Abstract
The purpose of the present work was the development and evaluation of stomach-specific controlled release mucoadhesive drug delivery system prepared by ionotropic gelation of gellan beads, containing acid-soluble drug amoxicillin trihydrate, using 32 factorial design with concentration of gellan gum and quantity of drug as variables. The study showed that beads prepared in alkaline cross-linking medium have higher entrapment efficiency than the acidic cross-linking medium. The entrapment efficiency was in the range of 32% to 46% w/w in acidic medium, which increased up to 60% to 90% w/w in alkaline medium. Batches with lowest, medium, and highest drug entrapment were subjected to chitosan coating to form a polyelectrolyte complex film. As polymer concentration increases, entrapment efficiency and particle size increases. Scanning electron microscopy revealed spherical but rough surface due to leaching of drug in acidic cross-linking solution, dense spherical structure in alkaline cross-linking solution, and rough surface of chitosan-coated beads with minor wrinkles. The in vitro drug release up to 7 h in a controlled manner following the Peppas model (r = 0.9998). In vitro and in vivo mucoadhesivity study showed that beads have good mucoadhesivity and more than 85% beads remained adhered to stomach mucosa of albino rat even after 7 h. In vitro growth inhibition study showed complete eradication of Helicobacter pylori. These results indicate that stomach-specific controlled release mucoadhesive system of amoxicillin gellan beads may be useful in H. pylori treatment.
Key words: acid-soluble drug, amoxicillin trihydrate, gellan gum, H. pylori, mucoadhesive controlled release drug delivery system
INTRODUCTION
In the last decade, the number of patients suffering from peptic ulcer and gastric cancer due to Helicobacter pylori infection has increased tremendously. H. pylori are spiral, gram-negative, microaerophilic rod-shaped bacteria with multiple flagella (1,2). H. pylori remains present on the luminal surface of the gastric mucosa under mucous gel layer, is highly motile, and produces enzyme urease to alter surrounding pH to protect itself from gastric acid (2). The current therapy for the treatment of H. pylori involves use of proton pump inhibitors with antibiotics and has drawbacks like poor patient compliance and increased bacterial resistance due to higher multiple dosage of antibiotics (3). Delivering drug at the site of infection for a longer period of time is one of the approaches to improve the efficacy of antibiotic therapy (4,5).
Mucoadhesive dosage forms have been widely used for site-specific targeting for both local and systemic drug delivery and have also been beneficial for the treatment of H. pylori infection (6–10). Mucoadhesion involves strong interaction between polymer and mucus lining of the tissue which increases contact time, permits localization, and prolongs drug absorption.
Hydrogels found applications in formulating controlled and mucoadhesive drug delivery systems. Their hydrophilic and three-dimensional network structure helps to encapsulate and regulate the release of the drug. Polysaccharide hydrogels have useful properties like biocompatibility, biodegradation, mucoadhesivity, and ease of formulations mostly by ionotropic gelation method. By controlling their degree of swelling and cross-linking, they can be useful as potential carriers of drugs for controlled release applications. Gellan gum is an extracellular anionic heteropolysaccharide consisting of a linear structure of repeating tetrasaccharide units of glucose, glucuronic acid, and rhamnose in a molar ratio of 2:1:1. It has a characteristic temperature and ionic-dependent gelling property (11). The mechanism of gelation involves the formation of double helical junction zones followed by aggregation of double helical segments to form a three-dimensional network by complexation with cations and hydrogen bonding with water. Another polysaccharide, chitosan, is a linear random copolymer of d-glucosamine and N-acetyl-d-glucosamine and is obtained by N-deacetylation of chitin (12–14). Chitosan hydrogel can be formed by covalent cross-linking or ionic cross-linking. Chitosan is a well-known polycation that reacts with negatively charged components, either ions or molecules, leading to the formation of polyelectrolyte complex through ionic bridges between polymer chains. Their mucoadhesive property is mainly based on the ionic interaction with anionic substructures of mucous layer.
Amoxicillin is the model drug for the treatment against H. pylori (15). Due to its acid-soluble nature, it is very difficult to control its drug release for an extended period in the acidic environment of stomach. In the present study, beads were prepared using gellan gum and chitosan by ionotropic gelation method. Initially, drug-loaded gellan beads were prepared using 32 factorial design to ensure high entrapment efficiencies by employing acidic and alkaline cross-linking mediums. All the batches showed complete drug release within 2 h in acidic media. Therefore, to obtain controlled drug release, the drug-loaded gellan beads with lowest, medium, and highest drug entrapment were subjected to chitosan coating. Uncoated and coated beads were evaluated and characterized for yield, entrapment efficiency, micromeritic properties, scanning electron microscopy (SEM), thermal properties, swelling behavior, in vitro drug release, in vivo and in vitro mucoadhesive properties of beads, and in vitro growth inhibition study.
MATERIALS AND METHODS
Materials
Amoxicillin trihydrate was gifted by Alkem (Daman, India). Gellan gum LA was gifted by C.P. Kelco Pvt. Limited (Mumbai, India). Chitosan was a gifted by A & E Connock (UK). Calcium chloride was purchased from Sisco Research Lab Pvt. Ltd., Mumbai, India. All other chemicals and reagents were of analytical grade.
Method of Preparation
Amoxicillin Gellan Beads
Beads were prepared by the cation-induced ionotropic gelation method using acidic and alkaline cross-linking medium. Gellan solution (1.5%, 1.75%, and 2.0% w/v) was prepared by dissolving the gellan in double-distilled water by heating at 90°C. Different quantities of amoxicillin were dispersed uniformly in 10 ml of gellan solution with constant stirring (300 rpm) at 40°C until a homogeneous solution was formed. The homogeneous bubble-free solution was extruded dropwise through 18 G syringe needle into the 100 ml cross-linking solution containing 5% calcium chloride of pH 5 or pH 9 with constant stirring (100 rpm) and curing time 5 min to provide sufficient mechanical strength (16).The beads were separated by filtration, washed with double-distilled water, and dried at 25–30°C for 24 h. All formulations were prepared in triplicate. The pH of the cross-linking media was adjusted using dilute HCl and 10% ethylamine solution. A total of nine formulations were prepared and the assigned formulation codes are given in Table I.
