Abstract
The studies reported in this work are aimed to elucidate the ternary inclusion complex formation of gemfibrozil (GFZ), a poorly water-soluble drug, with β-cyclodextrin (β-CD) with the aid of auxiliary substances like different grades of povidone(s) (viz. PVP K-29/32, PVP K-40, Plasdone S-630, and Polyplasdone XL), organic base (viz. triethanolamine), and metal ion (viz. MgCl2·6H2O), by investigating their interactions in solution and solid state. Phase solubility studies were carried out to evaluate the solubilizing power of β-cyclodextrin, in association with various auxiliary substances, to determine the apparent stability constant (KC) and complexation efficiency (CE) of complexes. Improvement in KC values for ternary complexes clearly proves the benefit of the addition of auxiliary substances to promote CE. Of all the approaches used, the use of polymer Plasdone S-630 was found to be the most promising approach in terms of optimum CE and KC. GFZ–β-CD (1:1) binary and ternary systems were prepared by kneading and lyophilization methods. The ternary systems clearly signified superiority over binary systems in terms of CE, solubility, KC, and reduction in the formulation bulk. Optimized ternary system of GFZ–β-CD–Plasdone S-630 prepared by using lyophilization method indicated a significant improvement in intrinsic dissolution rate when compared with ternary kneaded system. Differential scanning calorimetry, X-ray diffraction, Fourier transform infrared, scanning electron microscopy, and proton nuclear magnetic resonance were carried out to characterize the binary and optimized ternary complex. The results suggested the formation of new solid phases, eliciting strong evidences of ternary inclusion complex formation between GFZ, β-CD, and Plasdone S-630, particularly for lyophilized products.
Key words: auxiliary substances, complexation efficiency, cyclodextrins, gemfibrozil, intrinsic dissolution rate, stability constant
INTRODUCTION
Complexation with cyclodextrin (CD) is known as an effective method for enhancing dissolution properties and bioavailability of poorly soluble drugs. CDs are capable of forming inclusion complexes with many drugs by including a whole drug molecule or only some nonpolar part of it inside their cavity. In an aqueous solution, the complexes are readily dissociated, and free drug molecules are in relatively rapid dynamic equilibrium with drug molecules bound within the CD cavity (1,2). These noncovalent complexes show new physicochemical characteristics when compared with the guest molecules. They include better stability, increased bioavailability, and less undesirable side effects (3–5). In general, complexation efficiency of cyclodextrins is rather low, and thus, relatively large amounts of cyclodextrins are needed to complex small amounts of drug. Therefore, in cases where low complexation efficiency (CE) would require larger amounts of CD than is acceptable for solid dosage forms, the enhancement of the complexation efficiency of the chosen CD is of practical importance. Several approaches which can be applied in order to enhance the complexation efficiency were evaluated. Moreover, several factors including toxicity, cost and dosage, and the amount of CD in the dosage form has to be reduced as far as possible.
Gemfibrozil [5-(2, 5-dimethylphenoxy)-2, 2-dimethylpentanoic acid], an antihyperlipidemic drug and also a lipid and cholesterol modifying drug belonging to BCS Class Π, was choosen as a model drug. It is used in the treatment of people with very high serum triglycerides who are at a risk of developing pancreatitis and can also be used to reduce the risk of coronary heart disease (6). The major drawback of gemfibrozil (GFZ) is its poor aqueous solubility. The enhancement of solubility and dissolution rate of GFZ through complexation with β-cyclodextrin (β-CD) has already been reported (7).
Investigators (3,8,9) have shown that various methods can be employed to enhance the complexation efficiency of CDs namely by drug ionization, salt formation, cosolvents, ion pairing, and by the combination of two or more methods. In all the cases, a synergistic effect on the solubilizing power of cyclodextrins was seen. In the present work, the effect of auxiliary substances on the complexation and dissolution rate of GFZ with β-CD was investigated. The first approach was the addition of water-soluble polymers like polyvinyl pyrrolidones (PVP) with the aim to increase the solubility of both the complex and the drug itself. The second approach was the use of a basic ternary compound, for instance, triethanolamine (TEA), which promotes the solubilization of the guest molecule both by forming a salt and by increasing the stability constant of the complex. In the third approach, metal salts were added, as the combined use of Mg ions and β-CD had a synergic effect on the solubility of the drug. In the present study, the possibility of improving inclusion efficiency of β-cyclodextrin in the presence of some polymers, metal ion, and organic base were investigated using gemfibrozil as a model drug.
MATERIALS AND METHODS
Materials
Gemfibrozil was obtained as a gift sample from Torrent Pharmaceuticals Ltd., Indrad (Gujrat). Pharmaceutical grade (CAVAMAX W7), Polyplasdone XL, Plasdone S-630, and PVP K-29/32 were supplied as gift samples from ISP Technologies Inc. (Wayne, NJ). PVP K-40 was purchased from Thomas Baker Chemicals Ltd., Mumbai. All other materials used were of analytical grade.
Phase Solubility Studies
Excess amount of drug was added to 10 mL of double-distilled (DD) water containing increasing concentration of β-CD (0–15 mM) with or without fixed auxiliary substance concentration. The concentrations were selected on the basis of preliminary studies carried out between GFZ and various auxiliary substances. The optimized concentrations were 0.2% (w/v) for Polyplasdone XL, Plasdone S-630, and PVP K-29/32; 0.1% (w/v) for PVP K-40; 1.5% (w/v) for MgCl2·6H2O; and 6.25% (w/v) for TEA (the studies on TEA were done in phosphate buffer pH 6.80). For binary systems, suspensions in the glass containers were sealed and mechanically stirred until reaching equilibrium (about 72 h), while in case of ternary systems suspensions in the glass containers were sonicated on an ultrasonic bath at 60°C for 1 h, allowed to equilibrate for 72 h, and analyzed spectrophotometrically at 274 nm.
Apparent stability constant and complexation efficiency were estimated from the straight line obtained from the phase solubility diagram using the following equation (10–12).
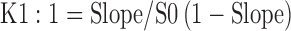 |
1 |
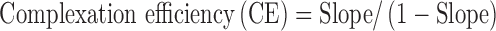 |
2 |
Preparation of GFZ–β-CD Binary and Ternary Systems
Kneading and lyophilization techniques were used to obtain GFZ–β-CD solid complexes. Physical binary and ternary mixtures were also prepared for further comparison.
