Abstract
The aim of this study was to modify pectin by covalent attachment of the water-insoluble ligand 4-aminothiophenol to its polymeric backbone. 4-Aminothiophenol is a ligand which is highly prone to oxidation. Therefore, this ligand allows oxidative cross-linking of pectin under mild oxidative conditions. Additionally, hydrophobization of pectin can be achieved by the mentioned modification which offers certain advantages over highly hydrophilic native pectins. 4-Aminothiophenol was covalently attached to pectin via amide bond formation between carboxylic moieties of pectin and the amino-group of 4-aminothiophenol. Two different pectin–4-aminothiophenol conjugates were synthesized and investigated regarding the amount of coupled ligand, rheological behavior under oxidative conditions, swelling behavior, and cytotoxic effects. Within this study, 557.3 ± 49.0 and 158.8 ± 23.1 μmol 4-aminothiophenol have been coupled per gram pectin. Within both conjugates, around 75% of the bound ligand appeared in its reduced form. Within rheological studies, a 500-fold increase in viscosity was achieved by addition of hydrogen peroxide as an oxidizing agent. Investigations on the swelling behavior revealed that this hydrophobic modification of pectin results in decelerated water uptake on the one hand and improved cohesive properties after oxidation of thiol groups to disulfide bonds on the other hand. Thereby, the maximum amount of water which can be uptaken by pectin matrices could be increased. According to these results, Pec-ATP conjugates could be valuable tools for several pharmaceutical applications due to the established method of gelation and the altered swelling and disintegration behavior.
Key words: 4-aminothiophenol, gel formation, pectin, swelling behavior, viscosity
INTRODUCTION
Pectin is an acidic, water-soluble polysaccharide present in cell walls of land plants. Most pectins available on the market are extracted from lemon peel or apple pomace (1). It is an ionic heteropolysaccharide which predominantly consists of blocks of α-(1,4)-linked galacturonic acid (“smooth regions”) and its methyl ester. These homogalacturonan blocks are periodically interrupted by 1,2-linked α-l-rhamnose units (“hairy regions”) (2). In native pectin, the degree of esterification of galacturonic acid amounts to at least 50%. Pectins with a lower degree of esterification can be obtained by controlled acid de-esterification, treatment with alkali, or pectin methyl esterases of microbial or plant origin (3). Pectins with high and low degrees of esterification are commercially available.
Pectin is widely used in food technology because of its favorable gelling properties. Furthermore, it can positively affect hemostasis and lipid metabolism. Additionally, it is widely used and investigated as a carrier and coating material in pharmaceutical sciences (4).
Gelation of pectin can be achieved via several methods. In food technology, the addition of high concentrations of confectioner’s sugar is a common method to gel pectins. Moreover, gelation of pectin can be achieved by lowering the pH of pectin solutions. Thus, the carboxylate groups of pectin are protonated, and repellation of pectin chains is reduced which induces the formation of a gel. At higher pH values, polycarboxylate groups are present which can be cross-linked by the addition of calcium ions which leads to macromolecular aggregates (5). Pectins from sugar beet are slightly different in structure since feruloylester substituents are found on the side chains of pectin. The feruloylester residues allow oxidative cross-linking of these pectins by addition of peroxide, peroxidase, or persulfate (6). More recently, Guilherme et al. functionalized pectin by immobilization of vinyl groups to its backbone. The vinyl groups were than polymerized, and a pH-sensitive hydrogel was obtained (7).
Another promising approach to obtain pectin hydrogels with good cohesive properties might be the coupling of thiol-bearing moieties to the polymer backbone and subsequent oxidative cross-linking of thiol groups. This approach has been established successfully for several polymers, such as chitosans (8), poly(acrylic acid) (9), and alginates (10) in our workgroup. In a previous study, our work group succeeded in covalently attaching l-cysteine to pectin (11).
In this present work, 4-aminothiophenol (4-ATP) was chosen as a ligand since aromatic thiol compounds display some interesting features that might improve the properties of pectin. Aromatic thiol compounds have been shown to be excellent electron donors within enzymatic reduction–oxidation processes (12). If this is the case, the use of 4-aminothiophenol could lead to improved in situ gelling properties of a pectin–4-ATP (Pec-ATP) conjugate under the influence of physiological enzymes. Moreover, the introduction of a hydrophobic ligand might lead to stronger gels and altered swelling behavior. Changed rheological properties and altered swelling behavior could be demonstrated for hydrophobically modified hydroxyethyl cellulose and dextran (13). Hence, it was the aim of this study to generate a new modification of pectin by immobilizing 4-ATP to the backbone of pectin. Compared to unmodified pectins, this modification should allow in situ formation of more viscous gels under physiological conditions due to oxidation of thiol groups. Furthermore, it was expected that coupling of a hydrophobic ligand to pectin would decelerate swelling which might have a positive influence on the cohesiveness of pectin (14). Thus, the stability of a potential drug carrier could be improved when oxidized Pec-ATP is used. Furthermore, formation of disulfide bonds by oxidation should improve the cohesiveness of a Pec-ATP matrix and contribute to its stability.
