Abstract
The aim of this work was the formulation and characterization of alginate (ALG)–doxycycline (DOX) hydrogel microparticles (MPs) embedded into Pluronic F127 thermogel for DOX intradermal sustained delivery. ALG–DOX MPs were formed by adding a solution of the drug into a 1.5% polymer solution while stirring. The MPs were cross-linked by dispersion into a 1.2% CaCl2 solution. Free MPs were characterized in terms of size, drug content, and release behavior by HPLC and UV–vis. DOX and hydrogel MPs were embedded into PF127, PF127-HPMC, and PF127-Methocel thermogels. The thermogels were characterized in terms of gelling time, morphology, and release behavior. A target release period of 4–7 days was considered optimal. The hydrogel MPs were about 20 µm in size with 90% of the population <59 µm. Drug content was about 35% (w/w). DOX released rapidly from the MPs, 90% within 2 days. An expected faster release was observed for free DOX from the thermogels with 80–90% of drug released after 3.5–4 h even in the presence of 1% HPMC or Methocel. The release was sustained after embedding the MPs into PF127 and PF127-HPMC thermogels. In particular, the PF127-HPMC thermogel showed an almost linear release, reaching 80% after 3 days and 90% up to 6 days. Although a further characterization and formulation assessment is required to optimize MP characteristics, ALG/DOX-loaded hydrogel MPs, when embedded into a PF127-HPMC thermogel, show a potential for achieving a 7-day sustained release formulation for DOX intradermal delivery.
Key words: doxycycline, hydrogel microparticles, sustained release, thermogel
INTRODUCTION
Doxycycline (DOX) is a member of the tetracycline antibiotics group derived from oxytetracycline, which is frequently used to treat chronic prostatitis (http://en.wikipedia.org/wiki/Prostatitis), sinusitis, syphilis, chlamydia (http://en.wikipedia.org/wiki/Chlamydia), pelvic inflammatory disease, acne (http://en.wikipedia.org/wiki/Acne_vulgaris), and rosacea (http://en.wikipedia.org/wiki/Rosacea) (1,2) and in the treatment and prophylaxis of Bacillus anthracis and malaria (3). DOX was shown to be effective against several other pathogens causing a number of diseases and, since it is at sub-antimicrobial doses an inhibitor of matrix metalloproteinases, DOX can be proposed for a number of purposes (4).
DOX is well absorbed after oral administration, and bioavailability is 90–100% in humans. DOX is lipid-soluble and penetrates body tissues and fluids better than tetracycline HCl or oxytetracycline, including distribution to the cerebrospinal fluids, prostate, and eye. Cautions and side effects are similar to other members of the tetracycline antibiotic group. DOX is available as hyclate (5), calcium, and monohydrate salts (6). It is formulated as injections, oral tablets, capsules, oral powders for reconstitution, and oral syrups (6). DOX has been reported to be a sclerosing agent (7,8), and this effect can be advantageous in the treatment of pathologies and disorders provoking tissue lesions and tumors. In this regard, DOX may be employed against dermal neurofibromas (DNFs) cutaneous lesions or subcutaneous nodules (9,10). Neurofibromatoses are a set of inherited autosomal dominant genetic disorders that tend to result in the development of benign tumors of the nerve sheath, but transformation to malignant peripheral nerve sheath tumors is also a risk (11). DNFs usually first appear in the preadolescent years and continue to occur throughout life (11). DNFs may reside within the skin or project above the surface of the skin as typically small, 1–2 cm, but numerous lesions that can produce pain, itching, significant disfigurement, and social stigmata. There are no approved pharmacological therapies, and the only treatments available require surgical removal or radiotherapy. The superficial location of the DNFs makes them a good target for topical therapy, i.e., direct injection into the lesion, so as to allow for DNF involution without scar formation or to prevent them from growing in size. Presumably, the DOX sclerosing action may cause thrombosis and interruption of blood supply to the DNFs with consequential inflammation, necrosis, and lesion falling off. Pilot studies on rats revealed that 0.2–0.4 mL of a 20 mg/mL DOX solution administered weekly for 4 weeks would cause necrosis after one to two injections and disappearance of the majority of the lesions (data not published). Therefore, intradermal administration of DOX may be helpful indeed to treat DNF lesions. In order to achieve this goal, formulation of a thermogel able to deliver the drug directly to the desired site of action upon injection may be desirable. This thermogel may represent either a controlled release device or merely a vehicle to deliver controlled release devices such as microparticles (MPs) loaded with DOX. This work was meant to provide a proof-of-principle on the possibility to obtain a sustained release of DOX over a period of 4–7 days aimed at the improvement of the treatment of DNF lesions. According to the literature addressing the use of thermogels as controlled release delivery systems, several strategies conceive the use of polysaccharides and some copolymers as gel-forming systems able also to bind physically DOX to the polymer (12–14). In the present work, two strategies were followed: (1) use of copolymer systems such as poly(oxy-ethylene)–poly(oxy-propylene) copolymers, which provide a thermogel behavior and (2) use of cross-linked polysaccharides, such as alginate (ALG), to form stable hydrogel systems able to steadily bind and/or entrap the drug (15). The development of a new thermogel formulation containing DOX was pursued by choosing a well-established thermogel polymer such as Poloxamer 407 (16,17) also known as Pluronic F-127 (PF127). PF127 is a polyethylene oxide–polypropylene oxide copolymer well recognized for gel-forming properties in a range of temperatures close to body temperature. It is also widely used due to physicochemical characteristics and safety (18–20). Generally, at concentrations above 20%, PF127 aqueous solutions are flowable liquids at 4°C, but become a solidified gel in the temperature range 25–40°C. Therefore, a PF127 thermogel formulation was selected in order to provide a proof-of-principle on the possibility of employing such systems for DOX delivery and controlled release. Different pH buffers, polymer concentrations, and DOX loadings were employed in order to establish the conditions in which a thermogel system possessing a sol–gel transition in the 32–37°C range can be formed. PF127, although widely accepted as a pharmaceutical excipient with a thermo-reversible property, has been found to ensure drug release only for a few hours (21,22); therefore, a combination of cross-linked ALG–DOX hydrogel MPs with PF127 thermogels was thereby employed in order to achieve a 4- to 7-day DOX sustained release behavior.
