Abstract
The objective of this study was to compare a novel sustained release tablet formulation that has the potential to be used for drugs of different physicochemical properties using a binary mixture of polymethacrylate polymers in their salt forms with the polymethacrylate interpolyelectrolyte complex (IPEC) tablets in terms of drug release and compactness. Also, we aimed to compare this formulation with an IPEC tablet in terms of drug release. Tablets prepared using Eudragit E-Citrate and Eudragit L-Sodium were more convenient, easier to prepare, and showed better sustained release and compactness characteristics compared to IPEC tablets of similar concentrations and preparation methods.
Key words: Eudragit E-100, Eudragit L-100, FTIR, IPEC, pH profile, sustained release
INTRODUCTION
Modern controlled-release dosage forms require reliable excipients to ensure a drug release rate which is reproducible in a narrow range. Eudragit® (polymethacrylates) polymers fulfill these requirements to a very high extent and enable the research and development to create tailor-made solutions (1–4). Eudragits are synthetic cationic and anionic copolymers of dimethylaminoethyl methacrylates, methacrylic acid, and methacrylic acid esters in varying ratios. These copolymers act as polyelectrolytes which are suitable for many purposes, from gastric or intestinal soluble drug formulations to insoluble but swellable delivery forms. This is usually regulated by the number of ionized and non-ionized groups in the structure of these copolymers. Eudragit polymers have been proven to be suitable for a wide variety of pharmaceutical applications; they are primarily used in oral capsule and tablet as film-coating agents for protective purposes and provide sustained release formulations (7–10). Depending on the type of polymer used, films of different solubility characteristics can be produced (2). They are also used as binders in both aqueous and organic wet granulation processes (4–6).
Several different types of Eudragits are commercially available and may be obtained as dry powder, granules, aqueous dispersion, or as an organic solution. Some of them are soluble in gastric fluid as well as in weakly acidic buffer solutions up to pH 5 such as Eudragit E (EE), which is a cationic polymer based on dimethylaminoethyl methacrylate and neutral methacrylic acid esters (3). Others such as Eudragit L (EL) and Eudragit S (ES) are soluble in neutral to weakly alkaline conditions (pH 6–7) and form salts with alkalis. EL and ES are anionic copolymers based on methacrylic acid and methyl methacrylate. Both EL and ES enjoy similar physicochemical properties except that the ratio of free carboxyl groups to the ester is approximately 1:1 in EL and approximately 1:2 in ES (2). They are used as enteric coating agents due to their resistance to gastric fluid (7,11–13).
Since most of these copolymers are polyelectrolytes, one possible way of modification of their structure is via inclusion of such polymers into so-called interpolyelectrolyte complexes (IPEC) (14,15).
Tablets were prepared from IPEC precipitates which were obtained by mixing cationic with anionic polymers in aqueous solutions. It is known that the stoichiometry of the components in IPEC depends on the pH values of the media, ionic strength, concentration, and in some cases on the order of mixing (16). The newly formed interpolyelectrolytes should possess properties different from those of the individual components. The interaction between the two oppositely charged polymers must occur in an environment of a suitable pH value where a significant degree of ionization on polymers exists. The resulted IPEC should serve as a pH-independent polymer carrier that can be formulated into controlled-release tablets.
Few papers were devoted to the possibility of involving mixtures of anionic and cationic polymethacrylate polymers in an ionic interaction in order to prepare a new complex. This complex has pH-independent characteristics which can be used in sustained release solid dosage forms (17–21).
Kabanova and Mustafine (22) have focused on the formation of IPEC complex between Eudragit E and Eudragit L and investigated the release profile from ibuprofen tablets containing about 30% w/w of IPEC. Results confirmed the formation of an interpolyelectrolyte complex between EE and EL at pH 5.5. The release rate of ibuprofen greatly decreased when the matrix tablet was prepared by the IPEC compared to that prepared by physical mixture of EE and EL polymers under the same conditions.
The combination of anionic and cationic polymethacrylate polymers (EE-Citrate and EL-Na) was found to be effective in formulating paracetamol into matrices and controlling the release of paracetamol from those matrices. The release behavior was almost independent of the pH of the surrounding environment especially at higher polymer concentrations, suggesting the formation of a new ionic compound between EE-Citrate and EL-Na with characteristics different from the individual polymers (23).
The aim of the current work was to compare the novel tablet formulation composed of a mixture of anionic and cationic polymethacrylate copolymers (EE-Citrate and EL-Na) presented in our previous work (23) to a tablet formulation made of polymethacrylate IPEC. The comparison was in terms of the drug release and tablet compactness using paracetamol as a model drug.
MATERIALS AND METHODS
Materials
The different types of Eudragit polymers of various grades (Eudragit E-PO and Eudragit L-100) were obtained from Röhm Pharma, Darmstadt, Germany. Methocel® E5, which is a low-viscosity grade of hydroxyl propyl methyl cellulose, was obtained from Colorcon Inc., West Point, Pennsylvania, USA. Monobasic potassium phosphate, tribasic sodium phosphate, sodium hydroxide, and glacial acetic acid were provided by Scharlau, Barcelona, Spain. Hydrochloric acid 37% was obtained from Merck, Darmstadt, Germany. Isopropyl alcohol was obtained from Tedia, Fairfield, Ohio, USA. Phosphoric acid 85% was provided by Riedel-de Haen, Seelze, Germany. Lactose monohydrate, citric acid monohydrate, magnesium stearate, and talc were obtained from Hikma Pharmaceutics, Amman, Jordan. Potassium bromide (UVasol®) for Fourier transform infrared (FTIR) spectroscopy was provided by Merck. Acetaminophen (paracetamol) was gifted by JOSWE Medicals, Amman, Jordan.
Methods
The Method of Analysis
The analysis for paracetamol was carried out using a UV/VIS spectrophotometer (Model V-530 Jasco, Tokyo, Japan) at the λmax of paracetamol which is 243 nm using a quartz cuvette cell and against an appropriate blank. The solvents used were the same as the dissolution media. The dilution factor was 25 (2 ml of the aliquot diluted in a 50-ml volumetric flask). The dilution was necessary in order to get UV absorbance readings below 1. The absorbance readings were converted to concentration (μg/ml) by using an appropriate calibration curve.