Within the present work, 4-ATP was covalently attached to the pectin backbone by formation of amide bonds between the galacturonic acid moieties of pectin and the aromatic amine of 4-ATP. Conjugates with different degrees of substitution were generated. Subsequently, the obtained conjugates were investigated for their thiol and disulfide content. Moreover, Pec-ATP conjugates were examined concerning cytotoxic effects, rheological properties, and swelling behavior.
MATERIAL AND METHODS
Materials
High-methoxy pectin from lemon peel with a degree of esterification of approximately 70% was kindly supplied by Herbstreith & Fox KG, Neuenbürg, Germany. CellTiter-Fluor™ Cell Viability Assay was purchased from Promega, Mannheim, Germany. All other chemicals were purchased from Sigma-Aldrich, Steinheim, Germany.
Methods
Synthesis of Pectin–4-Aminothiophenol Conjugates
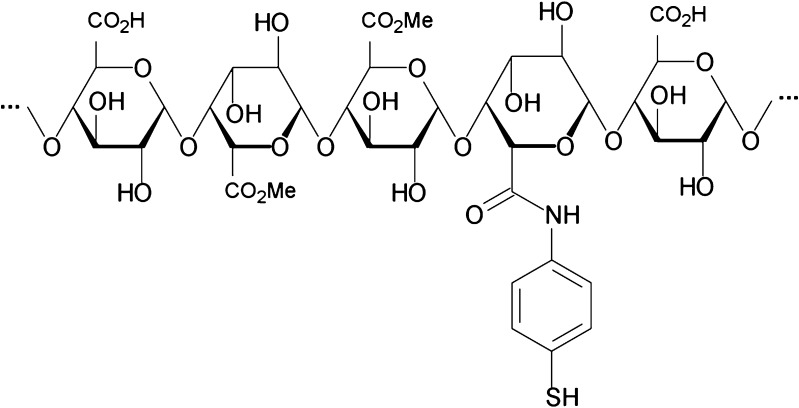
Covalent attachment of 4-ATP to pectin was achieved by formation of amide bonds between the carboxylic groups of the galacturonic acid moieties of pectin and the aromatic amine structures of 4-ATP. A schematic image of the substructure of Pec-4-ATP is provided in Fig. 1.
Fig. 1.
Schematic diagram of a substructure of pectin–4-aminothiophenol conjugates
In order to provide full reactivity and solubility of all reagents, a modification of a previously described method was necessary (11). The exact amounts of the utilized reagents for the different conjugates are supplied in Table I. In brief, pectin was suspended in 5 ml dioxane, and 65 ml of distilled water was added. This mixture was stirred until pectin was completely dissolved. Another 15 ml of dioxane was added dropwise. 4-ATP was dissolved in 5 ml dioxane and added dropwise to the pectin solution. Afterward, the pH was adjusted to 5.0 with 1 M NaOH and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC) was added after being dissolved in 5 ml distilled water. EDAC was added at last to minimize intra- and intermolecular ester formation of pectin. The reaction mixture was kept stirring at room temperature. After 3 h of stirring, 0.3 g of sodium borohydride was added, and the pH was adjusted to 7.0 with 1 M HCl in order to reduce disulfide bonds and stirred for another hour. The reaction was executed on ice, and the pH was readjusted regularly. The conditions for the reduction of thiol groups were chosen in order to minimize base-induced β-elimination of pectin on the one hand and reduction of galacturonic moieties on the other hand. To remove unbound 4-ATP from the conjugate, the reaction mixture was extracted in a separating funnel with ethyl acetate thrice. Afterward, the product was precipitated by adding 300 ml of isopropanol under magnetic stirring. Subsequently, the precipitate was washed twice with isopropanol, twice with acetone, and finally ground in a mortar until the product was dry.
Table I.
Composition of Reaction Mixtures and Basic Characterization of Pec-ATP Conjugates
| Conjugate | Polymer (g/100 ml) | EDAC (g) | 4-ATP (g) | Bound 4-ATP (µmol/g) | Free SH (µmol/g) | Unbound 4-ATP (µmol/g) |
|---|---|---|---|---|---|---|
| Pec-ATP I | 0.4 | 0.4 | 0.2 | 557.3 ± 49.0 | 423.4 ± 27.8 | 39.9 ± 3.1 |
| Pec-ATP II | 0.4 | 0.2 | 0.2 | 158.8 ± 23.1 | 121.7 ± 3.2 | 29.6 ± 2.3 |
Characterization of Pectin–4-Aminothiophenol Conjugates
The amount of covalently attached 4-ATP with free thiol groups on the conjugates was determined with Ellman’s reagent 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) as described previously (15). The total amount of covalently attached 4-ATP including its oxidized form was determined after reduction with NaBH4 and subsequent addition of DTNB (16). For safety reasons, the content of free 4-ATP in the obtained product was determined with 2,4,6-trinitrobenzene-1-sulfonic acid (17).
In order to ascertain the absence of EDAC, a high-performance liquid chromatography (HPLC) method for this compound was developed. For EDAC analysis via HPLC, a ProntoSIL C18-column (250 × 4.6 mm, 5 μm; Bischoff Chromatography, Leonberg, Germany) was used. A mixture of a 50-mM phosphate buffer, pH 6.8, and acetonitrile (90:10) served as mobile phase.