Table I.
Experimental Variables of Factorial Design with Their Coded Levels and Actual Values of Amoxicillin Gellan Beads, Results of Percent Yield, Percent Entrapment Efficiency, and Particle Size
| Batch | Gellan (X 1) | Amoxicillin (X 2) | Yield (%)a | Entrapment efficiency (%)a | Diameter (mm)a | |||
|---|---|---|---|---|---|---|---|---|
| Acidic | Alkaline | Acidic | Alkaline | Acidic | Alkaline | |||
| AM-1 | 1.5 (−1) | 700 (−1) | 53.87 ± 1.2 | 78.66 ± 1.1 | 35.78 ± 1.76 | 68.25 ± 1.77 | 0.70 ± 0.41 | 0.80 ± 0.4 |
| AM-2 | 1.5 (−1) | 800 (0) | 48.78 ± 1.1 | 76.25 ± 1.2 | 33.88 ± 2.1 | 66.25 ± 1 | 0.73 ± 0.5 | 0.83 ± 0.6 |
| AM-3 | 1.5 (−1) | 900 (+1) | 40.89 ± 1 | 70.45 ± 1.5 | 32.45 ± 2.13 | 60.45 ± 1.6 | 0.81 ± 0.4 | 0.84 ± 0.34 |
| AM-4 | 1.75 (0) | 700 (−1) | 45.34 ± 1.4 | 73.43 ± 1.2 | 42.08 ± 1.98 | 63.43 ± 1.88 | 0.82 ± 0.8 | 0.85 ± 0.78 |
| AM-5 | 1.75 (0) | 800 (0) | 57.98 ± 1.3 | 91.3 ± 0.97 | 39.45 ± 1.43 | 81.3 ± 1.33 | 0.83 ± 0.5 | 0.88 ± 0.6 |
| AM-6 | 1.75 (0) | 900 (+1) | 56.45 ± 0.9 | 89.27 ± 0.76 | 37.56 ± 1.89 | 79.27 ± 1.67 | 0.85 ± 0.5 | 0.89 ± 0.5 |
| AM-7 | 2.0 (+1) | 700 (−1) | 54.04 ± 1.8 | 79.74 ± 0.89 | 45.79 ± 2.1 | 69.74 ± 2 | 0.94 ± 0.3 | 0.98 ± 0.4 |
| AM-8 | 2.0 (+1) | 800 (0) | 65.34 ± 0.8 | 97.57 ± 0.5 | 40.01 ± 1.5 | 89.57 ± 1.54 | 0.99 ± 0.21 | 1.1 ± 0.4 |
| AM-9 | 2.0 (+1) | 900 (+1) | 63.45 ± 1.4 | 94.89 ± 0.9 | 38.2 ± 1.98 | 86.89 ± 1.78 | 1.1 ± 0.52 | 1.2 ± 0.5 |
aMean ± SD, n = 3
Gellan Beads Coated with Chitosan
Drug-loaded gellan beads obtained using alkaline cross-linking medium were selected for chitosan coating. Chitosan solution was prepared by dissolving 1% w/v chitosan in 5% v/v acetic acid solution containing 1% w/v CaCl2. The drug-loaded beads were dispersed in chitosan solution with continuous stirring at 100 rpm for 15 min, filtered, and dried at 25°C to 30°C for 24 h. All formulations were prepared in triplicate.
Factorial Design Experiments
A 32 randomized reduced factorial design was used in this study where two factors each at three levels were evaluated to find the interaction between the selected variables. The variables selected were concentration of gellan gum (X1) and quantity of drug (X2) at three different levels. The coded and the actual values of the experimental design are given in Table I.
Evaluation and Characterization of Beads
Yield and Entrapment Efficiency of Beads
The weight of the dried beads was recorded as yield of the process. For determination of entrapment efficiency, the dried beads (100 mg) were placed in 50 ml of phosphate buffer (pH 7.4) for 2 h and sonicated at 125 W for 30 min (Imeco Ultrasonics, India). The obtained dispersion was filtered through a 0.45-μm filter. The drug content of filtrate was determined spectrophotometrically at 272 nm (Shimadzu, UV/Visible 1601, Japan). Each sample determination was made in triplicate:
 |
1 |
Particle Size Determination
The microscopic photographs of beads (n = 100) was taken using a stereomicroscope (Carl Zeiss, Oberkochen, Germany) attached with a digital camera (Watec, Wat-202, Tokyo, Japan). The captured images were analyzed using the Biovis Image Plus software (Expert Tech Vision, Mumbai, India). Average diameter and different surface factors such as circulatory factor, elongation, roundness, and perimeter ratio were determined.
Differential Scanning Calorimetric (DSC) Analysis
Thermograms of amoxicillin trihydrate, gellan beads, drug-loaded gellan beads and chitosan-coated beads were obtained using a Mettler–Toledo differential scanning calorimeter (DSC) 821e instrument (Mettler Toledo, Greifensee, Switzerland) equipped with an intracooler. Indium standard was used to calibrate the DSC temperature and enthalpy scale. The samples were separately weighed and hermetically sealed in perforated aluminum pans. The system was purged with nitrogen gas at a flow rate of 80 ml/min, and heating was performed from 25°C to 250°C at a rate of 5°C/min.
Powder X-ray Diffraction
Powder X-ray diffraction (PXRD) patterns of amoxicillin trihydrate, gellan beads, drug-loaded gellan beads, and chitosan-coated beads of different batches were recorded by using a Philips PW1729 X-ray diffractometer (Philips PW 1729 X-ray diffractometer, Netherlands). Samples were irradiated with monochromatized Cu Kα radiation (1.542 Å) and analyzed at 2θ between 2° and 60°. The voltage and current used were 30 kV and 30 mA, respectively. The data were recorded over a range of 2° to 60° at a scanning rate of 5 × 103 cps using a chart speed of 10 mm/2θ.
Fourier Transform Infrared Analysis
Fourier transform infrared analysis (FTIR) measurements of amoxicillin trihydrate, gellan beads, drug-loaded gellan beads, and chitosan-coated beads of different batches were obtained on JASCO V5300 FTIR (Tokyo, Japan). The pellets were prepared on KBr-press (Spectra Lab, Pune, India) under hydraulic pressure of 150 kg/cm2. The spectra were scanned over the wave number range of 3,600 to 400 cm−1 at ambient temperature.
