Abstract
Sparingly, water-soluble drugs such as candesartan cilexetil offer challenges in developing a drug product with adequate bioavailability. The objective of the present study was to develop and characterize self-microemulsifying drug delivery system (SMEDDS) of candesartan cilexetil for filling into hard gelatin capsules. Solubility of candesartan cilexetil was evaluated in various nonaqueous careers that included oils, surfactants, and cosurfactants. Pseudoternary phase diagrams were constructed to identify the self-microemulsification region. Four self-microemulsifying formulations were prepared using mixtures of oils, surfactants, and cosurfactants in various proportions. The self-microemulsification properties, droplet size, and zeta potential of these formulations were studied upon dilution with water. The optimized liquid SMEDDS formulation was converted into free flowing powder by adsorbing onto a solid carrier for encapsulation. The dissolution characteristics of solid intermediates of SMEDDS filled into hard gelatin capsules was investigated and compared with liquid formulation and commercial formulation to ascertain the impact on self-emulsifying properties following conversion. The results indicated that solid intermediates showed comparable rate and extent of drug dissolution in a discriminating dissolution medium as liquid SMEDDS indicating that the self-emulsifying properties of SMEDDS were unaffected following conversion. Also, the rate and extent of drug dissolution for solid intermediates was significantly higher than commercial tablet formulation. The results from this study demonstrate the potential use of SMEDDS as a means of improving solubility, dissolution, and concomitantly the bioavailability.
Key words: candesartan cilexetil, lipid formulation, medium chain triglycerides, particle size, self-microemulsifying drug delivery system (SMEDDS)
INTRODUCTION
Most of the new drug candidates in development today are sparingly soluble and associated with poor bioavailability (1). There were various formulation strategies reported to address these problems; these include the use of surfactants (S), cyclodextrins, drug nanoparticles, solid dispersions, micronization, lipids, and permeation enhancers (2). Majority of these approaches have resulted in limited success because of the need for specialized equipments, complicated manufacturing process, longer processing time, and regulatory complexity. In recent years, an area that is gaining popularity with formulation scientists is using lipid-based careers to develop self-emulsifying drug delivery systems (SEDDS) to improve oral bioavailability of many lipophilic drugs (3,4).
SMEDDS are isotropic and thermodynamically stable solutions consisting of an oil, surfactant, cosurfactant (CoS; or solubilizer), and drug mixtures that spontaneously forms oil-in-water microemulsions when mixed with water under gentle stirring. The digestive motility of stomach and intestine provides the agitation required for self-emulsification in vivo (5). The advantages of these systems include not only improved drug solubilization but also enhanced release and absorption properties, due to the already dissolved form of drug in formulation and the resulting small droplet size thus providing a large interfacial surface area (6–8). The SEDDS formulation typically produce emulsions with a droplet size between 100 and 300 nm, while SMEDDS form transparent microemulsions with a droplet size that is less than 50 nm. The droplet size of the emulsion is a critical factor in self-emulsification performance because it determines the rate and extent of drug release as well as absorption (9). When compared with emulsions, which are sensitive and metastable dispersed forms, SMEDDS are physically stable formulations that are relatively easy to manufacture. Thus, for lipophilic compounds that have dissolution-limited absorption, these systems offer a significant enhancement in the rate and extent of absorption and concomitantly providing more reproducible blood concentration time profiles (10). The superior performance of self-microemulsifying formulations may be attributed to the following factors: (a) larger surface area provided by the fine emulsion droplets and subsequent lipolysis and formation of mixed micelles, (b) improved diffusion of the fine emulsion droplets: mixed micelles across the unstirred aqueous layer, (c) increased mucosal permeability due to presence of surfactants, and (d) improved lymphatic absorption due to the long-chain oil. It has also been reported that the long-chain oils incorporated in the formulation may promote lipoprotein synthesis and subsequent lymphatic absorption (11).
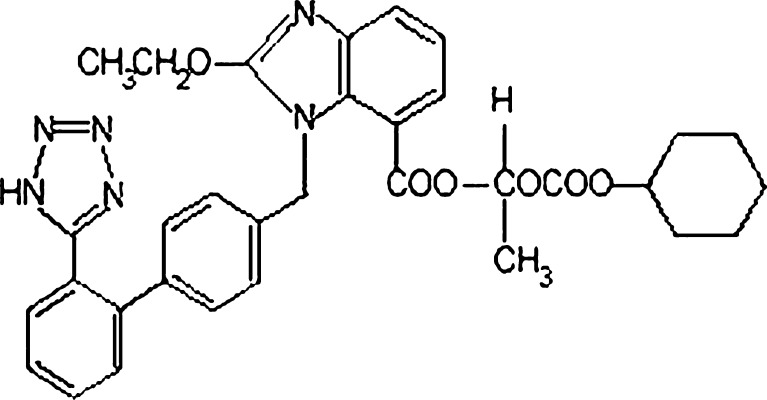
In the present study, an attempt was made to enhance the solubility and in vitro dissolution of candesartan cilexetil by formulating it as SMEDDS for filling into hard gelatin capsules. Candesartan cilexetil is an esterified prodrug of candesartan, a nonpeptide angiotensin II type 1 (AT1) receptor antagonist used in the treatment of hypertension (Fig. 1). Based on its solubility across physiologically relevant pH conditions and absorption characteristics, candesartan cilexetil is classified in the Biopharmaceutics Classification System as a class II drug. Low solubility of candesartan cilexetil across the physiological pH range is reported to result in incomplete absorption from the gastrointestinal (GI) tract and hence is reported to have an oral bioavailability of about 15%. Candesartan cilexetil is a highly lipophilic compound and has good solubility in tri- and diglyceride oils. These factors, therefore, may contribute toward absorption via the lymphatic route. This paper provides the first instance at developing a SMEDDS formulation of candesartan cilexetil using a combination of medium chain triglycerides and polyglycolyzed glycerides as surfactants. The formulation was characterized for its ability to form microemulsions based on droplet size, zeta potential, and dissolution characteristics.
