Abstract
In powder technology, it is often important to directly measure real powder flow rate from a small amount of powder. For example, in pharmaceutical industry, a frequent problem is to determine powder flow properties of new active pharmaceutical ingredient (API) in an early stage of the development when the amount of API is limited. The purpose of this paper is to introduce a new direct method to measure powder flow when the material is poorly flowing (cohesive) and the amount of material is about 1 to 2 g. The measuring system was simple, consisting of a flow chamber and electronic balance and an automated optical detection system, and for each measurement, only 1 to 2 g of sample was required. Based on the results obtained with this testing method, three selected sugar excipients, three grades of microcrystalline cellulose, and APIs (caffeine, carbamazepine, and paracetamol) can be classified as freely flowing, intermediate flowing, and poorly flowing powders, respectively. The average relative standard deviation for the flow time determinations was not more than 2–10%. The present novel flowability testing method provides a new tool for a rapid determination of flowing characteristics of powders (e.g., inhalation powders) and granules at a small scale.
Key words: cohesive powders, inhalation powders, powder flow, small-scale measurement
INTRODUCTION
The flowability of powders is of great importance in production of various pharmaceutical solid dosage forms such as inhalation powders, granules, pellets, tablets, and capsules. For example, to ensure reproducible filling of tablet dies or capsule shells, and subsequently, to control the weight variation of the dosage units in fast operating manufacturing processes, it is necessary to know the flowing properties of the powders accurately. Limited or uneven powder flow may result in excess entrapped air within powders thus promoting also capping or laminating, or cause even lubrication problems in high-speed tableting processes (1).
Powder flow is greatly dependent on single particle properties and bulk properties (i.e., particle size, shape, surface texture, density, and moisture content), as well as design and process operating conditions (hopper design, wall angle, and orifice diameter). The interparticular forces affecting powder flow may be roughly classified according to their origin as electrostatic interactions and molecular interactions. Electrostatic interactions are interactions of relatively highly charged particles (or faces), and they are active over relative long distance. Electrostatic forces typically arise from particle contacts or frictional charging. Molecular interactions are based on short-range nonspecific forces (secondary bonds or van der Waals attractions), and these interactions are increased as particle size is decreased, and they vary with changes in relative humidity (1,2). In addition, gravitational forces affect powder flow. In very fine or even micronized grade powders, the interparticle cohesive forces dominates over gravitational forces, and consequently, flow problems become evident. Powder flowability can be regarded as the resultant of the relative influences of all above-mentioned factors. Recently, the analysis of the flowability, cohesion, and adhesion forces, as well as shear behavior using computer simulation, is attracting increasing attention (3–7).
The flowability of powders has been determined by either directly using dynamic or kinetic methods, or indirect methods (8). Widely used direct methods include hopper flow rate method (in which the rate at which powder discharges from a hopper is measured) and recording flowmeter. Furthermore, a number of special direct techniques to study avalanching in powders have been described (9–12). Indirect methods include, e.g., angles of repose, shear cell determinations (5,8,11,13,14), and measurements of critical orifice diameter (10,15–18). Recently, flow properties of powders were measured using a more advanced ring shear cell tester which makes it possible to substantially reduce the amount of powder and labor required to characterize flow properties (19,20). Determination of a ratio of bulk and tap density (i.e., Carr’s compressibility index or Hausner’s ratio which have a correlation to flowability) has been also exploited as an indirect method in assessment of powder flow. These approaches, however, cannot provide accurate information on flowability since in determination of bulk and tap density, the shape of the particles affects more than electrical forces.
To date, only a few reports are available on development of a direct flowability testing method for poorly flowing powders in a small scale (21–23). The reason why the direct methods intended for measuring flowability of poorly flowing powders have not been introduced is because the powder forms a vault structure. When a vault structure is formed, it is very difficult to break it. This is the reason why there are no very accurate measurement devices available on the market that determines the flowability of poorly flowing powders using small sample amounts. Recently, Jiang et al. (23) developed an automatic small-scale measurement system for evaluating powder flowability based on the vibrating capillary method.