Selectivity
UV scanning was performed on standard solutions of paracetamol (10 μg/ml) in 0.1 N HCl pH 1.2 and in 0.2 N phosphate buffer pH 6.8, Eudragit E (5 μg/ml) in 0.1 N HCl pH 1.2, and Eudragit L (5 μg/ml) in 0.2 N phosphate buffer pH 6.8, in addition to a mixture of paracetamol with either Eudragit E or Eudragit L with the same concentrations mentioned above.
Preparation of Interpolyelectrolytes (IPEC)
Eudragit E (EE) solution was prepared by gradually dissolving 3.75 g of EE into a mixture of 5 ml of 0.1 N of CH3COOH and 5 ml distilled water and mixed well using a magnetic stirrer for 24 h until it completely dissolved. Then the solution was titrated by 0.1 N NaOH to the final pH value of 6.0. Eudragit L (EL) solution was prepared by gradually dissolving 3.375 g of EL into a mixture of 5 ml of 0.1 N of NaOH and 5 ml distilled water and mixed well using a magnetic stirrer for 24 h until it completely dissolved. Then the solution was titrated by 0.1 N CH3COOH to the final pH value of 6.0. EE solution was subsequently mixed with EL solution at room temperature for 24 h at pH 6.0 ± 0.5. The mixture was then filtered using a Buchner funnel and washed well using distilled water. The final precipitate was then dried for 48 h at 40°C, ground, and sieved via a 1-mm sieve to minimize variation in granule size. The granules were stored in a glass bottle and kept away from sunlight.
Preparation of Matrix Tablets Containing Eudragit Polymers in Different Forms
For the purpose of this research, two different methods were used for the preparation of matrix tablets: direct compression and wet granulation for comparative purposes with IPEC. At least 90% of Eudragit E-100 powder had a particle size <0.315 mm according to Ph. Eur. 2.1.4 or United States Pharmacopoeia (USP) <811>. Similarly, 95% of Eudragit L-100 powder had a particle size less than 0.25 mm (1,2).
Direct Compression:
The technique involved direct and geometric blending of paracetamol with other excipients depending on the desired formula (Table I) using a plastic beaker while gradually adding a specified amount of EE (base), EL (base), or IPEC as powders.
Table I.
Composition of Tablet Formulations Containing Different Forms of Eudragit Polymers
| No. | Component | Formula no. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||||||
| mg/tab | % (w/w) | mg/tab | % (w/w) | mg/tab | % (w/w) | mg/tab | % (w/w) | mg/tab | % (w/w) | mg/tab | % (w/w) | mg/tab | % (w/w) | ||
| 1 | Paracetamol | 233.92 | 44.03 | 233.92 | 41.03 | 233.92 | 44.79 | 233.92 | 61.88 | 233.92 | 61.88 | 233.92 | 61.88 | 233.92 | 61.88 |
| 2 | Lactose | 233.92 | 44.03 | 233.92 | 41.03 | 233.92 | 44.79 | ||||||||
| 3 | Methocel | 23.39 | 4.40 | 23.39 | 4.10 | 23.39 | 4.48 | 23.39 | 6.19 | 23.39 | 6.19 | 23.39 | 6.19 | 23.39 | 6.19 |
| 4 | Mg stearate | 2.63 | 0.50 | 2.82 | 0.50 | 2.59 | 0.50 | 1.88 | 0.50 | 1.88 | 0.50 | 1.88 | 0.50 | 1.88 | 0.50 |
| 5 | Talc | 2.63 | 0.50 | 2.82 | 0.50 | 2.59 | 0.50 | 1.88 | 0.50 | 1.88 | 0.50 | 1.88 | 0.50 | 1.88 | 0.50 |
| 6 | EE-Citrate | 18.45a | 3.47 | 38.81c | 6.81 | ||||||||||
| 7 | EL-Na | 16.31b | 3.07 | 34.37d | 6.03 | ||||||||||
| 8 | IPEC | 25.87 | 4.96 | 116.96 | 30.94 | 116.96 | 30.94 | ||||||||
| 9 | EE | 58.48 | 15.47 | 58.48 | 15.47 | ||||||||||
| 10 | EL | 58.48 | 15.47 | 58.48 | 15.47 | ||||||||||
| 11 | H2O | q.s. | q.s. | q.s. | q.s. | ||||||||||
| Total (mg) | 531.25 | 100 | 570.05 | 100 | 522.28 | 100 | 378.03 | 100 | 378.03 | 100 | 378.03 | 100 | 378.03 | 100 | |
aEquivalent to 12.94 mg of EE
bEquivalent to 12.94 mg of EL
cEquivalent to 27.26 mg of EE
dEquivalent to 27.26 mg of EL
For tablet compression, two tablet formulations were prepared. The first formulation is composed of one portion of paracetamol/EE powder and one portion of paracetamol/EL powder that were mixed at a 1:1 ratio (formula #6). The second formulation contains a single portion of IPEC powder only (formula #5). In each formulation, the powder mixture was manually mixed with magnesium stearate and talc for 5 min in a plastic beaker using a flat spatula. The mixing time was kept constant for all formulations in order to eliminate the impact of mixing time on the release profile.
The final mixture was then compressed into tablets using a flat-faced Carver® hydraulic single press (Carver®, Wabash, Indiana, USA) at 2,000 kg for 30 s. To study the effect of tablet hardness on paracetamol release, the above procedure was repeated using a compression force of 5,000 kg. The diameter of the tablet was 13 mm, and the amounts of paracetamol, lactose, and Methocel in each tablet were kept constant; the percentage of magnesium stearate and talc per tablet was kept at 0.5% each. Table I shows the amounts and percentages of components of the tablets in each formula in addition to the weight of each tablet. The batch size for the prepared granules was sufficient to produce 25–40 tablets.
-
2.
