio of PVAc–PVP to the specific diluent.
CPM, PVAc–PVP, the specific filler excipients used (LAC, MCC, or DCP), and colloidal silica were passed through a 25-mesh sieve (710 μm) and then blended manually in a plastic bag for 10 min. Afterwards, Mg stearate, sieved through a 25-mesh sieve, was added and mixed for an additional 2 min.
The blend was compressed on a Manesty Model F single punch tableting machine (Thomas Engineering Inc., Hoffman Estates, IL, USA) using 9 mm round-shaped flat punches to a target tablet hardness of 100–200 N.
Neat PVAc–PVP tablets were prepared in order to study the physical properties and response to thermal curing of a PVAc–PVP matrix without interference from the fillers. The neat PVAc–PVP tablets were prepared by compressing accurately weighed 300 mg portions of PVAc–PVP using 9 mm round-shaped flat punches in a hydraulic press (Karl Kolb, Germany) at a compression force of 10 kN for 10 s. The punches were lubricated with 5% (w/v) magnesium stearate suspension in methanol before each compression and allowed to dry.
Exposure to Thermal Stress Conditions
Tablets from the 12 prepared formulas were put in open Petri dishes and split in two groups. The first group was placed in a stability chamber (Binder KBF 240, Binder, Germany) pre-equilibrated to 60°C/75% RH. The second group was placed in a dry heat oven at 60°C (Philip Harris Ltd, Shenstone, England).
At predetermined time points (1 day, 4 days, 1 week, 2 weeks, 1 month, 2 months, and 3 months), samples of tablets were collected and stored in closed glass bottles for dissolution testing and hardness measurements.
Weight measurements were performed on the same set of 20 tablets from each formula throughout the study.
Dissolution testing, weight, and hardness measurements were performed after the tablets were allowed to cool down to room temperature.
Neat PVAc–PVP tablets and PVAc–PVP powder were also stored under the abovementioned thermal conditions for periods up to 1 month. At predetermined time points (1 day, 1 week, and 1 month), samples were withdrawn for density measurements using helium pycnometry and thermal analysis using differential scanning calorimetry (DSC).
Tablet Testing
Tablet weight variation
Twenty tablets from each formula were selected, individually weighed (Shimadzu AY120 analytical balance, Japan), put in open Petri dishes, and placed under thermal stress conditions (previous section). At predetermined time points (previous section), the tablets were withdrawn and reweighed. The average weights and standard deviations were calculated.
Tablet hardness
Hardness was determined for ten tablets (of known weights) of each formula at zero time and at predetermined time points during the thermal stress studies (previous section) using Dr. Schleuniger Hardness tester (Schleuniger Pharmatron AG, Solothurn, Switzerland). The average hardness and standard deviation were calculated.
Tablet dissolution testing
Dissolution studies were conducted in a USP dissolution apparatus II (Erweka DT600, Germany) over a 12-h period using 900 mLs of deionized water as dissolution medium. The stirring rate and temperature were adjusted to 100 rpm and 37 ± 0.5°C, respectively.
At predetermined time intervals, 5 mL samples were withdrawn for analysis and immediately replaced with an equal volume of fresh medium maintained at the same temperature. Samples were filtered using 0.45 μm syringe filters before the absorbance of CPM was measured at 261 nm (SpectroScan® 80D, SpectroScan, USA). CPM concentration was calculated from linear calibration plots. The dissolution tests were performed in triplicates, and the mean values as well as standard deviations were calculated.
In order to quantify the changes in the drug release profiles as a function of the duration and type of thermal exposure, the release profiles were fitted to the Higuchi square root of time model (20) given in Eq. 1:
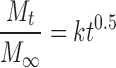 |
1 |
where Mt/M∞ is the fraction of drug released at time t, and k is the apparent release rate constant.
One of the most important assumptions in the Higuchi model is that the only release mechanism is diffusion of the dissolved drug through a reasonably intact matrix. Although the model may be less than ideal for describing a “real” matrix system, it is still considered the most famous and most often used mathematical equation to describe the release rate of drugs from such systems. This is probably due to its simplicity in describing the release process using a single value of the apparent release rate constant (21).
This simplicity is of great value in our situation where we are not interested in the mechanism of the drug release as we are in a quantitative measure of the drug release in the form of an apparent release rate constant.
The percentage change in the apparent release rate constant after storage under elevated temperature conditions was calculated according to Eq. 2.
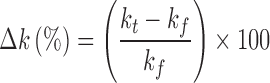 |
2 |
where kt is the apparent release rate constant from tablets stored for time t, and kf is the apparent release rate constant from freshly prepared tablets.
Differential Scanning Calorimetry
The evaluation of the thermal properties was performed on PVAc–PVP powder and neat PVAc–PVP tablets after storage, gently triturated in a porcelain mortar, using Mettler Toledo DSC 823 (Mettler, Switzerland).
Samples (4–5 mg) were weighed and sealed into aluminum pans with pierced lids. The samples were heated from 25°C to 250°C at a heating rate of 10°C/min under nitrogen purge (80 ml/min).
Helium Pycnometry
Density measurement of neat PVAc–PVP tablets was carried out using Ultrapycnometer 1000 (Quantachrome Instruments, USA). Before use, the instrument was allowed a minimum of 1 h for warm-up and thermal equilibration; a circulating water bath was used to provide constant temperature conditions (25°C).
The instrument was calibrated before measurements using a small calibration sphere (volume, 7.0699 cm3 in a sample holder with a nominal volume of 10.8 cm3).
For measurement, three to four neat PVAc–PVP tablets were weighed using an analytical balance (SHIMADZU AY120, Japan) then placed in the sample cell after removing the calibration sphere, a minimum of six runs were used to obtain accurate results, and averages and standard deviations were calculated. The same procedure was applied to PVAc–PVP powder.
RESULTS AND DISCUSSION
Tablets were successfully prepared from all of the prepared formulations. Due to different compaction properties of the used filler excipients, it was decided not to use a constant compression force for the preparation of the tablets but instead targeting an acceptable level of hardness (between 100 and 200 N). Tablets of consistent target hardness were most successfully prepared when LAC was used as a filler excipient, while in tablets containing MCC or DCP as fillers, the hardness decreased with increasing the content of the filler excipient in the mixture even when using high compression forces.
The prepared tablets were then placed under humid or dry thermal stress conditions (60°C/75% RH and 60°C, respectively). These are relatively harsher conditions than the ones usually used for the curing of film coatings; however, they were decided upon considering the fact that they are intended to modify a thick polymeric matrix rather than a thin film on the surface of a tablet or a pellet. Interestingly, a recent report (22) showed that similar, unconventionally harsh, curing conditions (60°C/75% RH or 80°C) were successful in improving the storage stability of Aquacoat-coated pellets at accelerated stability test conditions in comparison to other “milder” curing conditions.
