Abstract
Extrudates based on varying ratios of the triglyceride tripalmitin and the hydrophilic polymer polyethylene glycol as matrix formers were produced as oral dosage forms with controlled release characteristics. The extrudates were processed below the melting points of the excipients and contained the hydrophobic model drug chloramphenicol. The influence of the ratio of the matrix formers on drug dissolution was investigated, with an increase in the water-soluble polymer content increasing the drug release rate. In addition, the effect of varying the extrusion process on the extrudate structure and drug dissolution was investigated. Two-step extrusion was performed, which comprised an initial extrusion step of drug and one matrix component followed by milling these extrudates and a second extrusion step for the milled extrudates mixed with the second matrix component. Initial extrusion with polyethylene glycol led to increased dissolution rates, while initial extrusion with tripalmitin led to decreased dissolution rates compared to the dissolution characteristics of extrudates containing the same composition produced by one-step extrusion. Thus, two-step solid lipid extrusion can successfully be used as a process to modify the dissolution behavior of extrudates.
Keywords: dissolution, polyethylene glycol, process variations, solid lipid extrusion, triglyceride
INTRODUCTION
As active pharmaceutical ingredients (APIs) are usually administered orally, there have been many approaches for the development of new oral controlled release systems. In general, such dosage forms aim to avoid peaks in drug plasma concentration, sustain the release of the drug, and increase patient convenience. Established formulation approaches include the production of less soluble forms of the API, ion exchangers (1), and the generation of membranes (2) or matrices (3) as drug diffusion barriers. The release of the API is controlled by diffusion, erosion of the dosage form, or osmotic pressure (4). Matrix dosage forms, in which the API is embedded in suitable pharmaceutical excipients, can be produced with different processes. Such dosage forms cover a broad range of structures ranging from monolithic dosage forms (5) to nanoparticles (6,7) and films (8–10). Additional release barriers can be formed by coatings (11). The release characteristics of the final dosage form can be influenced by the choice of the excipients (12) as well as by the alteration of process parameters (13,14).
Besides the versatile group of polymers (15), lipids show appropriate properties as excipients for controlled release dosage forms (16–19). In addition to controlling drug release, lipids can increase the bioavailability of drugs (20,21) and mask unpleasant taste (22). Lipid-based dosage forms can be processed by different methods. A relatively new approach is solid lipid extrusion, in which powder mixtures of API and lipids are extruded below their melting points and the crystalline structures of the substances are maintained (23–26).
Hydrophilic polymers like polyethylene glycol have been used as pore formers and thus release modifiers in non-eroding matrices (27,28), and therefore, matrices consisting of lipid and polyethylene glycol are promising dosage forms with tailor-made dissolution profiles over a broad range of different profiles. Studies have been performed for a hydrophilic model drug, with the resulting dosage forms being physically stable, and by varying the ratio of the two matrix formers, a broad range of different dissolution profiles could be obtained (29,30).
The aim of this study was to apply the lipid/polyethylene glycol matrix system to a hydrophobic model drug (chloramphenicol) in different ratios using the process of solid lipid extrusion. Solid-state characterization with differential scanning calorimetry (DSC) and X-ray powder diffraction (XRPD) was performed on the powders and extrudates, as the physical stability is a crucial prerequisite for predictable drug release. In a second step, the extrusion process was modified as the variation of the manufacturing process parameters can have a pronounced effect on the dissolution behavior of the dosage form. In this study, three batches with the same chemical composition (chloramphenicol, tripalmitin, and polyethylene glycol in a 2:1:1 w/w/w ratio) were extruded using three different methods. One batch was extruded in a one-step extrusion process. The other two batches were produced in a two-step extrusion process, a method which is applied in polymer sciences (31). The powdered API was initially extruded with one of the matrix formers (lipid or polyethylene glycol). The extrudate was then milled down to granules and extruded again in combination with the second powdered matrix component. The three batches were investigated with regard to their physical structure. Dissolution testing was performed to investigate the influence of the process variation on dissolution characteristics. In addition, water vapor sorption testing and stability testing in accelerated conditions (40°C/75% relative humidity (RH)) were performed on each batch.