Fig. 1.
Chemical structure of candesartan cilexetil
MATERIAL AND METHODS
Materials
Candesartan cilexetil was procured from Dr. Reddy’s Laboratories Ltd., Hyderabad, India, and medium chain triglyceride oils (Miglyol 810 and 812) were purchased from Sasol (Germany). Macrogolglycerides (Labrasol), Tween 80, Labrafac CC, Labrafil 1944 CS, Labrafil 2125 CS, Lauroglycol 90, and Transcutol were obtained from Gattefosse (Westwood, NJ, USA). Cremophor RH 40 and Cremophor EL were obtained from BASF (Ludwigshafen, Germany). Polyethylene Glycol 400 was obtained from Fisher Scientific (Fair Lawn, NJ, USA).
Excipient Screening—Saturation Solubility Studies
The saturation solubility of candesartan cilexetil was evaluated in various oils, surfactants, and cosurfactants. In this study, an excess amount of candesartan cilexetil (approximately 500 mg) was added to 2 ml of each of vehicle in screw-capped glass vials and the mixture heated to 60°C in a water bath under continuous stirring using a vortex mixture to facilitate drug solubilization. The mixture was kept at ambient temperature for 72 h to attain equilibrium. The equilibrated sample was centrifuged at 2,000 rpm for 10 min to remove the undissolved drug. Aliquots of supernatant were diluted with methanol, and drug content was quantified using an high-performance liquid chromatography (HPLC) technique.
Saturation Solubility of Drug in Mixture of Surfactants and Cosurfactants
The saturation solubility of candesartan cilexetil was evaluated in predetermined ratios of selected surfactant and cosurfactants using the procedure as described earlier in the text.
Pseudoternary Phase Diagram
The pseudoternary phase diagrams of oil, surfactant–cosurfactant, and water were developed to optimize the formulation using a water titration method (12). The mixtures of oil and surfactant–cosurfactant ratios were diluted with water in a dropwise addition. Phase diagrams were constructed in the presence of drug to obtain the optimum concentrations of oil, surfactant, and cosurfactant. SMEDDS form fine oil–water emulsions upon addition to an aqueous media under gentle agitation. Since the free energy required to form an emulsion is very low, the formation is thermodynamically spontaneous. The surfactants used in the formulation form a layer around the emulsion droplets thus reducing the interfacial energy and providing a mechanical barrier to coalesce. The apparent spontaneity of emulsion formation was measured by visual observation wherein a series of SMEDDS formulations were prepared and their self-emulsifying properties observed visually. After identifying the microemulsion region in the phase diagrams, formulations were selected at desired ratio of component based on its ability to form microemulsion.
Design of SMEDDS Formulation
Based on ternary phase diagrams, a series of self-emulsifying systems were prepared with varying concentrations of oil, surfactant, and cosurfactant. For all formulations, the level of candesartan cilexetil was constant (i.e., 11.42%, w/w of the vehicle). The concentration of candesartan cilexetil was maintained at this level taking into account filling a therapeutic dose (16 mg) of drug into a size “2” hard gelatin capsule. At this level, the fill volume of a size “2” capsule can represent a maximum of 16 mg of candesartan cilexetil. The saturation solubility of SMEDDS formulations were evaluated using a procedure as described earlier in the text.
Characterization of SMEDDS
Visual Observations
A visual test to assess the self-emulsification properties reported by Craig et al. (7) was modified and adopted in the present study. In this method, a predetermined volume of formulation (0.2 ml) was introduced into 300 ml of water in a glass beaker that was maintained at 37 °C, and the contents mixed gently using a magnetic stirrer. The tendency to emulsify spontaneously and progress of emulsion droplets were observed. The tendency to form emulsion was judged qualitatively as “good” when droplets spread easily in water and formed a fine transparent emulsion, and it was rated “bad” when there was milky or no emulsion formation with immediate coalescence of oil droplets, especially when stirring was stopped. All the trials were carried out in duplicate, with similar observations being made between repeats.
Determination of Droplet Size and Zeta Potential
The average droplet size and zeta potential of candesartan cilexetil SMEDDS formulations were determined by photon correlation spectroscopy using Malvern Zetasizer (Malvern Instruments, UK). The selected formulations were dispersed in water (diluted 100 times) and placed in an electrophoretic cell for measurement.
Direct Liquid Filling in Hard Gelatin Capsules
The preconcentrates of optimized formulations containing the active were filled into size “2” hard gelatin capsule shells using semiautomatic direct liquid fill in hard gelatin capsule machine (Pam Machinery, Mumbai, India). The fill weight of the capsule formulation is 140 mg ± 10.0% that contained a therapeutic dose (16 mg). The filled capsules were sealed at the junction of body and cap with a gelatin band to prevent potential leaks using a capsule-banding machine (Pam Machinery, Mumbai, India).
Conversion to Solid Intermediates of Self-Microemulsifying Formulation
The optimized liquid SMEDDS formulation was converted into free flowing powder by adsorption of liquid SMEDDS onto solid carriers. The solid carriers used for adsorption comprised of materials that provided a high surface area with good disintegration characteristics. The solid carriers used include microcrystalline cellulose and colloidal silicon dioxide. The carrier’s chosen can adsorb at high levels up to 70% (w/w) (13). The conversion process involved addition of liquid formulation onto carriers under continuous mixing in a blender. The powder was dried and filled directly into capsules or alternately blended with suitable excipients for tabletting.
Solid-State Characterization of Solid SMEDDS
The solid-state properties of candesartan in the solid SMEDDS was investigated using differential scanning calorimetry (DSC) and X-ray powder diffraction (XRPD) techniques since this would influence the in vitro and in vivo dissolution characteristics.