Tablet Weight and Hardness
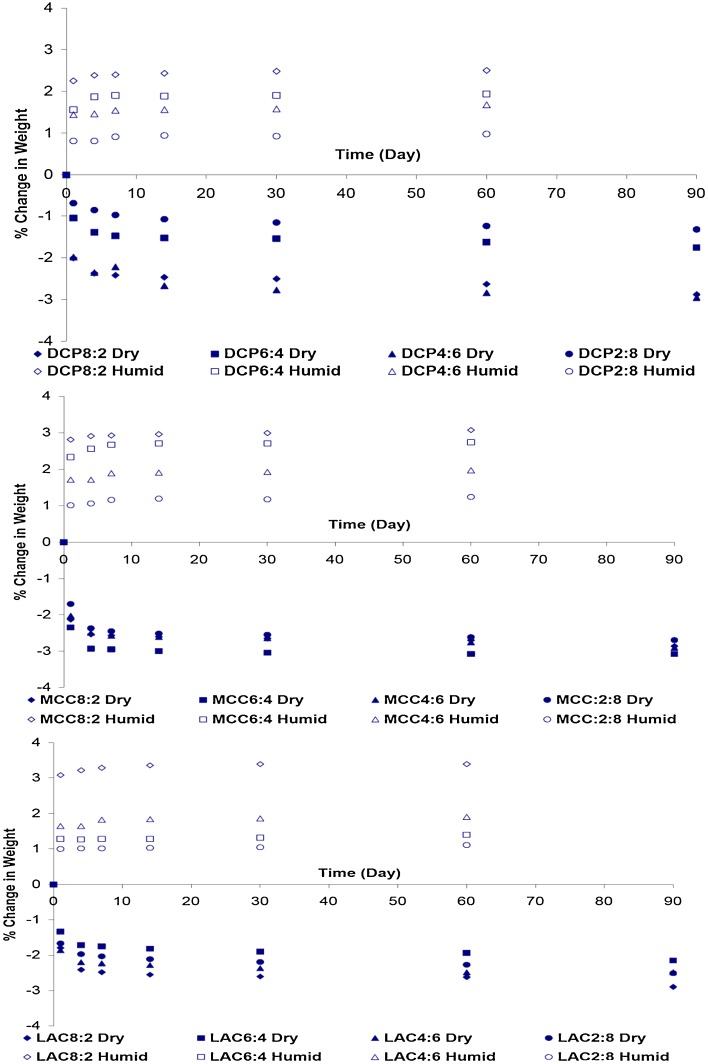
Figure 1 shows the percentage change in the tablet weight as a function of thermal exposure time under dry and humid conditions.
Fig. 1.
Effect of storage under dry heat (60°C) and humid heat (60°C/75% RH) conditions on the weight change of polyvinyl acetate–polyvinyl pyrroloidone-based tablets
Tablets exposed to dry heat conditions showed a reduction in their mass corresponding to removal of residual moisture associated with the tablet components. This process of weight loss is fastest in the early stages of thermal exposure reaching an average value for all formulations of 66.49% (±7.03) and 82.85% (±7.53%) of the 90-day (plateau) level after 1 and 4 days, respectively.
On the other hand, tablets stored under high temperature and humidity conditions showed an increase in their weight with time as a result of water sorption. Similar to the weight loss process, the tablets showed a quick weight gain in the early stages of exposure to high humidity with an average weight gain for all formulations reaching 86.98% (±3.91) and 90.48% (±4.59) of the 60-day (plateau) level after 1 and 4 days, respectively.
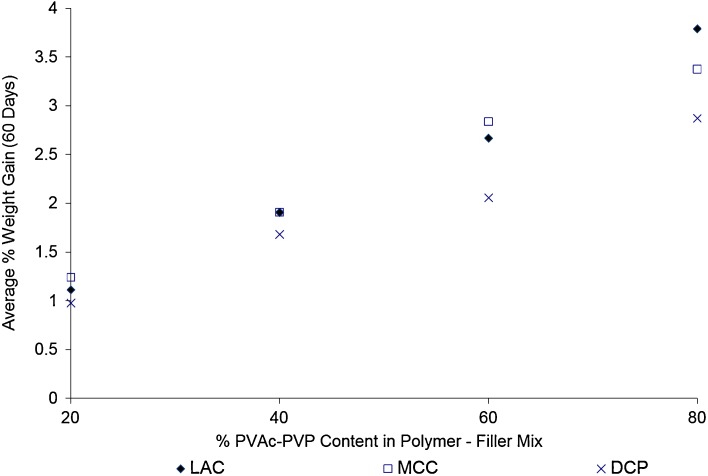
In addition, the weight gain seems to show a direct linear relationship with the content of PVAc–PVP in the tablet formulations as can be seen in Fig. 2. The linear relations have R2 values of 0.9905, 0.9825, and 0.9912 for matrices prepared using MCC, DCP, and LAC as filler excipients. This points to PVAc–PVP as the main water sorping component of the tabletted formulas which is expected based on the high hygroscopicity of the PVP component in PVAc–PVP (23,24).
Fig. 2.
Effect of polyvinyl acetate–polyvinyl pyrroloidone content on the average weight gain by tablets stored under high temperature–high humidity conditions (60°C/75% RH)
These results are in agreement with the previous reports where tablets with highest level of PVAc–PVP exhibited greatest moisture uptake (19).
Tablets prepared with DCP as a filler excipient showed the smallest percentage weight gain in comparison to those prepared with MCC and LAC, this may be explained by the nonhygroscopicity of DCP in comparison to the hygroscopic MCC and LAC.