MATERIALS AND METHODS
Analytical Methods
In order to quantify DOX in the thermogel system, HPLC (23) and UV methods were developed.
The system employed was a Waters 1525 Binary HPLC pump equipped with an in-line degasser AF, a Waters 2487 dual λ adsorbance detector, and a Waters 717 plus autosampler. A BDS Hypersil C8 (5 µm, 4.6 × 250 mm) column was used, and the analytical conditions consisted in the use of (1) 40% deionized water containing 0.02 M oxalic acid (Sigma, USA), 0.0005 M EDTA (Sigma) overall concentrations (pH = 2.05–2.10): 60% acetonitrile (Baker, USA) as a mobile phase, (2) isocratic mode, (3) flow rate 1.25 mL/min, and (4) UV–vis detection at 351 nm. The injected volume was 20 µL. This setup allowed obtaining a calibration curve in water in the 1.5–100 µg/mL DOX concentration range (r2 = 0.9999). All analyses were performed in triplicate and the error expressed as SD.
A UV method was also developed to allow analysis of DOX in the presence of polymers which may interact with the HPLC column, producing several analytical issues. Analysis was accomplished at 351 nm in a single wavelength mode using a Hitachi2000 double beam UV–vis spectrophotometer. Calibration was performed in 0.1 M phosphate (Sigma) buffer, pH 7.4, in the 1.2–30 µg/mL DOX concentration range (r2 = 0.9999). All analyses were performed in triplicate and the error expressed as SD.
DOX Solubility
DOX solubility in 0.05 M phosphate buffers at pH 5–7.4 was evaluated either at 4°C and 37°C. About 500 mg of DOX was placed in a scintillation vial and 1 mL of buffer added. The resulting suspensions were maintained at 4°C and 37°C for 1 h while stirring. The suspensions were then filtered through 13 mm Millipore GVHP 0.22-µm filters (Millipore, MA) to separate the dispersed solid from the dissolved drug. The filters were preliminarily validated to check for any possible drug absorption or other form of interaction. The obtained solutions were analyzed by HPLC upon proper dilution.
DOX Stability in Solution
DOX stability was investigated in order to establish molecule susceptibility to the working conditions employed. Briefly, weighed amounts of DOX were dissolved in 0.05 M phosphate buffers, pH 5 and 7, and incubated at 37°C over several days. DOX stability was studied also in water at 37°C over the same period of time. Samples were withdrawn at predetermined time points and submitted to HPLC analysis upon proper dilution. All experiments were performed in triplicate and the error expressed as SD.
PF127 Thermogel Preparation
The thermogel preparation was accomplished, according to standard procedures (14), in scintillation vials by simply dissolving increasing amounts of PF127 (Sigma) into 2 mL of 0.05 M phosphate buffers, pH 5–7, at 4°C. After complete dissolution, DOX amounts matching 10–30% target loadings were allowed to dissolve in the polymer solution maintained at 4°C. All batches were subjected to overnight stabilization at 4°C. The batches obtained and the corresponding conditions are reported in Table I.
Table I.
Thermogel Batches Loaded with DOX
| Batch no. | PF127 concentration (%) | DOX loading (%, w/w) | Phosphate buffer pH |
|---|---|---|---|
| 1 | 20.0 | 10.0 | 6.0 |
| 2 | 30.0 | 10.0 | 6.0 |
| 3 | 20.0 | 30.0 | 6.0 |
| 4 | 30.0 | 30.0 | 6.0 |
| 5 | 20.0 | 20.0 | 5.0 |
| 6 | 30.0 | 20.0 | 5.0 |
| 7 | 20.0 | 20.0 | 7.0 |
| 8 | 30.0 | 20.0 | 7.0 |
| 9 | 25.0 | 10.0 | 5.0 |
| 10 | 25.0 | 30.0 | 5.0 |
| 11 | 25.0 | 10.0 | 7.0 |
| 12 | 25.0 | 30.0 | 7.0 |
| 13 | 25.0 | 20.0 | 6.0 |
Modified PF127 thermogels were produced by adding 1% of carboxymethylcellulose (CMC, DOW, MI), hydroxypropylmethylcellulose (HPMC, E4M, DOW, MI), and Methocel (methyl cellulose, DOW, MI) to the PF127 solution and were characterized in terms of gelling capacity, DOX solubility in the polymer solution, stability in solution, and DOX in vitro release.
