Abstract
The Food and Drug Administration (FDA) approved the New Drug Application for Wellbutrin sustained release (SR) 100 mg tablets on October 4, 1996. However, by 1998, the FDA expressed concern about the stability of this drug product based on an increase in the dissolution profile on storage. Data submitted in the annual report showed that this drug product could not meet the expiry of 18 months at the International Committee on Harmonization storage condition of 25°C/60% relative humidity. The FDA mandated a 12-month expiry and GlaxoWellcome tightened this further by instituting an expiry of 9 months. The FDA also requested a long-term solution to the stability of Wellbutrin SR 100 mg tablets. Investigations via colloidal solutions revealed that the dissolution rate increase on storage occurred due to acid hydrolysis of the release controlling polymer. This drug product was successfully reformulated by slowing the initial dissolution rate and having an increased ratio of release controlling polymer to acid stabilizer. The reformulation used the same ingredients and manufacturing unit processes as the original formulation. The reformulated drug product was approved by the FDA on October 11, 2000 with an 18-month shelf-life. The shelf-life was extended to 36 months in an annual update to the FDA on December 1, 2005.
Key words: bupropion, dissolution, hypromellose, stability, Wellbutrin
INTRODUCTION
The Food and Drug Administration (FDA) approved the New Drug Application for Wellbutrin sustained release (SR) 50, 100, and 150 mg tablets on October 4, 1996. Subsequently, the 100- and 150-mg strengths were launched commercially. The 50-mg strength was never launched due to changes in this strength’s dissolution specifications stipulated in the approval by the FDA. In the approval, the FDA mandated the dissolution specifications be 25–45% released at the first hour, 60–85% released at the fourth hour, and not less than 80% released at the eighth hour for all strengths.
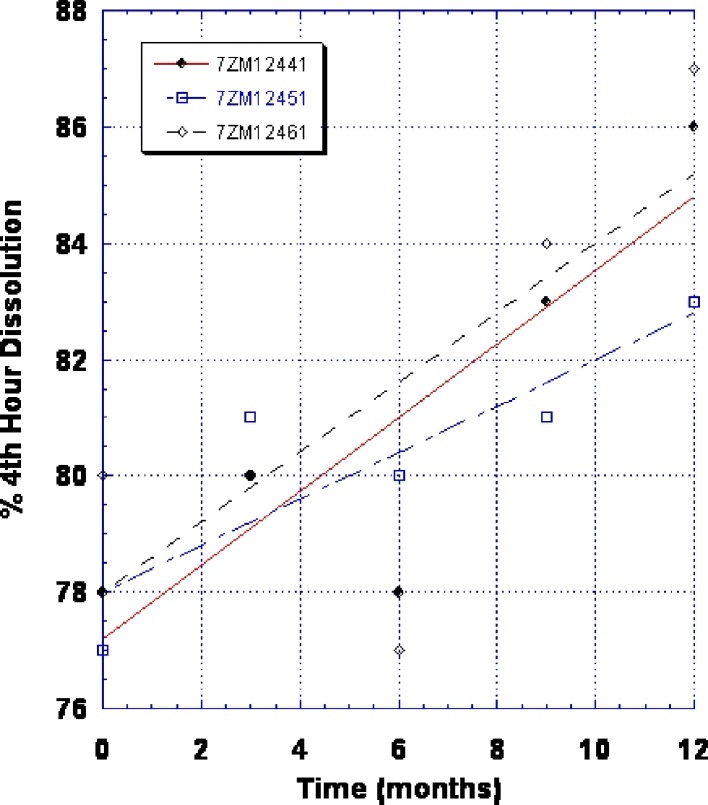
By 1998, the FDA expressed concern about the stability of the 100-mg SR strength based on this tablet’s increase in dissolution profile on storage. Figure 1 shows the increase in dissolution upon storage at 25°C/60% relative humidity (RH) for the original Wellbutrin SR 100 mg tablets. The agency had no concerns with the 150-mg SR strength. Data submitted in the annual report for the 100-mg SR tablet showed that it could not meet the expiry of 18 months at the International Committee on Harmonization storage condition of 25°C/60% RH. The FDA mandated a 12-month expiry. GlaxoWellcome instituted an internal expiry of 9 months. Packaging alternatives were investigated but were not successful in alleviating this problem. The FDA then requested a long-term solution to the stability of Wellbutrin SR 100 mg tablets.