Surface Topography
Photomicrographs of the beads were observed at ×50 magnification using SEM Cambridge Stereoscan 120 (Cambridge, UK) operated with an acceleration voltage of 10 kV. The beads were mounted on the standard specimen mounting stubs and were coated with a thin layer (20 nm) of gold by sputter coater unit (VG Microtech, Uckfield, East Sussex, UK).
Swelling Study
Chitosan-coated beads were studied for swelling characteristics. One hundred milligrams of beads were placed in the wire basket of the USP-26 dissolution test apparatus II (Electrolab TDT-06P Mumbai, India). The basket containing beads were put in a beaker containing 100 ml of 0.1 N HCl (pH 1.2) and phosphate buffer IP (pH 7.4) maintained at 37°C. The beads were periodically removed at predetermined intervals and weighed. Then the swelling ratio was calculated as per the following formula:
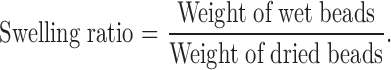 |
2 |
In Vitro Drug Release Studies
The release rate of drug-loaded gellan beads and chitosan-coated beads was studied using USP-26 Type II dissolution test apparatus (Electrolab TDT-06P Mumbai, India) containing 900 ml of 0.1 N HCl (pH 1.2) and maintained at 37 ± 0.5°C and stirred at 100 rpm. Samples were collected periodically and replaced with a fresh dissolution medium. Analysis of data was done using the PCP Disso v2.08 software (Poona College of Pharmacy, Pune, India).
Evaluation of Mucoadhesiveness of Beads
In Vitro Evaluation of Mucoadhesiveness of Beads
In vitro mucoadhesion of beads was tested on rat stomach tissue using bioadhesive tester (Memesin with AFG England). The tissue was glued on the surface of the lower plate and beads were glued on the surface of the upper plate. The surface of the plates was facing each other. The upper plate holding the beads was lowered slowly ensuring proper contact of beads with tissue surface without application of external pressure. After 10 min contact time, the detachment force was determined by measuring the force required for separation of the beads from the tissue surface.
In Vivo Evaluation of Mucoadhesiveness of Beads
The beads with highest in vitro mucoadhesivity were selected for the in vivo mucoadhesivity study (17). Three groups (six rats in each group) of albino rats (Sprague–Dawley strain, 200–300 g) of either sex were fasted for 24 h before the experiments but were allowed free access to water. Ten beads were orally administered with 2 ml of water to conscious rats. The rats were kept fasted and killed after 2, 4, and 7 h. The beads that remained adhered to the gastrointestinal tract were counted. The gastric mucoadhesion was expressed as the percentage of beads remaining in the stomach after perfusion.
In Vitro Growth Inhibition Studies
The bacterial strain used in the study was isolated from a hospitalized human patient (age 45 years) with gastric ulcer in Owaisi Hospital, Hyderabad, India. In vitro growth inhibition studies were performed for chitosan-coated gellan beads using a broth culture of H. pylori. H. pylori broth culture was incubated in a brain–heart infusion containing 0.25% yeast extract and 10% fetal calf serum and supplemented with 0.4% Campylobacter-selective supplement (Skirrow supplement). H. pylori strain was grown in Brucella broth at 37°C for 7 days in a microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2). Growth of the bacteria was monitored by measuring the optical density of broth cultures spectrophotometrically at 600 nm. The number of bacteria was determined by optical density with one optimal density unit corresponding to108 colony-forming units (CFU) ml−1. The colonies were identified as H. pylori by morphology and urease activity (9). To study the effect of formulations on H. pylori growth inhibition, 10 ml of nutrient broth was inoculated with a loopful of the H. pylori from stock culture to make a final culture of 100 CFU ml−1 (18). Amoxicillin suspension and chitosan-coated beads were incubated at 37°C in a microaerophilic atmosphere. Amoxicillin was used at a concentration of 8 µg/ml (this concentration is approximately fourfold of the reported MIC50 for H. pylori urease) (19). The culture containing tubes were shaken at 100 rpm at 37°C in a microaerobic atmosphere in an incubator. One hundred microliters of nutrient broth sample were removed at various time intervals and serial dilutions were plated on modified Skirrow’s medium. The agar plates were incubated for 4 days at 37°C under microaerobic conditions. The viable cell counts for each sample were calculated by counting the number of colonies on the agar plates.
RESULTS AND DISCUSSION
Process Design
Gellan beads were prepared by ionotropic gelation that involved interaction between negatively charged gellan gum with positively charged calcium ion. Preliminary study to obtain cross-linked gellan beads was carried out by using 0.5–2.5% w/v gellan solution and 1–5% w/v calcium chloride solution as cross-linking medium (20,21). The 1.5–2% w/v gellan gum solutions in 5% w/v calcium chloride solution produced satisfactory beads at agitation speed of 100 rpm. Therefore, 1.5%, 1.75%, and 2% w/v gellan concentrations were used for further studies.
Yield and Entrapment Efficiency of Beads
The yield of drug-loaded gellan beads obtained using acidic and alkaline cross-linking medium was found to be in the range of 40% to 65% w/w and 70% to 97% w/w and the entrapment efficiency was in the range of 32% to 40% w/w and 60% to 89% w/w, respectively. Comparatively lower values of responses obtained using acidic cross-linking medium may be due to loss of drug present on the surface of beads (Table I).
The data of values obtained for yield and entrapment efficiencies were subjected to multiple regression analysis using statistical software UNISTAT® (statistic version 3, Meglon US). The equation fitted was:
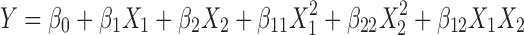 |
3 |
where Y represents the measured response, X represents the levels of factors, and β represents the coefficient computed from the responses of the formulations.
