Abstract
In this study, some single-layer and double-layer transdermal drug delivery systems (TDDSs) with different functional and non-functional acrylic pressure-sensitive adhesives (PSAs) were prepared. For this purpose, fentanyl as a drug was used. The effects of PSAs type, single-layer and double-layer TDDSs on skin permeation and in vitro drug release from devices were evaluated using a hydrodynamically well-characterized Chien permeation system fitted with excised rat abdominal skin. The adhesion properties of devices such as peel strength and tack values were obtained as well. It was found that TDDS with –COOH functional PSA showed the lowest steady-state flux. Double-layer TDDS displayed a constant flux up to 72 h. In double- and single-layer devices after 1 and 3 h, respectively, drug release followed Higuchi’s kinetic model. Formulations with the highest percentage of –COOH functional PSA have displayed the lowest flux. The double-layer TDDSs with non-functional PSA demonstrated the suitable skin permeation rate close to Duragesic® TDDS and suitable adhesion properties.
Key words: adhesion properties, fentanyl, peel strength, pressure-sensitive adhesive, skin permeation, tack, transdermal drug delivery system
INTRODUCTION
Transdermal drug delivery systems (TDDSs) offer an attractive alternative to conventional oral and injection therapies as a means of achieving constant therapeutic levels of drugs (1,2). Fentanyl is a synthetic opioid, an analgesic which is about 75–100 times more potent than morphine. Fentanyl is one of the most powerful drugs for use in pain treatment (3–7). In 1991, transdermal reservoir patches of fentanyl were approved for the treatment of chronic and cancer pains (4,8). There are four types of fentanyl transdermal patches in the market (Duragesic®). Their average flux is 2.5 μg cm−2 h−1 (1). Preparation of TDDSs consists of three basic designs including: reservoir, matrix, and drug in adhesive (8). Nevertheless, these devices could be grouped into two main categories: reservoir-type and matrix-type devices. The transdermal devices of reservoir-type consist of a reservoir that contains active pharmaceutical ingredients (API). From the reservoir, the API diffuses through the controlling membrane into the absorption site. The main advantage of this type of device is that the rate of drug delivery is maintained practically constant for a long period of time. Nevertheless, these devices are usually bulky. The matrix-type transdermal devices generally comprise a non-permeable backing liner, a polymeric adhesive matrix in which the active drug or drugs are dissolved or dispersed and a release liner. They have a total surface area that is the same as that of the active surface. One disadvantage of the matrix-type device is that for some active substances, it is difficult to maintain a constant dose for an extended period of time. Generally, in this type of device, the delivery rate diminishes with time as a consequence of the decreasing concentration of the API in the matrix (9–11).
All TDDSs include a pressure-sensitive adhesive (PSA) layer to hold the patches on the skin. PSAs are materials that adhere to substrate by application of a light force and leave no residue when removed. Usually, the PSAs used for TDDSs are based on an acrylic, silicone, and polyisobutylene. Lauryl alcohol, as a skin permeation enhancer, has the best performance in fentanyl TDDS (12). In literature, several documents can be found that disclose devices for transdermal administration comprising two or more layers. Accordingly, the preferred embodiment consists of four layers that include: protective backing layer, reservoir layer comprising the pharmaceutical active drug dispersed in a mixture of a mineral oil and polyisobutylene, microporous membrane that controls the rate at which the drug is released, and an adhesive layer (9,13–15). The addition of polymeric layers acting as controlling membranes for the drugs has not been totally successful because they are usually less comfortable to be used by the patient due to reduced mechanical properties with the addition of polymeric layer. Additionally, to control drug flux, these devices need the addition of a controlling membrane as they cannot perform adequately without it. Recently, a TDDS device with two superimposed adhesive layers is reported (9). This device consists a skin contact layer having non-functional PSA and a reservoir layer containing a mixture of functional (–COOH) PSA and a non-functional PSA.
The objective of the present work was to design a new TDDS having two acrylic adhesive layers with different adhesive compositions and functionalities. The layer adhered to the backing is a drug reservoir and the other is a rate-controlling layer which is in contact with the skin. The two layers have different affinities toward the drug. The difference in affinity allows the delivery rate to be controlled. For this purpose, the devices having acrylic adhesive with different functionalities (–COOH and –OH) and non-functional group containing fentanyl and lauryl alcohol were prepared. The influence of the double- and single-layer devices as well as PSA functionality on drug release, skin permeation, and adhesion properties have been evaluated.