Assessment of Cytotoxicity and Cell Viability
With the Celltiter-Fluor™ Cell Viability Assay, protease activity within live cells was measured. This protease activity is restricted to viable cells and is measured by a fluorogenic substrate called glycyl-phenylalanyl-aminofluorocoumarin (GF-AFC). This substrate is cell permeant and will be cleaved by the mentioned live cell proteases. Due to removal of the dipeptide aminofluorocoumarin (AFC) is generated. AFC delivers a fluorometric signal which is proportional to the number of live cells. Loss of membrane integrity and subsequent leakage of the mentioned proteases into the surrounding culture medium would result in inactivation of these proteases and absence of substrate cleavage (18).
The assay was performed on Caco-2 cells which were cultured on 96-well plates at a density of 1 × 104 cells/ml minimum essential medium (MEM) for 24 h. After attachment of Caco-2 cells to the plate, they were incubated with 100 µl of a 0.5% solution of Pec-ATP conjugates or unmodified pectin controls dissolved in MEM medium. MEM served as vehicle control. A digitonin solution was used as a positive control according to the supplied protocol. The pH value of all samples and controls was adjusted to 7.4. After 3 h of incubation at 37°C and 5% CO2, 100 µl of the provided reagent was added as described in the manual. After further 30 min of incubation, the resulting fluorescence was measured (emission 390 nm and excitation 505 nm). Cell viabilities were calculated as percentages of the vehicle control.
Rheological Investigations
The rheological properties of Pec-ATP hydrogels were investigated with a plate–plate combination rheometer (Haake Mars Rheometer, 379-0200, Thermo-Electron GmbH, Germany; Rotor C35/10, d = 35 mm). Viscosities were measured across a shear stress range of 0.5–500 Pa to determine the optimum shear stress for comparison between different conjugates and native pectin. Measurements were performed at room temperature.
Pec-ATP hydrogels were prepared by dissolving the polymer in distilled water at a concentration of 1% (w/v). The pH was adjusted to 7.0 in order to provide ideal conditions for oxidation by increasing the activity of thiol anions (19). Hydrogen peroxide was then added as an oxidizing agent in a final concentration of 0.5 nmol/ml. After addition of the oxidizing agent, the dynamic viscosity was measured as a function of time in order to follow the progress of oxidation and the resultant increase in viscosity. Unmodified pectin controls were treated in the same manner. In order to provide comparability, viscosities at a shear stress of 11.08 Pa were used to investigate the influence of oxidation by hydrogen peroxide on Pec-ATP gelation.
Swelling Behavior
The water absorbing capacities of different Pec-ATP conjugates and controls were determined by a gravimetric method as described previously (8). Thirty milligrams of each conjugate and unmodified pectin control was compressed into 5.0 mm diameter flat-faced disks (Paul Weber, Remshalden-Grünbach, Germany). The compaction pressure was kept constant during the preparation of all disks. Test disks were fixed on a paper clip and incubated in a 100-mM phosphate buffer solution (pH 7.4) at 37°C. At predetermined time points, the swollen test disks were taken out of the incubation medium, and the amount of water uptake was determined gravimetrically after removal of water from the surface by patting them onto filter paper; the same disk was used at each time point. Water uptake was calculated according to the following equation:
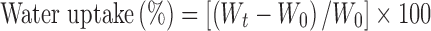 |
where Wt is the disk weight at a given time point and W0 is the initial weight of a disk. On the one hand, the maximum capacity of water absorption without disintegration was investigated, and on the other hand, water uptake was investigated as a function of time. Maximum water uptake was determined at different time points according to the last measurement before disintegration occurred. The different time points are provided in Table II.
Table II.
Times of Final Water Uptake Measurements for Investigated Species
| Investigated conjugate | Time of final measurement before disintegration (min) |
|---|---|
| Pectin | 120 |
| Pec-ATP I | 150 |
| Pec-ATP I (ox.) | 360 |
| Pec-ATP II | 120 |
| Pec-ATP II (ox.) | 240 |
These studies were carried out with the Pec-ATP conjugates as obtained from the described synthesis and with fully oxidized Pec-ATP conjugates. The latter were obtained by oxidation with hydrogen peroxide in aqueous solution at pH 7.4 and subsequent precipitation as described above.
Statistical Data Analysis
Results are expressed as the mean of at least three experiments ± SD. Statistical data analysis was performed using an unpaired Student t test with a confidence interval of 95% as the minimum level of significance.
RESULTS
Synthesis and Characterization of Pectin–4-Aminothiophenole Conjugates
Figure 1 shows the schematic diagram of a thiolated substructure of the newly formed Pec-ATP conjugate. Two batches of the conjugate, differing in the amount of immobilized 4-ATP, were synthesized following the composition given in Table I. The amount of coupled 4-ATP was successfully controlled by varying amounts of EDAC within the reaction mixture. Amounts of free thiol groups, immobilized on the polymer, total amounts of covalently attached 4-ATP, as well as amounts of remaining unbound 4-ATP are also given in Table I. A control being prepared in the same manner as the Pec-ATP conjugates but omitting EDAC during the coupling reaction displayed only traces of unbound 4-ATP. After being ground in a mortar, both products appeared as white and odorless powders which are easily soluble in water. The average yield of this synthesis amounted to 68.3 ± 2.1% of the utilized amount of pectin.