The regression equation for yield (acidic) was:
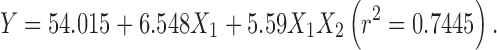 |
4 |
The regression equation for yield (alkaline) was:
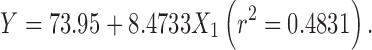 |
5 |
The regression equation for entrapment efficiency (acidic) was:
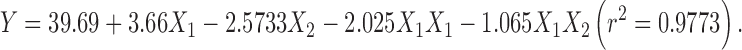 |
6 |
The regression equation for entrapment efficiency (alkaline) was:
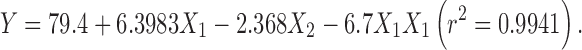 |
7 |
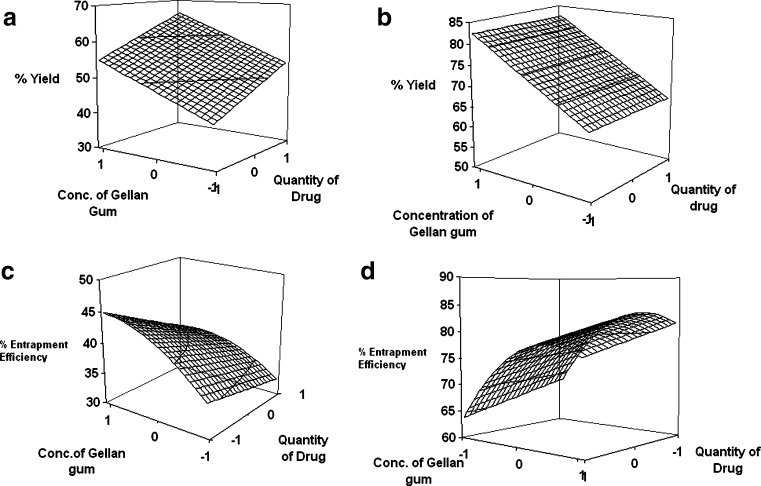
The regression analysis for yield and entrapment efficiency shows a positive effect of polymer concentration (Table II; Fig. 1). The increase in gel viscosity with polymer concentration develops cohesive interactions among gellan monomers and provides resistance to diffusion and leaching of drug, thus increasing the extent of agglomeration. The intramolecular cohesive interactions in polymers (X1X1) reduce space for accommodation of drug, resulting to lower drug entrapment. It is also reflected by negative effect of X2 (Eqs. 6 and 7). Higher value of yield and drug entrapment for beads obtained using alkaline medium can be explained on the basis of decreased solubility of drug.
Table II.
Regression Analysis Data of 32 Factorial Design
| Coefficient | Percent yield | Percent entrapment efficiency | ||
|---|---|---|---|---|
| Acidic | Alkaline | Acidic | Alkaline | |
| β 0 | 54.015 | 73.95 | 39.69 | 79.4 |
| β 1 | 6.548 | 8.4733 | 3.66 | 6.3983 |
| β 2 | – | – | −2.5733 | −2.368 |
| β 11 | – | – | −2.025 | −6.7 |
| β 22 | – | – | – | – |
| β 12 | 5.59 | – | −1.065 | – |
| R 2 | 0.7445 | 0.4831 | 0.9773 | 0.9941 |
| Significance | 0.014 | 0.0377 | 0.0015 | 0.0000 |
| F | 8.745 | 6.543 | 43.172 | 285 |
Fig. 1.
Response surface plot showing effect of factorial variables on a percent yield (acidic), b percent yield (alkaline), c percent entrapment efficiency (acidic), and d percent entrapment efficiency (alkaline)
The beads were spherical with circulatory factor in the range of 1.1 to 1.2 and roundness in the range of 0.4 to 0.6, but no significant difference was observed in these parameters. The mean particle size of beads obtained from acidic cross-linking medium and alkaline cross-linking medium was in the range of 0.70 to 1.1 mm and 0.80 to 1.2 mm, respectively (Table I). Size of the beads increased with increase in the concentration of gellan gum and amount of drug. This could be attributed to the increased diffusion resistance and agglomerating properties in viscous polymeric dispersion. The pH-dependent swelling of gellan gum in alkaline medium and, in turn, loose packing can also enlarge bead size (22).The mean diameter of chitosan-coated beads marginally increased with an increase in drug loading and chitosan concentration (Table III). This could be attributed to formation of a thick chitosan coating over the beads due to its ionic interaction.
Table III.
Result of Chitosan-Coated Amoxicillin Gellan Beads Yield, Entrapment Efficiency, and Particle Size
| Batch | Yield (%)a | Entrapment efficiency (%)a | Diameter (mm)a |
|---|---|---|---|
| AM-3 | 76.85 ± 1 | 71.67 ± 1.1 | 0.86 ± 0.6 |
| AM-6 | 91.2 ± 0.52 | 84.23 ± 1.2 | 0.91 ± 0.7 |
| AM-8 | 98.23 ± 0.4 | 91.76 ± 0.82 | 1.3 ± 0.5 |
aMean ± SD, n = 3
Characterization of Beads
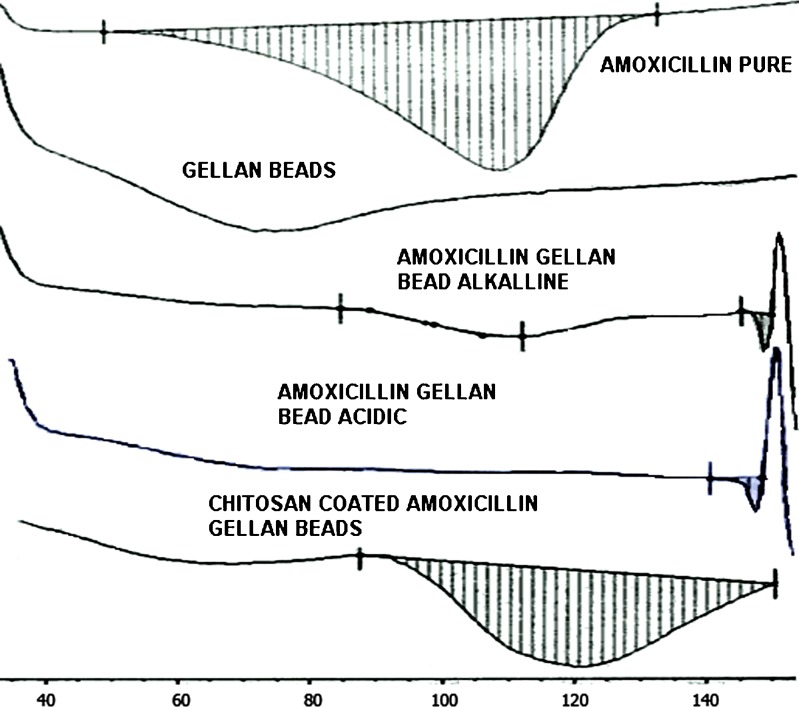
The DSC thermogram of pure amoxicillin showed exothermic peak at 109.6 ± 1.0°C which disappeared in cross-linked gellan beads; this may be due to dispersion of drug in molecular state. The endothermic peak of drug observed in chitosan-coated beads and shifting of peak to higher temperature (125.5°C), which may be attributed to recrystallization of drug during chitosan coating and slower heat transfer within beads, respectively, are given in Fig. 2.