MATERIALS AND METHODS
Materials
The pure powdered monoacid triglyceride tripalmitin (Dynasan 116®, Sasol, Witten, Germany) and powdered polyethylene glycol with a mean molecular weight of 10,000 (Polyglykol 10000 P®, Clariant, Sulzbach, Germany) were used as excipients as received. Chloramphenicol base, which was provided by Northeast General Pharmaceutical Factory (Shenyang, Liaoning, China), was used as a model drug as received.
Methods
Extrusion
Extrusion was performed with a co-rotating twin-screw extruder (Mikro 27GL-28D, Leistritz, Nürnberg, Germany). The powdered excipients and the powdered model drug were blended for 15 min in laboratory mixer (LM20 Bohle, Ennigerloh, Germany) at a rotational speed of 25 rpm. With a feeding rate of 40 g min−1, these blends were fed from a gravimetric dosing device (KT20K-Tron Soder, Lenzhard, Switzerland) into the barrel of the twin-screw extruder. The screw speed was set at 30 rpm. Depending on the composition of the blend, the temperature of the barrels was chosen individually in a temperature range below the melting ranges of the components. The mass was forced through a die plate containing 23 holes of 1 mm diameter and 2.5 mm length.
Milling and Sieving
Milling was performed on the initial extrudates in the two-step extrusion process with a centrifugal mill ZM 200 (Retsch GmbH, Haan, Germany). A push-fit rotor with 12 teeth was used in combination with a ring sieve with conidur holes (1.5 mm) at 6,000 rpm. The milled extrudates were sieved and the fraction above 200 μm was used for further extrusion processing.
Differential Scanning Calorimetry
Thermogravimetric characterization of the powders and extrudates was conducted using a DSC 821e calorimeter (Mettler-Toledo, Gießen, Germany). The samples (approximately 5 mg) were heated in hermetically sealed aluminum pans in the range of 5°C to 200°C with a heating rate of 10°C min−1. Each sample was measured twice.
X-ray Powder Diffraction
The samples were measured using an X’Pert PRO X-ray diffractometer, PANalytical (MPD PW3040/60XRD; CuKα anode; λ = 1.54443). The samples were gently consolidated in an aluminum holder and scanned at 40 kV and 30 mA from 5° to 30° (2θ) using a scanning step time of 1.9296 s and a step size of 0.0080° (2θ). Each sample was measured in triplicate.
Dissolution
Dissolution testing was performed using a dissolution tester (Sotax AT7 smart, Sotax, Lörrach, Germany) according to the USP 29 method 1. Demineralized water (900 mL) containing 0.001% polysorbate 20 for increased wetting was used as the dissolution medium. The extrudates were cut into cylinders of approximately 1 cm and weighed in portions of 60 mg. The temperature was set at 37 ± 0.5°C and the baskets stirred with a speed of 50 rpm. The dissolved drug was detected using a spectral photometer (Lambda 40, Perkin Elmer, Rodgau-Juegesheim, Germany) at a wavelength of 278 nm by measuring the absorption of the medium each 5 min for 24 h. The dissolution curves were based on the mean of three measurements. The standard deviation was below 3% in all cases.
Storage
The extrudates were stored in a climate chamber (KBF, Binder, Tuttlingen, Germany) in open Petri dishes for a period of 3 months at a temperature of 40°C and a relative humidity of 75%.
Scanning Electron Microscopy
Micrographs were recorded with a scanning electron microscope (Leo 1430VP, Leo Elektron Microscopy, Cambridge, UK) at a working voltage of 20 kV. The extrudates were fixed on aluminum holders using a double-sided carbon tape and were sputter-coated with gold for 180 s (Agar Manual Sputter Coater B 7340, Agar Scientific, Stansted, UK).
Water Vapor Sorption
Using a water vapor sorption analyzer SPS 11-10μ (Projekt Messtechnik, Ulm, Germany), the extrudates were analyzed with regard to their affinity to water. Samples of 1,500 mg were placed in aluminum cups. Measurements were conducted at 25°C in a range of 0–80% RH.