Wet Granulation:
The following solutions and solvents were prepared and used for preparation of granules:
1 g of EE dissolved in 7 g of 0.33 N citric acid solution
1 g of EL dissolved in 7 g of 1 N NaOH
The procedure for granule preparation involved manual blending of paracetamol with other excipients using a plastic beaker while gradually adding a specified amount of EE-Citrate and EL-Na using the solutions (a) and (b), respectively. Distilled water was used as a binder for granulation of the IPEC powder and EE/EL mixture (formulas #5 and #7). Binder solutions were added until an adequate degree of agglomeration was visible. The granules were then dried for 15 min in an oven at 50°C, screened through 3-, 2-, and 1-mm sieves, and each screening was followed by 15-min drying at 50°C. The key parameter that determines the drying time was the value of weight loss on drying (WLD, %), which was kept constant for all formulations (below 1%). It was found that granules that were dried for 30 min at 50°C showed a WLD below 1%. For tablet compression, two tablet formulations were prepared. The first formulation is composed of one portion of paracetamol/EE-Citrate granules and one portion of paracetamol/EL-Na granules mixed at a 1:1 ratio. The second formulation contains a single portion of IPEC granules only. In each formulation, the granules were mixed with magnesium stearate and talc for 5 min in a plastic beaker using a flat spatula. The mixing time was kept constant for all formulations in order to eliminate the impact of mixing time on the release profile. The final granules were then compressed into tablets using a flat-faced Carver® hydraulic single press at 2,000 kg for 30 s. To study the effect of tablet hardness on paracetamol release, the above procedure was repeated using a compression force of 5,000 kg. The diameter of the tablet was 13 mm, and the amounts of paracetamol, lactose, and Methocel in each tablet were kept constant; the percentage of magnesium stearate and talc per tablet was kept at 0.5% each. Table I shows the amounts and percentages of components of the tablets in each formula in addition to the weight of each tablet. The batch size for the prepared granules was sufficient to produce 25–40 tablets.
Swelling Studies
The degree of swelling was conducted on various formulations in an attempt to explain the mechanism of release of paracetamol in conditions which simulated the gastrointestinal tract; the first hour was in acidic medium (0.1 N HCl, pH 1.2), and then transferred into buffer solution (0.2 N phosphate buffer pH 6.8) (16).
Tablets for swelling study were prepared by compressing 250 mg of polymer carrier (without paracetamol) using a flat-faced manual hydraulic single press at 5,000 kg for 30 s. The polymeric matrix tablet was placed in a tarred basket which was then immersed into a thermostated bath (37 ± 0.5°C). The volume of the swelling medium was 40 ml. After every 15 min, the basket was removed from the medium, accurately dried by filter paper, and weighed. For determination of equilibrium degree of swelling, a final weighing was performed after 24 h. Three tablets from each formula were used and the mean and standard deviation were calculated. The polymer carriers used for the preparation of tablets were EE/EL powder mixture, EE powder, EL powder, and EE-Citrate/EL-Na powder and IPEC powder. EE-Citrate/EL-Na powder was obtained by grinding the dried precipitate obtained by mixing EE-Citrate and EL-Na solutions. The ratio of Eudragit mixtures in all formulations was 1:1 and the degree of swelling (H%) was calculated as:
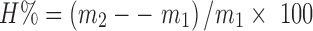 |
Where m1 is the weight of the dry sample and m2 the weight of the swollen samples (16). Statistical analysis using one-way ANOVA test was also carried out to check whether the difference in the degree of swelling for various formulas was significant or not. The confidence limit was set at 95%.
Drug Release Studies
Dissolution tests were performed using Electrolab USP basket dissolution apparatus (TDT-08L, Mumbai, India) with autosampler accessories (peristaltic pump, temperature controller, and fraction collector) according to the rotating basket method described in USP XXVIII (Apparatus I). The rotating speed was 100 rpm and the temperature was 37 ± 0.5°C. The volume of the dissolution medium was 1,000 ml. The dissolution media used were distilled water pH 6.5, 0.1 N HCl pH 1.2, and 0.2 N phosphate buffer pH 6.8. Distilled water was used in order to check for the presence of interaction between the two different Eudragit polymers in the absence of significant ionic charges on their functional groups usually present in acidic or basic dissolution media. The effect of pH profile on the release of paracetamol from matrix tablets was also investigated. The release of paracetamol was investigated at three different stages; the first stage lasted for 1 h at pH 1.2 and the volume of the dissolution medium was 500 ml. The second stage was conducted at pH 5 for 2 h. The volume of the dissolution medium in the second stage was increased to 740 ± 5 ml by adding about 235 ml of tribasic sodium phosphate (Na3PO4) and the pH was adjusted using NaOH or phosphoric acid. The final stage was conducted for 5 to 24 h and the pH was adjusted to 6.8 by adding about 80 ml tribasic sodium phosphate and completing the volume of the dissolution medium to 1,000 ml using distilled water. Tribasic sodium phosphate solution was prepared by dissolving 190 g of Na3PO4 powder into 5 l of distilled water.
At appropriate time intervals, 10 ml of samples was withdrawn and replaced with an equal volume of fresh dissolution medium. Samples were filtered using white cannula filters made of cellulose, diluted, and spectrophotometrically analyzed at λmax = 243 nm. Three tablets from each formulation were subjected to the dissolution test, and the results were presented as the mean values of three determinations, standard deviation, and coefficient of variation (RSD). Standard deviation was represented as error bars at each data point. The duration of the study ranged from 8 to 24 h or until the cumulative paracetamol release reached 90–100% for two consecutive time points. The percentage of paracetamol released was plotted versus time. Preliminary experiments had shown that Eudragit polymers did not interfere with the quantization of paracetamol.
Physical Tests
Crushing Strength (Hardness)
Ten tablets from each formula were tested using a tablet hardness tester (Model Pharmatron 8M® Tablet Tester, Solothurn, Germany).
Thickness and Diameter
Diameter and thickness for all tablets were determined using an electronic digital caliper (Model LTF 327.01 LTF, Germany). Measurements were taken up to two decimal points.