Fig. 2.
DSC thermograms of amoxicillin, amoxicillin gellan beads, and chitosan-coated amoxicillin gellan beads
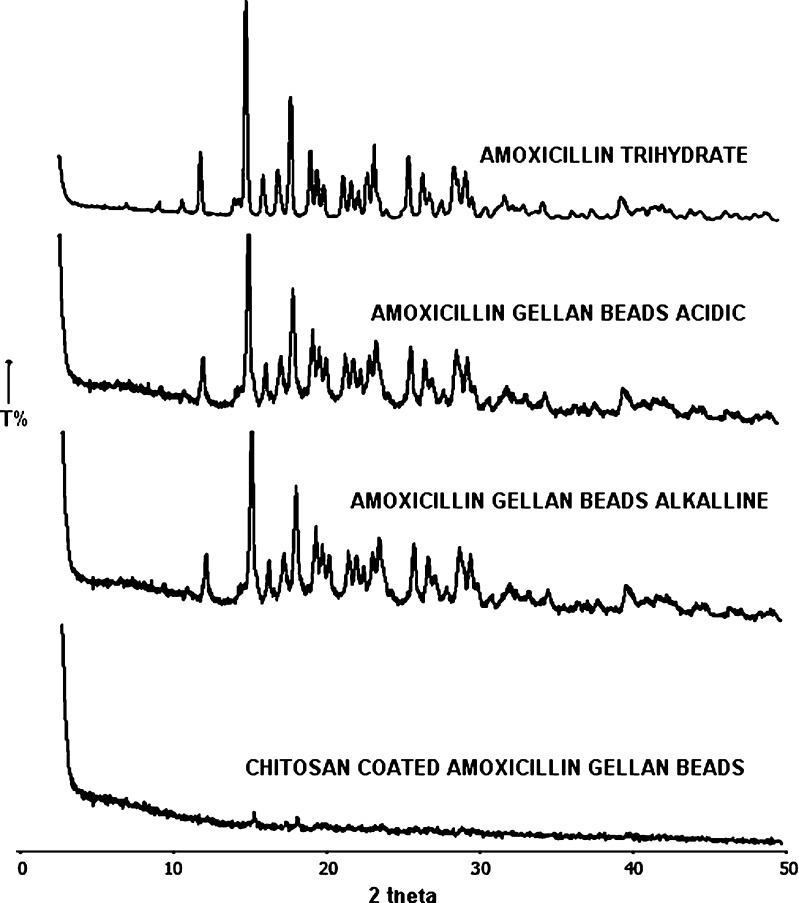
PXRD of amoxicillin gellan beads and chitosan-coated beads is shown in Fig. 3. The intensity of peaks referred for the crystalline nature of pure drug was decreased significantly in the formulations which can be due to the effect of polymer or formulation process. PXRD of amoxicillin showed characteristic peaks at about 12°, 14.5°, 18.5°, 23.5°, and 29° (2θ). Peak at 14.5° and 18.5° (2θ) was used to compare X-ray diffraction pattern of drug with beads. Significant reduction in peak intensities was observed in X-ray diffraction pattern of beads when compared with pure drug; PXRD of amoxicillin gellan beads (acidic and alkaline) shows peak at 14.5º and 18.5º (2θ) as in amoxicillin, but intensity of peak was decreased so it shows polymorphism. PXRD of 1% chitosan-coated amoxicillin gellan beads shows absence of characteristic peaks, indicating reduction in crystallinity of drug in the presence of thick coating of chitosan.
Fig. 3.
XRD patterns of amoxicillin, amoxicillin gellan beads, and chitosan-coated amoxicillin gellan beads
Fourier transfer infrared spectroscopy (FTIR) study that was carried out to determine interactions and structural changes in drug and excipient are given in Fig. 4. FTIR spectrum of pure amoxicillin showed characteristic peaks at 1,620 cm−1 (C=O stretching), 3,292 cm−1 (sec. –NH or –OH) and some prominent bands like 846–567 cm−1 (–CH aromatic ring bending and heteroaromatics). Characteristic peaks of drug were also present in FTIR spectrum of beads with some broadening and reduction in intensity, indicating the absence of chemical interactions between drug, polymer, and counterions after production of beads.
Fig. 4.
FTIR spectra of amoxicillin gellan beads
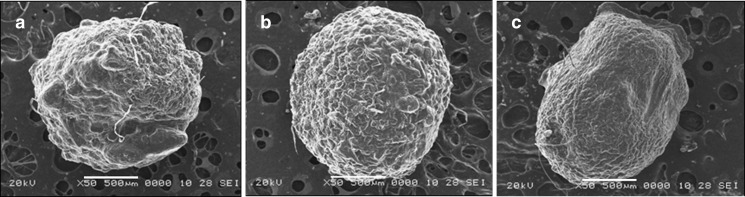
SEM photomicrographs of dried beads are shown in Fig. 5. The beads were spherical and dense with thick polymer coat. The drug-loaded gellan beads obtained using acidic medium was spherical but discrete with irregular rough surface due to leaching of drug in cross-linking solution forming large perturbations (Fig. 5a). The beads prepared in alkaline medium were large and spherical with more organized dense structure (Fig. 5b). The rough surface of chitosan-coated beads with minor wrinkles may be due to dehydration of the chitosan during drying (Fig. 5c). The increase in size of beads could be attributed to the increase in microviscosity of the polymeric dispersion with increase in gellan concentration.
Fig. 5.