Differential Scanning Calorimetry
Thermal properties of drug, placebo, and solid SMEDDS formulations were investigated using a Perkin-Elmer DSC-7 differential scanning calorimeter/TAC-7 thermal analysis controller with an intracooler-2 cooling system (Perkin-Elmer Instruments, USA). About 3 to 5 mg of product was placed in perforated aluminum sealed 50-μl pans, and the heat runs for each sample was set from 40°C to 200°C at 5°C/min, under an inert environment using nitrogen. The apparatus was calibrated using pure metals like indium with known melting points and heat of fusion (∆Hfusion).
X-Ray Powder Diffraction
XRPD diffractograms of drug, placebo, and solid SMEDDS formulations were recorded using a Panalytical Xpert Pro Diffractometer (PANalytical, The Netherlands) with a Cu line as the source of radiation. Standard runs using a 40-kV voltage, a 40-mA current, and a scanning rate of 0.02° min−1 over a 2θ range of 3–40° were used.
Morphological Analysis of Solid SMEDDS
The outer macroscopic structure of the solid SMEDDS was investigated by scanning electron microscope (SEM; FEI, The Netherlands), operating at 10 kV. The sample was fixed on a SEM stub using double-sided adhesive tape and then coated with thin layer of gold.
Dissolution Studies
The release of drug from liquid SMEDDS formulations, solid intermediates filled in capsules, and commercially available tablet formulation was determined using a US Pharmacopeia type II dissolution apparatus. The dissolution medium consisted of 0.02% Tween 20 in 0.05 M phosphate buffer, pH 6.5, the volume was 900 ml, and temperature of the dissolution medium was maintained at 37°C with the paddle operated at 50 rpm. An aliquot of 10 ml was withdrawn at predetermined intervals 5, 10, 15, 30, 45, and 60 min and filtered through 0.45-µm pore size membrane filters (Millipore, Bangalore, India). The amount of drug dissolved was determined using an HPLC method.
HPLC Analysis
The HPLC analysis was carried out using Waters Alliance high-performance liquid chromatography system (Agilent Technologies, USA). Chromatographic separation was accomplished using an Inertsil ODS-3, C18, 250 × 4.6-mm, 5-μm stainless steel column (Agilent Technologies, USA). The mobile phase consisted of a mixture of buffer (0.02 M monobasic potassium phosphate), acetonitrile, and triethylamine in the ratio of 40:60:0.2, with pH adjusted to 6.0 using phosphoric acid. The mobile phase was pumped isocratically at a flow rate of 2.0 ml/min during analysis, and the column temperature was maintained at 25°C. The amount of drug dissolved at each sampling time point was estimated using a UV wavelength of 254 nm.
RESULTS AND DISCUSSION
Excipient Screening—Solubility Studies
The solubility of candesartan cilexetil in various surfactants and oils are summarized in Table I. The drug substance showed good solubility in surfactants—Tween 80, Labrasol, Transcutol P, and Cremophor EL. Among oils, Miglyol 812 was the only oil in which the drug showed the desired solubility for dosage development.
Table I.
Solubility of Drug in Oils, Surfactants, and Cosurfactants
| Vehicle | Chemical composition | Solubility (mg/g), mean ± SD |
|---|---|---|
| Miglyol 810 | Caprylic/capric triglycerides | 1.16 ± 0.72 |
| Miglyol 812 | Caprylic/capric triglycerides | 2.50 ± 1.95 |
| Labrafac CC | Caprylic/capric triglycerides | 1.85 ± 0.46 |
| Labrafil 1944 CS | Oleoyl macrogolglycerides | 10.41 ± 0.95 |
| Lauroglycol 90 | Propylene glycol monolaurate | 76.51 ± 6.42 |
| Cremophor EL | Polyethoxylated castor oil | 106.26 ± 12.24 |
| Cremophor RH 40 | Polyoxyl 40 hydrogenated castor oil | 96.85 ± 1.12 |
| Transcutol P | Diethylene glycol monoethyl ether | 161.11 ± 12.18 |
| PEG 400 | Polyethylene glycol 400 | 105.94 ± 1.24 |
| Labrasol | Caprylocaproyl macrogolglycerides | 164.12 ± 1.56 |
| Tween 80 | Polysorbate 80/polyoxyethylene (20) sorbitan monooleate | 259.6 ± 22.64 |
| Labrafil 2125 CS | Linoleoyl macrogolglycerides | 12.14 ± 0.42 |
Solubility of drug substance in lipid carriers is a key criterion for selection of components for developing a SMEDDS formulation. The maximum amount that can be filled into a size “2” capsule is ∼300–310 μl containing the therapeutic dose; hence, the solubility of drug in these careers is critical to develop a capsule dosage form for in vivo studies.
Based on drug solubility and compatibility with hard gelatin capsule shell, Cremophor EL and Tween 80 were selected as surfactants, and Labrasol was selected as cosurfactant for further development. The components selected are miscible with each other and form a homogenous mixture with medium chain fatty acid glycerides (Miglyol). It is well established that medium chain fatty acids influence the tight junctions of the epithelial cells and long-chain fatty acids stimulate the lipoprotein synthesis and subsequent lymphatic absorption and thus enhancing drug bioavailability (14).
Solubility of Drug in Mixture of Surfactants
The solubility of candesartan cilexetil in different binary combinations of selected surfactants and cosurfactant (1:1, 1:2, and 2:1) is summarized in Table II. The solubility data were used to estimate the drug loading capacity to develop a dosage form for capsule filling.
Table II.