Gelling Time
The time required for the sol–gel transition to occur is essential to avoid drug leakage in the very first moments after injection. Therefore, all batches were evaluated at 32°C and 37°C for gelling time. To accomplish such, a water bath was used to control the temperature. All thermogel solutions were maintained at 4°C throughout the experiment, and the gelling time was measured from the moment in which the solutions were placed into the water bath so as to simulate injection into the body. Gelling was considered complete when no sliding was observed upon fast vial flip-over.
ALG–DOX Hydrogel MPs
The mixing of 1 mL of a 1.5% ALG solution with an excess of DOX in 0.05 M phosphate buffer, pH 5, or water led to the formation of an ALG–DOX precipitate. The precipitate was shaped into MPs by mixing ALG and DOX while homogenizing by Silverson stirrer (1,000 rpm) and then cross-linking with calcium to produce a hydrogel. This was accomplished by dispersing an amount of MPs in 5–10 mL of a 1.2% calcium chloride solution and stirring at room temperature for 10 min. The MPs were centrifuged, washed three times with deionized water, and freeze-dried overnight.
Some preparation parameters, such as pH and type of dispersing medium and the ALG/DOX ratio, were studied to assess the effect on the MP characteristics, as size and DOX content. Particle size was performed using a Mastersizer2000 (Malvern Instruments Inc., MA) by suspending the MPs in a 0.1% (w/w) Tween 80 solution, and population dispersion was calculated as span = D([D(v, 0.9) − D(v, 0.1)]/D(v, 0.5), where D(v, 0.5), D(v, 0.1), and D(v, 0.9) are the volumetric diameters at 50%, 10%, and 90% of the population distribution, respectively. DOX content was determined after solubilization of a weighed amount of MPs in a 1% (w/w) sodium citrate (Sigma) solution, and samples were analyzed by the HPLC method previously described.
The cross-linked MP stability in the release medium was evaluated as well in 0.05 M phosphate buffer, pH 5, over 2 days at 37°C.
ALG–DOX Hydrogel MPs in PF127 and PF127 Modified Thermogels
The ALG–DOX hydrogel MPs were embedded into PF127 thermogels by dispersing an amount of MPs corresponding to 20 mg of DOX into about 300 µL of preformed PF127 solution at 4°C. The thermogels employed were chosen according to the study conducted on the PF127 thermogel alone and PF127 thermogels modified with cellulose derivatives previously described. The system was evaluated in terms of capacity to release DOX in a sustained manner.
In Vitro Release Study
In vitro release analysis, either for the thermogels or the MPs and the combined systems, was performed on the batches that showed best characteristics. The method was based on a membraneless approach elsewhere reported (35,36) that closely mimics the actual in vivo conditions. Briefly, 200 µL of thermogel solution was placed in a 5-mL scintillation vial in order to let the solution spread on the bottom to maximize the contact surface with the release medium. This should mimic closer the actual in vivo conditions that do not present any barrier between the thermogel and the environment.
Three milliliters of 0.05 M phosphate buffers from pH 7.4 to 5 was employed as release medium. Either the scintillation vial or the release medium was equilibrated at 37°C prior to the start of the experiment. The buffer was added after checking the actual gel formation. One hundred microliters of aliquots was withdrawn and replaced with an equal volume of fresh buffer maintained at 37°C.
Initial time points were 10, 30, and 60 min, and the subsequent time points were established according to the results obtained from the initial release. All samples were submitted to UV analysis upon proper dilution. The experiment was performed on triplicate samples. Mass balances were also carried out at the end of the release study so as to establish whether DOX may undergo degradation or any other side interaction with the environment.
Release Mechanism
The release profiles obtained for ALG–DOX hydrogel MPs, alone and embedded into PF127 and PF127–1% HPMC thermogels, were fitted against Peppas, Higuchi, and Weibull models (24–27) to assess the release mechanism ruling DOX liberation from such systems. Lack of fit was determined as previously reported by applying the Levenberg–Marquardt method for the minimization of the reduced χ2 function and the analysis of the adjusted r2 (30,31). The Akaike information criterion (AIC) was also used to help determine the best fitting equation (32). The selected best model was used to establish DOX release mechanism according to the criteria reported in the literature (26–29).
RESULTS AND DISCUSSION
DOX Solubility and Stability
Due to an amphoteric nature, DOX solubility in a wide range of pH should resemble a V-shaped profile.
In fact, DOX possesses three different pKas around 3.4, 7.7, and 9.3 (33); a fourth deprotonation center is likely to exist even though not yet fully addressed (34). As a result, the molecule is positively charged at pH < pKa1, negatively charged at pH > pKa3 and zwitterionic in between, with an isoelectric point at about pH 5.5 (35). These characteristics are important as drug behavior depends strongly on physicochemical properties and the required experimental conditions. From the values obtained (Table II), it is evident that the solubility at 37°C was generally about fourfold greater than that at 4°C. Generally, drug concentrations were around 50 mg/mL at 4°C without significant variations with pH. On the contrary, at 37°C, DOX solubility increased significantly (p < 0.05) from 183 mg/mL at pH 5 to about 270 mg/mL at pH 7.4.