SEM photographs of a amoxicillin gellan bead (acidic), b amoxicillin gellan bead (alkaline), and c chitosan-coated amoxicillin gellan bead
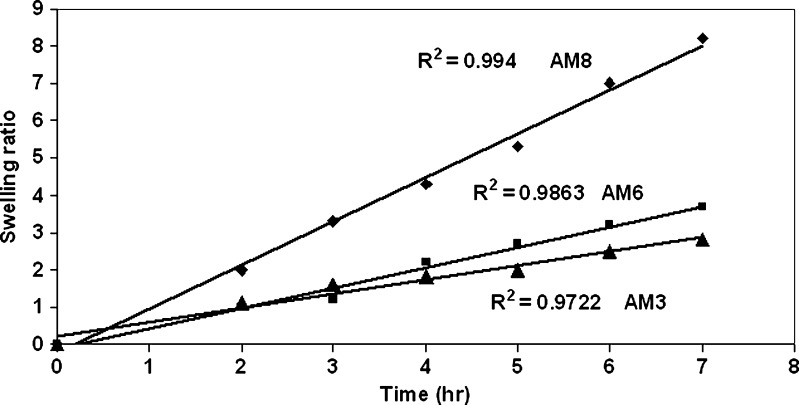
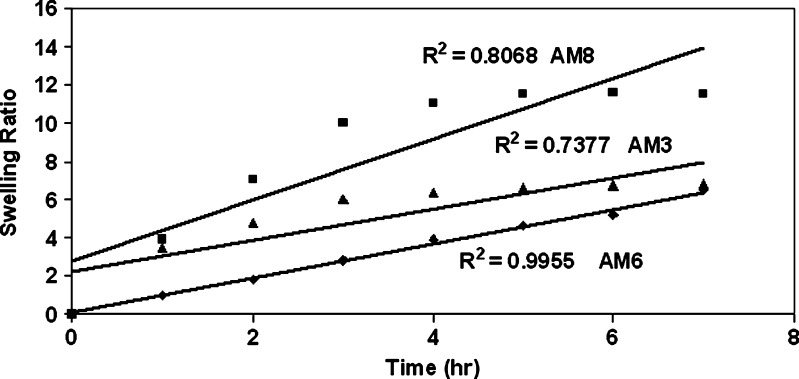
Chitosan-coated gellan beads were subjected to swelling studies in both acidic and alkaline medium same as the gastric pH and microenvironment at the H. pylori-infected area, respectively. Gellan being an anionic polyelectrolyte, pH affects the conformation of gellan chains in acidic medium by increasing the shielding effect of repulsion of carboxyl groups which is also enhanced by the addition of cations and change in anionic nature of gellan determined by the dissociation of carboxyl group (23), whereas in alkaline medium gellan has high swelling properties (24). Chitosan with a degree of deacetylation >87% bears positive charge with high charge density in acidic medium and exists in the neutral form in alkaline medium. In gastric fluid (pH 1.2), the positively charged matrix swells due to electrostatic repulsion between like charges and the osmotic effect of bound counterions. Chitosan have more protonation of amino groups in acidic medium and rapid swelling and erosion in alkaline medium (pH 7.4) (25,26). In acidic medium, chitosan-coated beads showed linear increase in swelling due to the combined effect of both the polymers (Fig. 6). In alkaline medium, chitosan-coated gellan beads have more swelling ratio than in acidic medium probably due to predominate swelling effect of gellan (Fig. 7).
Fig. 6.
Swelling study of chitosan-coated amoxicillin gellan beads at pH 1.2
Fig. 7.
Swelling study of chitosan-coated amoxicillin gellan beads at pH 7.4
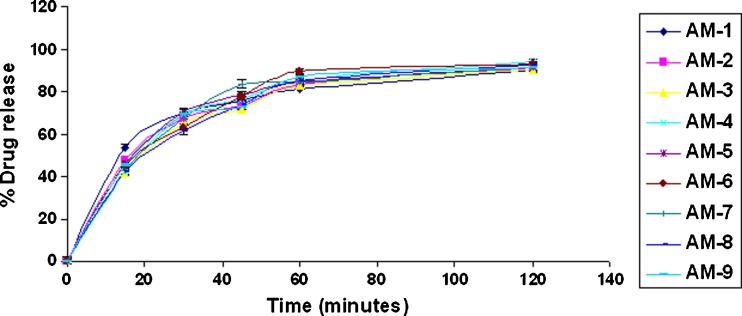
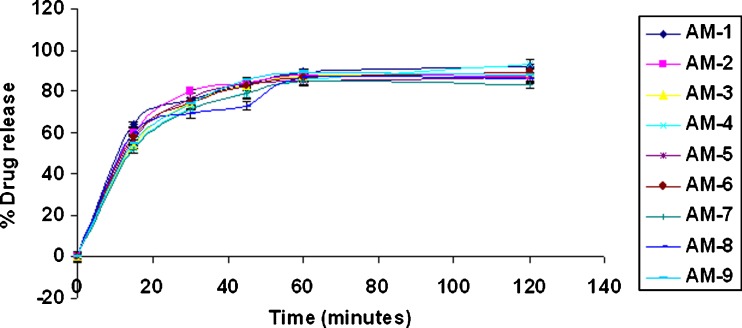
When amoxicillin gellan beads and chitosan-coated amoxicillin gellan beads were evaluated for drug release in 0.1 N HCl, pH 1.2 (Figs. 8, 9, and 10), the beads prepared in acidic and alkaline cross-linking medium show more than 80% drug release at the end of 1 h with t50% of 16 ± 3 min (acidic cross-linking medium) and 15 ± 1.5 min (alkaline cross-linking medium) which was mainly governed by acidic solubility of the drug. The delayed t50% values of 65 ± 15 min of the chitosan-coated beads may be due to thick chitosan coating that not only minimizes penetration of dissolution medium but slows down the drug diffusion (27,28). In addition, the increased crystallinity of drug during chitosan coating may contribute for slow drug release. The chitosan-coated beads showed controlled drug release over a period of 7 h following the Peppas model (r = 0.9998).
Fig. 8.
In vitro drug release of amoxicillin gellan beads (acidic) in 0.1 N HCl
Fig. 9.