Fig. 1.
Change in dissolution (% fourth-hour release) for original Wellbutrin SR 100 mg tablet (three commercial batches—lot nos. 7ZM12441, 7ZM12451, and 7ZM12461) after storage at 25°C/60% RH
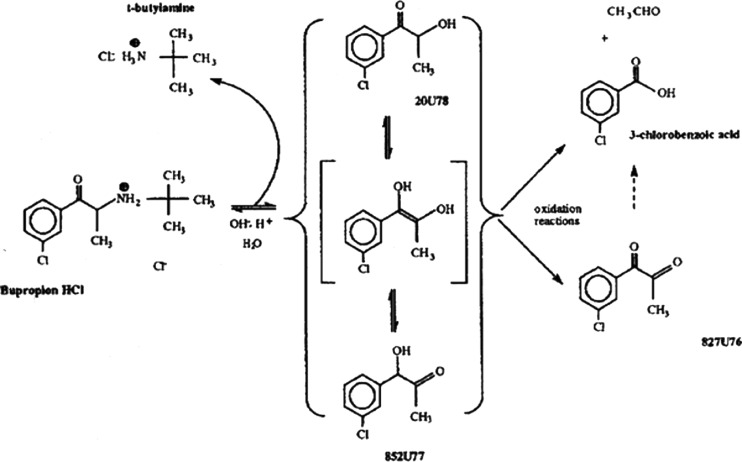
Reformulation of a product in an existing product line meant certain constraints on the reformulated product. In general, bupropion hydrochloride is very unstable when diluted with excipients. Figure 2 shows the degradation pathway for this molecule. Preventing the initial hydrolysis reaction is key to chemical stability. Cysteine hydrochloride stabilizes bupropion hydrochloride in the SR tablet formulations by providing an acidic environment, stabilizing the protonated amine. Even though the original Wellbutrin SR 100 mg tablet was unstable with respect to release rate, bupropion hydrochloride was chemically stable in this drug product. Therefore, any reformulation must not create instability in bupropion hydrochloride. Any reformulation would ideally conform to the product line image already established, i.e., round, biconvex tablets no larger in size than the commercial SR 150-mg strength. To speed reformulation and subsequent regulatory approval, any reformulation would also ideally involve little or no compositional or process changes.
Fig. 2.
Degradation pathway of bupropion hydrochloride
Intellectual property had been established for this drug product (1–6). These patents and patent applications disclosed chemically stabilizing bupropion hydrochloride and means of modifying its release from a dosage form. If compositional and processing changes were minimized, the reformulated product may be able to be protected by the existing patents and patent applications. The objective of this investigation was to understand the cause of this dissolution increase on storage and successfully reformulate this drug product to provide a minimum of 18 months shelf-life.
Literature Findings and Formulation Review
From a simplistic standpoint, any change in dissolution profile on stability would indicate some change in the release controlling excipient, hypromellose. Literature at that time from the vendor did give some indication of instability of hypromellose in the presence of very strong acids (7). This reference indicated stability even with hydrochloride or acid salt drugs. However, it also mentioned one customer getting hydrolysis of the ether linkages during storage when combined with a dihydrochloride salt form using a wet granulation process. The reduced viscosity from hydrolysis of the ether linkages resulted in faster dissolution. Alderman made similar statements on the acid stability and instability of hypromellose in a review article on cellulose ethers (8).
Other references also appeared to point to acid hydrolysis of hypromellose as a likely cause for faster dissolution. Shinetsu Chem. Ind. Co. of Japan holds a patent on manufacturing low molecular weight cellulose ether (9). The low molecular weight cellulose ether is prepared by reaction of high molecular weight cellulose ether in the presence of aqueous hydrochloric acid solution and/or gas.