Table II.
DOX Solubility Data at Different pHs and Temperature
| pH | Solubility (mg/mL) ± SD |
|---|---|
| 4°C | |
| 5.0 | 57 ± 15 |
| 6.0 | 54 ± 16 |
| 7.0 | 42 ± 13 |
| 7.4 | 53 ± 12 |
| 37°C | |
| 5.0 | 183 ± 10 |
| 6.0 | 208 ± 4 |
| 7.0 | 234 ± 8 |
| 7.4 | 270 ± 2 |
The presence on the molecule of two carbonyl and one amide groups allows tetracyclines to arrange into up to 64 possible tautomers that can interconvert by following a complex equilibrium in solution (34). This makes tetracyclines able to adapt and arrange themselves as a result of an environmental modification. Therefore, it is difficult to predict exactly the behavior. However, it has been determined that in aqueous solution, the predominant and therefore most stable form is the zwitterion (34) owing to the polar environment. Consequently, since the first pKa is around 3.4, it is conceivable to suppose that DOX, as much as other tetracyclines, could possess a higher stability at pH between the first and second pKa (3.4–7.7).
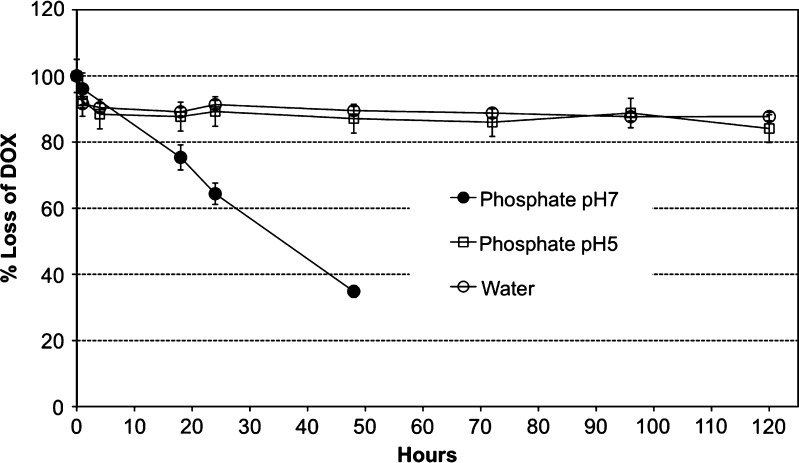
In this regard, DOX stability, investigated at different pH at 37°C, highlighted a fast decrease of the drug concentration in phosphate buffer at pH ≥ 7 (Fig. 1). Just slightly more than 30% of the initial amount remained after 2 days of incubation. On the contrary, at pH < 7, DOX was fairly stable over at least 4 days of incubation. Drug precipitation, although not observed, cannot be ruled out during incubation. Therefore, the reason of such a behavior may reside in the ability of the molecule to arrange into different epimers possessing also a different thermodynamic stability with pH as discussed above. In addition, DOX clearly demonstrated an increased instability when pH approaches 7.7, corresponding to the second pKa value. Moreover, being DOX quite susceptible to light, exposure of DOX solutions to light was carefully prevented over the entire experimental procedure. DOX solutions under light quickly became darker, predictably in accordance to DOX concentration. However, it was noticed that pH close to 7 caused increased darkening of DOX solutions, while at pH 5, this phenomenon was significantly delayed. This proves that pH is critical to DOX stability in a manner that yet needs to be understood. However, this hypothesis requires further investigation, and at this stage, it is not the aim of this work. Nevertheless, these results suggest that phosphate buffers with pH ≥ 7 should be avoided as far as storage and/or long-term studies are being concerned.
Fig. 1.
DOX stability in phosphate buffers and water at 37°C
PF127 Thermogels
In order to ensure an effective prolonged sustained release of DOX on the DNFs plaques, two main parameters have to be considered: (1) the copolymer nature of the polymer employed for the thermogel preparation and (2) stability in the intradermal environment. In fact, the copolymer nature is important to ensure a thermogel behavior, whereas stability, intended also as low solubility in physiological environments, may shift the overall DOX release mechanism toward a swelling/hydrolysis/diffusion driven process, which implies longer time for a complete release of the drug compared to a simple swelling/diffusion mechanism.
The solubility data can be useful to understand the characteristics of the thermogel batches obtained at different pHs. According to the gelling behavior, the best preparation among those reported in Table I was batch 2.
Batch 2 gelling time was about 3′30″ at 32°C and <2′ at 37°C. This result is satisfactory considering the mass of the sample analyzed of 2 mL. Thermogel injected volumes for intradermal administration can be as low as a few hundred microliters, and in this case, an average volume up to 200 µL may be employed. Such a small volume would have much lower gelling time of less than a minute. Also, the visual consistency of the gel was good, with no sliding or shifting of the gel mass upon vial flip-over and shaking.