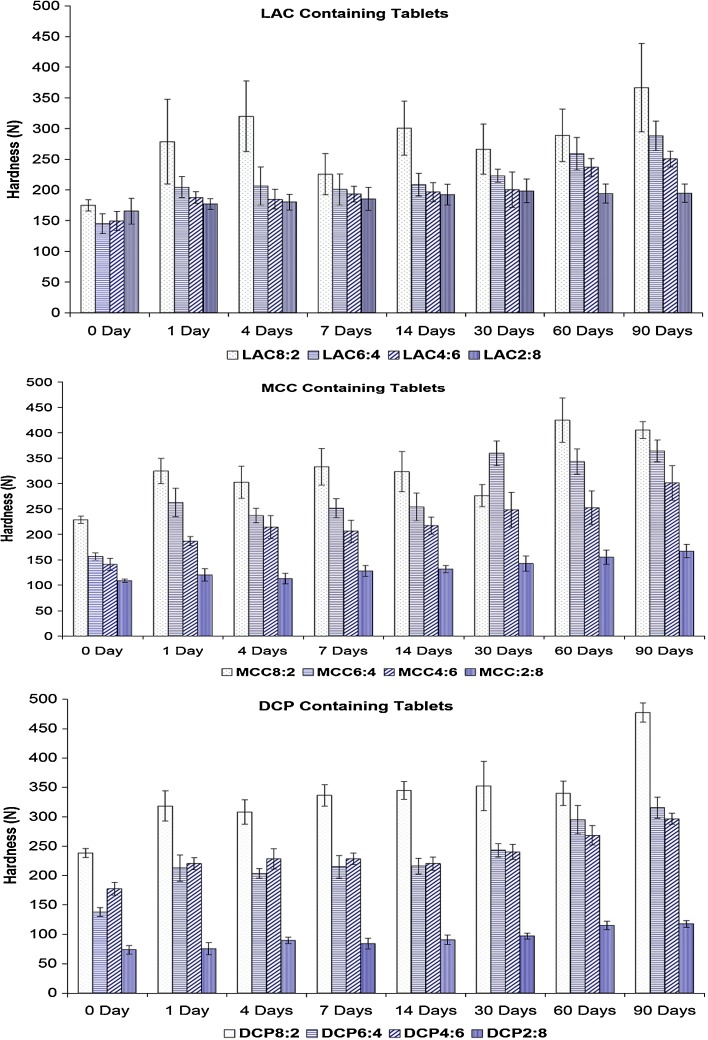
In regards to the tablet hardness, the storage under dry heat resulted in an increase in the tablet hardness as can be seen in Fig. 3. The increase in the tablet hardness was related directly to the PVAc–PVP content in the tablets indicating that a change in the structural properties in the PVAc–PVP component of the matrix is responsible for the increased mechanical strength of the tablets.
Fig. 3.
Effect of storage under dry heat (60°C) on the hardness of polyvinyl acetate–polyvinyl pyrroloidone-containing tablets
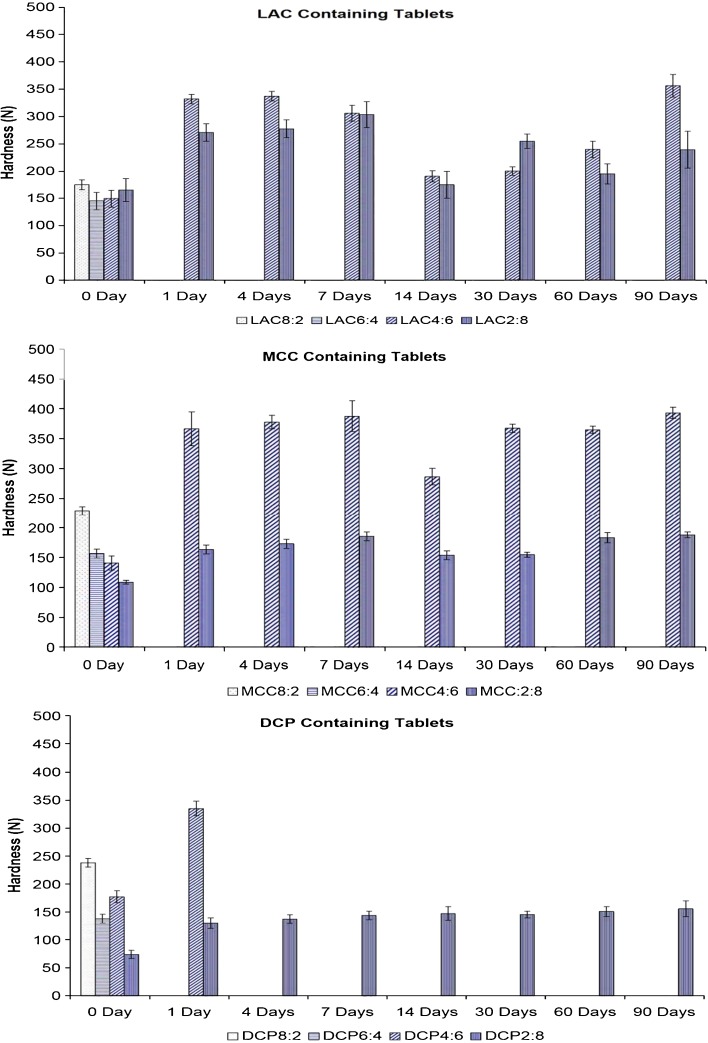
However, the most pronounced change in hardness was observed in the case of the tablets stored under elevated temperature and humidity conditions. Tablets with high PVAc–PVP content exposed to high temperature and humidity conditions for periods as short as 1 day showed increased hardness and exhibited pronounced elasticity; no reading was recorded by the hardness tester for those tablets.
The change in tablet hardness as a function of the storage time under humid heat conditions is shown in Fig. 4.
Fig. 4.
Effect of storage under humid heat (60°C/75% RH) on the hardness of polyvinyl acetate–polyvinyl pyrroloidone-based tablets
Differential Scanning Calorimetry
DSC was used to detect possible changes in the glass transition of the rate controlling PVAc component of Kollidon® SR which is expected to contribute to the molecular mobility of polymeric chains, plastic flow of polymeric particles, internal structural changes, as well as hardness and drug release changes of the Kollidon® SR matrices.
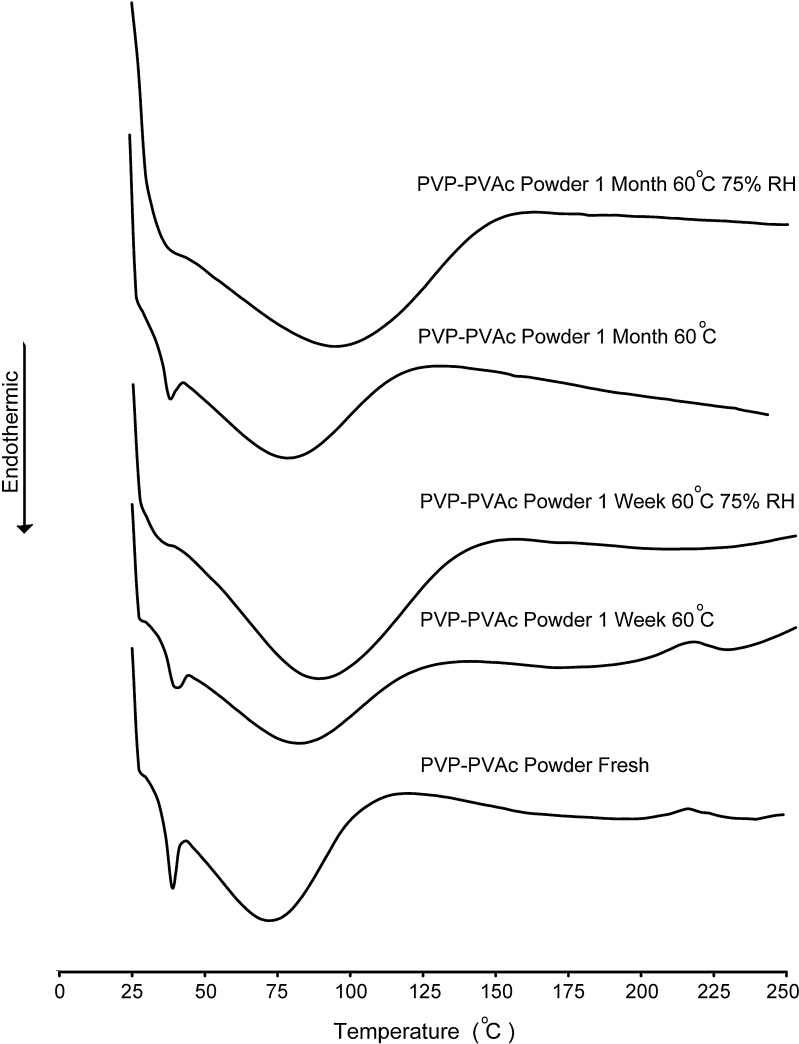
Fresh samples of PVAc–PVP powder showed a distinctive endothermic peak at around 40°C followed by a broad peak extending from around 50°C to 100°C. This observation is in accordance with the reported behaviors of the two polymeric components of the polymeric system with the first one corresponding to the glass transition temperature of PVAc (11,25,26) and the second one corresponding to dehydration of the hygroscopic PVP.