Mitchell et al. found that a 1% (w/v) aqueous solution of tetracycline hydrochloride in hypromellose (approximately pH 1.8) exhibited a decrease in intrinsic viscosity with time (10). Previous studies by Mitchell et al. showed that a 0.1-M hydrochloric acid solution would hydrolyze hypromellose (11). Therefore, they concluded that the reduction in intrinsic viscosity with the tetracycline hydrochloride solution was also due to a decrease in molecular weight caused by acid hydrolysis of hypromellose.
These findings certainly supported acid hydrolysis of hypromellose leading to an increase in dissolution for the original Wellbutrin SR 100 mg tablets. However, it also raised an important question—why didn’t the SR 150-mg strength show this same problem? In reviewing Table I, the SR 150-mg strength had a lower percent weight per weight of hypromellose. Therefore, the level of hypromellose in the tablet was not the issue in and of itself. Upon further examination, the SR 150-mg strength also had a lower level of cysteine hydrochloride. In computing the ratio of hypromellose to cysteine hydrochloride, one detects a significant difference between the original SR 100-mg strength and the SR 150-mg strength, 3.3 vs. 5.3, respectively. If having too low a ratio increased the hydrolysis of hypromellose, it may be possible to have a stable, reformulated product using the same excipients and same process. In reformulating, one would want to assure that the ratio of hypromellose to cysteine hydrochloride was at least as high as that for the SR 150-mg strength.
Table I.
Composition and Attributes of Wellbutrin SR 100 and 150 mg Tablets
| Ingredient | Original 100-mg strength (%) | Reformulated 100-mg strength (%) | 150-mg strength (%) |
|---|---|---|---|
| Bupropion hydrochloride | 37.0 | 30.8 | 37.5 |
| Hypromellose 2910, NF | 20.0 | 33.1 | 10.0 |
| Microcrystalline cellulose, NF | 36.0 | 29.0 | 49.6 |
| Cysteine hydrochloride, monohydrate, USP | 6.0 | 6.0 | 1.9 |
| Mg stearate, NF | 1.0 | 1.1 | 1.0 |
| Total core tablet weight (mg) | 270 | 325 | 400 |
| Core tablet diameter (mm) | 9.4 | 10 | 11 |
| Hypromellose 2910/cysteine hydrochloride ratio | 3.3 | 5.5 | 5.3 |
USP US Pharmacopeia, NF National Formulary
MATERIALS AND METHODS
Bupropion hydrochloride was obtained from Catalytica Pharmaceuticals Inc., Greenville, NC, USA. Hypromellose 2910, US Pharmacopeia (USP; Methocel™ E4M Premium CR Grade) was obtained from Dow Chemical Company, Midland, MI, USA. Cysteine HCl, USP was obtained from Sochinaz SA, Switzerland. Microcrystalline cellulose, National Formulary (NF; Avicel® PH 102) was obtained from FMC Corp., Pharmaceutical Division, Philadelphia, PA, USA. Magnesium stearate, NF was obtained from AKCROS Chemicals, New Brunswick, NJ, USA. Opadry® Blue YS-1-4282 was obtained from Colorcon, Inc., West Point, PA, USA. Carnauba wax, NF was obtained from Strahl & Pitsch Inc., West Babylon, NY, USA. Edible Black Ink was obtained from Colorcon Inc. or TEK Products & Services, Inc.
Tablet Formulation, Manufacturing, and Packaging
Table I gives the composition and attributes of the uncoated, original, and reformulated Wellbutrin SR 100 mg tablets, as well as that of the Wellbutrin SR 150 mg tablets. To manufacture these tablets, bupropion hydrochloride, microcrystalline cellulose, and hypromellose are weighed, sieved through a US standard 20 mesh screen, then placed into a cube blender, and blended for 20 min. This blend is then placed into a fluid bed, top spray granulated, and then dried. The initial spray solution is a 24.3% cysteine hydrochloride solution in Purified Water, USP followed by additional Purified Water, USP. The granulation is dried to a moisture endpoint of 0.5–1.5% loss on drying. Magnesium stearate is then added via a paddle to the granules in the bowl of the fluid bed, and this material is passed through a Quadro® Comil® with a 0.055″ screen into a cube blender. This material is then blended for 5 min. The lubricated blend is then compressed on a suitable rotary tablet press. Core tablets are then charged into a perforated coating pan and film-coated with Opadry YS-1-4282 until a theoretical application weight of ∼4% weight gain is achieved. After coating, tablets are allowed to cool in the coating pan until an exhaust temperature of less than 35°C is reached. Carnauba wax is then added (0.01% of core tablet weight) to the pan, and the tablets are rolled for an additional 10 min. The coated tablets are then printed with edible black ink specifying “WELLBUTRIN SR” and the strength of tablet in milligrams. Tablets are packaged in high-density polyethylene, 50 mL round, white, opaque bottles containing a combination charcoal/silica gel desiccant canister and closed with a child-resistant closure that includes an induction, heat-sealed membrane using suitable packaging machinery. Manufacturing and packaging occurs in a temperature- and humidity-controlled environment where the humidity is typically no greater than 50% RH at 20°C.