Kneaded Binary and Ternary Products
Solid inclusion complexes of GFZ–β-CD were prepared in a 1:1 M ratio by kneading method with and without addition of optimized concentration of different auxiliary substances. The different systems were kneaded for 45 min and then dried in a microwave at 320 W for 4 min. The dried mass was finally ground and sifted through a 100-mesh sieve (13).
Lyophilized Binary and Optimized Ternary Products
Freeze-dried product for binary systems were prepared by dissolving an equimolar mixture of gemfibrozil and cyclodextrin in double-distilled water and shaken for 24 h. Ammonia solution (25% v/v) was added to it dropwise till a clear solution was obtained. The solution was frozen overnight in Petri dishes at −45°C, lyophilized in a freeze drier at −45°C for 48 h, and subsequently lyophilized in a freeze drier for 72 h. For ternary products, equimolar amounts of β-CD and GFZ were dissolved in optimized concentration of auxiliary substance. The resulting solution was mixed and sonicated for 1 h and then allowed to equilibrate for 72 h at room temperature; the clear solution was frozen at −45°C and subsequently lyophilized in a freeze drier for 72 h (14) (Heto Dry Winner DW 1, Denmark).
Intrinsic Dissolution Rate for Binary, Ternary, and Ternary Optimized Complex
Intrinsic dissolution behavior for binary, ternary, and ternary optimized complex were investigated according to USP apparatus I, the rotating disk method. All the prepared binary, ternary, and optimized ternary complex were dissolved in 1 L of three different dissolution media of 0.1 N HCl (pH 1.25), double-distilled water (pH 6.80), and phosphate buffer (pH 7.50), which were stirred at 100 rpm and maintained at 37°C. At fixed time intervals, 5-mL aliquots were withdrawn and analyzed spectrophotometrically at 274 nm. The dissolution runs were done in triplicate, and the intrinsic dissolution rate (IDR) was determined (15).
DETECTION OF INCLUSION COMPLEXATION IN SOLID STATE OF BINARY AND TERNARY OPTIMIZED COMPLEXES
Differential Scanning Calorimetry
Differential scanning calorimetry (Perkin Elmer DSC6) measurements were conducted on 5 mg samples at a heating rate of 10°C/min over a 50–250°C temperature range. A nitrogen purge (20 ml/min) was maintained throughout the runs, using an empty sealed pan as a reference. Temperature and heat flow calibrations were performed using indium as a standard (8).
Scanning Electron Microscopy
The photomicrographs of the systems were obtained by scanning electron microscope (Zeiss EVO 50, UK). Samples were previously coated with silver, and obtained photomicrographs were examined at a magnification ratio of ×4,000.
Powder X-Ray Diffractometry
Powder X-ray diffraction patterns were obtained with an X-ray diffractometer (PW3040/60 X’Pert PRO, the Netherlands). The samples were irradiated with monochromatized Cu Ka radiation and analyzed between 2θ range of 5–50°.The voltage and current per setup used were 40 kV and 30 mA, respectively (16).
Fourier Transform Infrared Spectroscopy
A Jasco FT-761 apparatus (Japan) was used for Fourier transform infrared (FTIR) spectroscopy. Each sample was analyzed as a KBr disk in 400–4,000 cm−1 wave number range. The number of scans was adjusted automatically as a function of sample concentration in the disk.
Nuclear Magnetic Resonance Spectroscopy
The proton nuclear magnetic resonance (1H NMR) spectra of the samples were analyzed using Bruker Advance II DRX-400, Japan. The spectra were recorded at 300 MHz with dimethylsulphoxide as solvent and tetramethylsilane as an internal standard.
RESULTS AND DISCUSSION
Phase Solubility Studies
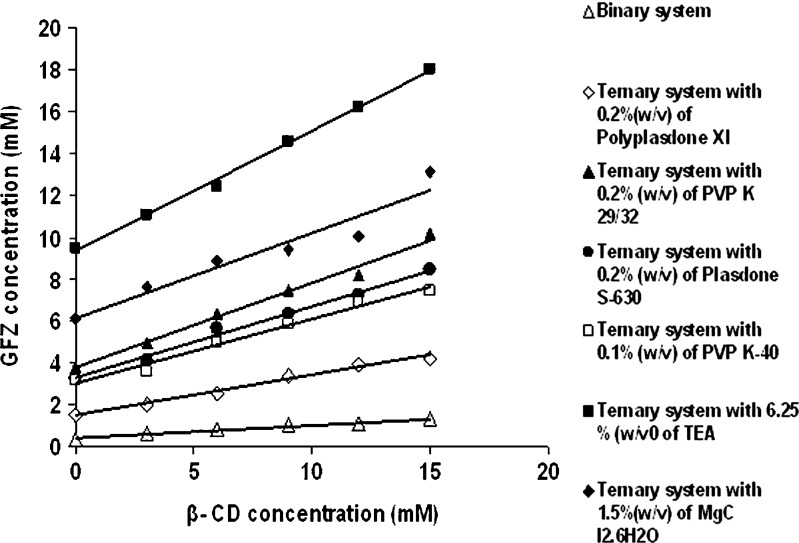
The phase solubility study diagrams obtained with β-CD with and without optimized concentration of different auxiliary substances like PVP K-29/32, PVP K-40, Polyplasdone XL, Plasdone S-630, TEA, and MgCl2·6H2O are shown in Fig. 1. They displayed an AL type equilibrium phase solubility diagram for both GFZ–β-CD binary and ternary systems showing that GFZ solubility increases linearly as a function of β-CD concentration and that soluble complexes were formed without occurrence of precipitation in the range of β-CD concentration used. The increment of GFZ solubility seems to be related to the inclusion ability of the β-CD molecules in water. The slope values in all diagrams were <1, suggesting the formation of 1:1 stoichiometry complexes in solution (8,12). The apparent stability constant (KC) and CE were calculated. The KC and CE values of GFZ binary and ternary complexes are presented in Table I. A synergistic effect on gemfibrozil solubility was observed in the presence of β-CD and different auxiliary substances, since the solubility values achieved in the presence of both CDs and auxiliary substances were higher than the sum of individual solubility values obtained with the CD and polymer solution. The addition of auxiliary substances to the β-CD solution did not change the type of phase solubility diagrams obtained for ternary systems and always resulted in an increase in the CE and KC values. A low stability constant value of 118 M−1 for MgCl2·6H2O indicated that interaction was weak and unstable between the components (17). In the case of TEA, GFZ was fully ionized due to pH alterations; therefore, it was possible to attain overall solubilization, and the absolute solubility enhancement is related to higher complexation efficiency value of 1.341, but is contradictorily affected by KC, which decreases with increasing ionization of drug molecule (18). Above all, enhancement of KC upon addition of the polymers was observed which may be attributed to the establishment of different interactions with CDs and drug molecules such as hydrophobic bonds, van der Waals forces, or hydrogen bonds (8,19). From the results obtained, Polyplasdone XL showed highest complexation efficiency and stability constant value followed by Plasdone S-630 as shown in Table I. A 4.24- and 4.28-fold decrease in the formulation bulk were documented in the case of Plasdone S-630 and Polyplasdone XL, respectively, when compared with binary mixture.