The aim of the present study was to develop a rapid flowability testing method for cohesive powders in a small scale. The basic idea in this new measurement system is to break the vault structure with a single upward motion, and consequently, let the powder flow through an orifice freely until it forms a vault structure again. This cycle is repeated until the powder has flowed completely. Hereby, the device described in this paper measures the powders ability to form a vault structure and the strength of that vault structure. This ability is directly proportional to the flowability of a powder. The resolution of the present flowability device has also turned out to be high.
MATERIALS AND METHODS
Materials
The materials tested were lactose monohydrate (100M and 200M, DMV, The Netherlands), glucose anhydrate (Ph. Eur.), xylitol (Ph.Eur.), mannitol (Ph.Eur.), sorbitol (Ph.Eur.), sucrose (Dan Sukker/Suomen sokeri Oy, Food Grade), microcrystalline cellulose (MCC; Avicel® PH-101, Avicel® PH-102, and Avicel® PH-200, FMC Corporation, USA), native maize starch (Ph.Eur.), native potato starch (Ph.Eur.), and partially pregelatinized maize starch (Starch 1500®, Colorcon Ltd., UK). As poorly flowable model active pharmaceutical ingredient (APIs), caffeine (Ph.Eur.), carbamazepine (Ph.Eur.), and paracetamol (Ph.Eur.) were studied. All above-mentioned materials are electrical insulators, and consequently, relative humidity is critical for powder flowing characteristics. Magnesium stearate (Ph.Eur.) was used for preparing binary powder mixtures of MCC. The results on flowability are summarized in Tables I and II.
Table I.
Comparison of Flow Rates (in Cubic Millimeters per Second and Milligrams per Second) and Carr’s Index of the Excipients Tested (n = 3)
| Excipient | Flow rate (mm3/s) | Flow rate (mg/s) | RH (%) | Carr’s index (%) | RH (%) |
|---|---|---|---|---|---|
| Sugars | |||||
| Lactose monohydrate 80 M | 90.8 (3.5) | 63.8 (2.7) | 55–56 | 13.1 (0.1) | 52 |
| Lactose monohydrate 200 M | 10.1 (0.7) | 4.0 (0.3) | 55–56 | 24.9 (0.5) | 54 |
| Glucose anhydrate | 130.9 (0.4) | 100.8 (1.4) | 55–56 | 14.3 (0.8) | 57 |
| Xylitol | 31.5 (1.6) | 24.2 (1.3) | 55–56 | 13.6 (1.0) | 53 |
| Sorbitol | 179.5 (3.5) | 101.4 (2.2) | 55–56 | 10.1 (0.9) | 50 |
| Mannitol | 99.4 (8.1) | 48.8 (4.3) | 55–56 | 10.9 (0.6) | 54 |
| Sucrose | 227.6 (31.9) | 195.6 (26.2) | 55–56 | 7.9 (1.1) | 57 |
| Celluloses | |||||
| Avicel PH–101 | 85.1 (3.1) | 22.9 (0.6) | 55–56 | 18.9 (1.4) | 63 |
| Avicel PH–102 | 195.1 (31.2) | 64.2 (10.6) | 55–56 | 16.3 (1.4) | 60 |
| Avicel PH-200 | 166.4 (4.0) | 55.4 (1.3) | 55–56 | 14.8 (1.2) | 59 |
| Starches | |||||
| Native maize starch | 37.7 (2.7) | 16.8 (1.1) | 55–56 | 17.2 (2.6) | 63 |
| Native potato starch | 80.5 (2.1) | 51.5 (1.2) | 55–56 | 12.8 (1.2) | 56 |
| Pregelatinized maize starch (Starch 1500) | 239.2 (14.0) | 137.6 (5.0) | 46 | 18.0 (2.3) | 59 |
Table II.