EDAC is well soluble in water as well as in isopropanol. Therefore, precipitation with isopropanol from an aqueous solution and subsequent washing by stirring the precipitate in isopropanol overnight was found to be a sufficient purification method for Pec-ATP conjugates. During HPLC analysis, the retention time for EDAC was found to be 5.8 min and concentrations between 30 µg/ml and 1 mg/ml could be detected according to a linear calibration curve. Test solutions of the produced modified pectins did not show any peaks of the carbodiimide.
Assessment of Cytotoxicity and Cell Viability
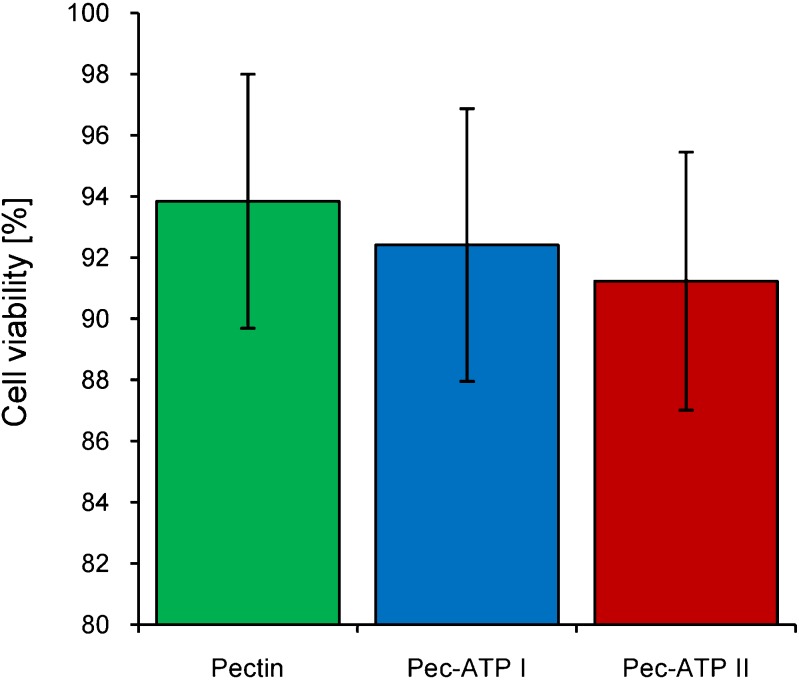
Cell viability of Caco-2 cells was investigated after treatment with 0.5% solutions of Pec-ATP conjugates by measuring the activity of so-called live cell proteases which cleave the fluorometric substrate GF-AFC. Cell viabilities provided in Fig. 2 are percentages of the fluorescence signal obtained after treatment of Caco-2 cells with MEM. Cell viability after treatment with Pec-ATP solutions remains higher than 90% within the time of investigation and at the utilized concentration. The observed cell viabilities of Pec-ATP conjugates do not differ significantly from native pectin which is part of an everyday diet and considered a safe polymer. During the experiments, no detachment of Caco-2 cells from the plates was observed. These results for cell viability after treatment with pectin conjugates correlate well with cell viability studies of previously synthesized thiolated pectin conjugates (11).
Fig. 2.
Cell viability according to cleavage of GF-AFC by live cell proteases after 3 h incubation with Pec-ATP and control test solutions. Indicated values are means of at least three experiments ± standard deviation
Rheological Investigations
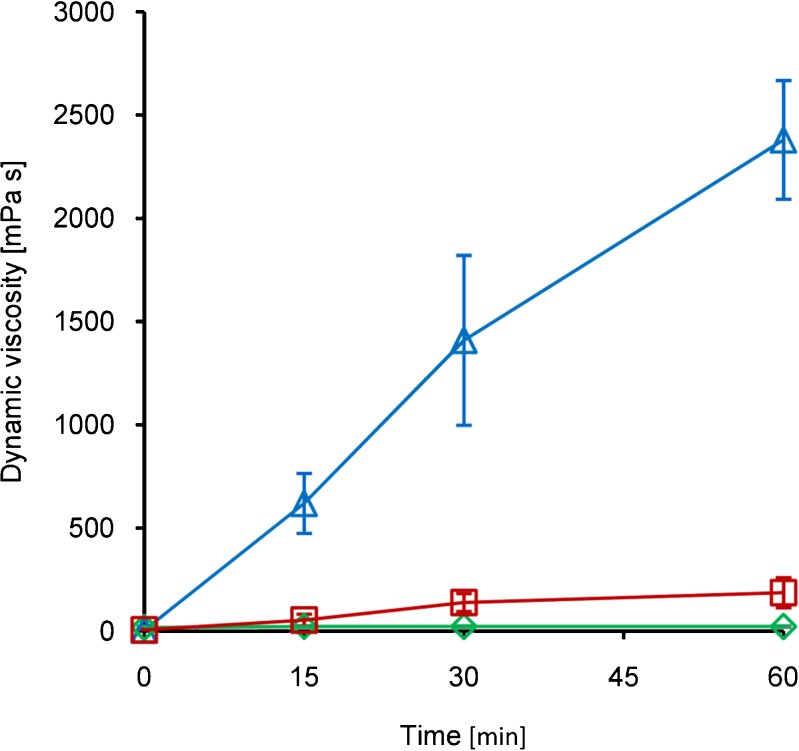
Viscosity measurements after addition of hydrogen peroxide to Pec-ATP conjugate solutions revealed that oxidative cross-linking of these conjugates can lead to highly viscous hydrogels. For Pec-ATP I, an almost 500-fold increase in dynamic viscosity was observed after 60 min; reduced viscosity was increased 634-fold compared to the initial viscosity. The viscosity of the utilized Pec-ATP II solution displayed a 28.7-fold increase in dynamic viscosity compared to the initial viscosity; reduced viscosity was increased 34-fold. Complete results of rheological investigations on the conjugates are presented in Fig. 3. Compared to unmodified pectin whose viscosity did not change after H2O2 addition, the viscosity of the conjugates was 10-fold (Pec-ATP II) and 138-fold (Pec-ATP I) increased. The shown increase in viscosity harmonizes well with the results of viscosity studies performed on oxidative gelation of thiolated chitosans (20).