MATERIALS AND METHODS
Materials
Acrylic adhesive Durotack 2287, 4098, 2196 (National Starch and Chemical, USA), Cotran9720 as a backing layer with thickness of 85 μm (3M, USA), release liner (Scotchpak 1022, 3M, USA), lauryl alcohol (Fluka, USA), fentanyl base (Diosynth B.V., The Netherlands), and all other materials were high-performance liquid chromatography (HPLC) grades.
Sample Preparation
Preparation of single- and double-layer devices is different, and therefore, each device is explained separately. Double-layer device includes two layers, reservoir layer (second layer) and skin contact layer (first layer). The formulations designated as numbers 1, 2, 3, 4, and 5 are related to double- layer devices, and numbers 6, 7, 8, 9, and 10 are related to single-layer devices. The total material composition in single-layer and double-layer formulations is similar.
Preparation of a Single-Layer Device
As presented in Table I, the components of single-layer devices were mixed together in a rotary mixer to obtain a solution which could be evenly applied on the backing layer to form a film with final specific thickness of 70 μm by using a film applicator (Elcometer 3580).
Table I.
Material Compositions of Single- and Double-Layer TDDSs
| Formulation number | Single- and double-layer device total component (%, w/w) | Total thickness (µm) | ||||
|---|---|---|---|---|---|---|
| Fentanyl | Lauryl alcohol | Pressure-sensitive adhesive | ||||
| Duro-Tak® 4098 | Duro-Tak® 2287 | Duro-Tak® 2196 | ||||
| 6 | 5.1 | 10 | 73.1 | – | 11.8 | 70 |
| 7 | 5.1 | 10 | 73.1 | 11.8 | – | 70 |
| 8 | 5.1 | 10 | 84.9 | – | – | 70 |
| 9 | 5.1 | 10 | 61.5 | – | 23.4 | 70 |
| 10 | 5.1 | 10 | 61.5 | 23.4 | – | 70 |
The samples were allowed to stand at room temperature and then further dried in an oven at 50°C for 45 min.
Preparation of Double-Layer Device
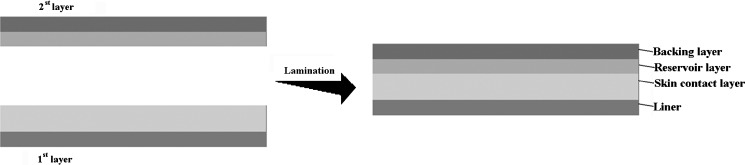
According to Table II, the components of the formulations for the skin contact (first) and the reservoir (second) layers were mixed with a rotary mixer. Formulation of skin contact layer for all devices is the same. First layer is coated on release liner and second layer is coated on backing layer by a film applicator at a controlled specified thickness (20 and 50 μm, respectively). The coated film was then placed into an oven at 50°C for 45 min to drive off all volatile processing solvents. Then, the first layer was laminated using a rolling ball by depositing the second layer on the exposed face of the adhesive matrix. The total thickness of the devices obtained was 70 μm (Fig. 1). Considering the equilibrium condition, the laminate was cut into patches in a required shape.
Table II.
Material Compositions of Double-Layer TDDSs
| Formulation number | Fentanyl | Lauryl alcohol | Pressure sensitive adhesive | Total thickness(µm) | |||
|---|---|---|---|---|---|---|---|
| Duro-Tak® 4098 | Duro-Tak® 2287 | Duro-Tak® 2196 | |||||
| Material composition of reservoir (second layer) of double-layer TDDSs | |||||||
| 1 | 8 | 10 | 41 | – | 20 | ||
| 2 | 8 | 10 | 41 | 41 | – | 20 | |
| 3 | 8 | 10 | 82 | – | – | 20 | |
| 4 | 8 | 10 | – | – | 82 | 20 | |
| 5 | 8 | 10 | – | 82 | – | 20 | |
| Material composition of skin contact layer (first) of double-layer TDDSs | |||||||
| 4 | 10 | 86 | 50 | ||||
Fig. 1.
Schematic of double-layer TDDs lamination
Preparation of Rat Abdominal Skin
Male Sprague–Dawley rats (150–170 g) obtained from Razi Vaccine & Serum Research Institute were killed using diethyl ether asphyxiation. Hair of the abdominal region was carefully removed, and a 5 × 5-cm full-thickness skin was excised from this region of each killed rat. The dermis side was wiped with isopropyl alcohol to remove the residual adhering fat. The skin was dipped and soaked in normal saline solution. It was then washed with distilled water, wrapped in aluminum foil, and stored in a deep freezer at −20°C for further use (16–18). One hour prior to the experiments, the samples were thawed.