Comparison of Flow Rates (in Cubic Millimeters per Second and Milligrams per Second) and Carr’s Index of Active Pharmaceutical Ingredients (APIs) Tested (n = 3)
| API | Flow rate (mm3/s) | Flow rate (mg/s) | RH (%) | Carr’s index (%) | RH (%) |
|---|---|---|---|---|---|
| Caffeine | 51.5 (0.8) | 9.0 (0.2) | 49 | 17.4 (0.3) | 52 |
| Carbamazepine | 28.4 (3.4) | 6.7 (0.8) | 47 | 26.0 (1.1) | 55 |
| Paracetamol | 24.6 (1.8) | 1.7 (0.2) | 52 | 25.0 (1.6) | 56 |
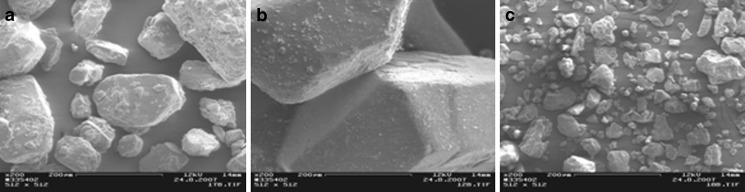
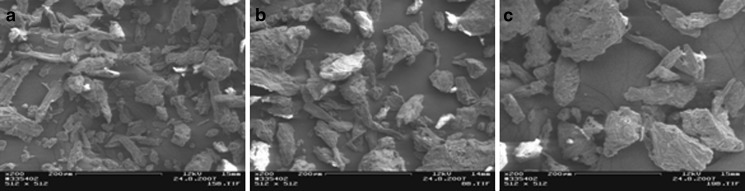
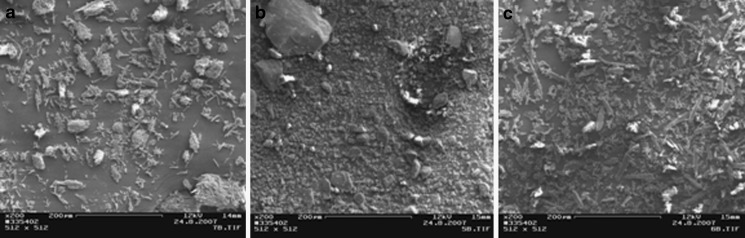
Scanning electron micrographs of the materials tested are illustrated in Figs. 1, 2, and 3. Sugar excipients (i.e., lactose monohydrate, glucose anhydrate, xylitol, sorbitol, mannitol, and sucrose) exhibited particles with a relatively round and spherical shape. As seen in Fig. 1, particle size of the sugars and starches ranges from the micronized scale (pregelatinized maize starch) to 400 µm (sucrose). The MCC grades (Avicel®) tested exhibited particles with an elongated and fiber-like shape. Particle size of MCCs was approximately 50 µm (Avicel® PH-101), 100 µm (Avicel® PH-102), and 200 µm (Avicel® PH-200; Fig. 2). The selected model APIs were composed of the particles with a particle size ranging from 5 to 50 µm (Fig. 3).
Fig. 1.
Scanning electron micrographs (SEMs) on glucose anhydrate a, sucrose b, and pregelatinized maize starch (Starch 1500) c
Fig. 2.
Scanning electron micrographs (SEMs) on microcrystalline celluloses grades: Avicel PH-101 a, Avicel PH-102 b, and Avicel PH-200 c
Fig. 3.
Scanning electron micrographs (SEMs) on the model active pharmaceutical ingredients: caffeine a, carbamazepine b, and paracetamol c
Methods
All materials were manually screened before testing by using a standard sieve (750 µm) to remove lumps. If the powder sample is composed of lumps, the real flowability value cannot be determined by this method or any method based on direct flowing of the powder through an orifice. Bulk and tap densities were determined by using an established Ph.Eur. method (24) and device (Erweka SVM tapping device, Erweka Apparatebau GmbH, Germany). For preparing binary powder mixtures of MCC and magnesium stearate (0.5% w/w), the powders were mixed for 2 min at 22 rpm in a glass jar using a laboratory-scale Turbula mixer (Turbula T2F, Willy A. Bachofen AG Machinenfabrik, Switzerland).