Dissolution Testing
Dissolution testing was performed according to the USP/NF official monograph for bupropion hydrochloride extended-release tablets (USP 31-NF 26), test 1—for products labeled for dosing every 12 h.
Colloidal Solution Preparation
Colloidal solutions of hypromellose were prepared by initially adding a portion of the total water needed to a beaker. After heating the water to approximately 80°C, hypromellose was slowly added with stirring to disperse and hydrate the polymer. In a separate container, any bupropion hydrochloride and/or cysteine hydrochloride needed for a solution was dissolved to form a clear solution with a minimum amount of water. This solution containing bupropion hydrochloride and/or cysteine hydrochloride was then added to the dispersed/hydrated hypromellose. To finish the solution preparation, the remaining water was added to the hypromellose solution, stirred, and then cooled to room temperature. These colloidal solutions were stored in 8 oz. polypropylene cups with lids at 30°C in a convection oven for up to 14 days.
Viscosity and pH Measurements
Colloidal solutions were allowed to cool to room temperature before any measurements were taken. Measurement of solution pH was done with an Orion Model 920A pH meter. Viscosity measurements were done with a Brookfield Viscometer with Spindle #2 and a spindle speed of 6 rpm. Viscosity and pH measurements were done at time 0 and after 14 days storage at 30°C.
Design of Experiments
To test the hypothesis that cysteine hydrochloride may be providing an acidic environment, resulting in hydrolysis of hypromellose, an experimental design using colloidal solutions was constructed. A D-optimal design was chosen for this study because two of the factors were measured at three levels and this design would allow fewer runs while still being able to estimate all the factors of interest. Factors and levels used in this study are given in Table II. Table III gives the composition of the solutions prepared for this study. Bupropion hydrochloride was also used in this experiment because it is a soluble component and is a salt of a strong acid and weak base, contributing to an acidic pH. The 2% concentration of hypromellose was chosen based on the use of 2% hypromellose for standard viscosity determinations. The lower level of 1% hypromellose was chosen to permit at least two levels of hypromellose to be tested. The high levels of bupropion hydrochloride and cysteine hydrochloride were chosen such that the ratio of bupropion hydrochloride/hypromellose/cysteine hydrochloride was identical to this ratio in the original Wellbutrin SR 100 mg tablet. The low levels of zero for these two ingredients were chosen to help differentiate the effect of bupropion hydrochloride vs. cysteine hydrochloride.
Table II.
Viscosity and pH D-Optimal Design Factors and Levels
| Factor | Low level (%, w/w) | Middle level (%, w/w) | High level (%, w/w) |
|---|---|---|---|
| Hypromellose 2910 | 1 | N/A | 2 |
| Bupropion hydrochloride | 0 | 1.85 | 3.7 |
| Cysteine hydrochloride | 0 | 0.3 | 0.6 |
N/A not applicable
Table III.