The thermograms of PVAc–PVP powder samples stored in open containers at 60°C for periods up to 1 month (Fig. 5) did not show significant change as expected.
Fig. 5.
Differential scanning calorimetry thermograms of polyvinyl acetate–polyvinyl pyrroloidone powder stored under dry heat (60°C) or humid heat conditions (60°C/75% RH)
On the other hand, PVAc–PVP powder stored in open containers placed in a stability chamber at 60°C and 75% RH showed a significant increase in the size of the PVP dehydration peak corresponding to increased energy investment in the removal of sorped water.
Unfortunately, this increase in the dehydration isotherm masked the PVAc glass transition, making it impossible to detect the plasticization effect of sorped water. However, the plasticization effect of water on PVAc has been reported for polymeric composites of PVAc and polystyrene (27).
The plasticization of PVAc in our case would be a direct result of our earlier observation of moisture uptake and a major component of the contribution of the hygroscopic PVP to the change in the structural properties of the PVAc–PVP matrices.
Helium Pycnometry
The density of fresh PVAc–PVP powder was determined by helium pycnometry to be 1.3099 ± 0.011 g/cm3 (average ± standard deviation); upon compressing the PVAc–PVP powder to produce neat PVAc–PVP tablets, the density dropped to 1.1997 ± 0.002 and 1.1789 ± 0.001 g/cm3 for two different sets of six units each. This is explained by the formation of dead spaces within the tablet matrix that could not be penetrated by helium during the pycnometric measurements resulting in higher apparent volume and lower density. The former set (initial density of 1.1997 ± 0.002 g/cm3) was then stored under humid heat conditions, and the latter (initial density of 1.1789 ± 0.001 g/cm3) was stored under dry heat conditions.
The results of density measurements after storage of the two tablet sets under dry and humid heat are shown in Table II.
Table II.
Effect of Storage under Dry Heat (60°C) and Humid Heat (60°C/75% RH) Conditions on the Density (g/cm3) of Neat Polyvinyl Acetate–Polyvinyl Pyrroloidone Compacts
| Density (g/cm3; average ± SD) | ||
|---|---|---|
| Fresh | 1.1997(±0.0010) | 1.1789(±0.0010) |
| Storage | 60°C ± 75% RH | 60°C |
| 1 day | 1.1237 (±0.0117) | 1.0661 (±0.0018) |
| 2 days | 1.1335 (±0.0078) | 1.0715 (±0.0019) |
| 4 days | 1.1916 (±0.0093) | 1.1214 (±0.0197) |
| 1 week | 1.1907 (±0.0099) | 1.0845 (±0.0011) |
| 2 weeks | 1.2604 (±0.0499) | 1.1157 (±0.0048) |
| 1 month | 1.2743 (±0.0088) | 1.1688 (±0.0033) |
Data presented as an average ± SD
Tablets stored under humid heat conditions showed a reduction in density after 1 day followed by a gradual increase in density with prolonged exposure to humid heat.
A qualitatively similar observation can be made with the set of tablets stored under dry heat conditions, which showed a reduction in its density upon storage for 1 day followed by a gradual increase with prolonged storage time.
The initial reduction in density is most probably due to the decompression and the elastic recovery of the tablets (28,29). On the other hand, the following increase in density upon prolonged storage is a reflection of changes in internal structure of the neat PVAc–PVP tablets matrix that is brought about by the plastic flow of the PVAc–PVP component within the tablet matrix encouraged by the elevated heat of storage.
In addition, the results show a difference in the response of the neat PVAc–PVP tablets when stored under different thermal stress conditions with the humid heat exerting a more pronounced effect on the density than the dry heat which could be explained by the plasticizing effect of water in the matrix allowing for easier rearrangement of the polymeric network by plastic flow.
Drug Release and Modeling
The amorphous nature of PVAc coupled with its low Tg of ≈35°C (11) imparts certain unique characteristics to this polymeric matrix. Upon compression, an insoluble plastic PVAc matrix structure is formed. The drug release from such a structure depends on the diffusion of the dissolved drug through channels created by the gradual leaching of the water-soluble PVP component. The presence of filler excipients with different water solubility/swellability can modulate release profiles from matrix systems.
The addition of water-soluble fillers like lactose was reported to accelerate the dissolution of soluble drugs by decreasing the tortuosity of the diffusion path of the drug (30).
On the other hand, the use of swellable, insoluble excipients (i.e., MCC or cross-linked carboxymethylcellulose) in tablet formulations containing 10% HPMC 2208 has been reported to cause an expansion of the gel layer resulting in a faster release of an insoluble model drug in the early stages of dissolution (burst effect) due to their disintegrating property (31).
Water insoluble filler excipients, such as DCP, tend to slow down drug release from HPMC matrices by getting entrapped within the matrix structure increasing the tortuosity of the matrix (32).