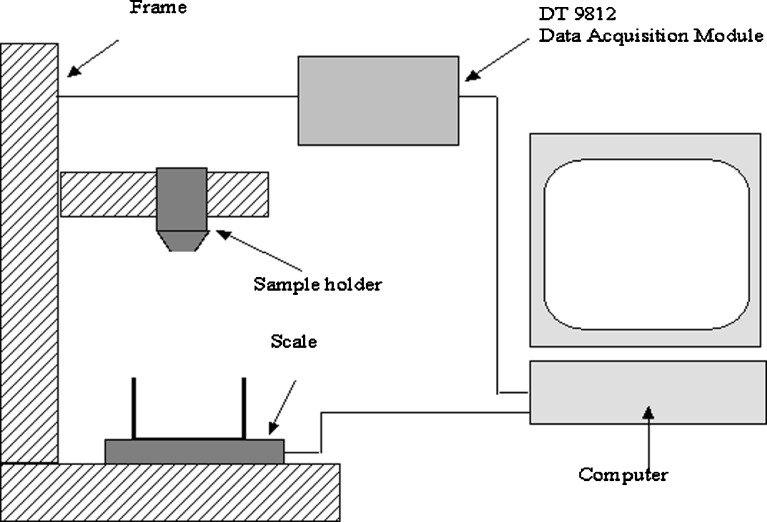
The schematic diagram of the present new flowability testing device designed for rapid flowability determinations of powders in a small scale is presented in Fig. 4. The system includes a frame, sample holder, orifice, and analytical scale. The volume of the sample holder is 5.96 ml, and the diameter of the orifice is 3.0 mm. The sample holder moves vertically, which makes the powder flow through an orifice and the breaking of the vault structure possible. With analytical scale connected to a computer, it is possible to calculate the flow rate (milligrams per second) and investigate the shape of the mass function. To minimize the non-linearity of the mass flow, 5% in the beginning and at the end of the mass flow function were not taken into count while calculating the flow rate. In flowability determinations, the sample holder was at a lower position 80% of the time and at an upper position 20% of the time. This makes it possible that the upward movement is powerful enough to break the vault structure of the sample. Furthermore, the potential flowing time (down position) was long enough to let the powder to flow freely until it forms a vault structure again with the frequency of 1 Hz.
Fig. 4.
Block diagram of a small-sample powder flow measuring device and testing set-up
RESULTS AND DISCUSSION
Tables I and II summarized comparison of flow rates (in cubic millimeters per second and milligrams per second) and Carr’s compressibility indexes of the materials tested. With few exceptions, the results on flowability obtained with the present flow rate testing method were in accordance with the calculated Carr’s indexes. Flowability of MCC powder (Avicel®) was greatly dependent on the grade of it. With respect to flowability, the MCC grades tested were ranked in the following decreasing order: Avicel PH-200, Avicel PH-102, and Avicel PH-101. However, contrary to Carr’s compressibility indexes, the measured flow rate values (cubic millimeters per second or milligrams per second) for Avicel® PH-200 powder was found to be lower than the respective values for Avicel® PH-102 (Table I). This is obviously due to the particle size (and size distribution) of Avicel® PH-200 which is just too large for the present orifice size (3 mm in diameter) used in the flow rate measurements.
As regards with sugar and starch excipients, sucrose exhibited clearly the best flowing characteristics. The other materials tested can be ranked in decreasing order (flow rate) as sorbitol, glucose anhydrate, mannitol, lactose monohydrate (80 M), and xylitol. As expected, lactose monohydrate (200 M) with a smaller particle size as well as maize starch presented the worst flowing capacity. The flowability results obtained with APIs were also in accordance with those obtained with the reference method (Carr’s compressibility index). With respect to flowability, the drugs tested were ranked in the following decreasing order: caffeine, carbamazepine, and paracetamol. With APIs, variation of the flowability results obtained with the present flow rate testing method was found to be very low.