Solubility of Drug in Mixture of Surfactants–Cosurfactants
| Combinations | Mixture of surfactant and cosurfactant | Solubility (mg/g) | ||
|---|---|---|---|---|
| 1:2 | 1:1 | 2:1 | ||
| 1 | Cremophor EL and Labrasol | 166.48 | 157.11 | 177.14 |
| 2 | Tween 80 and Labrasol | 236.24 | 215.10 | 231.23 |
| 3 | Cremophor EL and Transcutol P | 172.12 | 166.82 | 85.18 |
| 4 | Tween 80 and Transcutol P | 172.56 | 110.25 | 82.24 |
Pseudoternary Phase Diagram Study
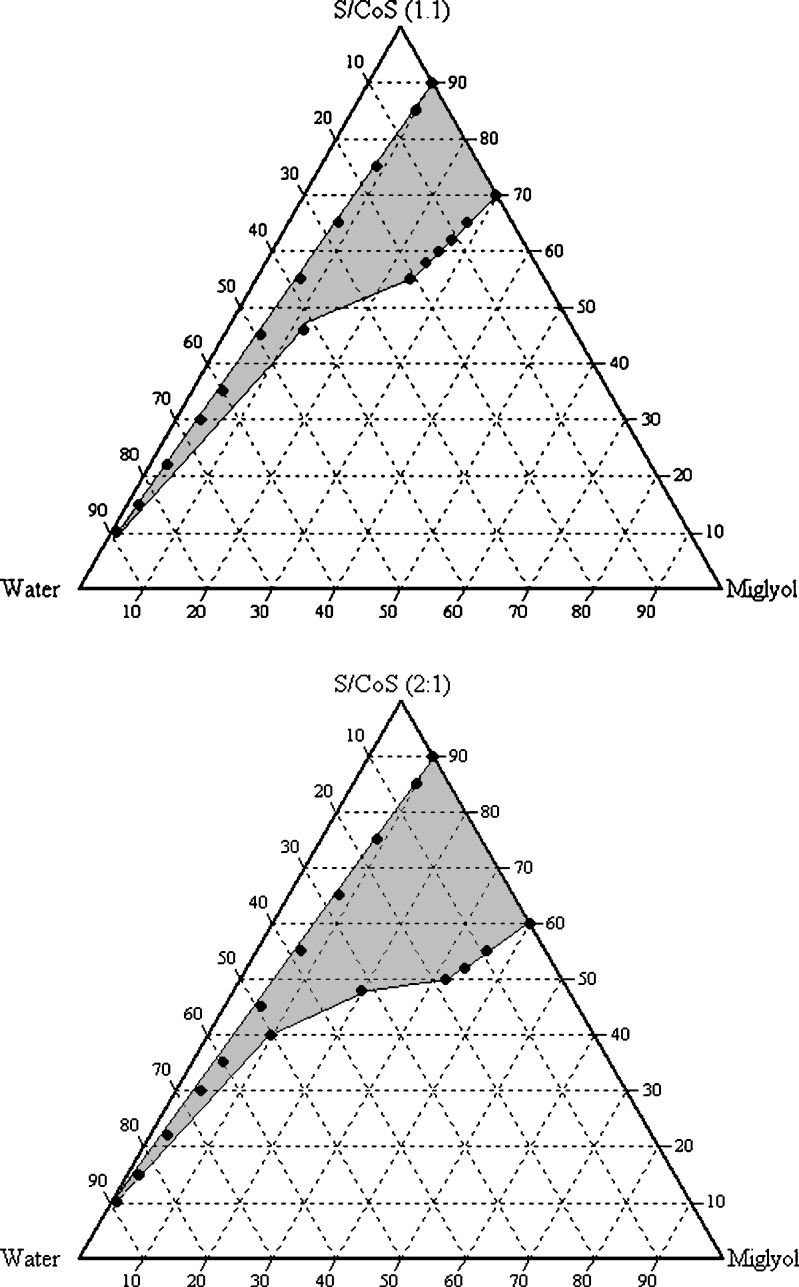
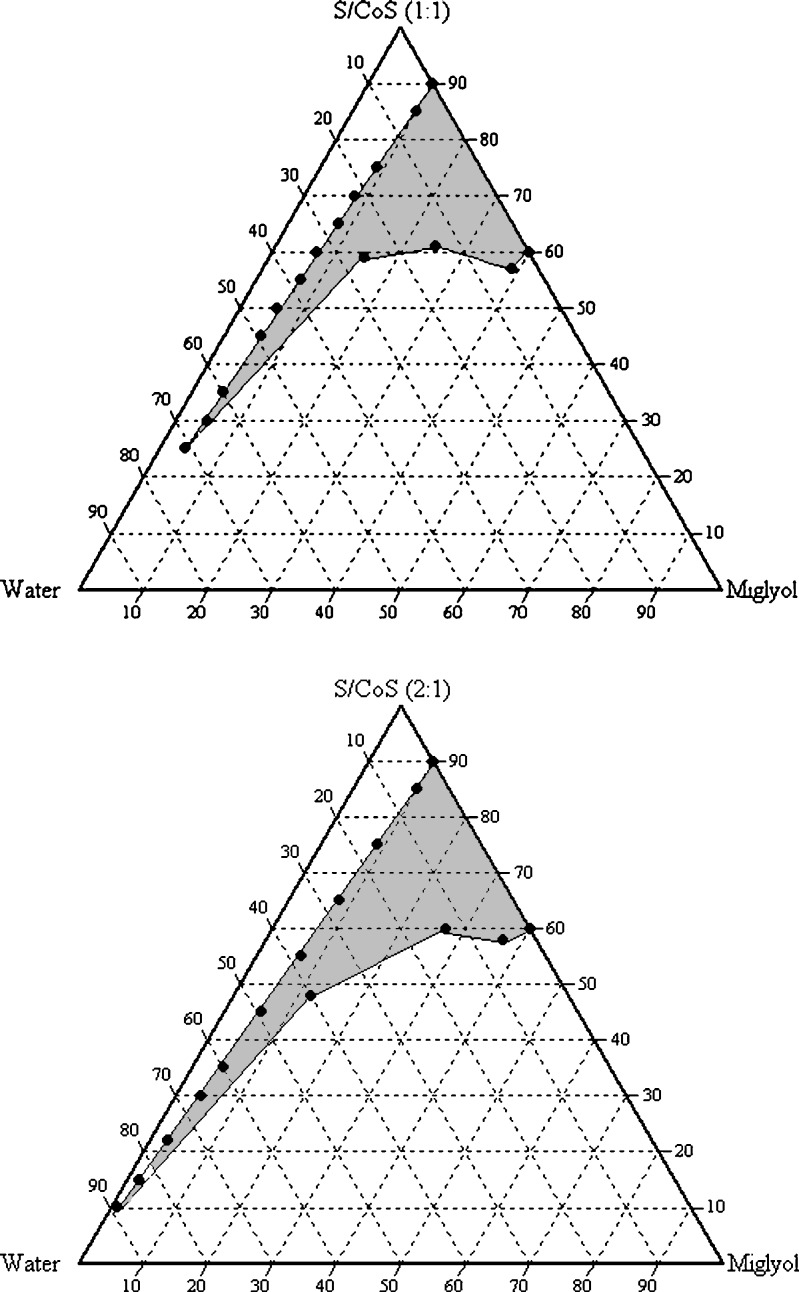
The solubility of drug substance in binary mixtures, one containing Miglyol 812, Tween 80, and Labrasol and other containing Miglyol 812, Cremophor EL, and Labrasol, selected for construction of phase diagram is summarized in Table III. Each of these combinations contained 11.42% (w/w) candesartan cilexetil. Phase diagrams at a specific ratio of surfactant/cosurfactant (1:1, 1:2, and 2:1) were constructed for each combination. The phase boundary was determined by visual observation (spontaneity of emulsification and clarity). The prototype formula compositions were selected within the microemulsion region of phase diagrams. The phase diagrams for combinations 1 and 2 are shown in Figs. 2 and 3, respectively (since the phase diagrams of 1:1 and 1:2 ratios are quite similar, only 1:1 and 2:1 ratios of the combinations were presented).
Table III.
Lipid Excipient Combinations Selected for Construction of Phase Diagram
| Excipients | Combination 1 | Combination 2 |
|---|---|---|
| Oil | Miglyol 812 | Miglyol 812 |
| Surfactant | Cremophor EL | Tween 80 |
| Cosurfactant | Labrasol | Labrasol |
Fig. 2.
Pseudoternary phase diagram with following components: oil (Miglyol), surfactant–cosurfactant (Labrasol–Cremophor EL 1:1 and 2:1), and water. The gray area indicates the microemulsion region
Fig. 3.
Pseudoternary phase diagram with following components: oil (Miglyol), surfactant–cosurfactant (Labrasol–Tween 80 1:1 and 2:1), and water. The gray area indicates the microemulsion region
In combination 1, when Miglyol 812, Tween 80, and Labrasol are mixed at 2:1 (weight ratio), the hydrophilic lipophilic balance value of the surfactants combination is ∼14.5. The microemulsion examined by photon correlation spectroscopy revealed that the volume-based diameters of particles were in range of 4–10 nm. Based on these results, it is feasible that a stable and transparent microemulsion with particle size below 50 nm can be obtained, indicating that the efficiency of emulsification was good when the S/CoS concentration was more than 40% of SMEDDS formulation. It was observed that increasing the concentration of surfactant such as Labrasol in SMEDDS formulation increased the spontaneity of the self-emulsification process.