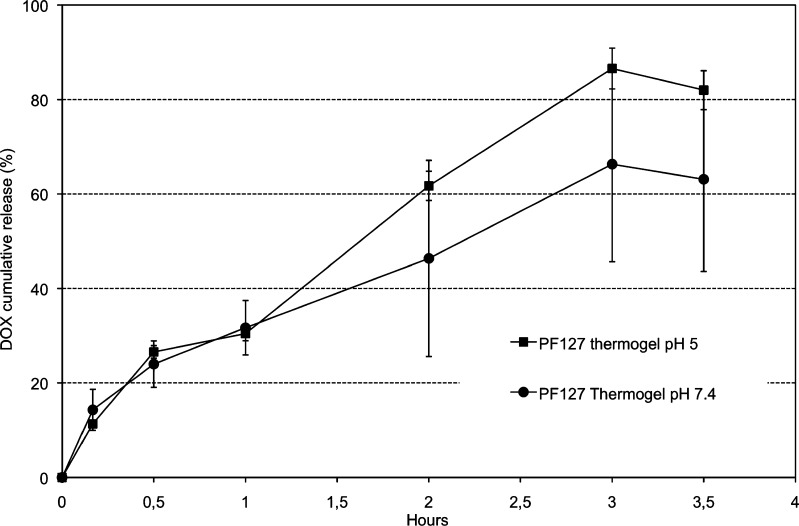
After overnight stabilization, some of the samples showed incomplete DOX solubilization. This was more evident, as expected, for batches having higher polymer and DOX concentrations. In general, batches prepared with phosphate buffer at pH 6 were seen to dissolve faster. This behavior may be linked to the DOX zwitterionic nature at pH 6 as well as to the presence of the surface-active polymer. In fact, DOX apparent solubility in the PF127 solution remarkably increased, and it was possible to dissolve amounts of DOX as high as 342 mg/mL, a value well above the highest DOX solubility (270 mg/mL at 37°C) previously recorded in the same buffers. From these results, batch 2 was chosen for further characterization in order to establish release performances. The in vitro release study was conducted initially in standard conditions (pH 7.4, 37°C) and compared with the release at pH 5. The thermogel dissolved rapidly and disappeared after about 3 h, and the release profiles plateaued within 3.5 h with a maximum of about 55% at pH 7.4, whereas at pH 5, the maximum was nearly 90% (Fig. 2).
Fig. 2.
DOX in vitro release from the PF127 thermogel in 0.05 M phosphate buffer, pH 7.4 and 5, at 37°C
The incomplete release at pH 7.4 was interpreted on the basis of the drug instability observed at pH ≥ 7. In particular, at pH 7.4, formation of crystals was observed starting from the second hour of release. These crystals were ascribed to DOX crystallization into a different form with respect to the hyclate (e.g., monohydrate). The mass balance performed at 3.5 h confirmed that the crystal formation was due to drug reprecipitation into an insoluble crystalline form as after dissolution of the crystals, an almost complete drug recovery was possible. This observation excluded drug chemical degradation and supports the hypothesis of a rearrangement of the molecule at different pH. Nonetheless, partial degradation of the molecule cannot be ruled out and further investigation is required.
From these observations, two main considerations may be drawn: (1) pH < 6.5 may be required in vitro as already suggested by the stability study and (2) the system used to test the release is mainly a static system that does not conceive drug consumption over time, thereby arousing possible crystallization and degradation. Therefore, such phenomenon is not likely to occur in vivo where the environment is characterized by a lower pH and a dynamic situation in which the drug is continuously eliminated from the release site.
In any case, the release profiles obtained, although expected, due to the high hydrophilicity of PF127 and DOX solubility in water is not corresponding to the desired 4- to 7-day sustained release targeted in this work. Moreover, pH 5 was considered the best choice to perform the in vitro release study for two reasons: (1) the observed DOX instability in buffers at pH ≥ 7 confirmed by the thermogel release study and (2) the actual in vivo environmental conditions that show pH from 4–5 in the stratum corneum to 7–7.3 in the lower epidermis, where it may also become acidic in the case of DNF appearance (36).
For the aforementioned reasons, the thermogel release behavior was investigated further in pH 5 phosphate buffers and compared with that obtained from modified thermogels in the same conditions. The addition of thickening agents, such as hydrophilic polymers, can be useful to increase thermogel stability over time in solution (17). Therefore, CMC, HPMC, and Methocel were mixed with PF127 at 1% (w/w) concentration to prolong DOX release that was previously observed to occur rapidly even though in a sustained manner.
The PF127 thermogel, corresponding to batch 2, added with 1% HPMC and Methocel showed better characteristics compared to that with 1% CMC. The addition of CMC generated a solution too viscous to be injected; moreover, CMC did not allow a proper dissolution of DOX inside the thermogel (data not shown). Therefore, PF127 with 1% HPMC and Methocel were chosen for further tests.