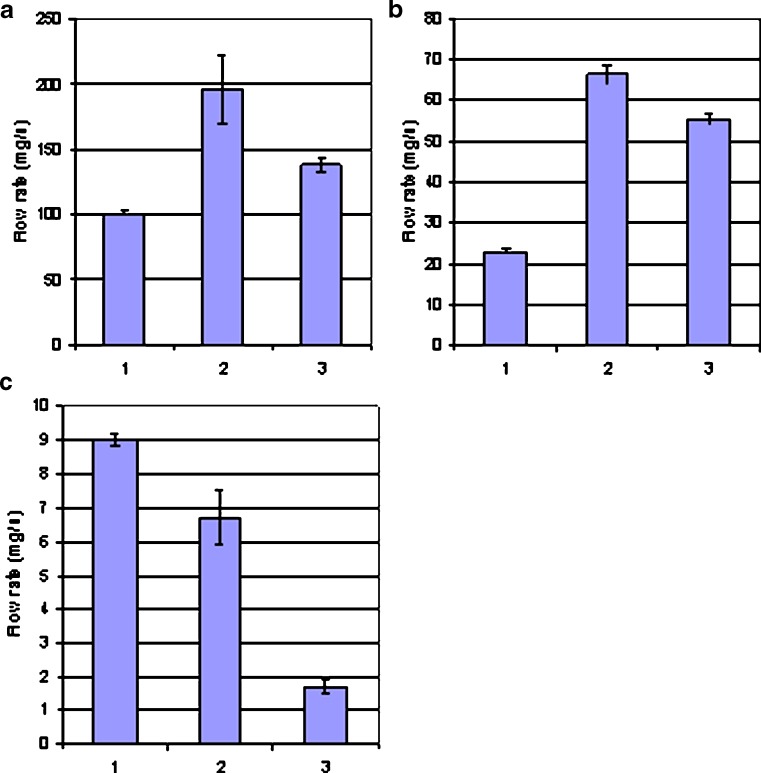
Based on the results obtained with the present flowability testing device, the test materials were classified as freely flowing, intermediate flowing, and poorly flowing powders as shown in Fig. 5. Sucrose, glucose, and pregelatinized maize starch were classified as typical free-flowing powders (with a flow rate over 100 mg/s), whereas caffeine, carbamazepine, and paracetamol (APIs) had very poor flow properties (a flow rate below 10 mg/s). MCCs (Avicel® PH-101, PH-102, and PH-200) presented relatively good flow properties, and consequently, they were classified as intermediately flowing powders (with a flow rate from 10 to 100 mg/s).
Fig. 5.
Classification of the test materials based on their powder flow properties. a Freely flowing powders: flow rate over 100 mg/s (key: 1 glucose, 2 sucrose, and 3 pregelatinized maize starch). b Intermediately flowing powders: flow rate from 10 to 100 mg/s (key: 1 MCC Avicel PH-101, 2 MCC Avicel PH-102, and 3 MCC Avicel PH-200). c Poorly flowing powders: flow rate under 10 mg/s (key: 1 caffeine, 2 carbamazepine, and 3 paracetamol)
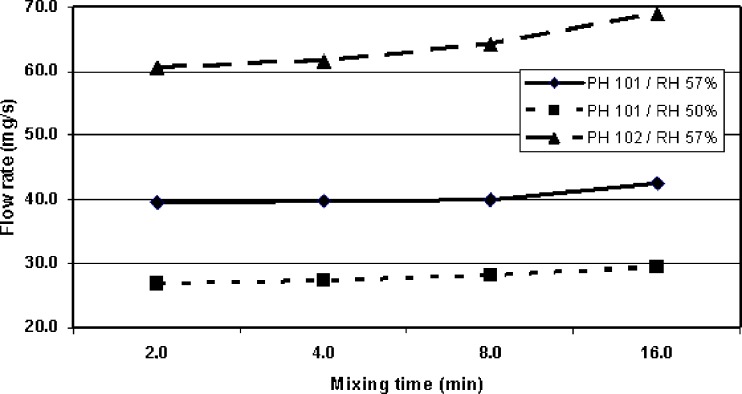
Potential effects of widely used tableting lubricant (magnesium state) on flow properties of MCC powders (Avicel® PH-101 and PH-102) were studied with the present flowing testing device. As seen in Fig. 6, mixing time of magnesium stearate slightly increased the flow rate of both MCC grades. Trend curves are not statistically significant, but they all have similar structure/curvature, which suggests that there is a real physical phenomenon behind. This is obviously due to the formation of a uniform lubricant film around the MCC particles, which in turn enhances flowing properties of the present binary mixture. As regards with flowing properties, Avicel® PH-102 MCC powder seems to be slightly more sensitive to the effects of mixing time of magnesium stearate than Avicel® PH-101 MCC powder.
Fig. 6.