In Vitro Skin Permeation
Permeation investigation was carried out using an excised rat abdominal skin in a well-characterized Chien permeation system with an effective diffusion area of 1 cm2 at 37°C. Receptor compartment of the diffusion cell was completely filled with 3 mL of filtered and degassed phosphate buffer solution (PBS) of pH 6 as receiver medium. The device with (1.5 × 1.5 cm) dimension was applied to the epidermal side of the rat skin with slight pressure and then mounted over the receptor compartment. The air bubbles that remained in the receptor compartment and below the skin were carefully removed by gentle tilting of the diffusion cell. The receptor medium was stirred by a magnetic stirrer. At predetermined time intervals (1, 4, 8, 12, 24, 32, 48, and 72 h), the receptor medium was completely withdrawn from the receptor compartment and was replaced with fresh PBS. The concentration of fentanyl was determined by a fully validated HPLC method, and the cumulative amount of fentanyl was calculated (11).
Permeation parameter, including the permeation rate or skin flux (Jss) was determined from Fick’s law of diffusion as follows:
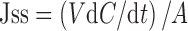 |
1 |
where Jss is the skin flux (µg/cm2/h), C is the cumulative drug concentration in the receiver fluid at time t, V is the receiver volume (mL), and A is active diffusion area (cm2) (19).
Steady-state flux is calculated from permeation profiles.
Drug Release
The release of fentanyl from the double-layer and single-layer devices was measured in a hydrodynamically well-characterized Chien permeation system at 37°C. Sodium phosphate buffer (pH 6) was prepared and heated to 37°C before being used as a release medium. For the determination of the fentanyl release profile, each sample with 1.5 × 1.5-cm dimension was mounted in the orifice of a half-cell of the Chien permeation system. At different intervals (0.25, 0.5, 1, 2, 3, 5, 24, 29, 48, 72, and 96 h), the release medium was completely withdrawn and was immediately replaced with a fresh solution and assayed by an HPLC-UV method.
Drug Analysis
Fentanyl was assayed by HPLC (Younglin, SDV30) with UV detector at 207 nm. HPLC separation system consisted of a PerfectSil Target, (column 150 × 4.6 mm id, 5 µm) equipped with a guard column. The mobile phase consisted of MeCN/K2HPO4 10 mM (80:20) which was adjusted to pH 6.0 ± 0.1 by addition of H3PO4. The flow rate of mobile phase was 1 mL/min. For preparation of the standard curve, solutions of 0.5, 1, 2, 3, 4, 5, 6, 8, and 10 µg/mL of fentanyl were prepared by spiking of materials at the above nine concentrations and drawing the linear calibration curve (R2 = 0.9998). The specificity for assay was established using the three sequential replicates of solution which were used in the standard curve (12).
Probe Tack Test
Tack tests were carried out for adhesive tapes according to ASTM D3121 using a Chemie Instruments Probe-Tack PT-500 (Fairfield, Ohio, USA) on at least four samples.
Peel Strength Measurement at 180°
Peel tests were carried out according to ASTMD3330 on adhesive-coated tapes with 25-mm width. After preparation of PSA tape/stainless steel joints, they were stored at room temperature for 20 min. Peel force in 180 directions was measured at a peel rate of 30.50 cm/min at room temperature using Chemie Instruments adhesive/release tester AR-1000 (Fairfield). The test was accomplished at least three times on each sample.
RESULTS AND DISCUSSION
Rat Skin Permeation
Influence of Different Functional and Non-functional PSAs on Skin Permeation
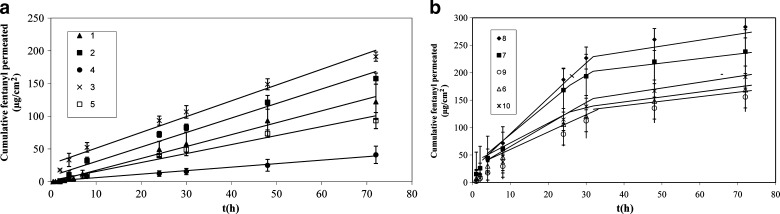
The skin permeation rate of fentanyl through excised rat abdominal skin was determined on different double-layer and single-layer devices prepared with different functional and non-functional acrylic PSAs. The permeation profiles of fentanyl are shown in Fig. 2. The steady-state flux is presented in Table III.
Fig. 2.