D-Optimal Design—Composition of Solutions
| Formulation no. | % Hypromellose | % Bupropion HCl | % Cysteine HCl |
|---|---|---|---|
| 1 | 1 | 3.70 | 0 |
| 2 | 2 | 0 | 0.3 |
| 3 | 2 | 0 | 0 |
| 4 | 1 | 1.85 | 0.3 |
| 5 | 1 | 3.70 | 0.6 |
| 6 | 2 | 3.70 | 0.6 |
| 7 | 1 | 1.85 | 0.3 |
| 8 | 2 | 0 | 0.6 |
| 9 | 2 | 1.85 | 0 |
| 10 | 1 | 0 | 0 |
| 11 | 1 | 0 | 0.6 |
| 12 | 2 | 3.70 | 0 |
HCl hydrogen chloride
RESULTS AND DISCUSSION
Viscosity and pH Study
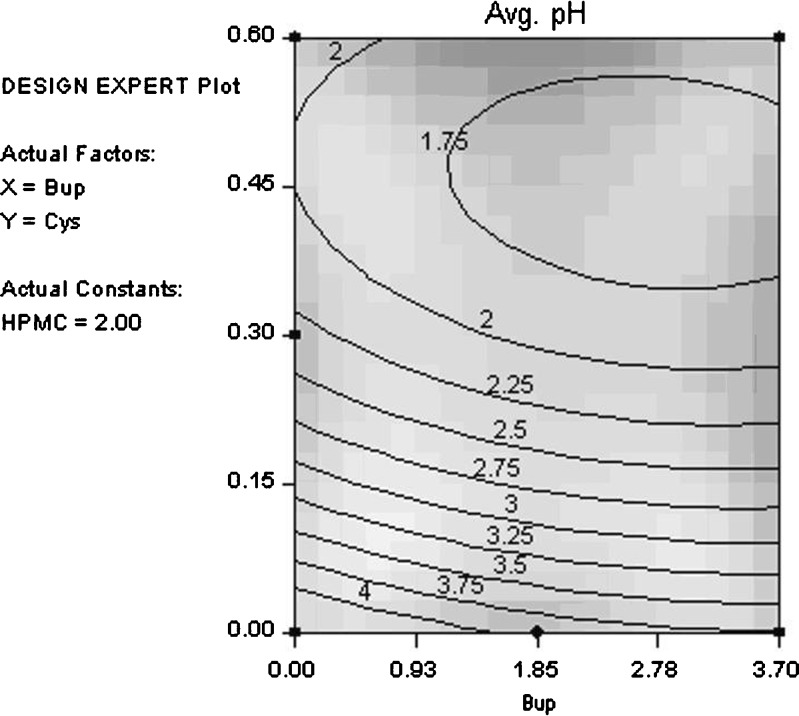
Table IV gives the statistical analysis for pH. No significant change in pH was observed in the solutions over the 14-day study period. Therefore, pH values measured in the study were averaged for statistical analysis. Table IV indicates that while all three components have some statistically significant effect on sample pH, cysteine hydrochloride has by far the biggest impact. This is expected because hypromellose is nonionic, bupropion hydrochloride has a pKa of 7.9, and cysteine hydrochloride has a pKa1 of 1.71. Figure 3 shows the average pH contour plot (cysteine hydrochloride vs. bupropion hydrochloride) for 2% hypromellose. Large changes in pH are seen with changes in cysteine hydrochloride concentration. Note that the shading in this contour plot and all contour plots in this article correspond to the degree of confidence of fit, i.e., light areas possess a higher degree of confidence in the model fit.
Table IV.
p Values for Statistically Significant Effects on pH: Adjusted R 2 = 0.9998, Lack-of-Fit p Value = 0.5361
| Effect | Coefficient | p value |
|---|---|---|
| Intercept | 5.3389 | |
| A | −0.4562 | <0.0001 |
| B | −0.3557 | <0.0001 |
| C | −11.7212 | <0.0001 |
| B2 | 0.0476 | 0.0003 |
| C2 | 10.5614 | <0.0001 |
| AB | ||
| AC | 0.7843 | <0.0001 |
| BC | 0.2027 | <0.0001 |
A hypromellose 2910, B bupropion hydrochloride, C cysteine hydrochloride
Fig. 3.