In combination II consisting of Miglyol 812, Cremophor EL, and Labrasol at 2:1 (weight ratio), the hydrophilic lipophilic balance value of the surfactants combination is ∼14.6; at this ratio, a decrease in mean effective droplet diameters from 19.6 to 14.5 nm was observed when the concentration of cosurfactant was ∼30%. Therefore, a ratio of S/CoS = 2:1 was selected for prototype development. When a cosurfactant is added (in addition to surfactant) to the system, it lowers the interfacial tension, fluidizes the hydrocarbon region of the interfacial film, and decreases the bending stress of interface (15).
Design of SMEDDS Formulation
The formula compositions of optimized SMEDDS formulations are summarized in Table IV. The efficiency of self-emulsification was assessed by adding the unit dose of the resulting formulation in 250 ml water under gentle agitation. The lipid formulations were assessed visually based on their rate of self-emulsification and emulsion clarity. The saturation solubility after formulating as SMEDDS is summarized in Table V. The results show higher solubility of drug after formulating into SMEDDS.
Table IV.
Formula Compositions of Optimized SMEDDS Formulations
| Component | Formulation A (mg) | Formulation B (mg) | Formulation C (mg) | Formulation D (mg) |
|---|---|---|---|---|
| Candesartan cilexetil | 16.00 | 16.00 | 16.00 | 16.00 |
| Tween 80 | – | 75.00 | – | 80.00 |
| Cremophor EL | 75.00 | – | 80.00 | – |
| Labrasol | 37.00 | 37.00 | 27.00 | 27.00 |
| Miglyol 812 | 12.00 | 12.00 | 17.00 | 17.00 |
Table V.
Saturation Solubility of SMEDDS Formulations
| Formulation Code | Solubility (mg/g) |
|---|---|
| A | 208 ± 2.79 |
| B | 256 ± 4.24 |
| C | 198 ± 6.12 |
| D | 230 ± 3.95 |
Droplet Size Analysis
The effect of formula composition of SMEDDS on droplet size distribution is shown in Table VI. The mean droplet size was relatively smaller for SMEDDS formulations containing Miglyol 812 as oil phase, Tween 80 as surfactant, and Labrasol as cosurfactant than the other formulations. Based on the formation of a stable microemulsion upon exposure to water, the optimized formulations (forms A, B, C, and D) were developed. The mean droplet size increased in formulation D when the concentration of oil was increased from 8.9 to 12.1% (w/w). This may be attributed to the amount of undissolved drug in the formulation.