Cumulative fentanyl permeated through excised abdominal rat skin versus time. a Double-layer formulation,1(R 2 = 0.98), 2(R 2 = 0.96), 3(R 2 = 0.99), 4(R2 = 0.97), 5(R 2 = 0.98) Each value represents mean of three tests with corresponding SD. b Single-layer formulation
Table III.
Lag Time and Steady-State Flux of Fentanyl Through Excised Rat Skin from Double-Layer and Single-Layer TDDS
| Number of formulation | Lag time (h) | Jss (µg/cm2) | |
|---|---|---|---|
| (1–24 h) | (24–72 h) | ||
| 1 | 0.50 | 1.8 ± 0.03 | |
| 2 | 0.23 | 2.3 ± 0.16 | |
| 3 | 0.08 | 2.4 ± 0.06 | |
| 4 | 4.10 | 0.5 ± 0.12 | |
| 5 | 1.00 | 1.3 ± 0.2 | |
| 6 | 0.12 | 4.1 ± 0.23 | 1.0 ± 0.08 |
| 7 | 0.97 | 6.5 ± 0.70 | 1.1 ± 0.20 |
| 8 | 0.04 | 7.8 ± 0.12 | 1.3 ± 0.09 |
| 9 | 0.34 | 3.7 ± 0.05 | 1.0 ± 0.18 |
| 10 | 1.05 | 4.8 ± 0.18 | 1.2 ± 0.20 |
The permeation rates of fentanyl from TDDS devices through rat skin for single-layer devices from 1 to 24 h were found in the range 3.7–6.5 μg cm−2 h−1 and for 24 to 72 h were found in the range 1–1.2 μg cm−2 h−1. The skin permeation fluxes for double-layer devices between 1 and 72 h were 0.5–2.4 μg cm−2 h−1. Among three different PSAs used in this study, formulations (4) and (9) with the highest percentage of Duro-Tak® 2196 with –COOH functional group resulted in the lowest fluxes, and formulations (3) and (8) containing Duro-Tak® 4098 (non-functional PSA) showed the highest fluxes. In formulations containing PSA with –COOH functional group, the delivery of fentanyl from the matrix was too low to provide an adequate permeation rate, but formulations with PSA comprising –OH functional group or with non-functional PSA showed higher permeation profiles compared with –COOH functional groups (11). This may be related to some interactions between acrylic adhesives and fentanyl. As reported by Stefano et al. (9) where a functional polymer is employed in the layer that acts as reservoir, a chemical interaction of energy higher than the attractive intermolecular forces, required for a simple solubility interaction between the functional group of the polymer and the active drug, may exist, and we found that it is dependent on the type of functionality. As shown in Fig. 3, fentanyl has amide (–CON) group. It was found that –COOH group of PSA gives stronger bond with –CON group of fentanyl than –OH group because –COO−δ is a stronger electron-withdrawing group than –O−δ. Therefore, the positive charge on H+δ of carboxyl group is greater than hydroxyl group, and it causes stronger hydrogen bonding between H+δ and –N−δ.
Fig. 3.
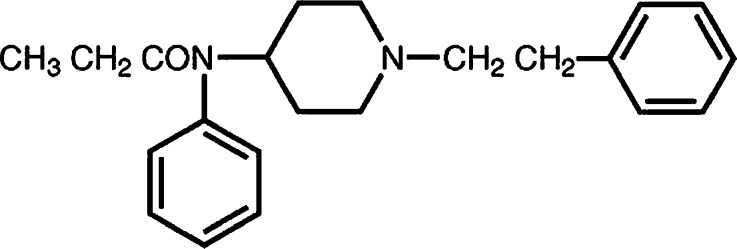
Chemical structure of fentanyl
Influence of Single-Layer and Double-Layer TDDs on Skin Permeation
Skin permeation profiles of single-layer and double-layer TDDS are shown in Fig. 2. In spite of the total compositions in single and double layers being the same, the rate of skin permeation of double-layer devices is constant up to 72 h, though it is changed for single layer. This has provided a suitable therapeutic delivery without using a rate-controlling membrane for the delivery of the active pharmaceutical ingredient. It is confirmed that the skin contact layer acts as a rate-controlling layer. Besides, for the formulations with the same total composition, the presence of the components distributed in a bilayer arrangement allows a control of the delivery of the active drug that is not obtained by the mixture of the same components arranged in a single layer.
Drug Release
In vitro drug release from double-layer and single-layer TDDS is studied, and the results are summarized in Table IV and related profiles shown in Figs. 4 and 5.