Moisture Content
The moisture content of the freshly prepared powders and granules was calculated using Halogen moisture analyzer (Model HR 85 Halogen Mettler, Columbus, Ohio, USA). One gram of the powder or granules was placed on an aluminum pan and the analysis was run automatically at 105°C for 1–2 min.
FTIR Spectroscopy
The interaction between EE and EL was studied using FTIR spectroscopy. The measurements were performed on EE and EL and on a 1:1 physical mixture of EE and EL in the powder base form in addition to IPEC powder. The IR absorption spectra of the samples pelletized with KBr were measured using FTIR spectrophotometer (Model 8400S, Shimadzu, Japan). Samples were dried at 40°C for 24 h before analysis. No baseline correction for the FTIR spectra was performed during or after the measurement.
RESULTS AND DISCUSSION
Analytical Method Validation
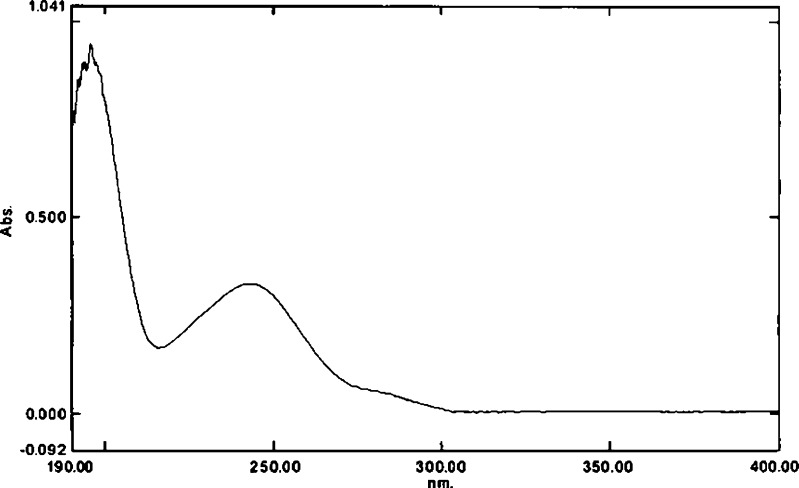
The UV scanning of paracetamol showed a maximum absorbance at 243 nm as shown in Fig. 1. There was no shifting in λmax for paracetamol at various pH values. The calibration curves of paracetamol was linear in different dissolution media at various pH values (distilled water, 0.1 N HCl, phosphate buffer pH 5 and 6.8); the correlation coefficient (r2) was higher than 0.99990. Eudragit polymers did not interfere with the analysis of paracetamol in the drug release studies because there were no significant peaks for EE and EL polymers observed in the UV range of 200–400 nm at concentrations found within the tablets, and the UV spectra of paracetamol in the presence and absence of Eudragit polymers were essentially similar to each other.
Fig. 1.
The UV scanning of paracetamol solution. The maximum absorbance occurs at 243 nm
Swelling Studies
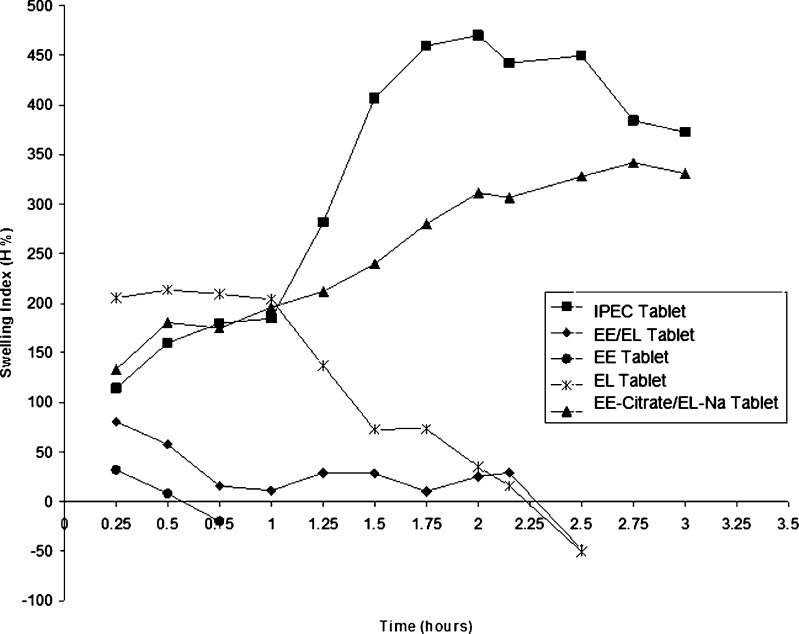
It is well known that the release properties of polymeric carriers can be somehow predicted by determination of their swelling characteristics (16). A comparative analysis of the swelling characteristics of these polymer carrier matrix systems is shown in Fig. 2. The negative values of the percentage of swelling indicated that tablets were partially eroded into the medium and, therefore, the weight of the tablet became smaller than the original weight of the tablet.
Fig. 2.
The swelling indices (H%) plotted against time for various formulations containing different types of Eudragit polymers. The ratio of polymers in mixtures is 1:1
Tablets containing only Eudragit E (EE base) were already dissolved after 30 min in acidic medium by protonating the dimethylamino groups in its structure. Oppositely, tablets prepared from Eudragit L (EL base) swelled in the acidic medium up to 200%. However, when they were transferred into phosphate buffer medium pH 6.8, they immediately started dissolution at different rates due to the deprotonation of their carboxylic acid groups.
Tablets containing a mixture of EE/EL (bases) showed a slight increase in weight and volume up to 90% after 15 min but rapidly disintegrated afterwards. When tablets were transferred into the buffer medium pH 6.8 after spending 1 h in the acidic medium, they showed a second increase in weight to 30% before they were completely disintegrated after about 2.5 h. This could be due to the presence of an interaction between EL with the swelled and positively charged EE. However, this interaction was kept at a minimum level because only one polymer was ionized in one medium at a time and the other was not; thus, no significant interpolymer interaction was present.