RESULTS AND DISCUSSION
One-Step Extrusion and Solid-State Characterization of Extrudates Containing Lipid and Polyethylene Glycol with a Hydrophobic Model Drug
It has previously been established that extruded matrices consisting of various ratios of the monoacid triglyceride tripalmitin and the hydrophilic polymer polyethylene glycol with the mean molecular weight of 10,000 provide a good basis for controlled release of drugs (26). The extrusion of the three components below their respective melting points led to physically stable matrices. By varying the ratio of the two substances forming the matrix, a broad range of dissolution profiles for the model drug theophylline anhydrate were obtained. In the present study, matrices containing chloramphenicol, a more hydrophobic model drug, were investigated. Extrudates were produced consisting of 50% matrix and 50% drug (w/w). Three different matrix compositions were produced: pure tripalmitin, tripalmitin/polyethylene glycol 9 + 1 (w/w), and tripalmitin/polyethylene glycol 5 + 5 (w/w). The optimal extrusion temperature was found to be 55°C for the pure lipid matrix and 48°C for the mixed matrices. At these temperatures, extrudates with a smooth external appearance could be obtained for each matrix composition.
Stable solid-state structures are required for reproducible drug release profiles from solid lipid extrudates and long-term stability (23). Solid-state characterization was performed on powders and on extrudates. Based on the results of former studies (26) with extrudates containing a combination of triglyceride and polyethylene glycol powders, chemical and physical interactions between the matrix components could be excluded. The results of the solid-state analysis are depicted in Fig. 1. The DSC melting endotherms of the pure powders (Fig. 1a) were detected as follows: tripalmitin in its stable β-form (onset 63°C), polyethylene glycol (onset 61°C), and chloramphenicol (onset 151°C). Each of these values is in good agreement to the previously reported melting points of 66°C for tripalmitin, 62°C for polyethylene glycol, and 151.8°C for chloramphenicol in the literature (32–34). Figure 1b depicts the corresponding extrudates produced using the three powdered substances. For the extrudate containing pure tripalmitin as a matrix former, the melting endotherm of the lipid is sharp with an onset at 62°C indicating that the lipid is in its stable β-form in the extrudate (32). Since the melting endotherms of the two excipients appear at quite similar temperatures (tripalmitin onset 62°C and polyethylene glycol onset 61°C), the specific melting endotherms of the extruded substances form only one combined peak (onset 62°C). The shape of this specific melting endotherm varies according to the mixing ratio, with the melting endotherm of the 5 + 5 (w/w) mixture exhibiting a more pronounced shoulder due to the fact that polyethylene glycol has a slightly lower melting point than tripalmitin (33). The 9 + 1 (w/w) mixture does not show such a pronounced shoulder as polyethylene glycol represents a smaller proportion of the matrix.
Fig. 1.
Physical characterization of powders and extrudates. a DSC thermograms of powders, b DSC thermograms of extrudates, and c XRPD diffractograms of powders and extrudates
In the extrudates, there was no sign of any solid-state changes for both matrix formers. The sharp melting endotherm (onset 151°C) suggests that the drug chloramphenicol remained in its stable crystalline form (34) in the tripalmitin extrudate (Fig. 1b). In the mixed extrudate, the melting endotherm for the drug is broader and flatter (onset 146°C in the 9 + 1 mixture and onset 112°C in the 5 + 5 mixture). This can be attributed to partial dissolution of the drug into the molten polyethylene glycol during the DSC measurement. The crystal structure of chloramphenicol after extrusion in the mixed matrices was confirmed with XRPD measurements (Fig. 1c), indicated by its significant peaks at 15.7°, 20.7°, 24.4°, and 26.1° (2θ) (35). When analyzing extrudates with X-ray diffraction, the sample has to be gently compressed into the sample holder to provide a flat surface, which is necessary for the measurement. Therefore, most extrudate pieces lie flat in the sample holder hence overrepresenting those surfaces to the detector in which the particles are oriented into the transportation direction of the extruder barrel. Differences in peak intensities in powder and extrudate are due to dilution and the mentioned orientation effects. In conclusion, physically stable solid lipid extrudates with varying matrix composition could be produced for the model drug chloramphenicol.