Table IV.
Drug Released Percents from Double-Layer and Single-Layer Devices
| Formulation number | Drug release (%) | R 2 |
|---|---|---|
| 1 | 59 | 0.99 |
| 2 | 76 | 0.99 |
| 3 | 90 | 0.99 |
| 4 | 50 | 1.00 |
| 5 | 66 | 0.98 |
| 6 | 77 | 0.97 |
| 7 | 88 | 0.97 |
| 8 | 92 | 0.97 |
| 9 | 74 | 0.99 |
| 10 | 85 | 0.97 |
Regression correlation coefficient (R 2) according to zero-order equation of double-layer and single-layer TDDSs
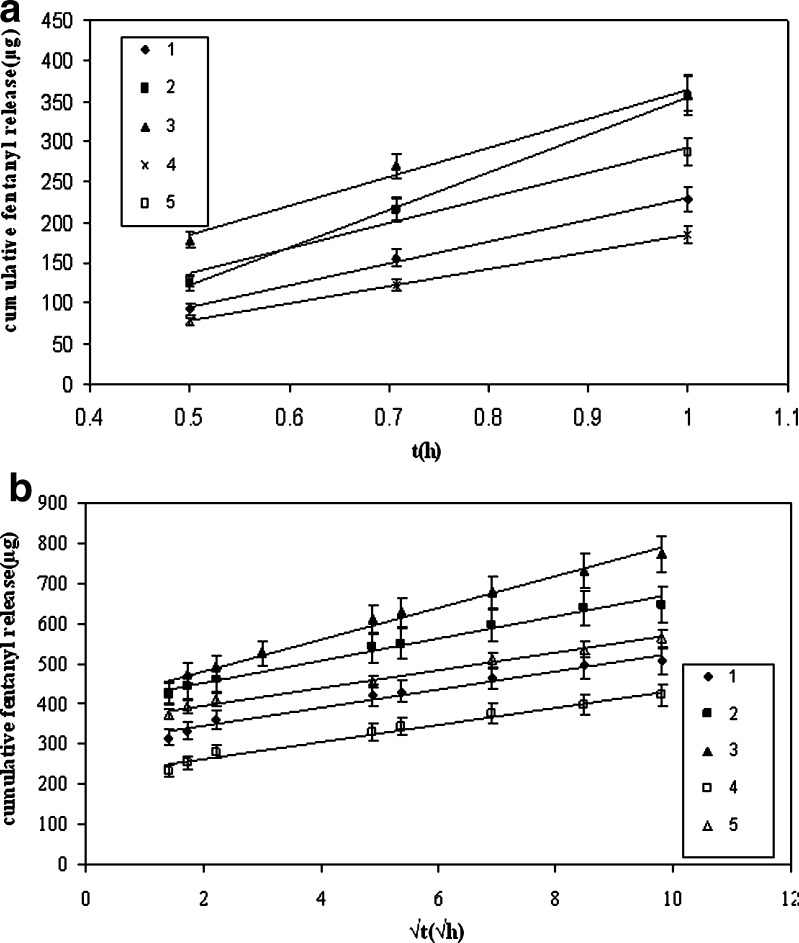
Fig. 4.