Tablets containing both polymers as salts (EE-Citrate/EL-Na) were ionized in all dissolution media; thus, a possible interaction between the positively and the negatively charged polymers was highly favored. These tablets showed high swelling index that was linearly increased throughout the swelling study until they reach an equilibrium value of about 350%. This swelling was probably due to the formation of a cross-linked matrix containing sufficient voids for swelling. In addition, both polymer structures might be changed to a more extended chain form. The continuous rise in swelling for EE-Citrate/EL-Na tablets can be further justified as follows: when the tablets come in contact with acidic medium, the free dimethylamino groups were charged by protonation while increasing the charge density on the polymer and cause a change on the polymer structure to a more extended chain form. Later, when the tablets were immersed into the buffer medium, the free carboxylic acid groups were ionized while EE retained most of its charge due to salt formation with citrate ions. This will lead to a further increase in charge density and polymer chain extension and consequently an increase in the degree of swelling.
The swelling behavior of IPEC has other characteristics. In acidic medium, the degree of swelling increased to approximately 100% after the first 30 min, which can be explained by a change in the interpolymer complex structure. When immersing the matrix into acidic medium, the free dimethylamino groups were protonated and their hydration increased the degree of swelling at the beginning of the experiment (15). Later, full ionization of all dimethylamino groups turned it into a polyelectrolyte with a relatively high charge density. As a result, the structure of the IPEC was changed because the ionic bonds were not fixed and they can move from one electrostatic site to another. When the pH of the medium changed to 6.8, the degree of swelling increased in 2 h and reached the equilibrium value of 400% after 24 h. The carboxylic acid groups of EL were ionized at pH 6.8 and consequently the degree of ionization increased. Oppositely, the protonation of the dimethylamino groups on EE was lost resulting in a lower degree of interaction and thus allowing partial disintegration (16).
Statistical analysis showed that the difference in the degree of swelling between IPEC tablets and EE-Citrate/EL-Na tablets was insignificant (P value > 0.05) during swelling in the acid stage. Swelling of IPEC tablets increased tremendously in the early stages in the buffer medium and it was significantly more than that of EE-Citrate/EL-Na tablets in the same medium. However, partial erosion and disintegration at later stages were observed with IPEC tablets compared to EE-Citrate/EL-Na tablets.
Drug Release Studies
A hypothesis of possible interaction between anionic and cationic polymers was suggested to control the release of paracetamol from a novel tablet preparation (23) mainly by restricting and regulating the diffusion of the drug from such matrices.
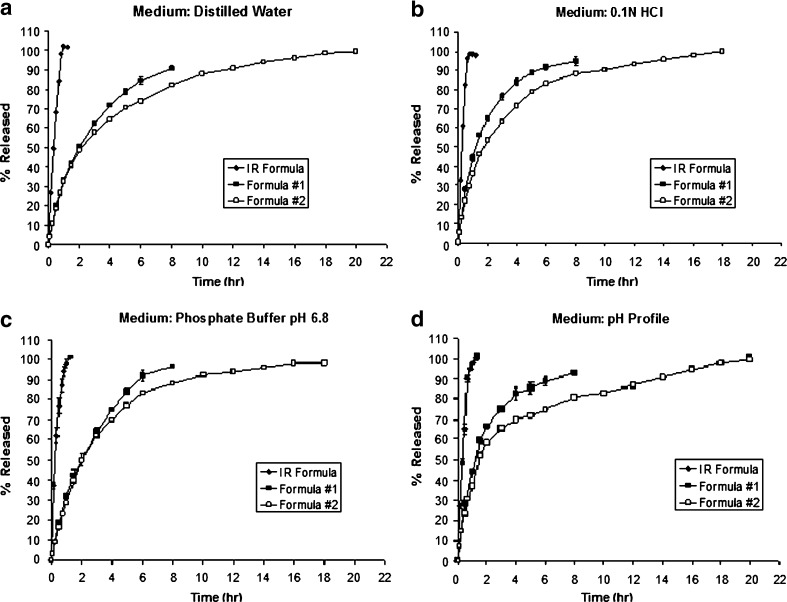
To study the effect of Eudragit polymers on the release of paracetamol from matrix tablets, an immediate release (IR) paracetamol formulation which is similar to formula #1 but devoid from Eudragit polymers has been prepared and analyzed for comparative purposes. The release profiles of paracetamol from the immediate release formula compared to sustained release formulas #1 and #2 are shown in Fig. 3. The release behavior of paracetamol complied with the USP specifications for immediate release paracetamol formulation and did not vary much in the three media because paracetamol is a weak acidic drug with a pKa of 9.51 (24,25); therefore, it was not ionized in an acidic, basic, or in neutral media. The solubility of paracetamol in the acidic medium was similar to that in basic medium, but it was slightly lower in distilled water as mentioned in the literature (about 14 mg/ml in distilled water, 20.2 mg/ml in 0.1 N HCl, and 20.5 mg/ml in phosphate buffer pH 6.8) (21,22). Thus, the release of paracetamol from matrix tablet can be considered to be independent of its solubility.
Fig. 3.