Release of the Model Drug from the Extrudates Containing Lipid and Polyethylene Glycol Produced with One-Step Extrusion
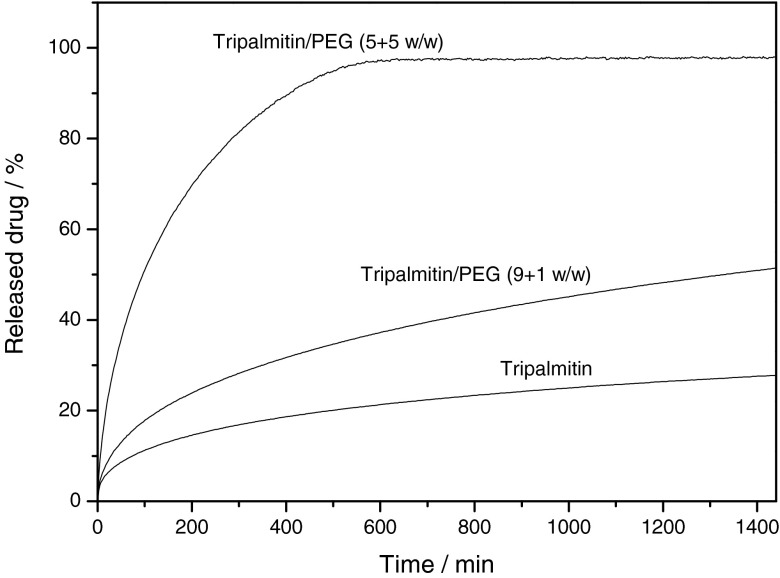
Dissolution testing was performed with the extrudates according to the USP 29 method 1 (basket) for 24 h (Fig. 2). The pure tripalmitin matrix resulted in the slowest release for the model drug. After 24 h less than 30% (w/w) of the embedded drug had been released. During dissolution testing, the lipid matrix stays intact and the drug is released through a pore network in the matrix (36). By adding the water-soluble polymer, polyethylene glycol, the release of the drug was accelerated. This is due to the fact that the polymer is additionally released when the dissolution medium penetrates the matrix, and therefore, the resulting porosity is increased and the tortuosity decreased. Even a small amount of polyethylene glycol in the matrix significantly accelerates the dissolution of the drug, and by varying the amount of polyethylene glycol, tailor-made dissolution profiles for chloramphenicol were obtained (Fig. 2).
Fig. 2.
Dissolution curves of extrudates containing 50% chloramphenicol (PEG polyethylene glycol), n = 3, mean, SD < 3%, not shown
Two-Step Extrusion and Solid-State Characterization of Extrudates Containing Lipid and Polyethylene Glycol with a Hydrophobic Model Drug
Based on the results of the extrudates obtained with the one-step extrusion, the extrusion process was modified to investigate its influence on the dissolution profiles for the drug. Three batches with the same composition were processed consisting of 50% drug and 50% matrix (tripalmitin/polyethylene glycol 5 + 5 (w/w)). In contrast to the one-step extrusion, the powdered drug and one of the two matrix formers were initially extruded and granulated. The third component was added to the pre-processed granules, and the mixture was extruded a second time resulting in the final dosage form.
Solid-state analysis was performed on the extrudates with DSC measurements (Fig. 3). The melting endotherms for the different extrudates suggested no solid-state differences between the extrudates. One combined melting endotherm for the matrix can be detected with an onset of about 62°C (32,33). The melting endotherm of the drug was less defined (onset 114°C) as the drug partially dissolved into the molten polyethylene glycol as already stated above (34). In conclusion, two-step extrusion including initial extrusion of drug and one matrix component followed by the extrusion of the milled extrudates with the second matrix component in powdered form leads to stable extrudates that exhibit the same solid-state behavior. No significant solid-state changes could be detected for matrix components and drug.