Cumulative release of fentanyl from double layer devices: a up to 1 h, b from 1 to 96 h
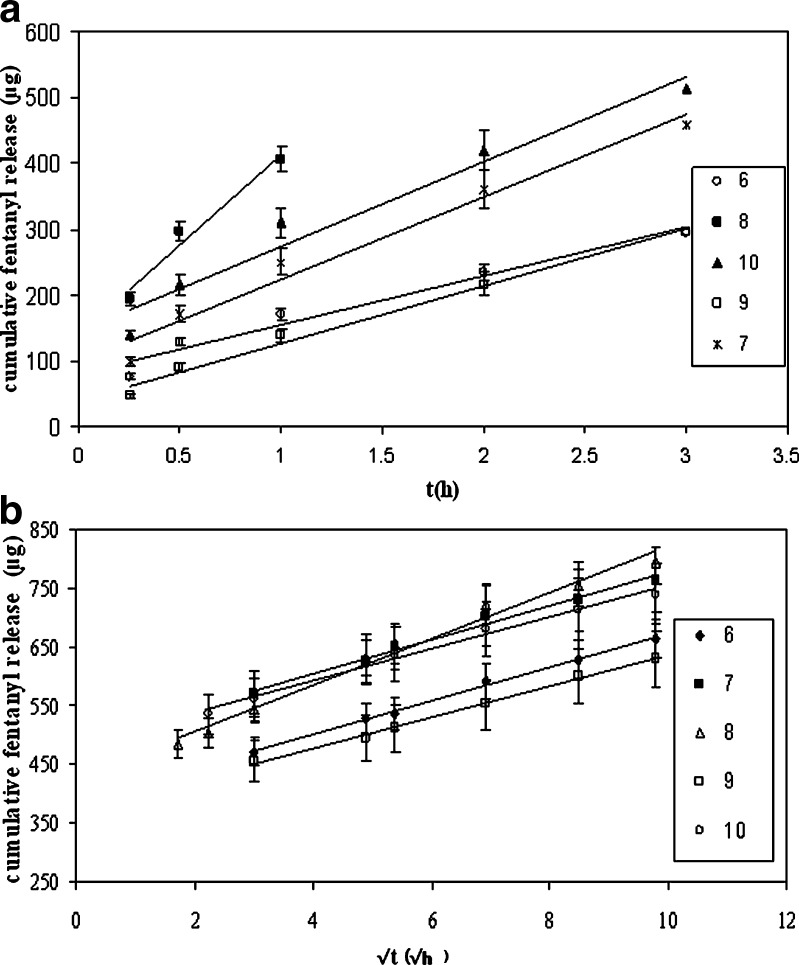
Fig. 5.
Cumulative release of fentanyl from double layer devices: a up to 3 h, b from 3 to 96 h
For this purpose, the cumulative released drug during 96 h is obtained, and the percent of released drug according to Eq. 2 is calculated and its result summarized in Table IV.
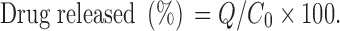 |
2 |
According to Table IV, the influence of different functional and non-functional PSAs on the amount of drug release is similar to their effect on skin permeation rate. It is found that the formulation which has the highest percentage of Duro-Tak® 2196 has the lowest drug release values.
A dispersed drug in an adhesive vehicle described by Higuchi showed that at the first interval, it followed zero-order equation, and after that, in quasi-steady-state condition, the vehicle’s release behavior conforms best to Higuchi model (20):
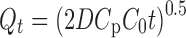 |
3 |
Where Qt as the cumulative amount of drug released at time t per unit of exposed area, D the constant diffusion coefficient, and C0 and Cp as the drug loading and drug solubility in the polymer matrix, respectively (21).
By considering the drug release profiles in Figs. 4 and 5, it was observed that in double- and single-layer devices after 1 and 3 h, respectively, their release behaviors follow Higuchi’s kinetic model (Figs. 4b and 5b) where initially, they conform to zero-order equation (Figs. 4a and 5a). The related regression correlation coefficients (R2) are summarized in Table V. Additionally, the slope of (Qt − t0.5) in double-layer devices is sharper than in single-layer devices. This can be related to C0 because according to Eq. 3, the cumulative drug release is directly proportional to C0, so by increasing C0, the slope (Qt − t0.5) is increased. By considering that the initial drug concentration in skin contact layer of double-layer devices is less sharp than the single-layer devices, it may be concluded that the slope (Qt − t0.5) becomes low in double layer.
Table V.
Slope of (Q t − t 0.5) According to Higuchi Equation and Regression Correlation Coefficient (R 2) of Double-Layer and Single-Layer TDDSs
| Number of formulation | Q/t 0.5(µg/h0.5) | R 2 |
|---|---|---|
| 1 | 23 ± 1.5 | 0.98 |
| 2 | 27.6 ± 1.9 | 0.99 |
| 3 | 39.4 ± 2.9 | 0.98 |
| 4a | 21 ± 1.4 | 0.98 |
| 5a | 22 ± 0.7 | 0.99 |
| 6b | 28.7 ± 2.3 | 0.99 |
| 7b | 28.8 ± 1.9 | 0.99 |
| 8 | 39.7 ± 1.4 | 0.98 |
| 9b | 26.5 ± 2.1 | 0.99 |
| 10b | 27.2 ± 2.5 | 0.99 |
aFor formulations 4 and 5, P < 0.05
bFor formulations 6, 7 and 9, 10, P > 0.05
In Fig. 4b, it is observed that in double-layer devices, the slopes of (Qt − t0.5) are different and according to Higuchi equation are equal to (2DCpC0) 0.5. Due to the same material compositions of skin contact layer in all formulations, the DCp value would be the same. Therefore, the different slopes are related to the change of C0 after diffusion equilibrium time because the affinity of fentanyl for diffusing from reservoir to skin contact layer is different.