Drug release profiles of immediate release paracetamol formulation compared to the release profile from formula #1 and formula #2 in three different dissolution media (distilled water pH 6.5, 0.1 N HCl pH 1.2, and phosphate buffer pH 6.8) in addition to pH release profile
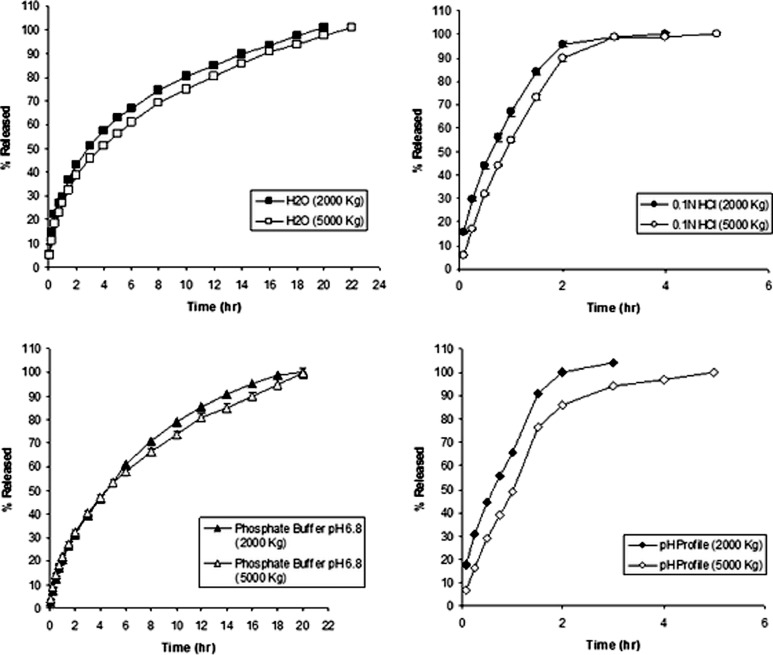
Figure 3 also showed the release profile of paracetamol from matrix tablet prepared by wet granulation method and containing 6.54% w/w of EE-Citrate/EL-Na which is equivalent to 5% w/w of Eudragit polymers in the base form (formula #1). These tablets showed very good control on the paracetamol release profile in the three dissolution media and pH profile even though the amount of Eudragit polymers per tablet was relatively small (less than 10% w/w). This is apparently due to the high degree of interaction that could exist since both polymers were ionized and maximum level charge density was obtained especially in acidic and buffer media. The interaction between the two polymers took place in distilled water dissolution medium because they were present as salts.
As the content of EE-Citrate/EL-Na mixture per tablet was raised from 6.54% to 12.84% (equivalent to 10% w/w of Eudragit polymers in the base form; formula #2), the release profile became more linear as shown in Fig. 4. It was observed that the variability in the release profiles for formula #2 in different dissolution media was kept at minimum levels suggesting the formation of an ionic compound between EE-Citrate and EL-Na that had pH-independent characteristics and provided sustained release effect with highest compressibility.
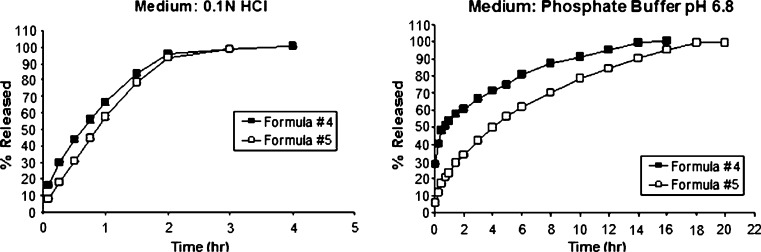
Fig. 4.
Comparison of drug release profiles of paracetamol formula #4 (IPEC 30% w/w direct compression) and formula #5 (IPEC 30% w/w wet granulation) in acidic and basic dissolution media. Both formulations were compressed at 2,000 kg
In order to evaluate the use of an IPEC prepared from EE and EL as a sustained release matrix carrier, a tablet formulation containing 5% w/w of IPEC has been prepared from EE and EL and directly compressed (formula #3). The IPEC tablets at 5% w/w were unable to produce a sustained release effect (paracetamol release completed in 15–30 min) compared to EE-Citrate/EL-Na tablets (data not shown). In addition, tablets containing IPEC showed poor compressibility and required careful handling and storage.
Moustafine et al. (15) investigated the potential of using IPEC to control the release of the model drug ibuprofen. Tablets containing 30% w/w IPEC were used, and the percentage ibuprofen released was less than 1% in acidic medium due to the fact that ibuprofen was not soluble in the acidic medium (pKa 5.2), which reached 50% after 4 h in phosphate buffer pH 6.8.
In the current study, increasing the percentage of IPEC per tablet to 30% w/w (formula #4) resulted in a decrease in the rate of paracetamol release as shown in Fig. 4. The decrease in paracetamol release rates was more pronounced in the buffer medium where only partial disintegration with significant degree of swelling was observed compared to the acidic or distilled water media where rapid and complete disintegration of tablets occurred within 1–2 h. The paracetamol release rates were increased as the compression force was reduced from 5,000 to 2,000 kg. This could be due to the increase in tablet porosities, i.e., the release was compression dependent. Tablet porosities could be evaluated using high-precision density measurements such as a gas pycnometer, if available. The IPEC tablets were much weaker than those containing mixture of EE/EL polymers at similar conditions as shown in Table II.
Table II.
The Mean Dimensions and Hardness Values for Different Tablet Formulas
| Parameter | Formula no. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Diameter (mm) | 13.09 | 0.01 | 13.05 | 0.02 | 13.08 | 0.01 | 13.07 | 0.01 | 13.02 | 0.01 | 13.03 | 0.02 | 13.01 | 0.01 |
| Thickness (mm) | 3.24 | 0.01 | 3.39 | 0.03 | 3.25 | 0.02 | 3.12 | 0.02 | 2.55 | 0.03 | 2.53 | 0.02 | 2.46 | 0.01 |
| Hardness (N) at 2,000 kg | 107 | 5.03 | 150 | 2.52 | 17 | 2.08 | 10 | 3.01 | 29 | 1.53 | 89 | 6.03 | 103 | 2.52 |
| Hardness (N) at 5,000 kga | N/A | N/A | 45 | 5.52 | 15 | 3.21 | 35 | 4.04 | 120 | 1.56 | N/A | |||
aHigh hardness values and suitable release profiles were achieved for formulas #1, #2, and #7 at low 2,000 kg compression force; therefore, it was not necessary to produce tablets at higher compression forces. Higher compression force was applied to subsequent tablet formulas since they showed extremely low strength at lower compression force
When formula #4 was prepared by wet granulation method through the addition of distilled water sufficient to form granules (formula #5), the paracetamol release rates were improved in both acidic and basic media (Fig. 4). This might be due to the improvement of the physical characteristics of tablets such as hardness and compactness in addition to the reduction of the tablet porosities. At the end of dissolution test, a rapid and complete disintegration in acid and pH profile was also observed. No data were available for dissolution in distilled water.