Fig. 3.
DSC thermograms of extrudates containing 50% chloramphenicol produced by varying extrusion processes
Release of the Model Drug from the Extrudates Containing Lipid and Polyethylene Glycol Produced with Two-Step Extrusion
The extrudates produced by two-step extrusion including either the initial extrusion of drug and lipid or the initial extrusion of drug and polyethylene glycol were subjected to dissolution testing according to the USP 29 method 1 (basket). Subsequently, the resulting dissolution profiles were compared to those obtained for the extrudates consisting of the same composition which were produced by one-step extrusion. The comparison of the dissolution profiles is depicted in Fig. 4. The release profile for the drug can be influenced by the method of extrusion. The extrudate that was produced using the initial extrusion step with polyethylene glycol exhibited the fastest release rate, and 100% of the embedded drug was released. In comparison, the extrudate produced using the one-step extrusion and the extrudate produced involving the initial extrusion step with tripalmitin, ended up releasing 98% of the drug load after 24 h. In these two cases, a small amount of drug particles is completely surrounded by lipid in the matrix and thus cannot be released. In addition, the extrudate produced with the initial extrusion with tripalmitin exhibited a slower dissolution rate than the extrudate produced by the one-step extrusion.
Fig. 4.
Dissolution curves of extrudates containing 50% chloramphenicol produced by varying extrusion processes (PEG polyethylene glycol), n = 3, mean, SD < 3%, not shown
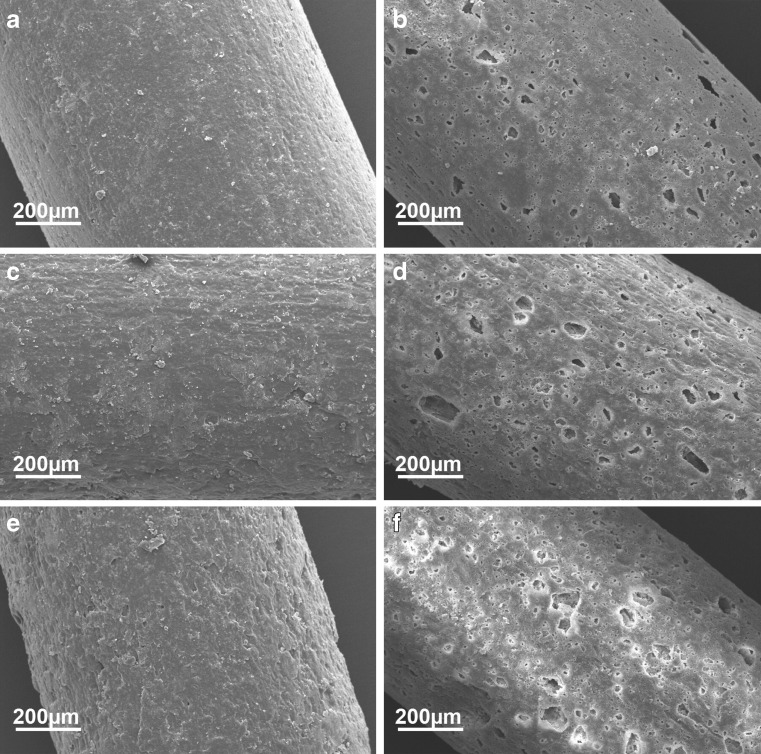
Scanning electron microscopy (SEM) images were recorded of each formulation before and after dissolution testing to investigate the surface structure (Fig. 5). Before dissolution testing, the extrudate surfaces were intact and non-porous. Interestingly, the matrix former used for the initial extrusion influenced the outer appearance of the extrudate. In general, extrudates that contain pure lipid as the matrix former exhibit a very smooth surface, whereas extrudates consisting of polyethylene glycol as the matrix former exhibit rather rough surface structures (29). In the present case, the extrudate which has been initially extruded with polyethylene glycol exhibits the roughest surface (Fig. 5e) whereas the extrudate which has been initially extruded with tripalmitin shows a smooth surface (Fig. 5a). The extrudate produced by one-step extrusion exhibited intermediate roughness (Fig. 5c).
