Abstract
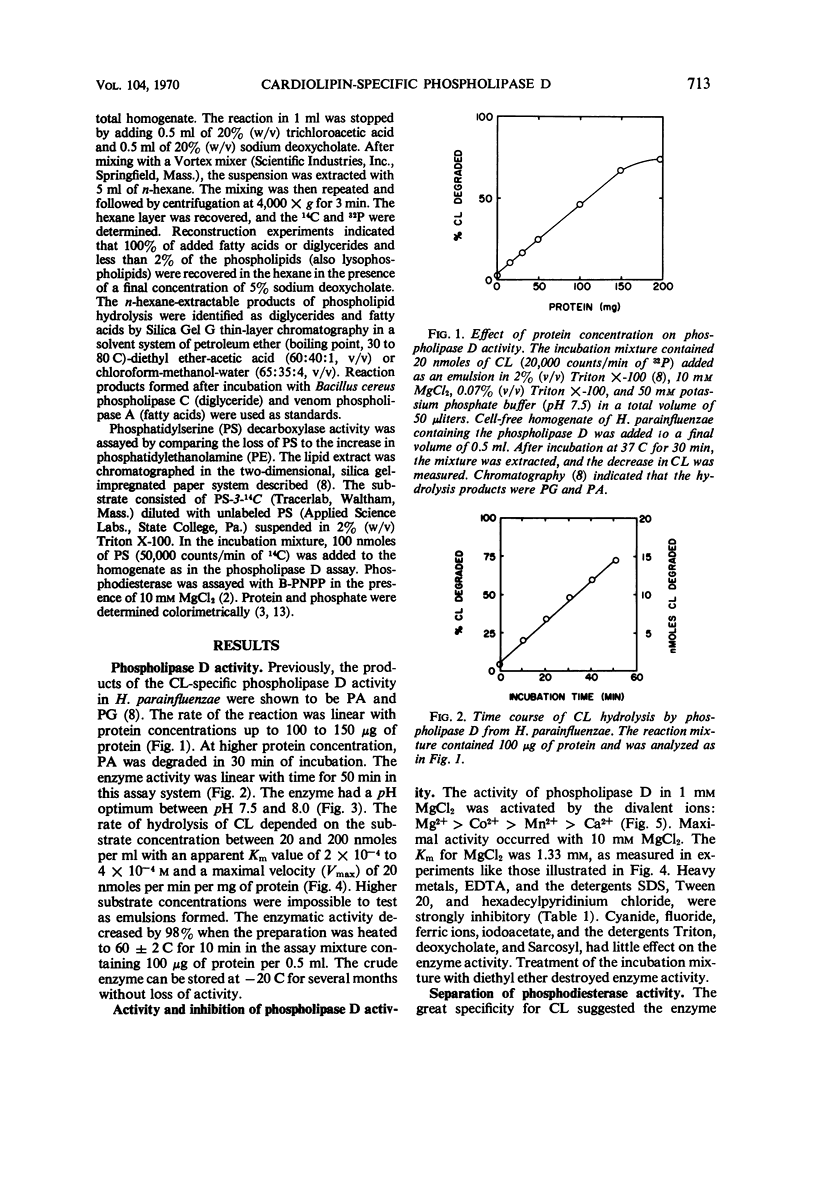
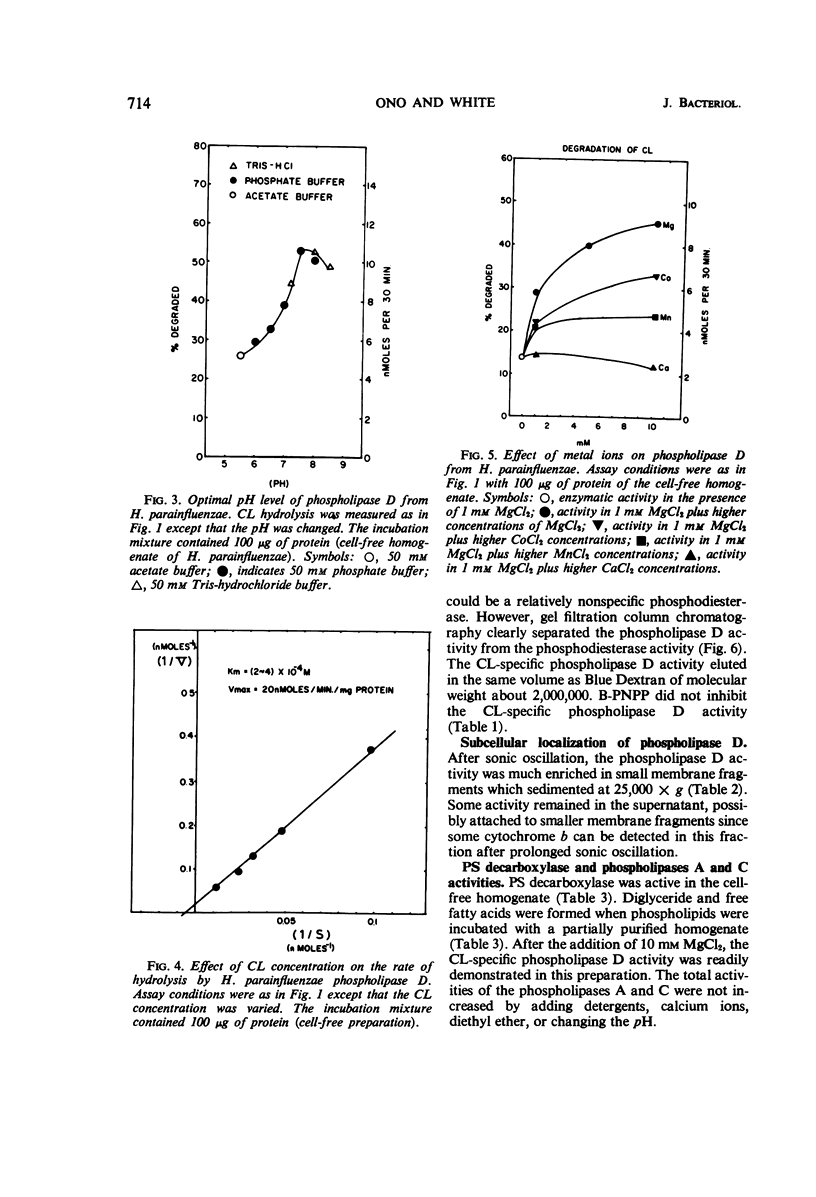
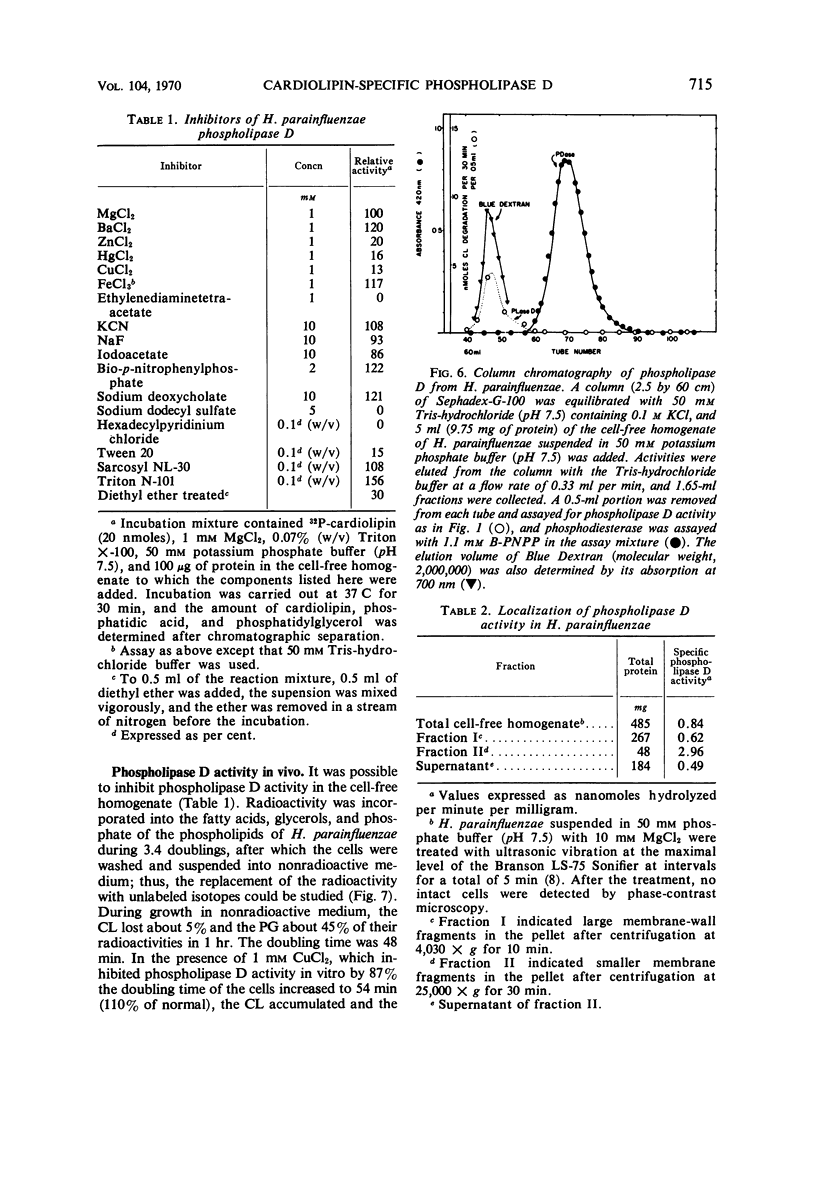
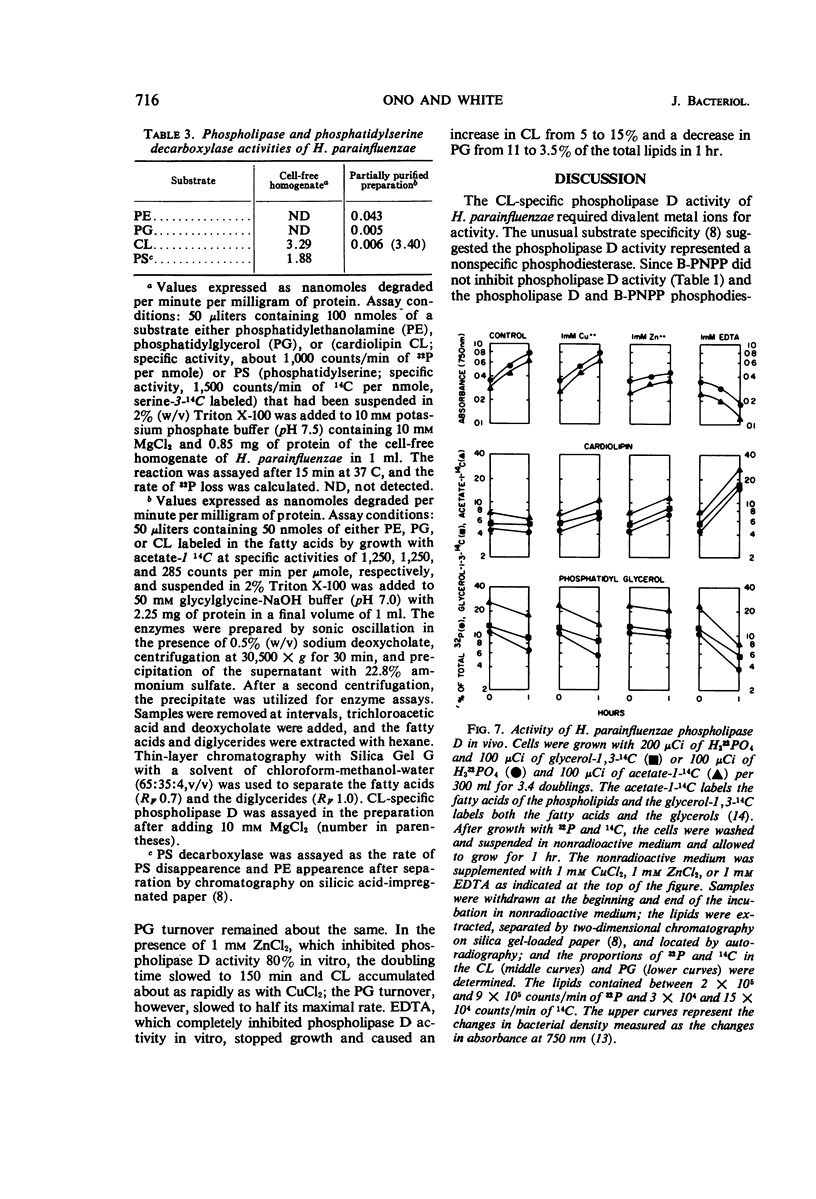
A phospholipase specific for cardiolipin (CL) was found in the membrane of Haemophilus parainfluenzae. The enzyme hydrolyzed CL to phosphatidic acid (PA) and phosphatidylglycerol (PG), indicating that it was a phospholipase D (an enzyme activity believed to be confined to higher plants). In addition to its substrate specificity, this enzyme was unusual in its requirement for Mg2+ (Km of 1.3 mm) for maximal activity and its inhibition by chelating agents, heavy metals, some detergents, and organic solvents. When inhibitors of phospholipase activity were added to the growth medium, CL accumulated and PG disappeared