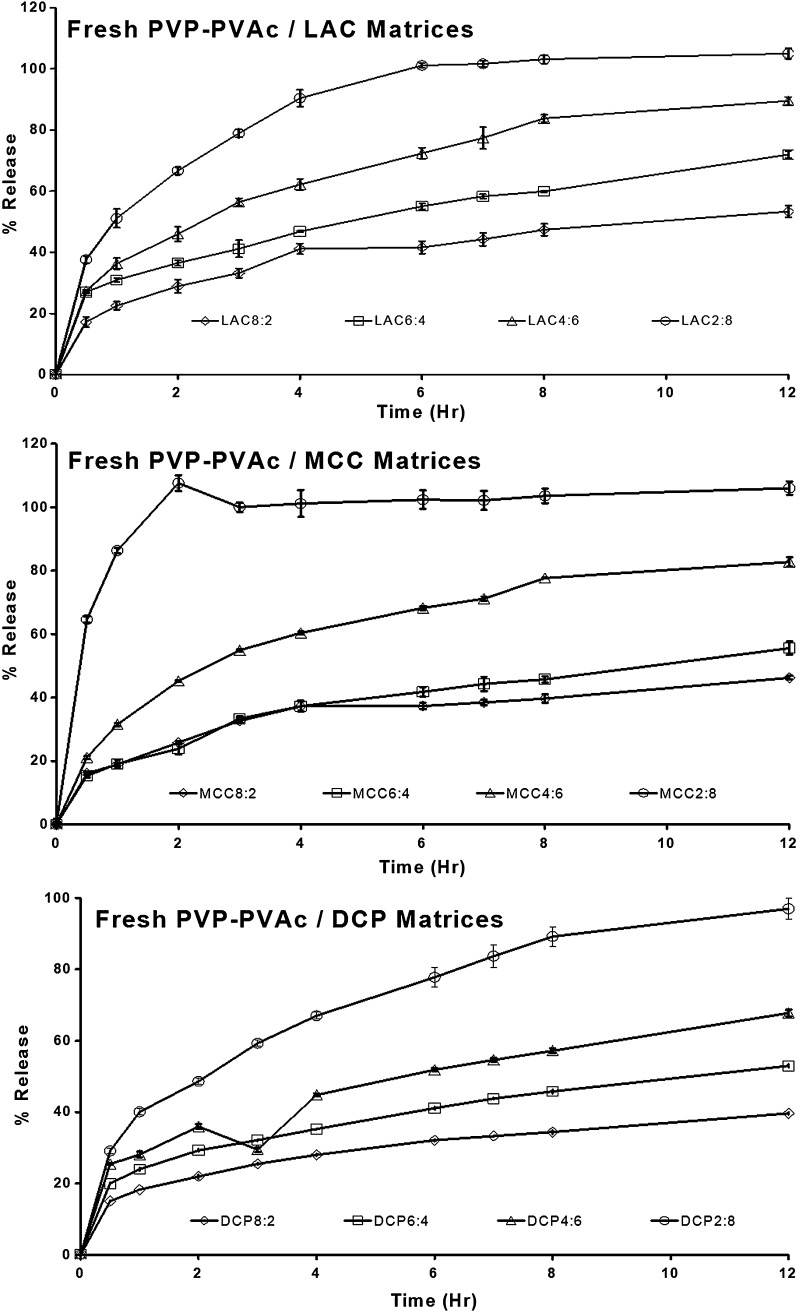
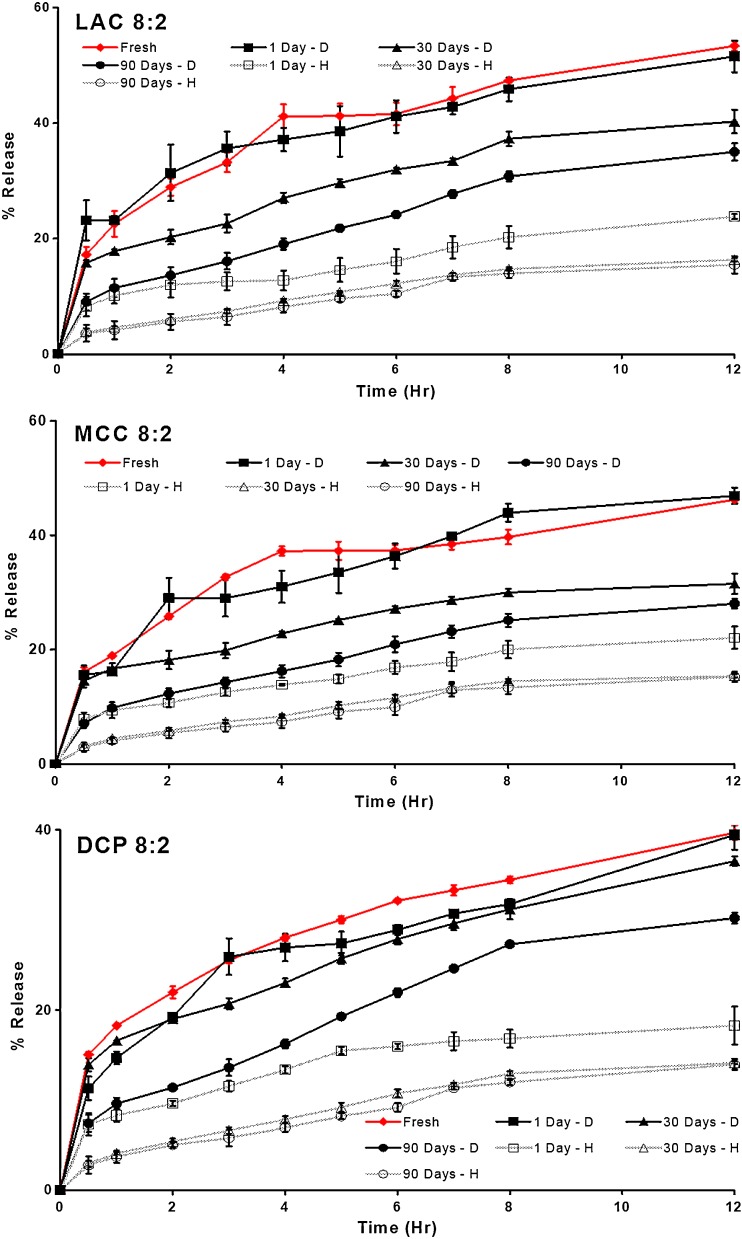
Figure 6 shows the drug release from freshly prepared PVAc–PVP tablets prepared with different excipients. It is evident that drug release from PVAc–PVP matrices can be modulated easily by changing the type and level of the filler excipient.
Fig. 6.
Effect of excipient type and level on drug release from freshly prepared polyvinyl acetate–polyvinyl pyrroloidone matrices formulated with lactose, microcrystalline cellulose, or dibasic calcium phosphate as filler excipient
Tablets prepared with the highest filler ratio (2:8 series) showed a fast release where MCC2:8 was fastest followed by LAC8:2, while DCP8:2 was the slowest of the three.
PVAc–PVP tablets did not gel upon exposure to the dissolution medium; instead, tablets containing LAC and MCC eroded gradually with the rate of erosion being directly proportional to the content of the filler excipient and inversely related to the duration of exposure to thermal stress. This can be explained based on the nature of these fillers being water-soluble (LAC) or water-swellable (MCC).