Fig. 5.
SEM images of extrudates before and after dissolution testing. Initially extruded with tripalmitin before (a) and after (b) dissolution, no initial extrusion before (c) and after (d) dissolution, initially extruded with polyethylene glycol before (e) and after (f) dissolution
After dissolution testing for 24 h, the extrudates exhibited pores at the surface which had been generated by the release of drug and polyethylene glycol particles from the inert lipid component of the matrix (Fig. 5b, d, f). The porosity of the surface appears to slightly depend on the method of extrudate manufacture. The extrudate initially extruded with lipid contained fewer pores (Fig. 5b) than the other two batches (Fig. 5d, f). In conclusion, the two-step extrusion could be used to influence the dissolution profile for the model drug, with initial extrusion involving polyethylene glycol leading to faster dissolution and initial extrusion with the lipid leading to slower dissolution compared to the extrudate processed by one-step extrusion. Due to the more intimate mixing of the drug with the matrix former which is used for the first extrusion step, the dissolution behavior can be modified.
Stability of the Formulations
Stability testing in accelerated conditions (40°C/75% RH) was performed on the extrudates for 3 months. DSC measurements were conducted on the stored samples to investigate their solid-state stability. In addition, water sorption experiments were performed to test the ability of the extrudates to absorb water vapor. The results of the DSC measurements are depicted in Fig. 6a. The thermograms suggest that the components of the extrudates remained in their stable solid-state structures. The water sorption experiments were performed in a dynamic water sorption analyzer which exposed the samples stepwise to different climatic conditions in the range of 0% to 80% water vapor. The results are depicted in Fig. 6b. None of the formulations exceeded a water uptake of 0.5% during the measurement.
Fig. 6.
Stability testing of extrudates containing 50% chloramphenicol. a DSC thermograms after 3 months storage in 40°C/75% RH and b vapor sorption analysis
CONCLUSION
Physically stable solid lipid extrudates including the hydrophilic polymer polyethylene glycol in different ratios and the hydrophobic model drug, chloramphenicol, were produced. The dissolution rate depended on the amount of polyethylene glycol in the matrix. Two-step extrusion consisting of initial extrusion of the model drug and one matrix former followed by milling of the extrudates and subsequent extrusion of the milled extrudates and the second powdered matrix former could be used to influence the dissolution behavior of the drug. The drug was more intimately mixed with one matrix component and the properties of this very substance influenced the drug release. Based on these results, the process of two-step extrusion can be used to modify dissolution profiles for drugs without altering the matrix composition.
Acknowledgments
The Sasol GmbH and Clariant are acknowledged for generous provision of their substances.
References
- 1.Jones C, Burtan MA, Gray BN, Hodgkin J. In vitro release of cytotoxic agents from ion exchange resins. J Control Release. 1989;8:251–257. doi: 10.1016/0168-3659(89)90046-1. [DOI] [Google Scholar]
- 2.Bagyalakshmi J, Vamskrishna RP, Manavalan R, Ravi TK, Manna PK. Formulation development and in vitro evaluation of membrane-moderated transdermal systems of ampicillin sodium in ethanol: pH 4.7 buffer solvent system. AAPS Pharm Sci Tech. 2007;8:E1–E6. doi: 10.1208/pt0801007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozguney I, Shuwisitkul D, Bodmeier R. Development and characterization of extended release Kollidon® SR mini-matrices. Eur J Pharm Biopharm. 2009;73:140–145. doi: 10.1016/j.ejpb.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Theeuwes F. Elementary osmotic pump. J Pharm Sci. 1975;64:1987–1999. doi: 10.1002/jps.2600641218. [DOI] [PubMed] [Google Scholar]