Table VI.
The Droplet Size and Zeta Potential of Optimized Microemulsion Formulations
| S. no | Formulation code | Droplet size (nm) | Zeta potential (mV) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d10 | d50 | d90 | Average | I | II | III | Average | ||
| 1 | A | 15.05 | 15.25 | 15.30 | 15.2 ± 0.13 | 62 | 68 | 65 | 65 ± 3.00 |
| 2 | B | 14.31 | 14.45 | 14.15 | 14.3 ± 0.15 | 132 | 119 | 109 | 120 ± 11.53 |
| 3 | C | 17.13 | 16.59 | 15.78 | 16.5 ± 0.68 | 51 | 60 | 54 | 55 ± 4.58 |
| 4 | D | 18.65 | 21.42 | 17.83 | 19.3 ± 1.88 | 147 | 126 | 132 | 135 ± 10.81 |
Zeta Potential Analysis
The results from zeta potential analyses of all four formulations are summarized in Table VI. Electrostatic forces of microemulsion droplets are critical for assessing the stability of the SMEDDS formulation. An increase in the electrostatic repulsive forces between microemulsion droplets prevents the coalescence of microemulsion droplets, and a decrease of electrostatic repulsive forces will result in phase separation. The formulations B and D consisting of Tween 80 showed higher zeta potential compared to formulations A and C which could be attributed due to high affinity of Tween 80 toward the surface of drug offering potential electrical double layer. All the formulations evaluated showed positive potential. Several studies have reported that the zeta potential played an important role in the interactions of formulation components with mucus of the gastrointestinal tract (16). The observations in these report suggest that a positively charged droplet could result in better interaction with the mucosal surface of GI tract because the intestinal cell interior carries a negative charge in the presence of mucosal fluid. Since the emulsified droplets produced by SMEDDS herein have a positive potential, they are likely to facilitate intestinal absorption of drug resulting in enhanced bioavailability.
Solid-State Characterization of Solid SMEDDS
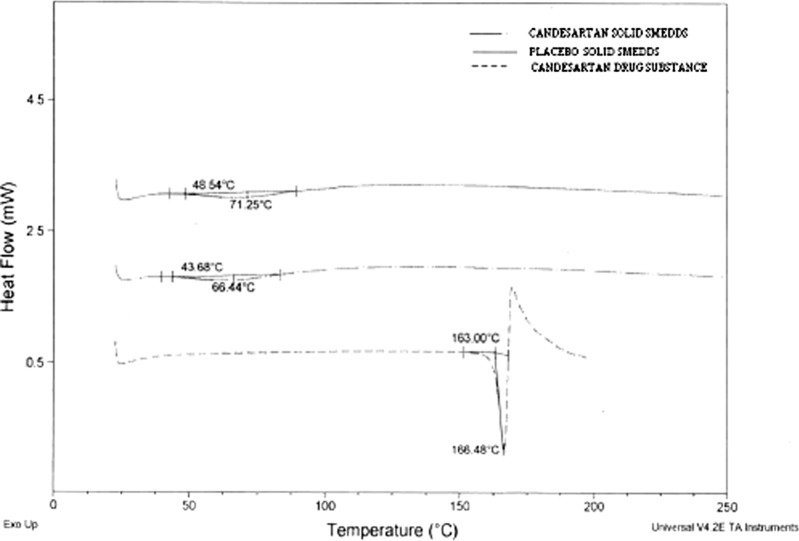
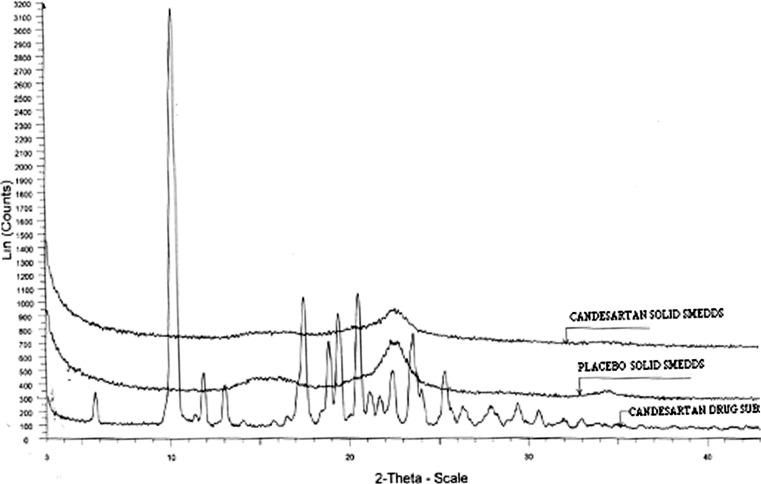
The DSC thermograms of pure candesartan cilexetil, placebo, and solid SMEDDS formulation are shown in Fig. 4. Pure drug substance showed sharp endothermic peaks at 166°C indicating that the drug is highly crystalline. The absence of drug peaks in the solid SMEDDS formulation indicates change in the melting behavior of drug and inhibition of crystallization following granulation using lipid surfactants and granulating materials.
Fig. 4.
DSC curves of candesartan drug substance, placebo and solid SMEDDS
The X-ray diffractograms of pure drug, placebo, and solid SMEDDS formulations are shown in Fig. 5. The results show absence of obvious peaks representing crystals of candesartan in solid SMEDDS indicating that the drug was in amorphous or disordered crystalline phase in the lipid matrix.
Fig. 5.