Mixing time of magnesium stearate slightly increases the flow rate of microcrystalline cellulose (MCC) powder (Avicel PH-101 and PH-102) (n = 3)
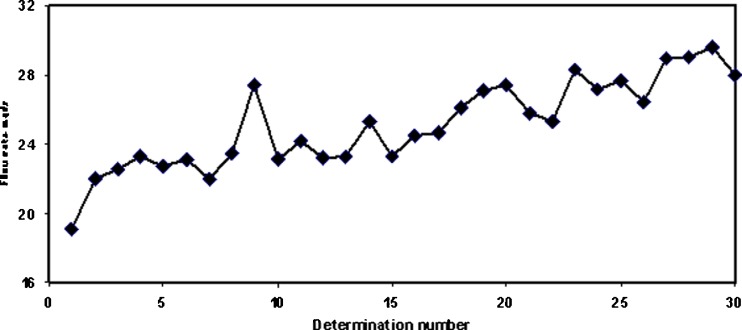
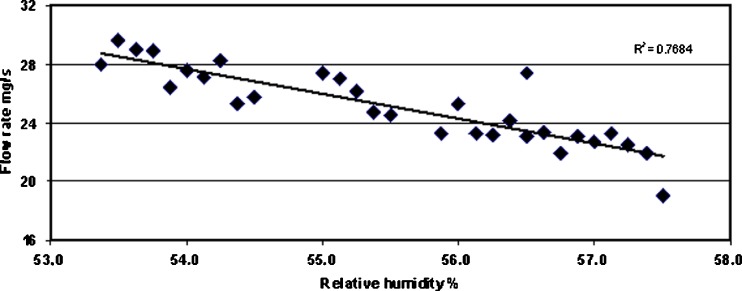
The effects of seasonal air humidity conditions on the flow rate of MCC powder (Avicel® PH-101) are shown in Fig. 7. Day to day fluctuation of the flow rate measured with a present powder flow testing method was very high. Since pharmaceutical excipients and APIs are in most cases electrical insulators, relative humidity is critical for powder flowing, and this should be taken into account when testing powder flow with the this method. As shown in Fig. 8, even a 1% change in relative humidity affects the flow rate of the present MCC powder (Avicel® PH-101). A linear correlation between relative air humidity and the flow rate of MCC powder (Avicel® PH-101) is shown in Fig. 8.
Fig. 7.
Effects of seasonal air humidity fluctuation on the flow rate of microcrystalline cellulose (MCC) powder (Avicel PH-101)
Fig. 8.
Linear correlation between relative air humidity and the flow rate of microcrystalline cellulose (MCC) powder (Avicel PH-101)
CONCLUSIONS
The present flowability testing method provides a new tool for a rapid determination of flowing characteristics of powders (e.g., inhalation powders) and granules at a small scale. The method is rapid, accurate, repeatable, and it provides wide measurement range. The present method is also capable to classify and rank the flow properties of pharmaceutical powders (excipients and APIs), and it could have interesting possibilities especially in pharmaceutical preformulation stage when amount of material (i.e., API) available is very limited. Based on the results of this study, pharmaceutical excipients and APIs can be classified as freely flowing, intermediate flowing, and poorly flowing powders. Powder flow is highly dependent on relative moisture.
References
- 1.Staniforth J. Powder flow. In: Aulton ME, editor. Pharmaceutics. The Science of Dosage Form Design, London: Churchill Livingstone, Elsevier Limited; 2002. pp. 197–210. [Google Scholar]
- 2.Rhodes MJ, Osborne CF, Hutton SR, Forsyth AJ. Effects of interparticle force on the packing of spherical granular material. Phys Rev Lett. 2001;87(244301):1–3. doi: 10.1103/PhysRevLett.87.244301. [DOI] [PubMed] [Google Scholar]
- 3.Antikainen OK, Rantanen JT, Yliruusi JK. Use of the Kohonen self-organizing map to predict the flowability of powders. STP Pharma Sci. 2000;10:349–354. [Google Scholar]
- 4.Thronton C, Zhang L. Numerical simulations of the direct shear test. Chem Eng Technol. 2003;26:153–156. doi: 10.1002/ceat.200390022. [DOI] [Google Scholar]
- 5.Gröger T, Tüzün U, Heyes DM. Modelling and measuring of cohesion in wet granular materials. Powder Technol. 2003;133:203–215. doi: 10.1016/S0032-5910(03)00093-7. [DOI] [Google Scholar]
- 6.Tomas J. Fundamentals of cohesive powder consolidation and flow. Granular Matter. 2004;6:75–86. doi: 10.1007/s10035-004-0167-9. [DOI] [Google Scholar]
- 7.Moreno-Atanasio R, Antony SJ, Ghadiri M. Analysis of flowability of cohesive powder using distinct element method. Powder Technol. 2005;158:51–57. doi: 10.1016/j.powtec.2005.04.029. [DOI] [Google Scholar]
- 8.Schwedes J. Review on testers for measuring flow properties of bulk solids. Granular Matter. 2003;5:1–43. doi: 10.1007/s10035-002-0124-4. [DOI] [Google Scholar]