In an attempt to study the behavior of EE/EL polymer mixture as bases under the same condition where IPEC was used, a 30% EE/EL polymer mixture was prepared by direct compression and wet granulation (formulas #6 and #7), and the drug release profiles are shown in Fig. 5 and were compared to those of IPEC. The release rates from formula #6 were significantly reduced in the buffer and distilled water media compared to the release profile in acidic medium and the acidic stage of the pH profile and were also compression independent in all dissolution media due to the high degree of compactness and minimum porosity. Release rate from formula #7 was found to be similar to #6 (data not shown). Error bars, which might be very small and non-apparent in some figures, represent the standard deviation in all the figures in this study.
Fig. 5.
Drug release profiles for paracetamol formula #6 (EE/EL 30% w/w direct compression) in three different media (distilled water pH 6.5, 0.1 N HCl pH 1.2, and phosphate buffer pH 6.8) in addition to pH release profile
Physical Tests
Tablets were compressed using single-press hydraulic Carver Press under the same conditions as shown in Table II to yield tablets of similar thicknesses and therefore sizes. Moreover, the effect of moisture content on the release profile has been eliminated because the values of moisture content for all formulations were set to be less than 1% (data not shown). Formulations #1, #2, #6, and #7 showed better hardness and compactness compared to #3 and #4 due to the better cohesion and ionic interaction between EE and EL in their ionized form.
FTIR Analysis
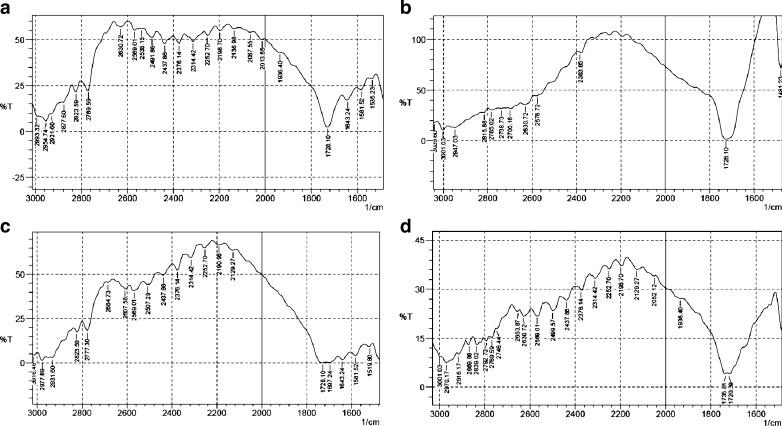
Figure 6a–c shows the FTIR spectra of EE powder (base), EL powder (base), and EE/EL physical mixture as bases in a 1:1 ratio, respectively. Since EE and EL belong to the same class—they are derivatives of methacrylic acid copolymers—the FTIR spectra would exhibit many common features. EE showed a characteristic band at 1,728 cm−1 which corresponds to absorption of ester groups in addition to two more absorption bands at 2,769 and 2,823 cm−1 which corresponds to the optical absorption due to non-ionized dimethylamino groups. On the other hand, the spectrum of EL showed similar but broader absorption band for the non-ionized carboxylic acid groups at 1,728 cm−1 than that found in EE due to the intra- and intermolecular hydrogen bonding between the carboxylic acid groups (26). Moreover, EL also showed a wide absorption range of the associated hydroxyl groups between 2,500 and 3,500 cm−1 but this was not clear from the figure; rather, several minor peaks have been observed in this range. As can be seen from the previous figure, the FTIR spectrum of the physical mixture EE and EL seemed to be a superposition of the spectra of the two polymers and no new peaks were observed suggesting that only minimum, if any, interaction could be found between EE and EL in their powdered base forms. However, a possible ionic interaction between EE-Citrate and EL-Na would be expected to have some impact on the shape of the FTIR spectrum.
Fig. 6.
FTIR spectra of a EE powder (base), b EL powder (base), c EE/EL physical mixture in the base forms at a 1:1 ratio, and d IPEC powder
Figure 6d showed the FTIR spectrum for IPEC powder prepared by precipitation of the complex formed between EE and EL. The spectrum demonstrated the formation of an IPEC similar to those published in the literature. The spectrum showed several new peaks at 1,751 and 1,542 cm−1 that might be assigned to the ionized carboxylate groups and hydrogen bonded carbonyl groups accompanied by the decrease in intensity of band corresponding to the non-ionized carboxylic acid group (15). Further, a decrease in the intensity of the non-ionized dimethylamino group in addition to the formation of weak bands in the range of 2,770–2,830 cm−1 might be due to the interaction of protonated dimethylamino group from EL with carboxylate group from EE.
CONCLUSIONS
Results from the swelling studies strongly supported the formation of ionic interaction between EE-Citrate/EL-Na as seen from the continuous and gradual rise in the degree of swelling for these tablets with much uniform shape and minimum disintegration compared to tablets prepared using IPEC, which showed maximum swelling after 2 h followed by a gradual decline due to significant disintegration that may be attributed to the poor compressibility of IPEC tablets compared to EE-Citrate/EL-Na tablets.
It is evident that a mixture of EE-Citrate/EL-Na in their salt form was effective in controlling the release of paracetamol from tablets with pH-independent characteristics and provided a better sustained release profiles than those prepared using IPEC in all dissolution media especially when they were prepared at lower polymer content (5–12% w/w) with lower variability and better physical properties of tablets.
The results of all experimental tests proved that the combination of EE and EL in their soluble salt form is suitable for formulating matrix tablets basically for neutral, unionized drugs. However, a large number of possible combinations of Eudragit polymers using the various types of salt can be tailored depending on the properties of drugs to be included within their matrix system. It can also be applied to acidic and basic drugs using one salt form to minimize the interaction between the drug and the polymer and to achieve desired release profiles.
It was found that our model drug (paracetamol) was compatible with the excipients investigated in this study, and the dissolution rates were better controlled using the EE-Citrate/EL-Na tablets compared to those from immediate release paracetamol tablets.