- 5.Gallardo D, Skalsky D, Kleinebudde P. Controlled release solid dosage forms using combinations of (meth)acrylate copolymers. Pharm Dev Technol. 2008;13:413–423. doi: 10.1080/10837450802202098. [DOI] [PubMed] [Google Scholar]
- 6.Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. CRC Crit Rev Ther Drug Carr Syst. 1987;3:233–261. [PubMed] [Google Scholar]
- 7.Oppenheim RC. Solid colloidal drug delivery systems: nanoparticles. Int J Pharm. 1981;8:217–234. doi: 10.1016/0378-5173(81)90100-9. [DOI] [Google Scholar]
- 8.Fites AL, Banker GS, Smolen VF. Controlled drug release through polymeric films. J Pharm Sci. 1970;59:610–613. doi: 10.1002/jps.2600590507. [DOI] [PubMed] [Google Scholar]
- 9.Samuelov Y, Donbrow M, Friedman M. Sustained release of drugs from ethylcellulose–polyethylene glycol films and kinetics of drug release. J Pharm Sci. 1979;68:325–329. doi: 10.1002/jps.2600680318. [DOI] [PubMed] [Google Scholar]
- 10.Zentner GM, Rork GS, Himmelstein KJ. Osmotic flow through controlled porosity films: an approach to delivery of water soluble compounds. J Control Release. 1985;2:217–229. doi: 10.1016/0168-3659(85)90047-1. [DOI] [Google Scholar]
- 11.Muhammed NA, Boisvert W, Harris MR, Weiss J. Modifying the release properties of Eudragit L30D. Drug Dev Ind Pharm. 1991;17:2497–2509. doi: 10.3109/03639049109048089. [DOI] [Google Scholar]
- 12.Thommes M, Kleinebudde P. Use of k-carrageenan as alternative pelletisation aid to microcrystalline cellulose in extrusion/spheronisation. II. Influence of drug and filler type. Eur J Pharm Biopharm. 2006;63:68–75. doi: 10.1016/j.ejpb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Henrist D, Remon JP. Influence of the process parameters on the characteristics of starch based hot stage extrudates. Int J Pharm. 1999;189:7–17. doi: 10.1016/S0378-5173(99)00229-X. [DOI] [PubMed] [Google Scholar]
- 14.Lyons JG, Blackie P, Higginbotham CL. The significance of variation in extrusion speeds and temperatures on a PEO/PCL blend based matrix for oral drug delivery. Int J Pharm. 2008;351:201–208. doi: 10.1016/j.ijpharm.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Davis SS, Illum L. Polymeric microspheres as drug carriers. Biomaterials. 1999;9:111–115. doi: 10.1016/0142-9612(88)90081-6. [DOI] [PubMed] [Google Scholar]
- 16.Hamdani J, Moes AJ, Amighi K. Development and evaluation of prolonged release pellets obtained by the melt pelletization process. Int J Pharm. 2002;245:167–177. doi: 10.1016/S0378-5173(02)00348-4. [DOI] [PubMed] [Google Scholar]
- 17.Prabhu S, Ortega M, Ma C. Novel lipid based formulations enhancing the in vitro dissolution and permeability characteristics of a poorly water-soluble model drug, piroxicam. Int J Pharm. 2005;301:209–216. doi: 10.1016/j.ijpharm.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Qi S, Deutsch D, Craig DQ. An investigation into the mechanism of drug release from taste-masking fatty acid microspheres. J Pharm Sci. 2008;97:3842–3854. doi: 10.1002/jps.21243. [DOI] [PubMed] [Google Scholar]
- 19.Hamdani J, Moes AJ, Amighi K. Physical and thermal characterization of Precirol® and Compritol® as lipophilic glycerides used for the preparation of controlled release matrix pellets. Int J Pharm. 2003;260:47–57. doi: 10.1016/S0378-5173(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 20.Humberstone AJ, Charman WN. Lipid based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25:103–128. doi: 10.1016/S0169-409X(96)00494-2. [DOI] [Google Scholar]