Average pH contour plot (cysteine hydrochloride vs. bupropion hydrochloride) for 2% hypromellose
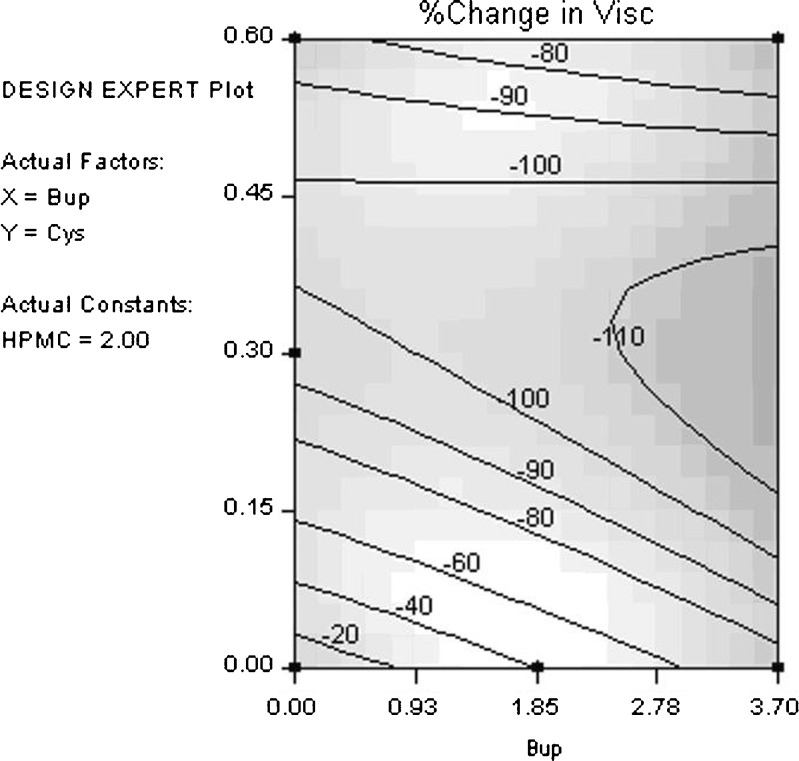
Table V gives the statistical analysis for percentage change in viscosity (average at 14 days vs. initial). Cysteine hydrochloride clearly has the largest impact on producing a significant reduction in viscosity with time. This reduction in viscosity indicates a loss in molecular weight of hypromellose (12). The most likely cause of this loss in molecular weight is hydrolysis of hypromellose. Figure 4 shows the percentage change in viscosity contour plot (cysteine hydrochloride vs. bupropion hydrochloride) for 2% hypromellose. Large changes in viscosity are seen with changes in cysteine hydrochloride concentration.
Table V.
p Values for Statistically Significant Effects on Percentage Change in Viscosity: Adjusted R 2 = 0.9568, Lack-of-Fit p Value = 0.7482
| Effect | Coefficient | p value |
|---|---|---|
| Intercept | 79.2050 | |
| A | −42.8383 | 0.0013 |
| B | −32.2734 | 0.0009 |
| C | −457.6078 | 0.0015 |
| B2 | ||
| C2 | 551.0650 | 0.0003 |
| AB | 7.0822 | 0.0554 |
| AC | ||
| BC | 39.1617 | 0.0005 |
A hypromellose 2910, B bupropion hydrochloride, C cysteine hydrochloride
Fig. 4.
Percentage change in viscosity contour plot (cysteine hydrochloride vs. bupropion hydrochloride) for 2% hypromellose
Tablet Reformulation
The most desirable reformulation would be one using the same excipients and process as the FDA-approved formulations. Another desired attribute would be for the reformulated tablet to have a slower dissolution profile. Having a slower dissolution profile would mean that even if some increase occurred, the shelf-life of the product may be extended because it is starting from a lower point in the dissolution specifications. The reformulation should have a hypromellose to cysteine hydrochloride ratio as high or higher than that for the SR 150-mg strength for physical stability of the dosage form. Ultimately, these changes should result in a reformulated tablet with a minimum acceptable shelf-life of 18 months.