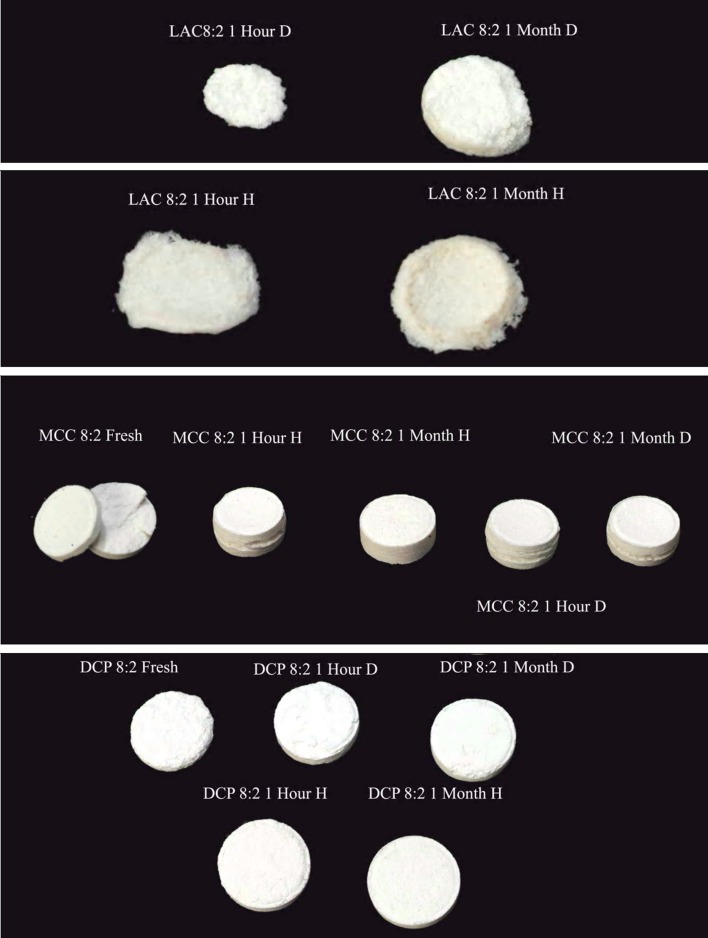
On the other hand, the insolubility and nonswellability of DCP explain the fact that DCP-containing matrices only showed minor erosion at the surface (Fig. 7).
Fig. 7.
Photos of polyvinyl acetate–polyvinyl pyrroloidone matrices exposed to (D) dry and (H) humid (75% RH) thermal stress (60°C) conditions for periods of 1 h and 1 month after dissolution testing for 12 h
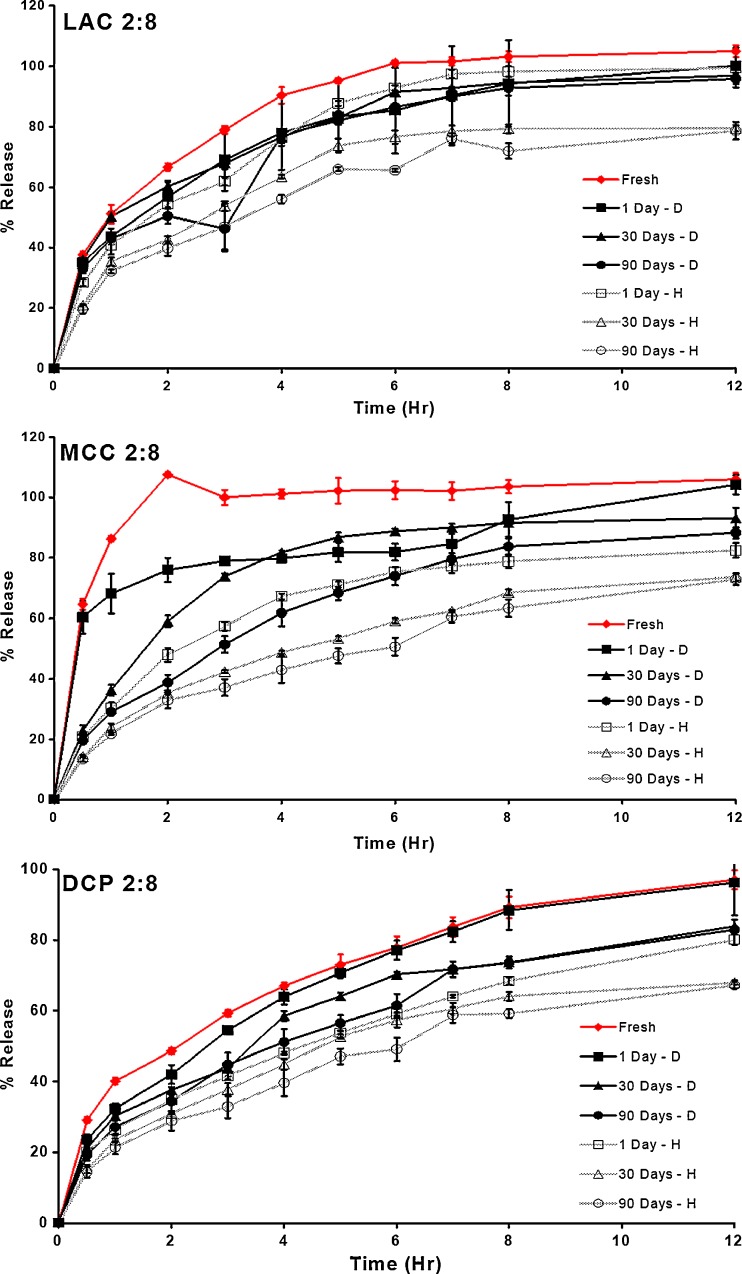
Figures 8 and 9 show the drug release profiles of formulations containing the lowest and highest ratios of PVAc–PVP to filler excipients (8:2 and 2:8 formulas) after exposure to dry and humid heat for different periods of time. These figures show that the change in the release profile of the matrices appears to be dependent on the type of storage conditions with tablets stored under humid heat conditions showing a more pronounced change in their drug release in comparison to those stored under dry heat conditions. In addition, the PVAc–PVP content of the matrices appears to play an important role with the most pronounced changes occurring in matrices with highest PVAc–PVP content.
Fig. 8.
Effect of thermal stress conditions on drug release from matrices containing the lowest level of polyvinyl acetate–polyvinyl pyrroloidone (2:8 formulas)
Fig. 9.