Fig. 1.
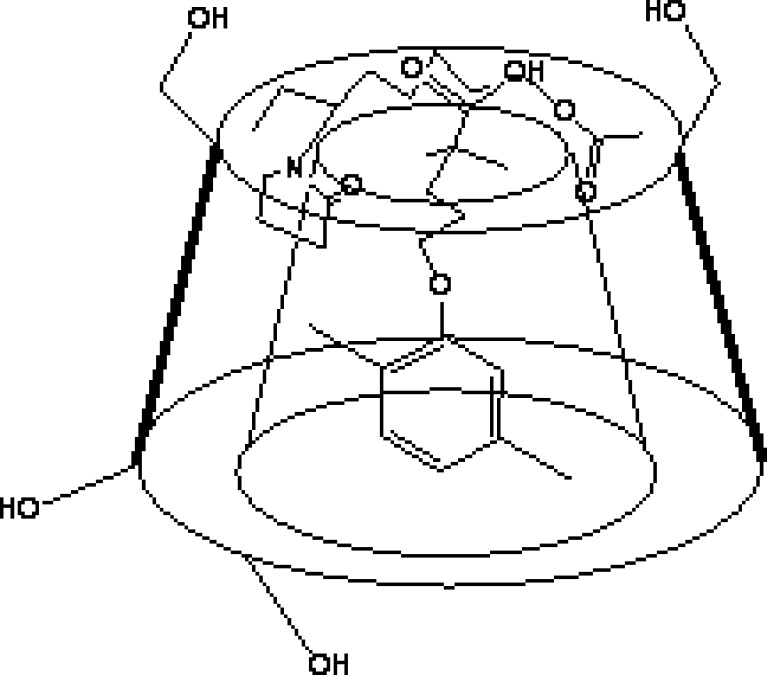
Cartoonic figure showing the mechanism of complexation of drug, polymer, and auxiliary substance
Table I.
Comparative Table Showing the Results of Phase Solubility Studies and Intrinsic Dissolution Rate for Binary and Ternary Systems Prepared by Kneading and Freeze-Drying Method
| Systems | CE | K C | Drug: β-CD ratio | IDR (mg/min/cm2) | ||
|---|---|---|---|---|---|---|
| DD water (pH 6.8) | 0.1 N HCl (pH 1.2) | Phosphate buffer (pH 7.5) | ||||
| GFZ–β-CD binary KN complex | 0.0667 | 153 | 1:16 | 0.103 | 0.079 | 0.185 |
| GFZ–β-CD–Polyplasdone XL | 0.366 | 241 | 1:3.73 | 0.091 | 0.053 | 0.175 |
| GFZ–β-CD–Plasdone S-630 | 0.359 | 180 | 1:3.77 | 0.161 | 0.108 | 0.478 |
| GFZ–β-CD–PVP K-29/32 | 0.354 | 161 | 1:3.81 | 0.112 | 0.081 | 0.275 |
| GFZ–β-CD–PVP K-40 | 0.352 | 141 | 1:3.84 | 0.122 | 0.101 | 0.290 |
| GFZ–β-CD–MgCl2·6H2O | 0.406 | 118 | 1:3.46 | 0.142 | 0.106 | 0.309 |
| GFZ–β-CDPlasdone S-630 LPh complex | – | – | – | 0.235 | 0.225 | 0.490 |
| GFZ–β-CD binary LPh complex | – | – | – | 0.165 | 0.162 | 0.215 |
| GFZ–β-CD–TEA | 1.341 | 138 | 1:1.74 | – | – | – |
Intrinsic Dissolution Rate of Binary and Ternary Kneaded Complexes
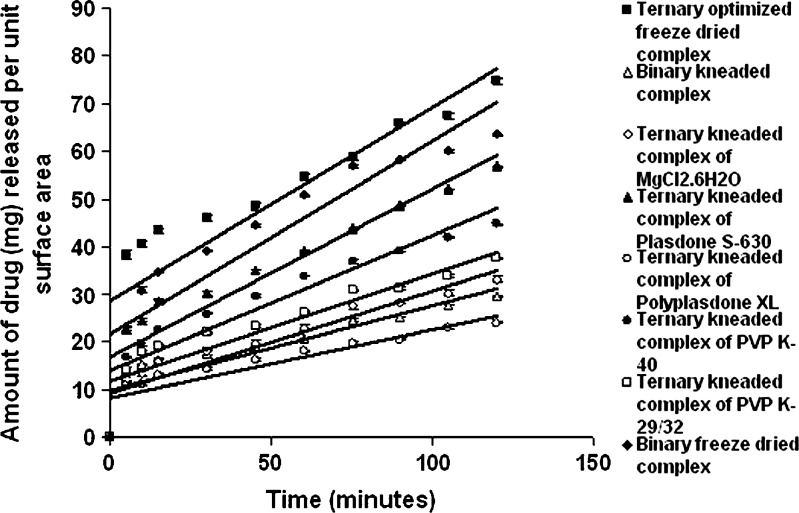
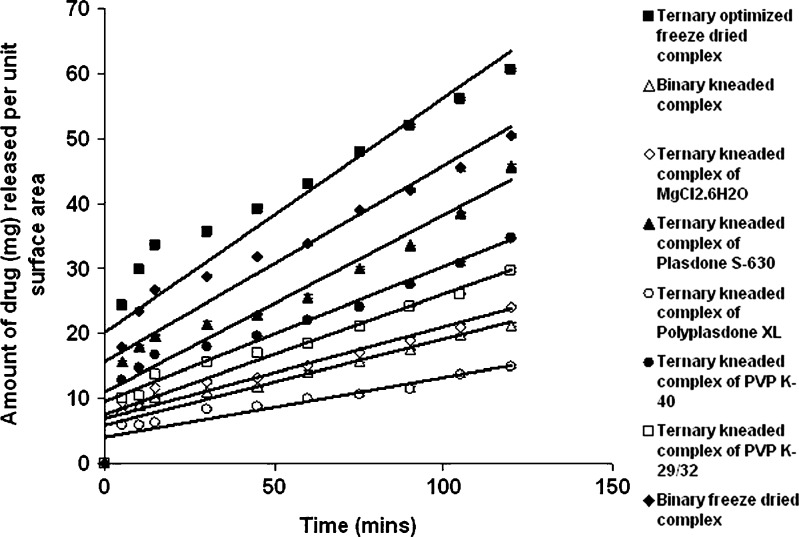
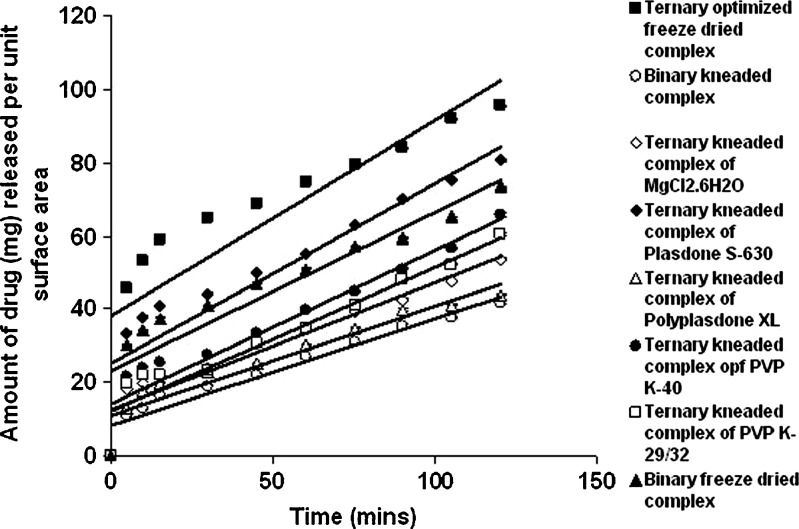
Dissolution studies were performed for binary and ternary kneaded complexes in three different media, i.e., 0.1 N HCl (pH 1.25), phosphate buffer (pH 7.50), and double-distilled water (pH 6.80) as shown in Figs. 2, 3, and 4. A marked increase in dissolution rate was evident in ternary mixture, which can be due to improvement in drug wettability, formation of readily soluble complexes in dissolution medium, and also due to the hydrophilic nature of polymer (20). The kneaded complexes for binary and ternary systems can be ranked according to the IDR value in double-distilled water in the order as shown in Table I. GFZ–β-CD–Plasdone S-630 > GFZ–β-CD–MgCl2·6H2O > GFZ–β-CD–PVP K-40 > GFZ–β-CD–PVP K-29/32 > binary system > GFZ–β-CD–Polyplasdone XL. The IDR values of these complexes in two other mediums were also in the same order. IDR values show that Plasdone S-630 was always more effective than the other auxiliary substances in enhancing IDR value. The low IDR values of Polyplasdone XL as shown in Table II may be attributed to the high stability constant which holds the drug intact within the polymer structure, while the use of MgCl2·6H2O as an auxiliary substance also resulted in significantly good values of IDR. It is assumed that in ternary