Fig. 3.
Dynamic viscosity of Pec-ATP I (triangles), Pec-ATP II (squares), and unmodified pectin (diamonds) solutions after addition of hydrogen peroxide. Indicated values are means of at least three experiments ± standard deviation
Swelling Behavior
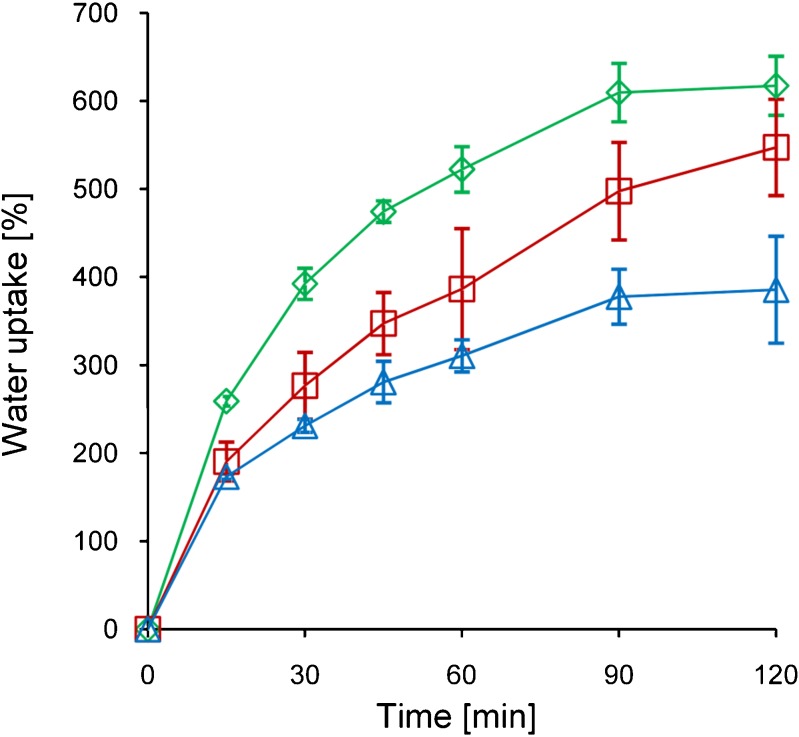
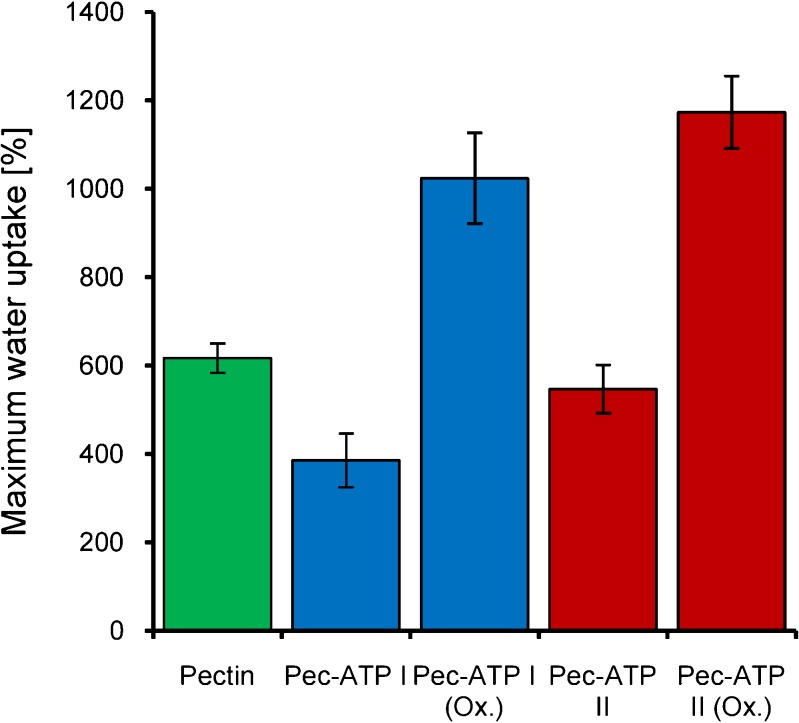
Native pectin was hydrophobically modified by covalent attachment of 4-aminothiophenol. While investigating the swelling behavior of the newly obtained polymers, it was the aim of this study to investigate the influence of the hydrophobic modification on the one hand. On the other hand, it was another aim to examine the influence of the altered cohesiveness due to disulfide formation on polymer hydration. During these experiments, it was found that hydrophobic thiolation of pectin has notable influence on swelling speed as well as total water absorbing capacities. In Fig. 4, the water uptake of the conjugates is shown as a function of time whereas the maximum water uptake of the utilized conjugates before disintegration is depicted in Fig. 5. It could be demonstrated that swelling speed of both hydrophobically modified conjugates is reduced compared to unmodified pectin, but only Pec-ATP I shows a significant difference after 120 min. Moreover, studies with oxidized Pec-ATP conjugates revealed that the total capacity of water uptake can be increased by this modification and subsequent oxidation.