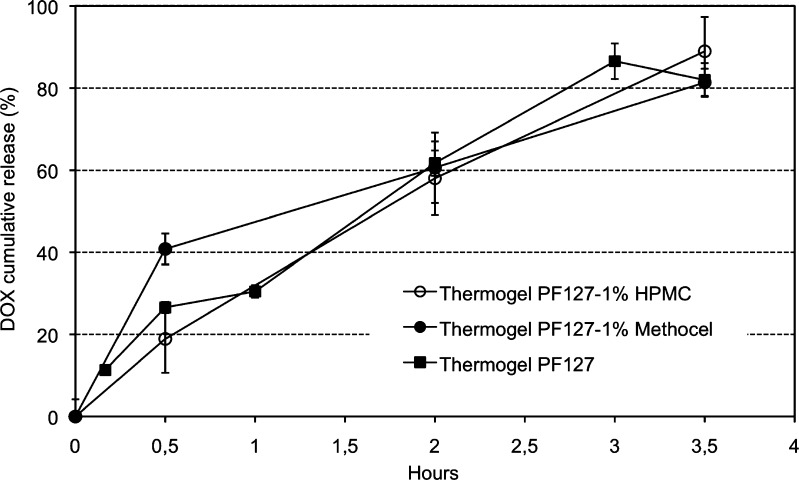
The gelling behavior of modified thermogels matched that of pure PF127 with a rapid gelling (<1 min) at 37°C. The release, although similar for all batches, was slightly more sustained for the thermogel with 1% HPMC (Fig. 3). In fact, it practically matched zero-order release kinetics (r2 = 0.990), reaching 90% of DOX released after 3.5 h.
Fig. 3.
DOX in vitro release from PF127 thermogel and modified thermogels in 0.05 M phosphate buffer, pH 5, at 37°C
Moreover, the consistency and stability against dissolution of the thermogels with HPMC and Methocel were slightly higher compared to PF127 alone. Yet, no improvement in release time was observed, but rather was comparable to that for the pure PF127 thermogel.
ALG–DOX Hydrogel MPs
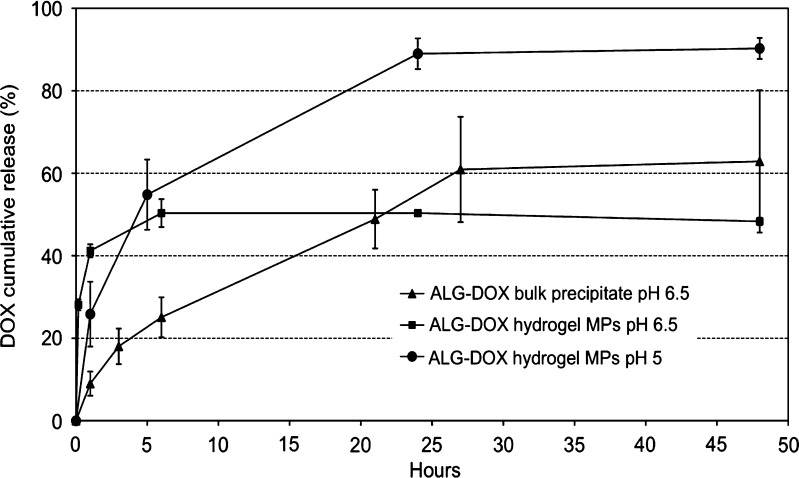
The ALG–DOX precipitate is likely generated by the interaction of the charged amine group on DOX with the acidic groups on the ALG backbone. Precipitate formation was observed immediately after injection of a concentrated DOX solution into the ALG solution upon mixing. The pH was always totally or partially acidic as a result of DOX first pKa (3.4). The DOX content following hydrolysis of the polymer with 1 M HCl for about 5 h was found to be in the range of 30–40% (w/w). The bulk precipitate was lowly soluble as the preliminary release test performed at pH = 6.5 and 37°C over 2 days showed a low release up to 40% and the presence of a consistent residual mass of the precipitate (Fig. 4). Mass balance analysis showed a total DOX recovery of barely 60% (data not shown); moreover, release after 1 day decreased. These observations were in agreement with those previously reported for the thermogels, thus suggesting that even pH 6.5 phosphate buffer may not be the right medium for the in vitro release studies, and even in this case, a pH 5 buffer was found more suitable.
Fig. 4.
DOX release in 0.05 M phosphate buffer, pH 6.5, from the bulk precipitate with ALG and comparison with the release from hydrogel MPs at pH 6.5 and 5
Formulation of the precipitate into MPs produced a DOX released at pH 6.5 with a maximum (50%) comparable to that of the bulk precipitate (60%; Fig. 4), however with a much higher burst in the first hour. This effect can be ascribed to the much larger surface area of the MPs compared to the bulk precipitate. In fact, MP size distribution was narrow and suitable for injection. In fact, volume mean diameter was about 27 μm with a D(v,0.5) = 21.2 μm, D(v,0.9) = 55.6 μm, and D(v,0.1) = 7.8 μm. The resulting dispersion (span) was 2.3, a value not too high considering that no particular homogenization process has been carried out apart from silverson stirring. Cross-linking with calcium chloride could enhance stability of the precipitate in the release medium as after 48 h of incubation in 0.05 M phosphate buffer, pH 6.5, at 37°C, no evident solubilization of the MPs was observed with residual DOX within the hydrogel.