system of povidone (s) the molecules of drug–β-CD inclusion complex are present in a more or less intimate dispersed state within the PVP matrix through interactions between the exterior of complex and PVP, and this state could be responsible for the higher dissolution rates with respect to the binary system (20). From the results obtained, Plasdone S-630 showed maximum value, which may be due to the fact that it acts as a polymeric surfactant which allows it to form a tie layer between hydrophobic and hydrophilic material (21,22) as shown in Fig. 5.
Fig. 2.
Intrinsic dissolution profile of pure drug, binary system complex, and ternary system complexes in double-distilled water (pH 6.80)
Fig. 3.
Intrinsic dissolution profile of pure drug, binary system complex, and ternary system complexes in 0.1 N HCL (pH 1.25)
Fig. 4.
Intrinsic dissolution profile of pure drug, binary system complex, and ternary system complexes in phosphate buffer (pH 7.50)
Table II.
Relative Degree of Crystallinity (RDC) Values for Binary and Ternary Systems
| Sample | RDC | |
|---|---|---|
| Binary system | Ternary system | |
| PM | 0.53 | 0.23 |
| KN | 0.46 | 0.20 |
| LPh | 0.38 | 0.09 |
Fig. 5.
Phase solubility diagrams for gemfibrozil in binary and ternary systems with optimized concentration of different auxiliary substances
Intrinsic Dissolution Rate of Optimized Freeze-Dried Complex
Enhanced IDR value was obtained with the freeze-dried product of GFZ–β-CD–Plasdone S-630 combination in all the three mediums (Figs. 2, 3, and 4), which may be due to decrease in drug (GFZ) crystallinity as a consequence of the specific GFZ–β-CD interactions produced by the preparation method. This was further demonstrated by the physicochemical characterization of the freeze-dried product (19). A marked increase in the release of drug from ternary freeze-dried complexes containing Plasdone S-630 was seen when compared with binary freeze-dried complexes due to formation of water-soluble drug polymer complexes since polymers mainly interact with drug molecules via electrostatic bonds, i.e., ion-to-dipole and dipole-to-dipole bonds and other forces such as van der Waals forces and hydrogen bridges (9,23). Significant enhancements in IDR of freeze-dried product may be also attributed to an increase in solubility upon complexation. A 49.9% and 17% enhancement in the release of drug from freeze-dried complex was investigated in double-distilled water (pH 6.80) and phosphate buffer (pH 7.50) when compared with the release obtained from the kneaded complex of gemfibrozil–β-CD–Plasdone S-630.