X-ray powder diffractometry of candesartan drug substance, placebo, and solid SMEDDS
Morphological Analysis of Solid SMEDDS
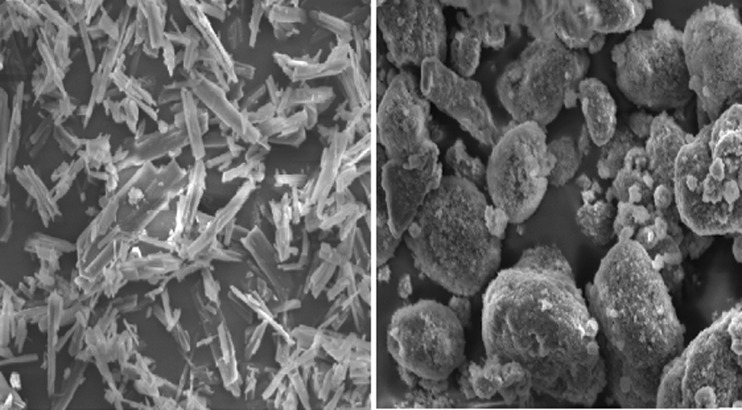
The SEM images of pure candesartan and solid SMEDDS formulations are shown in Fig. 6. The SEM images of solid SMEDDS show well-separated particles with no agglomeration.
Fig. 6.
SEM images of pure drug substance (left), solid SMEDDS (right; magnification ×5,000; scale = 50.0 μm)
In Vitro Dissolution Study
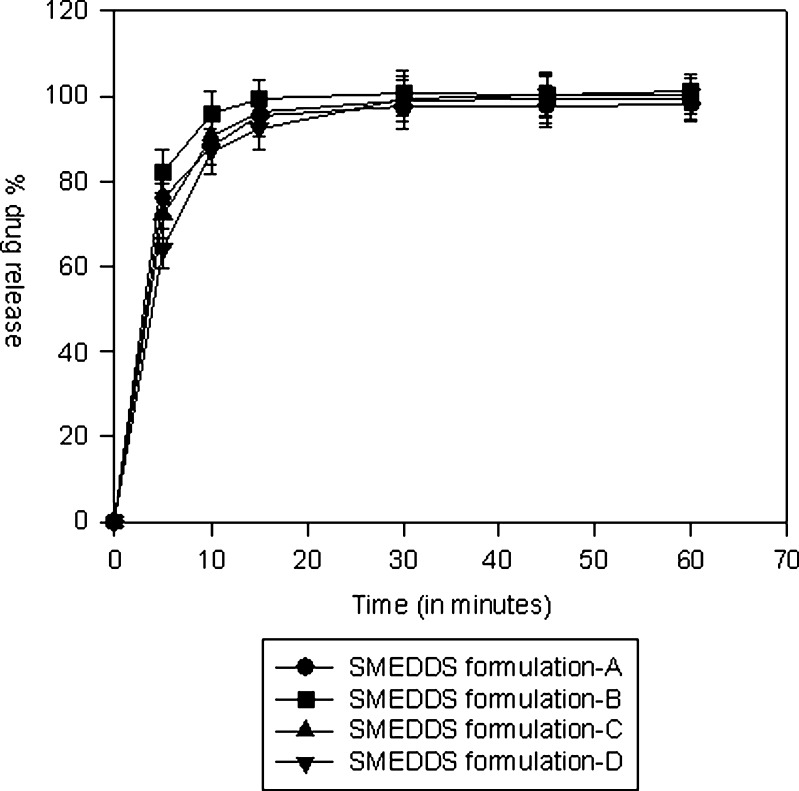
The in vitro dissolution comparisons of SMEDDS formulations in a discriminating dissolution medium (0.02% Tween 20 in 0.05 M phosphate buffer, pH 6.5) are shown in Fig. 7. In the dissolution media, 0.02% of Tween 20 was added since it provided better discrimination between the formulations. The faster dissolution from SMEDDS may be attributed to the fact that in this formulation, the drug is a solubilized form and upon exposure to dissolution medium results in small droplets that can dissolve rapidly in the dissolution medium. The release from liquid SMEDDS formulation B (mean droplet size 14.3 nm) was faster than SMEDDS formulation D (mean droplet size 19.3 nm) indicating influence of droplet size on the rate of drug dissolution.
Fig. 7.
In vitro dissolution comparisons of SMEDDS formulations in a discriminating dissolution medium
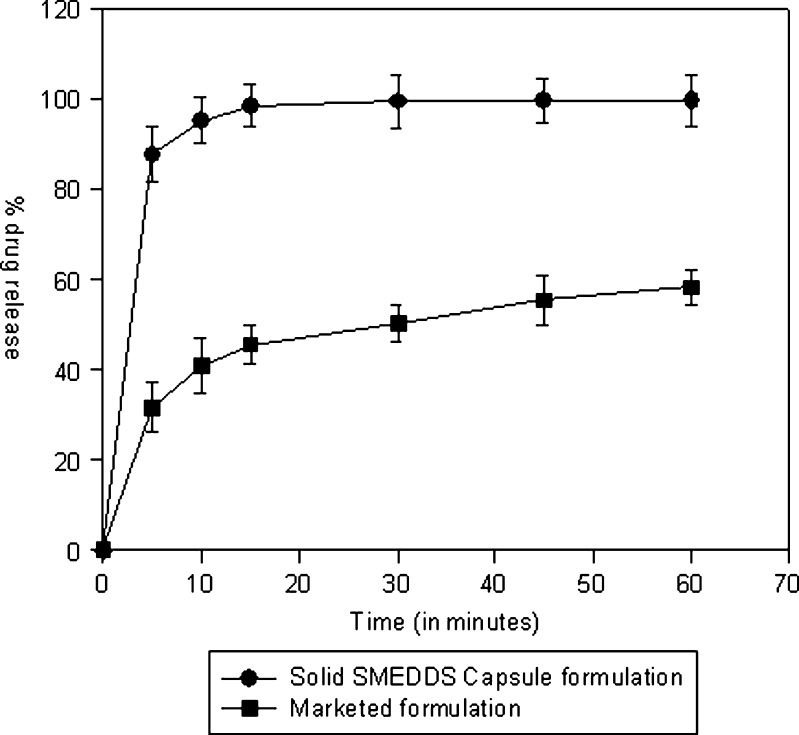
Formula composition of the selected solid SMEDDS formulation is shown in Table VII. The drug dissolution from solid intermediates of SMEDDS filled in hard gelatin capsules and commercially available tablet formulation of candesartan in a discriminating dissolution medium (phosphate buffer, pH 6.5) is summarized in Table VIII. The commercial tablet formulation Candelong® consists of candesartan cilexetil, hydroxypropyl cellulose, polyethylene glycol, lactose, corn starch, carboxymethylcellulose calcium, magnesium stearate, and ferric oxide. The drug dissolution from solid intermediates in capsules was comparable to liquid SMEDDS and significantly higher than the commercial product (Fig. 8), indicating that the self-emulsifying properties of SMEDDS were unaffected following conversion. The similarity factor (F2) calculated on the dissolution data of marketed formulation and self-emulsifying formulation is 15 which indicate that the marketed formulation is dissimilar to self-emulsifying formulation.