References
- 1.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opin Biol Ther. 2004;4(6):881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 2.Rowe RC. Handbook of pharmaceutical excipients. 5. Washington DC: American Pharmacists Association; 2005. [Google Scholar]
- 3.McGinity JW. Aqueous polymeric coatings for pharmaceutical dosage forms. 2. New York: Marcel Dekker; 1997. [Google Scholar]
- 4.Radtke G, Knop K, Lippold BC. Manufacture of slow-release matrix granules by wet granulation with an aqueous dispersion of quaternary poly(meth)acrylates in the fluidized bed. Drug Dev Ind Pharm. 2002;28(10):1295–1302. doi: 10.1081/DDC-120015363. [DOI] [PubMed] [Google Scholar]
- 5.Kidokoro M, Shah NH, Malick AW, Infeld MH, McGinity JW. Properties of tablets containing granulations of ibuprofen and an acrylic copolymer prepared by thermal processes. Pharm Dev Technol. 2001;6(2):263–275. doi: 10.1081/PDT-100002203. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C, Bodmeier R. Incorporation of polymeric nanoparticles into solid dosage forms. J Control Release. 1999;57(2):115–125. doi: 10.1016/S0168-3659(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 7.Leopold CS, Eikeler D. Eudragit E as coating material for the pH-controlled drug release in the topical treatment of inflammatory bowel disease (IBD) J Drug Target. 1998;6(2):85–94. doi: 10.3109/10611869808997884. [DOI] [PubMed] [Google Scholar]
- 8.Nagaprasad V, Sen H, inventors. Controlled drug delivery system for diltiazem. USA patent 6074669:2000.
- 9.Ibric S, Jovanovic M, Djuric Z, Parojcic J, Petrovic SD, Solomun L, et al. Artificial neural networks in the modeling and optimization of aspirin extended release tablets with Eudragit L 100 as matrix substance. AAPS PharmSciTech. 2003;4(1):E9. doi: 10.1208/pt040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takka S, Rajbhandari S, Sakr A. Effect of anionic polymers on the release of propranolol hydrochloride from matrix tablets. Eur J Pharm Biopharm. 2001;52(1):75–82. doi: 10.1016/S0939-6411(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 11.Leopold CS. Coated dosage forms for colon-specific drug delivery. Pharm Sci Tech Today. 1999;2(5):197–204. doi: 10.1016/S1461-5347(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 12.Di Colo G, Falchi S, Zambito Y. In vitro evaluation of a system for pH-controlled peroral delivery of metformin. J Control Release. 2002;80(1–3):119–128. doi: 10.1016/S0168-3659(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Fuentes J, Fernandez-Arevalo M, Gonzalez-Rodriguez ML, Cirri M, Mura P. Development of enteric-coated timed-release matrix tablets for colon targeting. J Drug Target. 2004;12(9–10):607–612. doi: 10.1080/10611860400013501. [DOI] [PubMed] [Google Scholar]
- 14.Dan Y, Wang Q. Homogeneous complex solution formed through interpolyelectrolyte complexation of poly(acrylamide-acrylic acid) with poly(acrylamide-dimethyldiallylammonium chloride) in aqueous media. Polym Int. 2000;49:551–560. doi: 10.1002/1097-0126(200006)49:6<551::AID-PI411>3.0.CO;2-G. [DOI] [Google Scholar]
- 15.Mustafine RI, Van den Mooter G, Kemenova VA. Eudragit E modified by inclusion into an interpolyelectrolyte complex. Pharm Chem J. 2005;39(1):39–42. doi: 10.1007/s11094-005-0076-1. [DOI] [Google Scholar]
- 16.Zaharov IM, Moustafine RI, Kemenova VA. Physicochemical characterization and drug release properties of Eudragit EPO/Eudragit L100-55 interpolyelectrolyte complexes. Eur J Pharm Biopharm. 2006;63:26–36. doi: 10.1016/j.ejpb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Becheran-Maron L, Peniche C, Arguelles-Monal W. Study of the interpolyelectrolyte reaction between chitosan and alginate: influence of alginate composition and chitosan molecular weight. Int J Biol Macromol. 2004;34(1–2):127–133. doi: 10.1016/j.ijbiomac.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Kabanova TV, Zhdanova ER, Moustafine RI. Characterization of Eudragit E100/Carbomer 940P interpolyelectrolyte complexes using swellability measurements. J Control Release. 2006;116(2):e33–e35. doi: 10.1016/j.jconrel.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Moustafine RI, Bobyleva OV. Design of new polymer carriers based of Eudragit E PO/Eudragit L100-55 interpolyelectrolyte complexes using swellability measurements. J Control Release. 2006;116(2):e35–e36. doi: 10.1016/j.jconrel.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Margulis EB, Moustafine RI. Swellability testing of chitosan/Eudragit L100-55 interpolyelectrolyte complexes for colonic drug delivery. J Control Release. 2006;116(2):e36–e37. doi: 10.1016/j.jconrel.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Mustafine RI, Protasova AA, Van den Mooter G, Kemenova VA. Modification of chitosan by inclusion into interpolyelectrolyte complex with Eudragit L. Pharm Chem J. 2006;40(6):325–328. doi: 10.1007/s11094-006-0120-9. [DOI] [Google Scholar]
- 22.Kabanova TV, Mustafine RI. Synthesis and characterization of an interpolyelectrolyte complex based on Eudragit E100 and L100 copolymers. Pharm Chem J. 2004;38(11):625–627. doi: 10.1007/s11094-005-0044-9. [DOI] [Google Scholar]
- 23.Obeidat WM, Abu Znait AH, Sallam AS. Novel combination of anionic and cationic polymethacrylate polymers for sustained release tablet preparation. Drug Dev Ind Pharm. 2008;34(6):650–660. doi: 10.1080/03639040701836578. [DOI] [PubMed] [Google Scholar]
- 24.University of the Sciences in Philadelphia. Analgesics, antipyretics, and anti-inflammatory drugs. In: Remington: The science and practice of pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. p. 939–51.
- 25.The United States Pharmacopoeia USP28/NF23, Rockville-Maryland, USA, 2005.
- 26.Coates J. Interpretation of infrared spectra, a practical approach. In: Meyers RA, editor. Encyclopedia of analytical chemistry. Chichester, USA: John Wiley and Sons; 2000. pp. 10815–10837. [Google Scholar]