In vitro drug release of amoxicillin gellan beads (alkaline) in 0.1 N HCl
Fig. 10.
In vitro drug release of chitosan-coated amoxicillin gellan beads in 0.1 N HCl
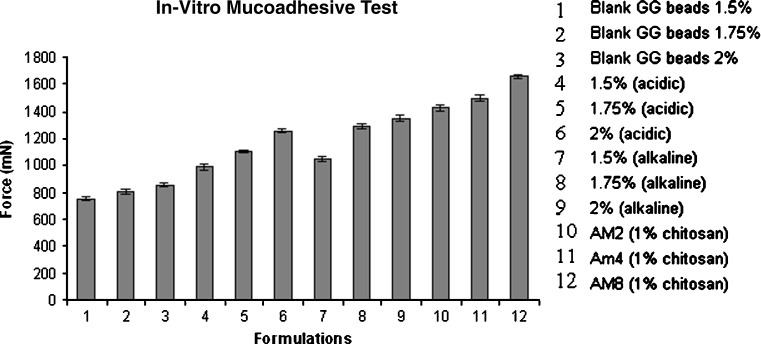
Mucoadhesive properties of amoxicillin gellan beads increased with increased concentration of gellan and drug, which was superior to the gellan beads without drug (Fig. 11). The gellan–gellan interactions produces compact beads reducing mucoadhesivity; this may also be the reason for poor mucoadhesivity of beads obtained using acidic medium than alkaline medium. Beads obtained from alkaline cross-linking medium showed improved mucoadhesivity; this might be due to (1) the decreased intramolecular cohesivity in gellan chains in the presence of drug and increase in adhesivity with stomach mucosa and (2) induced capillary forces due to channels formed by dissolution of drug (9,27). Chitosan-coated beads showed higher mucoadhesivity with increasing gellan amount, suggesting increased gellan–chitosan cross-linking and, in turn, availability of polymer chains for mucous membrane interactions (29,30). Batch AM8 having higher in vitro mucoadhesivity was further tested for in vivo mucoadhesion where more than 85% beads retained on stomach mucosa after 7 h.
Fig. 11.
In vitro evaluation of mucoadhesiveness of beads
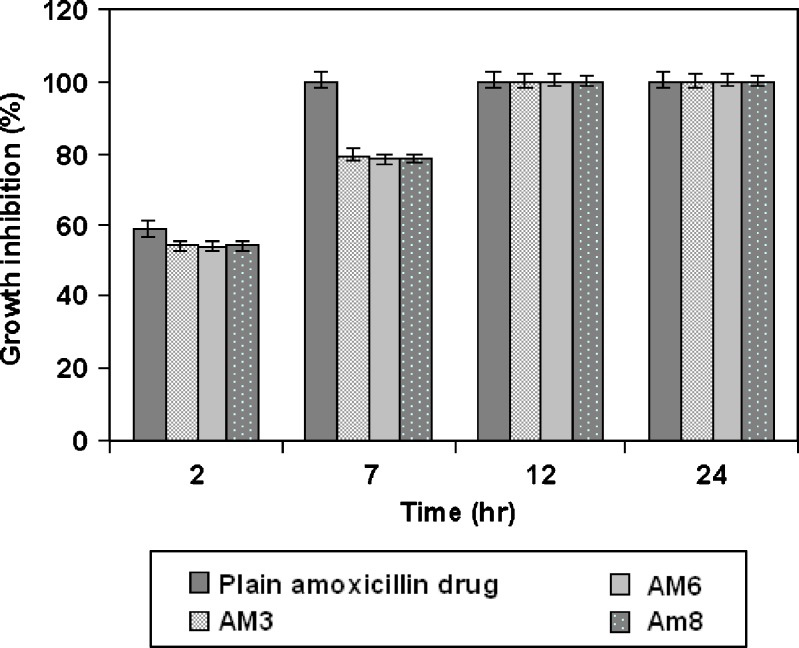
The chitosan-coated drug-loaded beads that were evaluated for in vitro H. pylori growth inhibition studies are given in Fig. 12. The antimicrobial effect of amoxicillin and beads were determined in terms of percentage growth inhibition, which was calculated as the ratio of colony counts of a given mixture against that of tubes containing H. pylori alone. The percentage growth inhibition values decreased gradually with increasing incubation time. Beads showed about 54% and 79% growth inhibition at the end of 2 and 7 h, respectively, where pure amoxicillin showed comparatively more growth inhibition of about 59% and 100% after 2 and 7 h, respectively. However, the usual gastric residence of pure drug may be up to 2 h only. All batches showed complete growth inhibition after 12 h of incubation. Overall, the results suggested that chitosan-coated amoxicillin gellan beads prolonged drug release and gastric retention and showed better H. pylori growth inhibition than plain drug.
Fig. 12.
In vitro growth inhibition study
CONCLUSION
The present study gave an overview of formulating ready to use controlled release drug therapy for the treatment of H. pylori using calcium–gellan beads coated with 1% chitosan. The alkaline cross-linking medium showed improvement in entrapment efficiency. Chitosan-coated gellan beads containing amoxicillin showed good mucoadhesivity and complete growth inhibition of H. pylori. Thus, chitosan-coated amoxicillin gellan beads might be a promising drug delivery system for the treatment of H. pylori infection.
References
- 1.Marshall BJ. Unidentified curved bacilli in gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 2.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 3.Vakil N, Cutler A. Ten-day triple therapy with ranitidine, bismuthcitrate, amoxicillin and clarithromycin in eradicating Helicobacter pylori. Am J Gastroenterol. 1999;94:1197–1199. doi: 10.1111/j.1572-0241.1999.01065.x. [DOI] [PubMed] [Google Scholar]
- 4.Amiji MM. Tetracycline-containing chitosan microspheres for local treatment of Helicobacter pylori infection. Cellulose. 2007;14:3–14. doi: 10.1007/s10570-006-9070-3. [DOI] [Google Scholar]
- 5.Umamaheshwari RB, Suman R, Jain NK. Anti Helicobacter pylori effect of mucoadhesive nanoparticle bearing amoxicillin in experimental gerbils. AAPS PharmSciTech. 2004;5:60–68. doi: 10.1208/pt050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardonnet PL, Faivre V, Pugh WJ, Piffaretti JC, Falson F. Gastroretentive dosage forms: overview and special case of Helicobacter pylori. J Control Release. 2006;111:1–18. doi: 10.1016/j.jconrel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, Jacob J, Klein AB, Chayapruks T, Mathiowitz E. Bioadhesive polymers for stomach targeted drug delivery. Proceedings of the 24th International Symposium on Controlled Release of Bioactive Materials, Stockholm; 1997. p. 259–260.