Effect of thermal stress conditions on drug release from matrices containing the highest level of polyvinyl acetate–polyvinyl pyrroloidone (8:2 formulas)
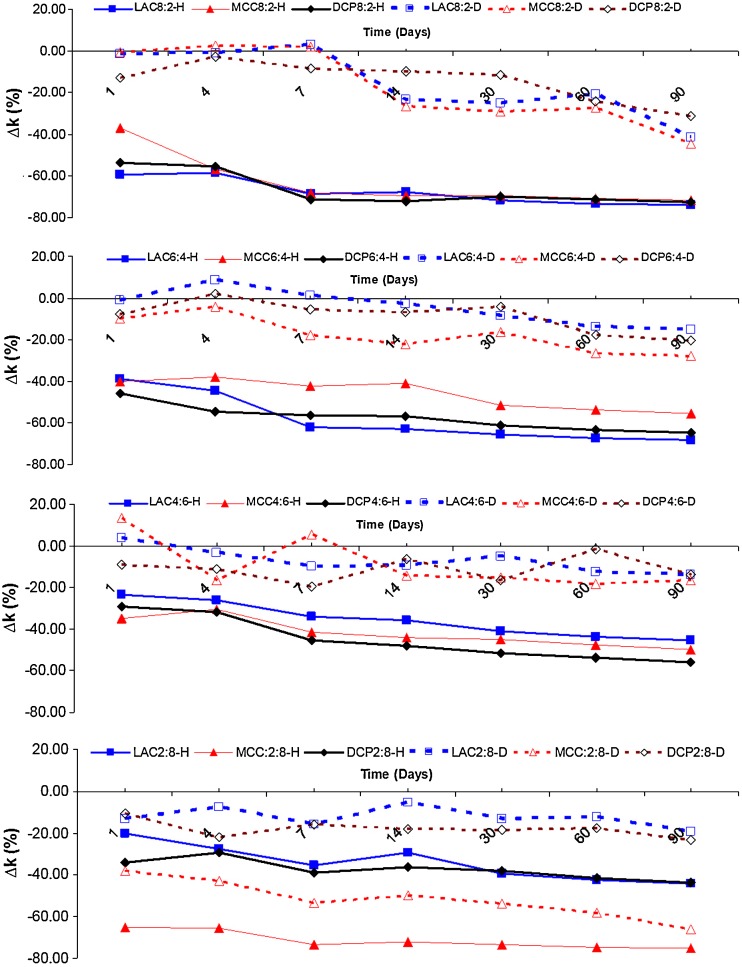
These observations are supported by Fig. 10 which shows the percentage change in the apparent release constant as a function of time of exposure to dry or humid heat of all the studied formulas.
Fig. 10.
Effect of excipient type and level on the percentage change in the apparent release rate constant (∆k%) of chlorpheniramine maleate from polyvinyl acetate–polyvinyl pyrroloidone matrices stored under (D) dry and (H) humide (75% RH) thermal stress (60°C) conditions
The changes in the apparent rate constants were highest in tablets stored under high humidity conditions with the ∆k% reaching up to −71.69% and −75.41% after 3 months of storage in the case of formulas MCC 8:2 and MCC 2:8.
The effect of humid heat on matrices with high PVAc–PVP content can be explained by the coalescence of polymeric particle and increased tortuosity of the matrices. The increased tortuosity results in increased diffusional path length and decreased rate of drug release from the matrix.
On the other hand, the effect of humid heat on tablets with low PVAc–PVP content can only be partially explained by the previous mechanism considering its low content of the polymeric matrix forming material; however, a more possible mechanism involves the action of the coalesced polymeric particles as a binding agent for the rest of the matrix components explaining the improved structural integrity of the tablets and the hindered erosion or disintegration upon exposure to dissolution media resulting in a slower drug release which was, otherwise, a relatively quick process mediated by tablet disintegration and erosion as can be seen in Fig. 7 where formulas MCC 2:8, LAC 2:8, and DCP 2:8 showed the fastest release in that specific order.
Both mechanisms are accelerated by the uptake of water from the high humidity environment by the hygroscopic PVP component of the matrix former and the action of water as a plasticizer of the PVAc component. Plasticization of the PVAc component allows polymeric chains and segments to diffuse past each other crossing particle boundaries and resulting in particle bonding and a more tortuous matrix.
Dry heat resulted in a change in ∆k% reaching up to −44.43% and −65.94% after 3 months of storage in the case of formulas MCC 8:2 and MCC 2:8. Similar scenarios to the ones described above are responsible for these changes in drug release; however, the absence of the plasticizing effect of sorped water slows down these changes.
It does not appear that the use of one specific filler excipient in combination with PVAc–PVP in formulating matrices offer much advantages in terms of stabilizing drug release upon thermal exposure.
It should be noted also that thermal stress whether dry or humid on drug release achieved most of its effect within the first day to the first week of exposure; afterwards, the effect reached a plateau. This indicates the potential of a curing step for reasonable periods of time (overnight) in stabilizing the drug release from PVAc–PVP matrices. However, it should be noted that the use of dry heat in the curing step may not be adequate as the process of aging or maturation of the PVAc–PVP matrices was highly dependent on the presence of humidity.
This could point to some formulation approaches that may be attempted, besides curing, to stabilize drug release from PVAc–PVP matrices including the use of moisture-protective coatings. Such approaches should be evaluated as methods of optimizing drug delivery using PVAc–PVP systems.
CONCLUSION
The effect of dry and humid thermal stresses, as candidates for curing conditions, on the physical and drug release properties of PVAc–PVP matrices was investigated. Both physical and drug release properties of the prepared matrices were affected by the studied stress conditions. None of the typically used filler excipients in controlled release matrix systems exerted a stabilizing effect on PVAc–PVP matrices.
The aging process was more pronounced under humid heat in comparison to dry heat. This was explained by the sorption of water by the hygroscopic PVP component of the system. The sorped water would then plasticize the PVAc component promoting a structural rearrangement of the matrix manifested by changes in tablet hardness, pycnometric density, and drug release properties.
The results suggest that dry heating may not be adequate as a curing procedure to stabilize drug release from PVAc–PVP matrices; alternative procedure may be heating under humid conditions.
Acknowledgements
The authors would like to acknowledge the financial support received from the Deanship of Scientific Research at the University of Jordan and the technical help in preparation and testing of matrices in the facilities of Triumpharma Inc., Amman, Jordan, and Specialized Pharma Inc., Amman, Jordan.
The authors also wish to acknowledge the technical help of Ms. Shorouq AlSotari, Ms. Amani Nimer, and Mr. Tareq Najjar. The authors also wish to thank Ms. Suha Muhaissen for help in taking photographs of tested tablets.