- 9.Kaye BH, Gratton-Liimatainen J, Lloyd J. The effect of flowagents on the rheology of a plastic powder. Part Part Syst Char. 1995;12:194–197. doi: 10.1002/ppsc.19950120406. [DOI] [Google Scholar]
- 10.Lee YSL, Poynter R, Podczeck F, Newton M. Development of a dual approach to assess powder flow from avalanching behaviour. AAPS PharmSciTech. 2000;1:44–52. doi: 10.1208/pt010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock BC, Vukovinsky KE, Brolley B, Grimsey I, Hedden D, Olsofsky A, Doherty RA. Development of a robust procedure for assessing powder flow using a commercial avalanche testing instrument. J Pharm Biomed. Anal. 2004;35:979–990. doi: 10.1016/j.jpba.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Freeman R. Measuring the flow properties of consolidated, conditioned and aerated powders—a comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007;174:25–33. doi: 10.1016/j.powtec.2006.10.016. [DOI] [Google Scholar]
- 13.Carr JF, Walker DM. An annular shear cell for granular materials. Powder Technol. 1968;1:369–373. doi: 10.1016/0032-5910(68)80019-1. [DOI] [Google Scholar]
- 14.Ramchandruni H, Hoag SW. Design and validation of an annular shear cell for pharmaceutical powder testing. J Pharm Sci. 2001;90:531–540. doi: 10.1002/1520-6017(200105)90:5<531::AID-JPS1010>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Walker DM. An approximate theory for pressures and arching in hoppers. Chem Eng Sci. 1966;21:975–997. doi: 10.1016/0009-2509(66)85095-9. [DOI] [Google Scholar]
- 16.Jolliffe IG, Newton JM, Waiters JK. Theoretical considerations of the filling of pharmaceutical hard gelatin capsule. Powder Technol. 1980;27:189–195. doi: 10.1016/0032-5910(80)85021-2. [DOI] [Google Scholar]
- 17.Tan SB, Newton JM. Powder flowability as an indication of capsule filling performance. Int J Pharm. 1990;61(1–2):145–155. doi: 10.1016/0378-5173(90)90053-7. [DOI] [Google Scholar]
- 18.Flemming J, Mielck JB. Requirements for the production of micro-tablets: suitability of direct-compression excipients estimated from powder characteristics and flow rates. Drug Dev Ind Pharm. 1995;21:2239–2251. doi: 10.3109/03639049509065904. [DOI] [Google Scholar]
- 19.Hou H, Sun CC. Quantifying effects of particulate properties on powder flow properties using a ring shear tester. J Pharm Sci. 2008;97:4030–4039. doi: 10.1002/jps.21288. [DOI] [PubMed] [Google Scholar]
- 20.Sun CC. Improving powder flow properties of citric acid by crystal hydration. J Pharm Sci. 2009;98:1744–1749. doi: 10.1002/jps.21554. [DOI] [PubMed] [Google Scholar]
- 21.Crowder TM, Hickey AJ. An instrument for rapid powder flow measurement and temporal fractal analysis (short communication) Part Part Syst Charact. 1999;16:32–34. doi: 10.1002/(SICI)1521-4117(199905)16:1<32::AID-PPSC32>3.0.CO;2-8. [DOI] [Google Scholar]
- 22.Rios M. Developments in powder flow testing. Pharm Technol Eur. 2006;30:38–49. [Google Scholar]
- 23.Jiang Y, Matsusaka S, Masuda H, Qian Y. Development of measurement system for powder flowability based on vibrating capillary method. Powder Technol. 2009;188:242–247. doi: 10.1016/j.powtec.2008.05.003. [DOI] [Google Scholar]
- 24.Council of Europe . European Pharmacopoeia 5th ed. Strasbourg: Council of Europe; 2004. [Google Scholar]