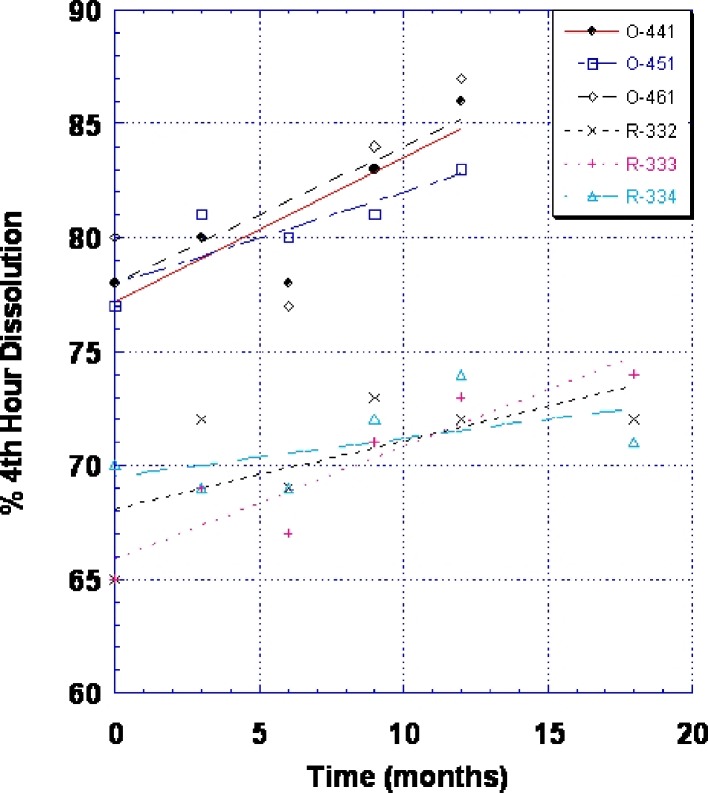
To slow the release, the tablet size and level of release controlling polymer were increased. Table I shows the details of these changes. An increase in the diameter of the reformulated tablet not only permitted the use of additional release controlling polymer but also decreased the surface area to volume ratio of the tablet, thereby reducing the dissolution rate, as shown in Fig. 5. The reformulated tablet was also similar in product image (round, biconvex) and smaller in size than the 150-mg SR strength.
Fig. 5.
Change in dissolution (% fourth-hour release) for original Wellbutrin SR 100 mg tablet (three commercial batches—lot nos. O-441, O-451, and O-461) and reformulated Wellbutrin SR 100 mg tablet (three commercial batches—lot nos. R-332, R-333, and R-334) after storage at 25°C/60% RH
One could propose that another way to increase the ratio of hypromellose to cysteine hydrochloride would be to lower the amount of cysteine hydrochloride. This change would then decrease the hydrolysis of the hypromellose. This approach was not taken for two reasons. First, development of Wellbutrin SR 50, 100, and 150 mg tablets occurred by different individuals at different times. Studies had been conducted indicating that the 100-mg strength required 6% cysteine hydrochloride to chemically stabilize bupropion hydrochloride. It was a conscious decision not to reopen this aspect of the work in reformulation since chemical stabilization of bupropion hydrochloride had been such a significant challenge. Second, because of the desire to slow the initial release rate of these tablets, it naturally leads the reformulation effort to increase the level of hypromellose in the tablet and to dilute the bupropion hydrochloride further in the rate controlling matrix via a larger tablet size.
Figure 5 also shows that the reformulated product not only had a lower initial percentage dissolved at 4 h but it also shows that the absolute slope of the change in percentage dissolved at 4 h vs. storage at 25°C/60% RH was smaller. Statistical analyses of these two factors show that they were both statistically significantly different than the original 100-mg SR strength. Details of the statistical analyses can be found in Tables VI, VII, VIII, and IX.
Table VI.
t Test Comparing Reformulation vs. Original Formulation Initial 4-h Dissolution, Assuming Equal Variances
| Difference | t test | df | Prob > |t| | |
|---|---|---|---|---|
| Estimate | −11.6667 | −6.187 | 4 | 0.0035 |
| Standard error | 1.8856 | |||
| Lower 95% | −16.9020 | |||
| Upper 95% | −6.4314 |
df degrees of freedom
Table VII.