References
- 1.Colombo R, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optical performance. Pharm Sci Technol Today. 2000;3:198–204. doi: 10.1016/S1461-5347(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 2.Kanjickal DG, Lopina ST. Modeling of drug release from polymeric delivery systems—a review. Crit Rev Therap Drug Carrier Syst. 2004;21:345–386. doi: 10.1615/CritRevTherDrugCarrierSyst.v21.i5.10. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds TD, Gehrke SH, Hussain AS, Shenouda LS. Polymer erosion and drug release characterization of hydroxypropyl methylcellulose matrices. J Pharm Sci. 1998;87:1115–1123. doi: 10.1021/js980004q. [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh N, Corrigan OI. Swelling and erosion properties of hydroxypropylmethylcellulose (Hypromellose) matrices—influence of agitation rate and dissolution medium composition. Int J Pharm. 2004;279:141–152. doi: 10.1016/j.ijpharm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Farhad T, Abrahmsen-Alami S, Hansen M, Larsson A. The impact of dose and solubility of additives on the release from HPMC matrix tablets-identifying critical conditions. Pharm Res. 2009;26(6):1496–1503. doi: 10.1007/s11095-009-9861-y. [DOI] [PubMed] [Google Scholar]
- 6.Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharmacy Pharmacol. 2005;57(5):533–546. doi: 10.1211/0022357055957. [DOI] [PubMed] [Google Scholar]
- 7.Khan GM, Zhu J. Studies on drug release kinetics from ibuprofen-carbomer hydrophilic matrix tablets: influence of co-excipients on release rate of the drug. J Control Release. 1999;57(2):197–203. doi: 10.1016/S0168-3659(98)00122-9. [DOI] [PubMed] [Google Scholar]
- 8.Khan GM, Meidan VM. Drug release kinetics from tablet matrices based upon ethylcellulose ether-derivatives: a comparison between different formulations. Drug Dev Ind Pharm. 2007;33(6):627–639. doi: 10.1080/03639040601179954. [DOI] [PubMed] [Google Scholar]
- 9.Gallardo D, Skalsky B, Kleinebudde P. Controlled release solid dosage forms using combinations of (meth)acrylate copolymers. Pharm Dev Technol. 2008;13(5):413–423. doi: 10.1080/10837450802202098. [DOI] [PubMed] [Google Scholar]
- 10.Tapia C, Escobar Z, Costa E, Sapag-Hagar J, Valenzuela F, Basualto C, et al. Comparative studies on polyelectrolyte complexes and mixtures of chitosan-alginate and chitosan-carrageenan as prolonged diltiazem clorhydrate release systems. Eur J Pharm Biopharm. 2004;57(1):65–75. doi: 10.1016/S0939-6411(03)00153-X. [DOI] [PubMed] [Google Scholar]
- 11.Bühler V, Kollidon SR. In Kollidon®—polyvinylpyrrolidone in pharmaceutical industry. 9th Ed. Ludwigshafen: BASF SE; 2008. pp. 255–270. [Google Scholar]
- 12.Draganoiu E, Andheria M, Sakr A. Evaluation of the new polyvinyl acetate/povidone excipient for matrix sustained release dosage forms. Pharm Ind. 2001;63(6):624–629. [Google Scholar]
- 13.Shao ZJ, Farooqi MI, Diaz S, Krishna AK, Muhammad NA. Effects of formulation variables and post-compression curing on drug release from a new sustained-release matrix material: poly(vinyl acetate)–povidone. Pharm Dev Technol. 2001;6(2):247–254. doi: 10.1081/PDT-100002201. [DOI] [PubMed] [Google Scholar]
- 14.Kranz H, Wagner T. Effects of formulation and process variables on the release of a weakly basic drug from single unit extended release formulations. Eur J Pharm Biopharm. 2006;62(1):70–76. doi: 10.1016/j.ejpb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Kranz H, Guthmann C, Wagner T, Lipp R, Reinhard J. Development of a single unit extended release formulation for ZK 811 752, a weakly basic drug. Eur J Pharm Sci. 2005;26(1):47–53. doi: 10.1016/j.ejps.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Omelczuk MO, McGinity JW. The influence of thermal treatment on the physical–mechanical and dissolution properties of tablets containing poly (DL lactic acid) Pharm Res. 1993;10(4):542–548. doi: 10.1023/A:1018993818206. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Shah NH, Malick AW, Infeld MH, McGinity JW. Influence of thermal processing on the properties of chlorpheniramine maleate tablets containing an acrylic polymer. Pharm Dev Technol. 2002;7(4):481–489. doi: 10.1081/PDT-120015050. [DOI] [PubMed] [Google Scholar]
- 18.AlKhatib HS, Sakr A. Modeling of the effect of triethyl citrate and curing on drug release from film coated tablets. Pharm Ind. 2005;67(2):237–242. [Google Scholar]
- 19.Engineer S, Shao ZJ, Khagani NA. Temperature/humidity sensitivity of sustained-release formulations containing Kollidon SR. Drug Dev Ind Pharm. 2004;30(10):1089–1094. doi: 10.1081/DDC-200040292. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi T. Mechanism of sustained-action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 21.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliver Rev. 2001;48(2–3):139–157. doi: 10.1016/S0169-409X(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 22.Körber M, Hoffart V, Walther M, Macrae RJ, Bodmeier R. Effect of unconventional curing conditions and storage on pellets coated with Aquacoat ECD. Drug Dev Ind Pharm. 2010;36(2):190-199. [DOI] [PubMed]
- 23.Sadek HM, Olsen JL. Determination of water-adsorption isotherms of hydrophilic polymers. Pharm Technol. 1981;5(2):42–48. [Google Scholar]
- 24.Callahan JC, Cleary GW, Elefant M, Kaplan G, Kensler T, Nash RA. Equilibrium moisture content of pharmaceutical excipients. Drug Dev Ind Pharm. 1982;8(3):355–369. doi: 10.3109/03639048209022105. [DOI] [Google Scholar]
- 25.Zhang F, McGinity JW. Properties of hot-melt extruded theophylline tablets containing poly (vinyl acetate) Drug Dev Ind Pharm. 2000;26(9):931–942. doi: 10.1081/DDC-100101320. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez Novoa GA, Heinämäki J, Mirza S, Antikainen O, Colarte AI, Paz AS, et al. Physical solid-state properties and dissolution of sustained-release matrices of polyvinylacetate. Eur J Pharm Biopharm. 2005;59(2):343–350. doi: 10.1016/j.ejpb.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Camino G, Polishchuk AY, Luda MP, Revellino M. Comparison of the roles of two shrinkage-controlled low-profile additives in water aging of polyster resin-glass fiber composites. Polym Composites. 2000;21(5):821–831. doi: 10.1002/pc.10237. [DOI] [Google Scholar]
- 28.Aulton ME, Travers DN, White PJ. Strain recovery of compacts on extended storage. J Pharmacy Pharmacol. 1973;25(Suppl):79P–86P. [PubMed] [Google Scholar]
- 29.Picker KM. Time dependence of elastic recovery for characterization of tableting materials. Pharm Dev Technol. 2001;6(1):61–70. doi: 10.1081/PDT-100000014. [DOI] [PubMed] [Google Scholar]
- 30.Rekhi GS, Nellore RV, Hussain AS, Tillman LG, Malinowski HJ, Augsburger LL. Identification of critical formulation and processing variables for metoprolol tartrate extended-release (ER) matrix tablets. J Control Release. 1999;59(3):327–342. doi: 10.1016/S0168-3659(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 31.Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled release dosage forms. Int J Pharm Technol Prod Manuf. 1984;5:1–9. [Google Scholar]
- 32.Vueba ML, de Carvalho LA Batista, Veiga F, Sousa JJ, Pina ME. Role of cellulose ether polymers on ibuprofen release from matrix tablets. Drug Dev Ind Pharm. 2005;31(7):653–665. doi: 10.1080/03639040500216360. [DOI] [PubMed] [Google Scholar]