Table VII.
Formula Composition of Solid SMEDDS Formulation
| Component | Formula per unit dose (mg) |
|---|---|
| Candesartan cilexetil | 16.00 |
| Tween 80 | 75.00 |
| Labrasol | 37.00 |
| Miglyol 812 | 12.00 |
| Colloidal silicon dioxide | 100.00 |
| Microcrystalline cellulose | 200.00 |
| Croscarmellose sodium | 10.00 |
| Total fill weight per capsule | 450.00 |
Table VIII.
In Vitro Dissolution of Commercial Formulation and Solid Intermediates of SMEDDS Filled in Capsules
| Time (min) | Cumulative % drug dissolved | |
|---|---|---|
| Marketed product | Solid intermediates of SMEDDS in capsules | |
| 5 | 31.54 | 87.70 |
| 10 | 40.80 | 95.05 |
| 15 | 45.49 | 98.40 |
| 30 | 50.17 | 99.42 |
| 45 | 55.33 | 99.55 |
| 60 | 58.17 | 99.58 |
Dissolution medium: pH 6.5, phosphate buffer containing 0.02% Tween 20
Fig. 8.
Comparative dissolution profile of candesartan cilexetil commercial formulation and solid intermediates of SMEDDS filled in capsules
CONCLUSION
A SMEDDS formulation of a poorly water-soluble drug, candesartan cilexetil was formulated for direct filling into hard gelatin capsules for oral administration. The formula composition of SMEDDS for capsule filling was obtained based on solubility evaluation, pseudoternary phase diagram, and droplet size analysis. The optimized formulation showed rapid self-microemulsification in an aqueous media. The SMEDDS formulation converted into solid intermediates for capsule filling showed faster rate of drug release than the marketed product in a discriminating dissolution media. The results from this study demonstrate the utility of SMEDDS to enhance solubility and dissolution of sparingly soluble compounds like candesartan which may result in improved therapeutic performance.
References
- 1.Robinson JR. Introduction: semi-solid formulations for oral drug delivery. Bull Tech Gattefosse. 1996;89:11–13. [Google Scholar]
- 2.Aungst BJ. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci. 1993;82:979–987. doi: 10.1002/jps.2600821002. [DOI] [PubMed] [Google Scholar]
- 3.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 4.Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Del Rev. 1997;25:47–58. doi: 10.1016/S0169-409X(96)00490-5. [DOI] [Google Scholar]
- 5.Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106:15–23. doi: 10.1016/0378-5173(94)90271-2. [DOI] [Google Scholar]
- 6.Farah N, Laforet JP, Denis J. Bull Tech Gattefosse. 1994;87:41–47. [Google Scholar]
- 7.Craig DQM, Barket SA, Banning D, Booth SW. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequencies dielectric spectroscopy. Int J Pharm. 1995;114:103–110. doi: 10.1016/0378-5173(94)00222-Q. [DOI] [Google Scholar]
- 8.Gershanik T, Benita S. Self-dispersion lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50:179–188. doi: 10.1016/S0939-6411(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 9.Tarr BD, Yalkowsky SH. Enhanced intestinal absorption of cyclosporine in rats through the reduction of emulsion droplet size. Pharm Res. 1989;6:40–43. doi: 10.1023/A:1015843517762. [DOI] [PubMed] [Google Scholar]
- 10.Neslihan GR, Simon B. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Charman WN, Stella VJ. Transport of lipophilic molecules by the intestinal lymphatic system. Adv Drug Del Rev. 1991;7:1–14. doi: 10.1016/0169-409X(91)90046-F. [DOI] [Google Scholar]
- 12.Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63:288–294. doi: 10.1016/j.ejpb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, et al. Oral solid gentamicin preparation using emulsifier and adsorbent. J Control Release. 2005;105:23–31. doi: 10.1016/j.jconrel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Lindmark T, Nikkila T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 monolayers. J Pharmacol Exp Ther. 1995;275:958–964. [PubMed] [Google Scholar]
- 15.Eccleston GM. Microemulsions encyclopedia of pharmaceutical technology, vol 9. New York: Marcel Dekker; 1992. pp. 375–421. [Google Scholar]
- 16.Wei LL, Sun PN, Nie SF, Pan WS. Preparation and evaluation of SEDDS and SMEDDS containing Carvedilol. Drug Dev Ind Pharm. 2005;31:785–794. doi: 10.1080/03639040500216428. [DOI] [PubMed] [Google Scholar]