Differential Scanning Calorimetry
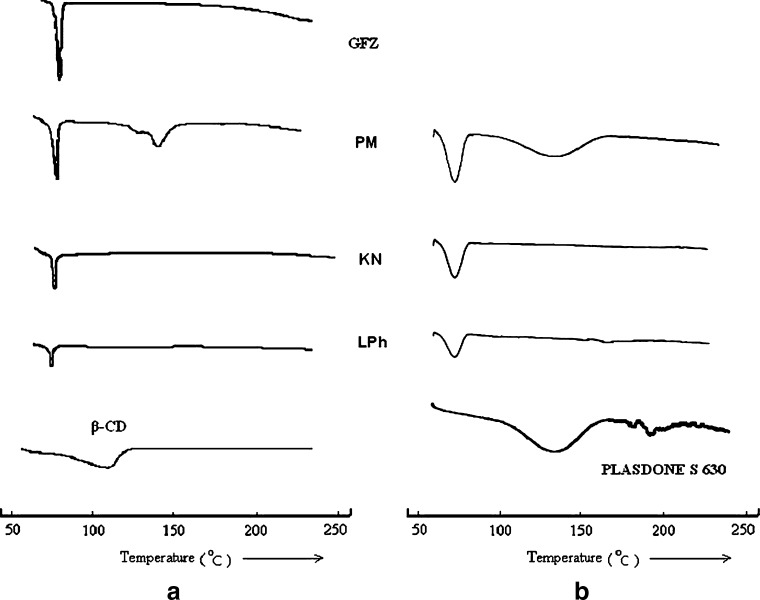
The differential scanning calorimetry (DSC) profiles of pure components of the respective binary and ternary systems in the melting range of the drug and dehydration of the carrier are shown in Fig. 6. Pure GFZ showed a typical behavior of anhydrous crystalline drug with a well-defined melting peak at 63°C, corresponding to its melting point in both the binary and ternary systems. The DSC curve of β-CD showed the liberation of crystal water as an endothermal effect peaked at about 135°C, whereas broader endotherms were associated with water loss from amorphous Plasdone S-630 (12). The comparison of DSC curves from binary systems with those belonging to ternary systems did not result in significant differences. Both characteristic peaks of GFZ (drug melting) and CDs (water loss) were clearly distinguishable in binary and ternary physical mixtures (PMs). As far as the kneading method is concerned, a small broad peak was still obvious for both binary and ternary kneaded products. Despite the lower size and shifts to lower temperatures, the small peak correspondent to the melting of free drug suggests that, as for PMs, there is no inclusion compound formed in either system even though drug–CD interaction was expressive. However, taking into account the results of scanning electron microscopy (SEM) photographs, where the presence of GFZ was still detectable, we can assume that a reduction in drug crystallinity would have occurred probably due to a partial dispersion at molecular level in the solid products (24,25). DSC curves of GFZ–β-CD binary and ternary lyophilized (LPh) products exhibited a complete disappearance of the endothermal melting peak of GFZ, and this may be attributed to an amorphous state and/or to an inclusion complexation, so these results suggest that only GFZ–β-CD LPh products can be considered as true inclusion complexes, differing from simple PMs (26,27).
Fig. 6.
Differential scanning calorimetry curves of a gemfibrozil (GFZ), β-cyclodextrin (β-CD), Plasdone S-630, and GFZ–β-CD binary and b ternary systems: physical mixtures (PM), kneaded (KN), and lyophilized (LPh) products
Scanning Electron Microscopy
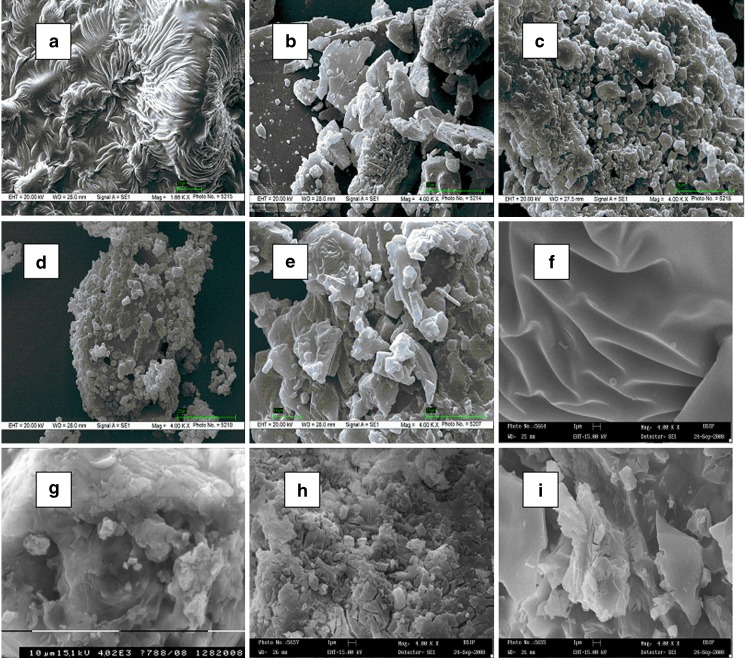
Although there is an existence of strong morphological similarities between binary and ternary products, the SEM microphotographs of both GFZ–β-CD binary and ternary products have been selected and are represented in Fig. 7. Scanning electron microscopy is used to study the microscopic aspects of the raw materials (i.e., CDs and drug substances) and the products obtained by different methods of preparation. Even if there is a clear difference in crystallization state of the raw materials and the products, this study is inadequate to affirm inclusion complexation but nevertheless helps to assess the existence of a single component in the preparations obtained. From SEM analysis, it can be seen that pure GFZ particles appeared as crystals in long tubular shape with smooth surfaces. β-CD particles consisted of three dimensional parallelogram crystals of irregular size. Microscopic examination of GFZ–β-CD PMs for both binary and ternary systems showed the presence of GFZ crystals mixed and adhered on the surface of β-CD particles, revealing no apparent interaction between species in solid state. On the contrary, a drastic change in the original morphology and shape of both GFZ and β-CD particles were observed in all the binary and ternary products, which may be attributed to preparation method adopted (12). In the kneaded (KN) binary and ternary products, it was still possible to distinguish GFZ crystals as agglomerates on the surface of β-CD particles that had lost their original shapes. Finally, the lyophilization technique gave rise to amorphous pieces of irregular size with a lamellate aspect common for binary and ternary products, which could just be distinguished as a single component. The drastic change of the particle shape and aspect in GFZ–β-CD LPh products indicates the presence of new solid phase, which could be due to a consequence of a crystalline habitus change in the system, or it may support the evidence of existence of single phase (28).
Fig. 7.