- 21.Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Del Rev. 2008;60:734–746. doi: 10.1016/j.addr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Michalk A, Kanikanti VR, Hamann HJ, Kleinebudde P. Controlled release of active as a consequence of the die diameter in solid lipid extrusion. J Control Release. 2008;132:35–41. doi: 10.1016/j.jconrel.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Windbergs M, Strachan CJ, Kleinebudde P. Understanding the solid-state behavior of triglyceride solid lipid extrudates and its influence on dissolution. Eur J Pharm Biopharm. 2009;71:80–87. doi: 10.1016/j.ejpb.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Breitkreutz J, El Saleh F, Kiera C, Kleinebudde P, Wiedey W. Pediatric drug formulations of sodium benzoate: II. Coated granules with a lipophilic binder. Eur J Pharm Biopharm. 2003;53:255–260. doi: 10.1016/S0939-6411(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 25.Pinto J, Silverio NP. Assesment of the extrudability of three different mixtures of saturated polyglycolysed glycerides by determination of the “specific work of extrusion” and cappillar rheometry. Pharm Dev Technol. 2001;6:117–128. doi: 10.1081/PDT-100000045. [DOI] [PubMed] [Google Scholar]
- 26.Reitz C, Strachan C, Kleinebudde P. Solid lipid extrudates as sustained-release matrices: the effect of surface structure on drug release properties. Eur J Pharm Sci. 2008;35:335–343. doi: 10.1016/j.ejps.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Verhoeven E, De Beer TRM, Schacht E, Van den Mooter G, Remon JP, Vervaet C. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: in vitro and in vivo evaluations. Eur J Pharm Biopharm. 2009;72:463–470. doi: 10.1016/j.ejpb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann S, Mohl S, Siepmann F, Siepmann J, Winter G. New insights into the role of polyethylene glycol acting as protein release modifier in lipidic implants. Pharm Res. 2007;8:1527–1537. doi: 10.1007/s11095-007-9271-y. [DOI] [PubMed] [Google Scholar]
- 29.Windbergs M, Strachan CJ, Kleinebudde P. Tailor-made dissolution profiles by extruded matrices based on lipid polyethylene glycol mixtures. J Control Release. 2009;137:211–216. doi: 10.1016/j.jconrel.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Windbergs M, Strachan CJ, Kleinebudde P. Influence of structural variations on drug release from lipid/polyethylene glycol matrices. Eur J Pharm Sci. 2009;37:555–562. doi: 10.1016/j.ejps.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Gonzalez FJ, Virgilio N, Ramsay BA, Favis BD. Influence of melt drawing on the morphology of one- and two-step processed LDPE/Thermoplastic starch blends. Adv Polym Tech. 2003;22:297–305. doi: 10.1002/adv.10057. [DOI] [Google Scholar]
- 32.Hagemann JW. Thermal behaviour and polymorphism of acylglycerides. In: Garti N, Sato K, editors. Crystallization and polymorphism of fats and fatty acids. New York: Marcel Dekker; 1988. pp. 9–95. [Google Scholar]
- 33.Craig DQM, Newton JM. Characterisation of polyethylene glycols using differential scanning calorimetry. Int J Pharm. 1991;74:33–41. doi: 10.1016/0378-5173(91)90405-D. [DOI] [Google Scholar]
- 34.Marciniec B, Stawny M, Kozak M, Naskrent M. The effect of ionizing radiation on chloramphenicol. J Therm Anal Calorim. 2006;84:741–746. doi: 10.1007/s10973-005-7545-3. [DOI] [Google Scholar]
- 35.Sarisuta N, Kunstitchai S, Pichert L, Mueller BW. Drug solubility in phospholipid carrier as a predictive parameter for drug recovery in microparticles produced by the aerosol solvent extraction system (ASES) process. Drug Dev Ind Pharm. 2007;33:932–944. doi: 10.1080/03639040601128670. [DOI] [PubMed] [Google Scholar]
- 36.Windbergs M, Jurna M, Offerhaus HL, Herek JL, Kleinebudde P, Strachan CJ. Chemical imaging of oral solid dosage forms and changes upon dissolution using coherent anti-Stokes Raman scattering. Anal Chem. 2009;81:2085–2091. doi: 10.1021/ac8020856. [DOI] [PubMed] [Google Scholar]