Fig. 4.
Time-related water uptake of Pec-ATP I (triangles), Pec-ATP II (squares), and unmodified pectin (diamonds) test disks within phosphate buffer, pH 7.4. Indicated values are means of at least three experiments ± standard deviation
Fig. 5.
Maximum water uptake of unmodified pectin and Pec-ATP conjugates before and after complete oxidation. Indicated values are means of at least three experiments ± standard deviation
DISCUSSION
Native pectin is widely used in various technological fields. In food technology as well as in pharmaceutical applications, it is mostly utilized as a gelling agent. Due to its gelling properties, it can also be utilized as a stabilizer and thickener for liquid formulations like emulsions and suspensions.
In the past, several methods to achieve pectin gelation have been described. Among these methods, addition of sugar, acid, or calcium ions can be found. Covalent cross-linking of pectin with resultant gelation or decreased water solubility could be achieved with epichlorohydrin (21) and vinyl modification of pectin with subsequent polymerization (7). Oxidative cross-linking could be achieved with pectin from sugar beet by oxidizing the feruloylester moieties (6). A similar approach has been followed within this study by covalent attachment of 4-ATP to pectin. 4-ATP is a thiol-bearing compound with a strong notion of oxidation. Hence, this modification allows cross-linking of pectin by oxidation under comparably mild conditions.
In order to assess unexpected risks of Pec-ATP as a potential adjuvant in pharmaceutical, medical, or food technology, the impact of these conjugates on the membrane integrity of Caco-2 cells was investigated. Within the Celltiter-Fluor™ assay, the observed effect on cell viability did not differ significantly from that of unmodified pectin, and more than 90% of the cells remained viable. Due to the high survival rate of Caco-2 cells and the nonsignificant difference between modified and unmodified pectin, a toxic effect on cell membranes of Pec-ATP can be excluded. 4-ATP itself was reported to show considerable cytotoxic effects (22). It is also reported that electron-accepting substituents can notably reduce the toxicity. Amide formation between the amino group of 4-ATP and carboxylic moieties of pectin results in significantly reduced nucleophilicity, reactivity, and toxicity of the amino function.
Two Pec-ATP conjugates with significantly different amounts of attached amounts of coupled 4-ATP were investigated regarding their gelling behavior after addition of hydrogen peroxide. From Fig. 4, it becomes obvious that the degree of 4-ATP conjugation has a strong impact on the gelation. Regarding the degree of conjugation, viscosity increases in an overproportional manner. Oxidation of the conjugate bearing 158.79 ± 23.12 μmol 4-ATP/g polymer leads to a 34-fold increased reduced viscosity, whereas oxidation of the conjugate bearing 557.34 ± 49.03 μmol/g polymer leads to a 634-fold increased reduced viscosity compared to the initial viscosity. Within solutions of native sugar beet pectins, reduced viscosity could be increased not more than 2.5-fold by means of oxidation (6). Compared to gelation by calcium pectinate formation, covalent cross-linking by disulfide formation is clearly superior when a sufficient amount of thiol groups is present on the modified polymer. Viscosities of high-methoxy pectin solutions increase by a factor of approximately 250 in the presence of calcium ions (23). A 10- to 20-fold increase in viscosity could be achieved by another chemical modification. Methyl esters of galacturonic acid moieties were reduced to galactose units in order to allow these moieties to participate in the gelling process in the presence of 50% sugar (24).
Unmodified pectin showed rapid swelling and rapid disintegration within the experiments performed within this study. This observation can be explained by its high hydrophilicity. Water uptake of the test disks leads to formation of a hydrogel which can disintegrate according to its cohesive forces. Unmodified pectin matrices display weak cohesive forces and are characterized by rapid disintegration in aqueous environments which is a key limitation for several applications, especially in pharmaceutical sciences where pectin modifications could be potential carrier materials. Within this study, it could be shown that hydrophobic modification of pectin with 4-ATP leads to decelerated swelling. Otherwise, the total water uptake prior to disintegration of the pectin matrix could be significantly increased before disintegration of both oxidized conjugates. Decelerated swelling can be ascribed to the increased hydrophobicity of the new conjugates. Increased water uptake can be attributed to improved cohesive forces within test disks due to disulfide bond formation. Furthermore, improved disintegration behavior of Pec-ATP matrices compared to native pectin is supported by decelerated water uptake. Recently, the swelling behavior of calcium pectinate matrices was investigated, and it was found that the swelling of calcium pectinate is pH dependent (25). At low pH values, the ionic interactions between pectin and calcium ions will be reduced which limits the cohesive strengths of test disks and reduces the water absorbing capacities. Compared to calcium pectinate, Pec-ATP offers the possibility of covalent cross-linking. Therefore, cohesiveness of these conjugates does not rely on ionic interactions. Disintegration of pectins by pectinases in the colon should not be influenced as the general glycosidic structure of pectin has not been altered. Hence, its advantageous features regarding colonic drug delivery remain unaffected.