Scanning electron micrographs of GFZ–β-cyclodextrin binary system; a gemfibrozil, b β-cyclodextrin, c physical mixture, d kneaded, e lyophilized products; and GFZ–β-cyclodextrin ternary system containing optimized concentration of Plasdone S-630, f Plasdone S-630, g physical mixture, h kneaded, e lyophilized products
X-Ray Diffractometry Studies
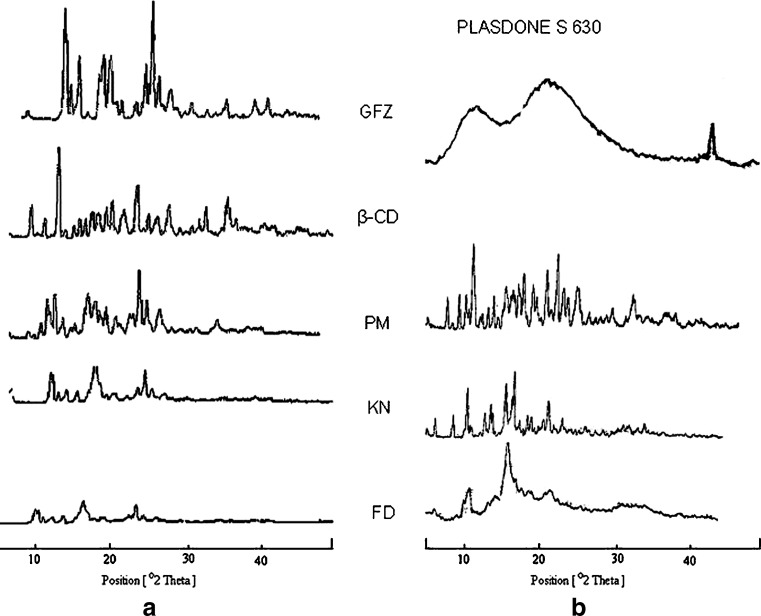
Powder X-ray diffractometry (XRD) is a useful method for the detection of cyclodextrin complexation in powder or microcrystalline states. The diffraction pattern of the complex is supposed to be clearly distinct from that of the superposition of each of the components if a true inclusion complex is formed. Crystallinity was determined by comparing some representative peak heights in the diffraction patterns of the binary systems with those of a reference. The relationship used for the calculation of crystallinity was relative degree of crystallinity (RDC) = ISam/Iref., where ISam = the peak height of the sample under investigation and Iref = the peak height at the same angle for the reference with the highest intensity (29,30). Pure drug peak at 24° 2θ was used for calculating the RDC of kneaded and lyophilized binary systems. The RDC values of corresponding binary systems are given in Table II. However, a lower intensity of their diffraction peaks and overlapping of some GFZ peaks with the peaks of β-CD was also observed (Fig. 7), which was attributed to the reduction in particle size during the preparation of the PMs (12). The diffraction patterns of KN binary and ternary products displayed a crystalline state, but in comparison with the diffractrogram of the correspondent PM it was possible to observe the disappearance of some diffraction peaks of β-CD and GFZ (Fig. 8). Furthermore, for binary and ternary GFZ–β-CD LPh products, the obtained patterns were diffused indicating the amorphous state reached by the lyophilization technique (12,31).
Fig. 8.
Powder X-ray diffraction patterns of a GFZ–β-CD binary systems and b GFZ–β-CD–Plasdone S-630ternary systems
Fourier Transform Infrared Spectroscopy
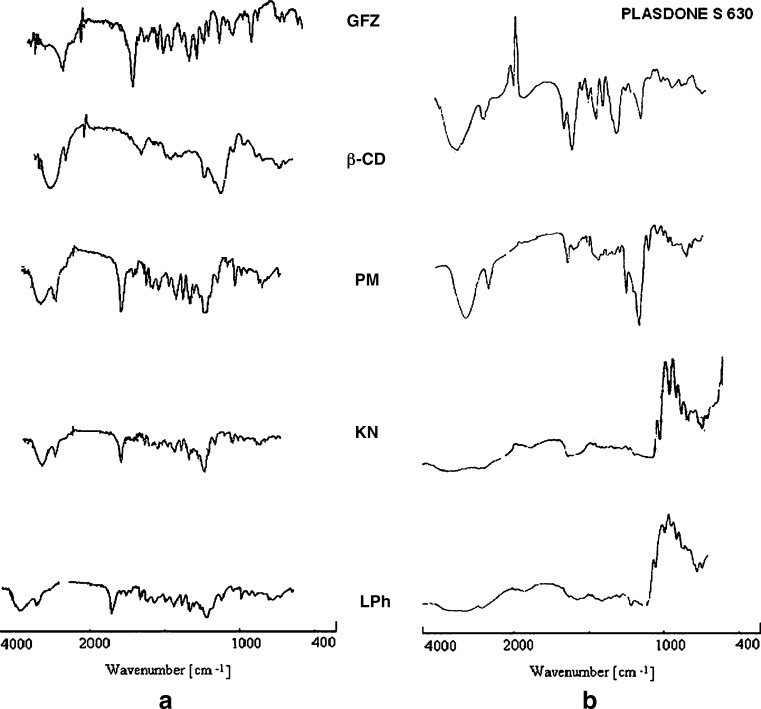
FTIR spectroscopy has also been used to assess the interaction between CD and guest molecules in the solid state, since upon complexation, shifts or changes in the absorption spectrum occur. Two bands, characteristic of GFZ IR spectra at 1,704.96 cm−1 (C=O stretching) and at 1,126 cm−1 (C–O stretching) vibrations (Fig. 9), were used for diagnostic purposes for the analysis of solid interactions in both the binary and ternary systems. No significant changes of the characteristic carbonyl-stretching band of GFZ were observed in any PM either in binary and ternary systems; i.e., in physical mixture, above bands are practically unchanged for position and intensity with respect to the IR spectrum of GFZ alone. Drug (O–H) stretching vibration and Plasdone S-630 (C–O) stretching vibration of kneaded complex shifts towards a lower wavelength confirms the existence of interaction of drug and β-CD, and these spectral changes can be explained by dissociation of intermolecular hydrogen bonds of GFZ through inclusion complexation. (28) Similarly, the IR C=O stretching band was highly diminished, broader, and shifted to lower frequencies in all spectral patterns of KN and LPh binary and ternary products. It was verified that the magnitude of alteration of the original C=O stretching band was clearly affected by the preparation method selected. Thus, the decrease in the intensity of C=O stretching band and the amplitude of its shift was in the following range KN < LPh. These results served to confirm the existence of strong interactions between GFZ, β-CD, and Plasdone S-630 (12). These also may be indicative of GFZ dispersion as a consequence of the interaction with β-CD and Plasdone S-630, which could result in GFZ inclusion into the hydrophobic cavity of the carrier. In IR spectra of inclusion complex of gemfibrozil–β-CD–Plasdone S-630 freeze-dried complex, absorption band at 1,126 cm−1 cannot be detected, which might be due to co-occurrence of C–O band with C–O ester intensified band at 1,261.36 cm−1. This indicated a strong interaction and complete complex formation of drug with β-CD and Plasdone S-630 (32,33).