- 8.Cuna M, Alonso MJ, Torres D. Preparation and in vivo evaluation of mucoadhesive microparticles containing amoxicillin–resin complexes for drug delivery to the gastric mucosa. Eur J Pharm Biopharm. 2001;51:199–205. doi: 10.1016/S0939-6411(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 9.Nagahara N, Akiyama Y, Tada M, Nakao M, Kitano M, Ogawa Y. Mucoadhesive microspheres containing amoxycillin for clearance of Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:2492–2494. doi: 10.1128/aac.42.10.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Tauchi Y, Deguchi Y, Morimoto K, Tabata Y, Ikada Y. Positively charged gelatin microspheres as gastric mucoadhesive drug delivery system for eradication of H. pylori. Drug Deliv. 2000;7:237–243. doi: 10.1080/107175400455173. [DOI] [PubMed] [Google Scholar]
- 11.Maoa R, Tanga J, Swansonb BG. Texture properties of high and low acyl mixed gellan gels. Carbohydr Polym. 2000;41:331–338. doi: 10.1016/S0144-8617(99)00108-3. [DOI] [Google Scholar]
- 12.Domard A, Cartier N. Glucosamine oligomers: IV. Solid state-crystallization and sustained dissolution. Int J Biol Macromol. 1992;14:100–106. doi: 10.1016/0141-8130(92)90006-T. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe-Seiler F. Chitin and chitosan. Ber Dtsch Chem Ges. 1994;27:3329–3331. doi: 10.1002/cber.189402703135. [DOI] [Google Scholar]
- 14.Roberts GAF. Structure of chitin and chitosan. In: Roberts GAF, editor. Chitin chemistry. Houndmills: MacMillan; 1992. pp. 1–53. [Google Scholar]
- 15.Rajinikanth PS, Balasubramaniam J, Mishra B. Development and evaluation of a novel floating in situ gelling system of amoxicillin for eradication of Helicobacter pylori. Int J Pharm. 2007;335:114–122. doi: 10.1016/j.ijpharm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Patel YL, Sher P, Pawar AP. The effect of drug concentration and curing time on processing and properties of calcium alginate beads containing metronidazole by response surface methodology. AAPS PharmSciTech. 2006;7:24–30. doi: 10.1208/pt070486. [DOI] [PubMed] [Google Scholar]
- 17.Liua Z, Lua W, Qianb L, Zhanga X, Zenga P, Pana J. In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J Control Release. 2005;102:135–144. doi: 10.1016/j.jconrel.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Rajinikanth PS, Mishra B. Preparation and in vitro characterization of gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm. 2007;57:413–427. doi: 10.2478/v10007-007-0033-5. [DOI] [PubMed] [Google Scholar]
- 19.Mergaud F, Trimoulet P, Lamouliatte H, Boyanova L. Bactericidal effect of amoxicillin on Helicobacter pylori in an in vitro model using epithelial cells. Antimicrob Agents Chemother. 1991;35:869–872. doi: 10.1128/aac.35.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawar AP, Gadhe AR, Venkatachalam P, Sher P, Mahadik KR. Effect of core and surface cross-linking on the entrapment of metronidazole in pectin beads. Acta Pharm. 2008;58:75–85. doi: 10.2478/v10007-007-0046-0. [DOI] [PubMed] [Google Scholar]
- 21.Huanga Y, Tanga J, Swansonb BG, Rasco BA. Effect of calcium concentration on textural properties of high and low acyl mixed gellan gels. Carbohydr Polym. 2003;54:517–522. doi: 10.1016/j.carbpol.2003.08.006. [DOI] [Google Scholar]
- 22.Agnihotri SA, Jawalkar SS, Aminabhavi TM. Controlled release of cephalexin through gellan gum beads: effect of formulation parameters on entrapment efficiency, size, and drug release. Eur J Pharm Biopharm. 2006;63:249–261. doi: 10.1016/j.ejpb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto F, Cunha RL. Acid gelation of gellan: effect of final pH and heat treatment conditions. Carbohydr Polym. 2007;68:517–527. doi: 10.1016/j.carbpol.2006.11.009. [DOI] [Google Scholar]
- 24.Patil S, Sharma S, Nimbalkar A, Pawar A. Study of formulation variables on properties of drug-gellan beads by factorial design. Drug Dev Ind Pharm. 2006;32:315–326. doi: 10.1080/03639040500518930. [DOI] [PubMed] [Google Scholar]
- 25.Chandy T, Sharma CP. Chitosan—as a biomaterial. Biomater Artif Cells Artif Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 26.Yao KD, Peng T. Swelling kinetics and release characteristics of crosslinked chitosan:polyether polymer network (semi-IPN) hydrogels. J Polym Sci Part A Polym Chem. 1994;32:1213–1223. doi: 10.1002/pola.1994.080320702. [DOI] [Google Scholar]
- 27.Chena S, Liu M, Jin S, Wang B. Preparation of ionic-crosslinked chitosan-based gel beads and effect of reaction conditions on drug release behaviors. Int J Pharm. 2008;349:180–187. doi: 10.1016/j.ijpharm.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Shu XZ, Zhu KJ. Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm. 2002;233:217–225. doi: 10.1016/S0378-5173(01)00943-7. [DOI] [PubMed] [Google Scholar]
- 29.Hagerstrom H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci. 2000;9:301–309. doi: 10.1016/S0928-0987(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 30.Keely S, Rullay A, Wilson C, Carmichael A, Carrington S, Corfield A, Haddleton DM, Brayden DJ. In vitro and ex vivo intestinal tissue models to measure mucoadhesion of poly(methacrylate) and N-trimethylated chitosan polymers. Pharm Res. 2005;22:38–49. doi: 10.1007/s11095-004-9007-1. [DOI] [PubMed] [Google Scholar]