At pH 5, a more sustained release from the MPs was observed with a maximum of about 90% of DOX released after 24 h (Fig. 4). This confirmed the increased stability of DOX at this pH even though this condition may not match perfectly the in vivo environment, in particular in the lower epidermis layers.
The variation of preparation parameters, such as ALG/DOX ratio and dispersing solvent, did not change ALG–DOX hydrogel MP characteristics (Table III). In detail, for the preparation of DOX100307, 0.05 M phosphate buffer, pH 5, was employed as dispersing medium and the ALG/DOX ratio was about 1:5. Increasing the ALG/DOX ratio to 2:5 and using water as dispersing medium did not alter particle characteristics. A very good content was recorded for all preparations with a value around 35% (w/w).
Table III.
Characterization of ALG–DOX Hydrogel MPs
| Batch no. | ALG/DOX ratio | Dispersing medium | %Drug content ± SD | Recovery (%) |
|---|---|---|---|---|
| DOX100307 | 1/5 | 0.05 M phosphate buffer, pH 5 | 35 ± 2 | 33 |
| DOX100407 | 2/5 | 0.05 M phosphate buffer, pH 5 | 32 ± 3 | 30 |
| DOX100807 | 1/5 | Water | 35.8 ± 0.9 | 21 |
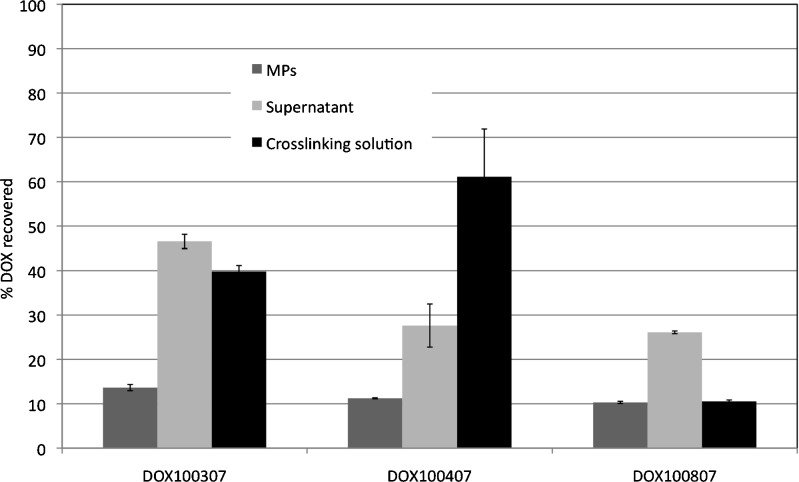
The low drug recovery was due mainly to DOX losses in the dispersing medium and in the cross-linking solution as well as during the washing steps between hydrogel MP recovery and cross-linking (Fig. 5). So a large part of the drug was lost during the different processing steps. This is conceivable as the amount added is in large excess with respect to ALG. Therefore, an attempt was made to calibrate the amount of DOX according to the content found in the MPs by increasing the ALG/DOX ratio to 1:3, but the visual character of the dispersion changed drastically, producing after freeze drying an aggregate system possessing a spongy consistency. Therefore, the preparation was found to possess proper characteristics only when DOX is in large excess with respect to ALG. Of course, further studies are needed in order to clarify this particular behavior.
Fig. 5.
DOX mass balance among the different preparation steps, compared to the amount measured in the MPs, for the three batches of Table III
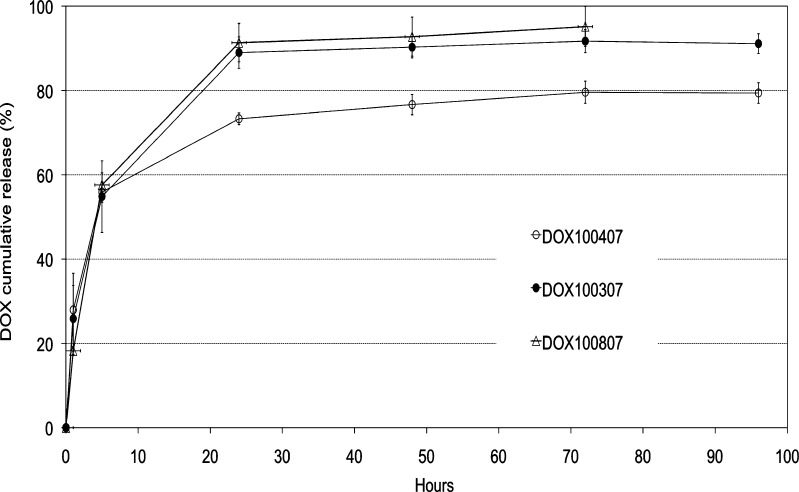
DOX in vitro release was carried out according to the conditions suggested by the stability study and previous release studies. As it was for the thermogels, pH 5 was found to satisfy either stability concerns or a plausible in vitro–in vivo correlation. The analysis of DOX release from the batches produced outlined fast diffusion in the first 24 h, leading to the release of about 70–90% of the drug. In the following period, a further slight release was observed up to day 3 (Fig. 6). It must be highlighted, however, that the conditions in which the release was performed do not represent the actual situation that may be observed in vivo. In fact, the experiment was performed in sink conditions that obviously produced fast hydration and swelling and then release of the water-soluble form of the drug. Such a situation is unlikely to occur in vivo as the intradermal environment, although it can contain up to 70% of water in the lower layers (36), is usually poor of fluids needed to efficiently hydrate and subsequently drain the drug from within the particles. Therefore, real conditions may favor a much slower release resulting in more sustained characteristics. Mass balance at 3 and 4 days of release allowed almost 100% recovery of the drug, proving that no DOX degradation or physical transformation occurred (data not shown).