Fig. 9.
Fourier transform infrared absorption bands in the range 400–4,000 cm−1 region. Gemfibrozil (GFZ), β-cyclodextrin (β-CD), Plasdone S-630, and GFZ–β-CD binary (a) and ternary systems (b): physical mixtures (PM), Kneaded (KN), and lyophilized (LPh) products
Nuclear Magnetic Resonance Spectroscopy
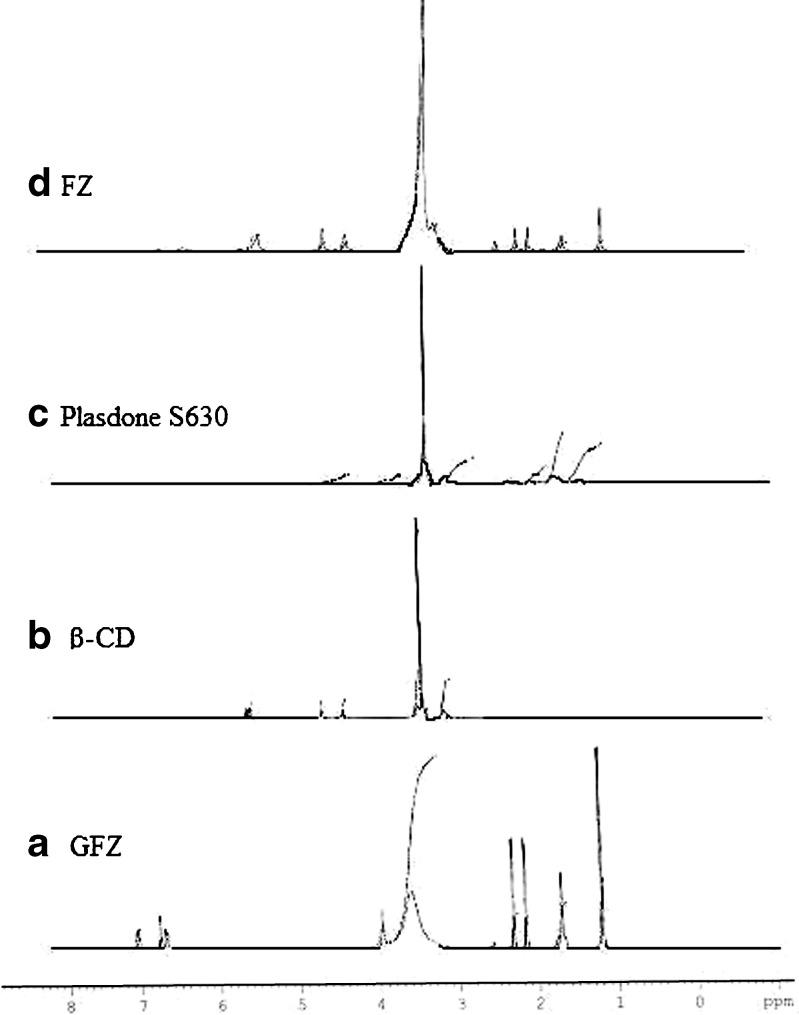
The 1H NMR spectra (Fig. 10) of GFZ showed chemical shift of 2.332 ppm (–COOH), while in the freeze-dried complex the chemical shift was 2.491 ppm (–COOH), which supported the possible hydrogen bonding between the drug and polymer. There seemed to be possible interaction between carboxylic group of drug and polymer due to hydrogen bonding. The inclusion of aromatic ring in the cavity evidenced by 1H NMR has a protective effect on the excited state of the drug.
Fig. 10.
Nuclear magnetic resonance spectra of a gemfibrozil (GFZ), b β-cyclodextrin (β-CD), c Plasdone S-630, and d ternary system
CONCLUSION
This study determined greater KC values for ternary complexes in comparison with the corresponding binary one suggesting a significant improvement in the complexation efficiency between GFZ and β-CD by addition of small amounts of auxiliary substances. Plasdone S-630 was to be the most suitable auxiliary substance in terms of superior complexation efficiency and stability constant. Furthermore, IDR studies indicated that ternary freeze-dried complex of Plasdone S-630 resulted in higher dissolution rate of GFZ when compared with the kneaded complex of the same formula. This was further confirmed by elucidations through SEM, XRD, FTIR, DSC, and 1H NMR studies that suggested the formation of new solid phases, some of them in amorphous state, allowing the conclusion of strong evidences of binary and ternary inclusion complex formation between GFZ–β-CD and Plasdone S-630,particularly for LPh binary and ternary products.
Acknowledgements
The authors wish to thank IIT Delhi for providing the facilities for SEM and XRD. We would also like to thank Mr.Yogesh Murti and Mr. Harjeet Singh for their spectral suggestions.