Means and Standard Deviations of Reformulation vs. Original Formulation Initial 4-h Dissolution
| Level | Number | Mean | Standard deviation | Standard error mean | Lower 95% | Upper 95% |
|---|---|---|---|---|---|---|
| Reformulation | 3 | 66.6667 | 2.88675 | 1.6667 | 62.039 | 71.294 |
| Original formulation | 3 | 78.3333 | 1.52753 | 0.8819 | 75.885 | 80.782 |
Table VIII.
t Test Comparing Reformulation vs. Original Formulation Absolute Slope Comparison, Assuming Equal Variances
| Difference | t test | df | Prob > |t| | |
|---|---|---|---|---|
| Estimate | −0.24468 | −3.087 | 4 | 0.0367 |
| Standard error | 0.079256 | |||
| Lower 95% | −0.46473 | |||
| Upper 95% | −0.02463 |
df degrees of freedom
Table IX.
Means and Standard Deviations of Reformulation vs. Original Formulation Absolute Slope Comparison
| Level | Number | Mean | Standard deviation | Standard error mean | Lower 95% | Upper 95% |
|---|---|---|---|---|---|---|
| Reformulation | 3 | 0.313493 | 0.059921 | 0.03460 | 0.21744 | 0.40954 |
| Original formulation | 3 | 0.558173 | 0.123507 | 0.07131 | 0.36019 | 0.75615 |
CONCLUSIONS
Wellbutrin SR 100 mg tablets were successfully reformulated. The reformulated drug product used the same ingredients and same manufacturing unit processes as the original formulation. The reformulated tablet had a significantly slower initial dissolution rate and a slower increase in dissolution rate on storage. Key to reformulating the product was the knowledge that hydrolysis of the release controlling polymer was occurring in the original formulation and that this could be minimized by increasing the ratio of hypromellose to cysteine hydrochloride.
A Prior Approval Supplement (PAS) was filed with the FDA on June 13, 2000 with 9 months stability data and pharmacokinetic data in humans showing it to be bioequivalent to the original Wellbutrin SR 100 mg tablets. This PAS was updated with 12 months stability data on August 29, 2000. The FDA approved the reformulation on October 11, 2000 and granted an 18-month shelf-life. This shelf-life was extended to 36 months in this drug product’s annual update to the FDA in December 2005.
Acknowledgments
The authors would like to thank GlaxoSmithKline for allowing publication of this manuscript and the numerous Burroughs-Wellcome, GlaxoWellcome, and GlaxoSmithKline employees who assisted in the commercialization of Wellbutrin SR tablets.
References
- 1.Ruff MD, et al. Pharmaceutical composition containing bupropion hydrochloride and a stabilizer. U.S. patent no. 5,358,970.
- 2.Ruff MD, et al. Stabilized pharmaceutical composition containing bupropion. U.S. patent no. 5,731,000.
- 3.Ruff MD, et al. Stabilized pharmaceutical. U.S. patent no. 5,541,231.
- 4.Baker RW, et al. Pharmaceutical delivery system. U.S. patent no. RE. 33,994.
- 5.Ludwig JSG, et al. Controlled sustained release tablets containing Bupropion. U.S. patent no. 5,427,798.
- 6.Ruff MD, et al. Stabilized pharmaceutical. U.S. patent no. 5,763,493.
- 7.The Dow Chemical Company . Formulating for controlled release with METHOCEL premium cellulose ethers, form no. 198-1029-997GW, page 19. Midland: The Dow Chemical Company; 1989. [Google Scholar]
- 8.Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm Tech & Prod Mfr. 1984;5(3):1–9. [Google Scholar]
- 9.Tanaka T, Tamura Y (Shinetsu Chem. Ind. Co., Japan). Manufacture of low molecular weight cellulose ether. Application JP 95-114619 950512.
- 10.Mitchell K, Ford JL, Armstrong DJ, Elliott PNC, Hogan JE, Rostron C. The influence of drugs on the properties of gels and swelling characteristics of matrices containing methylcellulose or hydroxypropylmethylcellulose. Int J Pharm. 1993;100:165–73. doi: 10.1016/0378-5173(93)90087-V. [DOI] [Google Scholar]
- 11.Mitchell K. A study of factors controlling drug release from matrices containing cellulose ethers. Ph.D. thesis no. DX186471, Liverpool J. Moores University, 1992.
- 12.The Dow Chemical Company . METHOCEL cellulose ethers—technical handbook, form no. 192-1062-791-JB. Midland: The Dow Chemical Company; 1988. p. 21. [Google Scholar]