Interestingly, a low amount of coupled 4-ATP does not lead to an enormous increase in viscosity after oxidation but appears to be sufficient to stabilize modified pectin matrices by oxidation. Hence, it could be possible to synthesize custom-made pectin conjugates for specific purposes by adjusting the coupling rate to the specific requirements.
At this stage, it is assumed that by modifying pectin in the described manner, general problems of pectin as a drug vehicle can be overcome. It could be demonstrated that due to oxidation, comparatively more viscous gels can be formed. Future studies might include investigations regarding in situ gelling properties in order to improve sustained release features of pectic matrices. Moreover, it was shown within this study that disintegration of test disks could be significantly delayed. Hence, it should be possible to develop tablets that are stable across a larger part of the intestine compared to unmodified pectin tablets which will probably erode or dissolve in the upper part of the intestine due to the limited cohesive properties. Regarding particulate dosage forms, stabilization of particulate drug carriers by oxidation of thiol groups to disulfide bonds could be demonstrated by our research group on other well water-soluble polymers than pectin (26). Hence, it can be assumed that this principle can be transferred to modified pectins. Tablets as well as particulate delivery systems which are stabilized via disulfide bonds could be able to reach the colon without premature erosion. These would be ideal requirements for colon-specific drug delivery. After passing the harsh environment of the small intestine without extensive degradation, it can be expected that Pec-ATP matrices are degraded by microbial pectinases as the general polymer backbone structure would remain unaffected. Furthermore, the environment of the colon could contribute to the disintegration of such dosage forms by reductive cleavage of disulfide bonds. This would allow colon-specific delivery of drugs that are supposed to act in the colon or drugs that need to be protected from the comparably harsh conditions of more proximal gastrointestinal segments.
CONCLUSION
In the current study, our workgroup accomplished the covalent attachment of a water-insoluble ligand, namely 4-ATP, to the highly hydrophilic biopolysaccharide pectin. The results of the performed characterizations suggest that the obtained conjugate does not show an altered toxicity profile compared to unmodified pectin. Beyond, Pec-ATP conjugates offer a gelation mechanism based on formation of disulfide bonds due to oxidation. Moreover, swelling experiments indicate that disintegration of pectin matrices caused by excessive water uptake can be reduced due to hydrophobic modification and improved cohesiveness. Oxidative gelation of the new conjugates and reduced disintegration of oxidized Pec-ATP matrices in particular make Pec-ATP a valuable tool for various applications. It could be of high interest in pharmaceutical technology since stabilization of pectin matrices could facilitate sustained as well as colonic drug delivery as the rapid disintegration of pectin matrices has always been a major drawback for these applications. Moreover, this modification could offer another possibility to use pectin as a coating material.
References
- 1.May CD. Industrial pectins: sources, production and applications. Carbohydr Polym. 1990;12:79–99. doi: 10.1016/0144-8617(90)90105-2. [DOI] [Google Scholar]
- 2.Thibault JF, Renard CMGC, Axelos MAV, Roger P, Crépau MJ. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr Res. 1993;238:287–339. doi: 10.1016/0008-6215(93)87019-O. [DOI] [Google Scholar]
- 3.Ralet MC, Dronnet V, Buchholt HC, Thibault JF. Enzymatically and chemically de-esterified lime pectins: characterization, polyelectrolyte behavior and calcium binding properties. Carbohydr Res. 2001;336:117–25. doi: 10.1016/S0008-6215(01)00248-8. [DOI] [PubMed] [Google Scholar]
- 4.Endress HU. Nonfood uses of pectin. In: Walter RH, Taylor S, editors. The chemistry and technology of pectin. USA: Academic; 1991. pp. 251–68. [Google Scholar]
- 5.Kohn K. Ion binding on polyuronates—alginate and pectin. Pure Appl Chem. 1975;42:371–97. doi: 10.1351/pac197542030371. [DOI] [Google Scholar]
- 6.Oosterveld A, Beldman G, Voragen AGJ. Oxidative cross-linking of pectic polysaccharides from sugar beet pulp. Carbohydr Res. 2000;328:199–207. doi: 10.1016/S0008-6215(00)00096-3. [DOI] [PubMed] [Google Scholar]
- 7.Guilherme RG, Moia TA, Reis AV, Paulino AT, Rubira AF, Mattoso LHC, et al. Synthesis and water absorption transport mechanism of a pH-sensitive polymer network structured on vinyl-functionalized pectin. Biomacromolecules. 2009;10:190–6. doi: 10.1021/bm801250p. [DOI] [PubMed] [Google Scholar]