References
- 1.Manca ML, Zaru M, Ennas G, et al. Diclofenac–β-cyclodextrin binary systems: physicochemical characterization and in vitro dissolution and diffusion studies. AAPS PharmSci Tech. 2005;6:64–72. doi: 10.1208/pt060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challa R, Ahuja A, Khar RK, Ali J. Cyclodextrins in drug delivery: an updated review. AAPS Pharm Sci Tech. 2005;6:E326–E338. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftsson T, Masson M, Siigurjonsdottir JF. Methods to enhance the complexation efficiency of cyclodextrins. STP Pharma Sci. 1999;9:237–242. [Google Scholar]
- 4.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 5.Ukama K, Otagiri M. Cyclodextrins in drug carrier systems. Chem Rev. 1987;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 6.Ulu ST. LC determination of gemfibrozil in tablets. Chromatographia. 2006;64:447. doi: 10.1365/s10337-006-0057-x. [DOI] [Google Scholar]
- 7.Ain S, Philip B, Pathak K. Preformulative assessment of inclusion complexes of Gemfibrozil, with cyclodextrins. PDA. 2008;62:300–308. [PubMed] [Google Scholar]
- 8.Patel AR, Vavia PR. Effect of hydrophilic polymers on solubilization of fenofibrate by cyclodextrin complexation. J Inc Phenom Macrocycl Chem. 2006;56:247–251. doi: 10.1007/s10847-006-9091-4. [DOI] [Google Scholar]
- 9.Loftsson T, Fridriksdottir H. The effect of water soluble polymers on aqueous solubility of drugs. Int J Pharm Sci. 1996;127:293–296. doi: 10.1016/0378-5173(95)04207-5. [DOI] [Google Scholar]
- 10.Higuchi T, Connors A. Phase solubility techniques. Adv Anal Chem Instrum. 1965; 117–211
- 11.Loftsson T, Hreinsdottir D, Masson M. The complexation efficiency. J Inc l Phenom Macrocycl Chem. 2007;57:545–552. doi: 10.1007/s10847-006-9247-2. [DOI] [Google Scholar]
- 12.Laura SSR, Dominos CF, Francisco JBV. Physicochemical investigation of the effects of water-soluble polymers on vinpocetine complexation with β-cyclodextrin and its sulfobutyl ether derivative in solution and solid state. Eur J Pharm Sci. 2003;20:253–266. doi: 10.1016/S0928-0987(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 13.Chowdary KPR, Srinivas SV. Effect of polyvinylpyrrolidone on complexation and dissolution rate of β and hydroxypropyl–β-cyclodextrin complexes of celecoxib. Ind J Pharm. 2006;68:631–634. doi: 10.4103/0250-474X.29632. [DOI] [Google Scholar]
- 14.Govindarajan R, Nagarsenkar S. Influence of preparation methodology on solid state properties of an acidic drug-cyclodextrin system. J Pharm Pharmacol. 2004;56:725–733. doi: 10.1211/0022357023538. [DOI] [PubMed] [Google Scholar]
- 15.Aulton M. Pharmaceutical preformulation: the physicochemical properties of drug substances. Pharmaceutics the design of dosage form design. 2. Philadelphia: Churchill Livingstone; 2002. pp. 122–123. [Google Scholar]
- 16.Chowdary KPR, Srinivas SV. Influence of hydrophilic polymer on celecoxib complexation with hydroxypropyl–β-CD. AAPS Pharm Sci Tech. 2006;7:E1–E5. doi: 10.1208/pt070379. [DOI] [PubMed] [Google Scholar]
- 17.Yamakawa T, Nishimura S. Liquid formulation of a novel non-fluorinated topical quinolone, T-3912, utilizing the synergistic solubilizing effect of the combined use of magnesium ions and hydroxypropyl–β-cyclodextrin. J Cont Release. 2003;86:101–113. doi: 10.1016/S0168-3659(02)00367-X. [DOI] [PubMed] [Google Scholar]
- 18.Granero G, Garnero C, Marcela L. The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl–β-cyclodextrin. Eur J Pharm Sci. 2003;20:285–293. doi: 10.1016/S0928-0987(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 19.Fridriksdottir H, Siiguroardottir AM, Ueda H. The effect of water soluble polymers on drug–cyclodextrin complexation. Int J Pharm. 1994;110:169–177. doi: 10.1016/0378-5173(94)90155-4. [DOI] [Google Scholar]
- 20.Mura P, Faucci MT, Bettinetti GP. The influence of polyvinyl pyrrolidone on naproxen complexation with hydroxypropyl β-cyclodextrin. Eur J Pharm Sci. 2001;13:187–194. doi: 10.1016/S0928-0987(01)00093-8. [DOI] [PubMed] [Google Scholar]
- 21.A PRODUCT GUIDE Performance Enhancing Products for Pharmaceuticals Available at www.ispcorp.com. Accessed March 8, 2008.
- 22.Kollidon BW. Polyvinylpyrrolidone for the pharmaceutical industry 4th edn. BASF; 1998.
- 23.Doijad RC, Kanakal MM, Manvi FV. Effect of processing variables on dissolution and solubility of piroxicam: hydroxypropyl–β-cyclodextrin inclusion complexes. Indian J Pharm S. 2007;69:323–326. doi: 10.4103/0250-474X.33175. [DOI] [Google Scholar]
- 24.Dollo CLC, et al. Improvement in solubility and dissolution rate of 1, 2-dithiole-3-iones upon complexation with β-cyclodextrin and its hydroxypropyl and sulfobutylether derivatives. J Pharm Sci. 1999;88:889–895. doi: 10.1021/js990067o. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed GEL. Effect of cyclodextrins on the physico-chemical properties and antimycotic activity of clotrimoxazole. Int J Pharm. 1998;171:111–121. doi: 10.1016/S0378-5173(98)00163-X. [DOI] [Google Scholar]
- 26.Kurazumi M, Nambu N, Nagri T. Pharmaceutical interactions in dosage form and processing for Inclusion compounds of non-steroidal anti-inflammatory and other slightly water soluble drugs with β-cyclodextrins in powdered form. Chem Pharm Bull. 1975;23:3062–3068. doi: 10.1248/cpb.23.3062. [DOI] [PubMed] [Google Scholar]
- 27.Veiga F, Fernandes C, Maincent P. Influence of the preparation method on the physicochemical properties of tolbutamide/cyclodextrin binary system. Drug Dev Ind Pharm. 2001;27:523–532. doi: 10.1081/DDC-100105177. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes C, Viera MT, Viega F. Physicochemical characterization and in vitro dissolution behaviour of nicardipine-cyclodextrin inclusion compounds. Eur J Pharm Sci. 2002;15:79–88. doi: 10.1016/S0928-0987(01)00208-1. [DOI] [PubMed] [Google Scholar]
- 29.Ryan JA. Compressed pellet X-RD monitoring for optimization of crystallinity in lyophilized solids: imipenem: cilastatin sodium case. J Pharm Sci. 1986;75:805–807. doi: 10.1002/jps.2600750817. [DOI] [PubMed] [Google Scholar]
- 30.Nalluri BN, Chowdhary KPR, Murthy KVR, et al. Physicochemical characterization and dissolution properties of nimesulide–cyclodextrin binary system. AAPS PharmSci Tech. 2003;4:1–12. doi: 10.1208/pt040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao VM, Haslam JL, Stella VJ. Controlled and complete release of model poorly water soluble drug, prednisolone, from hydroxypropylmethylcellulose matrix tablets using SBE–β-cyclodextrin as a solubilizing agent. J Pharm Sci. 2001;129:807–816. doi: 10.1002/jps.1034. [DOI] [PubMed] [Google Scholar]
- 32.Baboota S, Dhaliwal M, Kohli K, et al. Inclusion complexation of Rofecoxib with dimethyl β-cyclodextrin. J Pharm Sci. 2005;85:226–229. [Google Scholar]
- 33.Erden N, Celebi N. A study of inclusion complex of naproxen with β-cyclodextrin. Int J Pharm. 1988;48:83–89. doi: 10.1016/0378-5173(88)90250-5. [DOI] [Google Scholar]