Considering Figs. 4b and 5b Higuchi equation, the slope of (Qt − t0.5) was calculated and summarized in Table V. Due to hydrogen bonding between fentanyl and functional PSA, by addition of functional PSA to reservoir layer, the diffusion coefficients are reduced; therefore, the diffusion of drug to skin contact layer becomes more difficult. Considering the stronger hydrogen bonding between –COOH and fentanyl compared to –OH, the diffusion of fentanyl from reservoir with –COOH is less than –OH functional group of PSA. As a result, it is expected to have less change in the initial drug concentration of skin contact layer with functional PSA in reservoir layer compared to non-functional PSA. This phenomenon causes the decrease in the slope of Qt − t0.5 in devices containing non-functional, –OH functional, and –COOH functional PSA in the reservoir layer, respectively.
The slope of Qt − t0.5 in single-layer systems is shown in Table V. Accordingly, with the growing amount of functional PSA in the systems, the slope is decreased. By considering P value, it is found that the slope is not dependent on the type of functionality. As stated above, according to Higuchi equation, the slope is proportional to DCpC0. In all single-layer devices, C0 is constant, and consequently, the differences of slopes are related to Cp and D. By addition of functional PSAs, Cp is growing and D is decreased. It is observed that by addition of functional PSA, final DCp is decreased, and as a result, D is more effective than Cp.
Tack and Peel Studies
The result of adhesion properties are shown in Table VI. Generally, the adhesion properties of the single layer are higher than the double layer. It is also found that the interfacial adhesion of double layer is weak, especially when the PSA of double layer is different.
Table VI.
Adhesion Properties of Double-Layer and Single-Layer Devices
| Number of formulation | Peel strength (N/25 mm) | Tack (N/mm2) |
|---|---|---|
| 1 | 0.13 | 1.1 |
| 2 | 0.77 | 2.90 |
| 3 | 1.20 | 2.2 |
| 4 | –a | 0.9 |
| 5 | 0.30 | 1.25 |
| 6 | 3.75 | 3.5 |
| 7 | 3.5 | 3.5 |
| 8 | 1.8 | 2.0 |
| 9 | 4.9 | 3.5 |
| 10 | 5.6 | 3.5 |
aIt was too low for detection by instrument
In double-layer formulations, by increasing percentage of functional PSA, the peel strength and tack values of devices are decreased. In single-layer devices, by increasing the functional PSAs content, peel strength values grow. Tack values in single layer were constant and not dependent on functionality of PSA.
CONCLUSION
It is found that the membrane-controlling layer has a significant effect on drug release and rate of skin permeation. It is also found that the rate of skin permeation and drug release are decreased using functional PSAs in the formulations. The double-layer devices have nearly constant skin permeation rate. Formulations with the highest percentage of –COOH functional PSA have resulted in the lowest flux.
Release profiles of double- and single-layer devices follow the Higuchi’s kinetic model. The double-layer device with non-functional PSA has a suitable constant skin permeation rate and acceptable adhesion properties.
Acknowledgments
Support of this work from Iran Polymer and Petrochemical Institute is appreciated, and thanks are due to Ms. Houri Mivehchi for editing the manuscript.