- 8.Kast CE, Bernkop-Schnürch A. Thiolated polymers—thiomers: development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials. 2001;22:2345–52. doi: 10.1016/S0142-9612(00)00421-X. [DOI] [PubMed] [Google Scholar]
- 9.Marschütz MK, Bernkop-Schnürch A. Thiolated polymers: self-crosslinking properties of thiolated 450 kDa poly(acrylic acid) and their influence on mucoadhesion. Eur J Pharm Sci. 2002;15:387–94. doi: 10.1016/S0928-0987(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 10.Bernkop-Schnürch A, Kast CE, Richter MF. Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine. J Control Release. 2001;71:277–85. doi: 10.1016/S0168-3659(01)00227-9. [DOI] [PubMed] [Google Scholar]
- 11.Majzoob S, Atyabi F, Dorkoosh F, Kafedjiiski K, Loretz B, Bernkop-Schnürch A. Pectin–cysteine conjugate: synthesis and in vitro evaluation of its potential for drug delivery. J Pharm Pharmacol. 2006;58:1601–10. doi: 10.1211/jpp.58.12.0006. [DOI] [PubMed] [Google Scholar]
- 12.Burner U, Jantschko W, Obinger C. Kinetics of oxidation of aliphatic and aromatic thiols by myeloperoxidase compounds I and II. FEBS Lett. 1999;443:290–6. doi: 10.1016/S0014-5793(98)01727-X. [DOI] [PubMed] [Google Scholar]
- 13.Silioc C, Maleki A, Zhu K, Kjoniksen AL, Nystrom B. Effect of hydrophobic modification on rheological and swelling features during chemical gelation of aqueous polysaccharides. Biomacromolecules. 2007;8:710–28. doi: 10.1021/bm061090o. [DOI] [PubMed] [Google Scholar]
- 14.Mortazavi SA, Smart SD. An investigation into the role of water movement and mucus gel dehydration in mucoadhesion. J Control Rel. 1993;25(3):197–203. doi: 10.1016/0168-3659(93)90078-J. [DOI] [Google Scholar]
- 15.Bernkop-Schnürch A, Schwarz V, Steininger S. Polymers with thiol groups: a new generation of mucoadhesive polymers? Pharm Res. 1999;16:876–81. doi: 10.1023/A:1018830204170. [DOI] [PubMed] [Google Scholar]
- 16.Habeeb AF. A sensitive method for localization of disulfide containing peptides in column effluents. Anal Biochem. 1973;56:60–5. doi: 10.1016/0003-2697(73)90169-3. [DOI] [PubMed] [Google Scholar]
- 17.Satake K, Okuyama T, Ohashi M, Shinoda T. The spectrophotometric determination of amines, amino acids and peptide with 2, 4, 6-trinitrobenzene-1-sulfonic acid. J Biochem. 1960;47:654. [Google Scholar]
- 18.Niles AL, Moravec RA, Hesselberth PE, Scurria MA, Daily WJ, Riss TL. A homogenous assay to measure live and dead cells in the same sample by detecting different protease markers. Anal Biochem. 2007;366:197–206. doi: 10.1016/j.ab.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Bernkop-Schnürch A, Hornof M, Zoidl T. Thiolated polymers—thiomers: synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int J Pharm. 2003;260:229–37. doi: 10.1016/S0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 20.Sakloetsakun D, Hombach J, Bernkop-Schnürch A. In situ gelling properties of chitosan–thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials. 2009;30:6151–7. doi: 10.1016/j.biomaterials.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 21.Semdé R, Moes AJ, Devleeschouwer MJ, Amighi K. Synthesis and enzymatic degradation of epichlorohydrin cross-linked pectins. Drug Dev Ind Pharm. 2003;29:203–13. doi: 10.1081/DDC-120016728. [DOI] [PubMed] [Google Scholar]
- 22.Verma RP, Kapur S, Barberena O, Shusterman A, Hansch CH, Selassie CD. Synthesis, cytotoxicity and QSAR analysis of X-thiophenols in rapidly dividing cells. Chem Res Toxicol. 2003;16:276–84. doi: 10.1021/tx020103q. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien AB, Philp K, Morris ER. Gelation of high-methoxy pectin by enzymatic de-esterification in the presence of calcium ions: a preliminary evaluation. Carbohydr Res. 2009;344:1818–23. doi: 10.1016/j.carres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbohm C, Lundt I, Christensen TMIE, Young NWG. Chemically methylated and reduced pectins: preparation, characterisation by 1H NMR spectroscopy, enzymatic degradation, and gelling properties. Carbohydr Res. 2003;338:637–49. doi: 10.1016/S0008-6215(02)00440-8. [DOI] [PubMed] [Google Scholar]
- 25.Sriamornsak P, Kennedy RA. Swelling and diffusion studies of calcium polysaccharide gels intended for film coating. Int J Pharm. 2008;358:205–13. doi: 10.1016/j.ijpharm.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Krauland AH, Bernkop-Schnürch A. Thiomers: development and in vitro evaluation of a peroral microparticulate peptide delivery system. Eur J Pharm Biopharm. 2004;57:181–7. doi: 10.1016/j.ejpb.2003.09.011. [DOI] [PubMed] [Google Scholar]