in the membrane, suggesting that the phospholipase D was active in vivo. The activity of phospholipase D in cell-free homogenates was greater than expected from earlier studies of CL metabolism and greater than the other phospholipase activities detected in the homogenate. The high activity of the CL-specific phospholipase D suggests there might be a very active degradation of CL to PG and PA and an active resynthesis of CL from the hydrolysis products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Futai M., Mizuno D. A new phosphodiesterase forming nucleoside 5'-monophosphate from rat liver. Its partial purification and substrate specificity for nicotinamide adenine dinucleotide and oligonucleotides. J Biol Chem. 1967 Nov 25;242(22):5301–5307. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- Ono Y., Nojima S. Phospholipase A of Mycobacterium phlei: a regulatory membrane enzyme with ferric iron as effector. J Biochem. 1969 Jun;65(6):979–981. doi: 10.1093/oxfordjournals.jbchem.a129106. [DOI] [PubMed] [Google Scholar]
- Ono Y., Nojima S. Phospholipases of the membrane fraction of Mycobacterium phlei. Biochim Biophys Acta. 1969 Jan 21;176(1):111–119. [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D activity in Haemophilus parainfluenzae. J Bacteriol. 1970 Jul;103(1):111–115. doi: 10.1128/jb.103.1.111-115.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Heterogeneity of phospholipid composition in the bacterial membrane. J Bacteriol. 1970 May;102(2):508–513. doi: 10.1128/jb.102.2.508-513.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]