References
- 1.Mehdizadeh A, Ghahremani MH, Rouini MR, Toliyat T. Effects of pressure sensitive adhesives and chemical permeation enhancers on permeability of fentanyl through excised rat skin. Acta Pharm. 2006;56:219–229. [PubMed] [Google Scholar]
- 2.Boucand A, Machet L, Arbeille B, Machet MC, Sourance M, Mavon A, Patat F, Vaillant L. Ultrasound-enhanced transderam transport of fentanyl and caffeine across human and hairless rat skin. Int J Pharm. 2001;228:69–77. doi: 10.1016/S0378-5173(01)00820-1. [DOI] [PubMed] [Google Scholar]
- 3.Collaud S, Warloe T, Jordan O, Gurny R, Lange N. Clinical evaluation of bioadhesive hydrogels for topical delivery of hexylaminolevulinate to Barrett’s esophagus. J Control Release. 2007;2(3):203–210. doi: 10.1016/j.jconrel.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Cichewicz DL, Welch SP, Smith FL. Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal delta9-tetrahydrocannabinol. Eur J Pharmacol. 2005;525(1–3):74–82. doi: 10.1016/j.ejphar.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Goicoechea C, Sánchez E, Cano C, Jagerovic N, Martín MI. Algesic activity and pharmacological characterization of N-[1-phenylpyrazol-3-yl]-N-[1-(2-phenethyl)-4-piperidyl] propenamide, a new opioid agonist acting peripherally. Eur J Pharmacol. 2008;595:22–29. doi: 10.1016/j.ejphar.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Marinangeli F, Ciccozzi A, Aloisio L, Colangeli A, Paladini A, Bajocco Ch, Coaccioli S, Varrassi G. Improved cancer pain treatment using combined fentanyl-TTS and tramadol. Pain Practice. 2007;7(4):307–312. doi: 10.1111/j.1533-2500.2007.00155.x. [DOI] [PubMed] [Google Scholar]
- 7.Consuelo IDL, Falson F, Guy RH, Jasques Y. Application of microemulsions in dermal and transdermal drug delivery. J Control Rel. 2007;122:135–140. doi: 10.1016/j.jconrel.2007.05.017. [DOI] [Google Scholar]
- 8.Mehdizadeh A, Toliate T, Rouni MR, Abaszadeh SH, Dorkoosh F. Design and in vitro evaluation of new drug-in-adhesive formulations of fentanyl transdermal patches. Acta Pharm. 2004;54:301–317. [PubMed] [Google Scholar]
- 9.Stefano FJE, Scasso AF, inventors; Stites & Haribson PLLC, assignee. Transdermal delivery system with two superimposed adhesive layers having different affinities to the active substance comprised. United States Patent US 20050208116 A1. 2005 Sep 22.
- 10.Panchagnula R. Transdermal delivery of drugs. Ind J Pharmacol. 1997;29:140–156. [Google Scholar]
- 11.Stefano FJE, Scasso AF, inventors; Thalas Group Incorporated, assignee. Transdermal delivery device for the administration of fentanyl. WO Patent WO 03/097008 A2. 2003 Nov 27.
- 12.Taghizadeh SM, Soroushnia A, Mirzadeh H, Barikani M. Preparation and in vitro evaluation of a new fentanyl patch based on acrylic/silicone pressure-sensitive adhesive blends. Drug Dev Ind Pharm. 2009;35:487–498. doi: 10.1080/03639040802448638. [DOI] [PubMed] [Google Scholar]
- 13.Urquhart J,Chandrasekaran SK, Shaw JE, inventors, Alza Corporation, assignee. Bandage for transdermally administering scopolamine to prevent nausea. United States Patent US 4,031,894. 1976 June 28.
- 14.Urquhart J, Chandrasekaran SK, Shaw JE, inventors, Alza Corporation, assigne. Method and therapeutic system for providing chemotherapy transdermally. United States Patent US 4,060,084. 1977 Nov. 29.
- 15.Urquhart J, Chandrasekaran SK, Shaw JE, inventors, Alza Corporation, assignee. Method and therapeutic system for administering scopolamine transdermally. United States Patent US 4,262,003.1981 Apr.14.
- 16.Valenta C, Auner BG, Loibl I. Skin permeation and stability studies of 5-aminolevulinic acid in a new gel and patch preparation. J Control Release. 2005;107(3):495–501. doi: 10.1016/j.jconrel.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Fana Q, Sirkar KK, Wangb Y, Michniakc B. In vitro delivery of doxycycline hydrochloride based on a porous membrane-based aqueous-organic partitioning system. J Control Release. 2004;98:355–365. doi: 10.1016/j.jconrel.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Roy SD, Hou SY, Witham SL, Flynn GL. Transdermal delivery of narcotic analgesics: comparative metabolism and permeability of human cadaver skin and hairless mouse skin. J Pharm Sci. 1994;83:1723–1728. doi: 10.1002/jps.2600831215. [DOI] [PubMed] [Google Scholar]
- 19.Roy SD, Flynn GL, Gutierrez M, Cleary GW. Characterizations of pressure-sensitive adhesives for matrix patch design. J Pharm Sci. 1996;85(5):491–495. doi: 10.1021/js950415w. [DOI] [PubMed] [Google Scholar]
- 20.Kaliaa YN, Guya RH. Modeling transdermal drug release. Adv Drug Deliv Rev. 2001;48:159–172. doi: 10.1016/S0169-409X(01)00113-2. [DOI] [PubMed] [Google Scholar]
- 21.Papadokostaki KG, Petropoulos JH. Kinetics of release of a model disperse dye from supersaturated cellulose acetate matrices. J Control Release. 1998;54:251–264. doi: 10.1016/S0168-3659(97)00158-2. [DOI] [PubMed] [Google Scholar]