Fig. 6.
DOX in vitro release profiles from ALG–DOX hydrogel MPs in 0.05 M phosphate buffer, pH 5, at 37°C
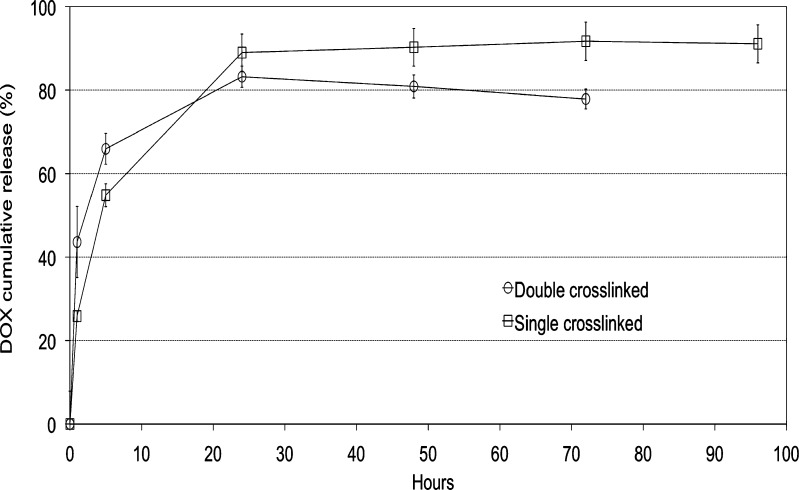
An attempt to extend the release of the drug was performed by introducing a second cross-linking step in the preparation process. However, this was not considered successful as the DOX content dropped to 12% and the release showed an even higher burst compared to the single cross-linking step (Fig. 7). This is likely a consequence of the repetition of the cross-linking process which by itself, as previously shown, can favor drug extraction.
Fig. 7.
Comparison of DOX in vitro release profiles from ALG–DOX hydrogel MPs before and after the second cross-linking step (0.05 M phosphate buffer, pH 5, at 37°C)
According to the longer release obtained using the ALG–DOX MPs, it could be hypothesized that an even longer release period may be achievable by embedding the hydrogel particle system into the PF127 thermogels. In fact, the presence of the viscous environment represented by the thermogel should slow the hydration, swelling, and diffusion processes, leading to a more prolonged DOX release.
ALG–DOX Hydrogel MPs Embedded into PF127 Thermogels
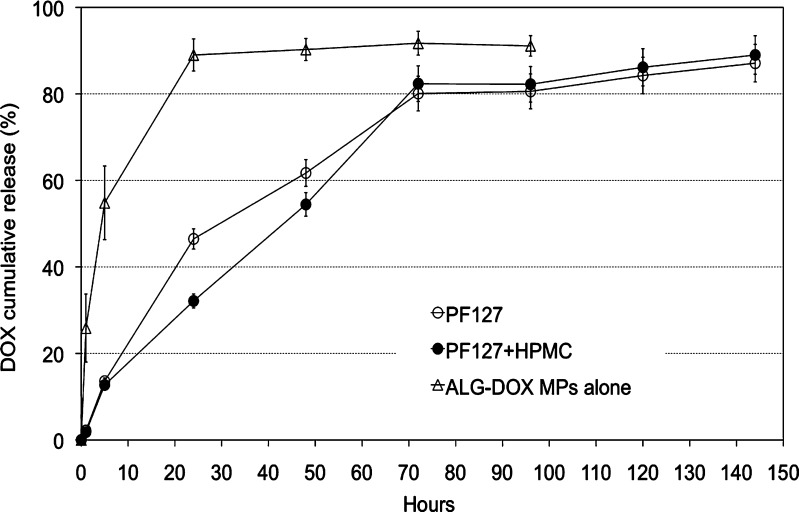
In agreement with the previous hypothesis, the extension of DOX release was investigated by dispersing the MPs inside the PF127 thermogel system. The PF127- and HPMC-added thermogels were chosen as vehicles, while the MP batch DOX100307 was selected to be embedded inside the thermogels. Both thermogels showed unaltered gelling capacity and consistency upon MP inclusion in the system (data not shown). The release profiles obtained in pH 5 phosphate buffer at 37°C confirmed a remarkably extended and sustained release of DOX over a period of 3 days followed by a period of slower but progressive release that reached about 90% after 6 days (Fig. 8). The thermogel with HPMC confirmed sustained release compared to PF127 alone, even though after 3 days, the profiles overlapped.
Fig. 8.
Comparison of DOX in vitro release profiles from ALG–DOX hydrogel MPs alone and embedded into PF127 thermogels (0.05 M phosphate buffer, pH 5, at 37°C)
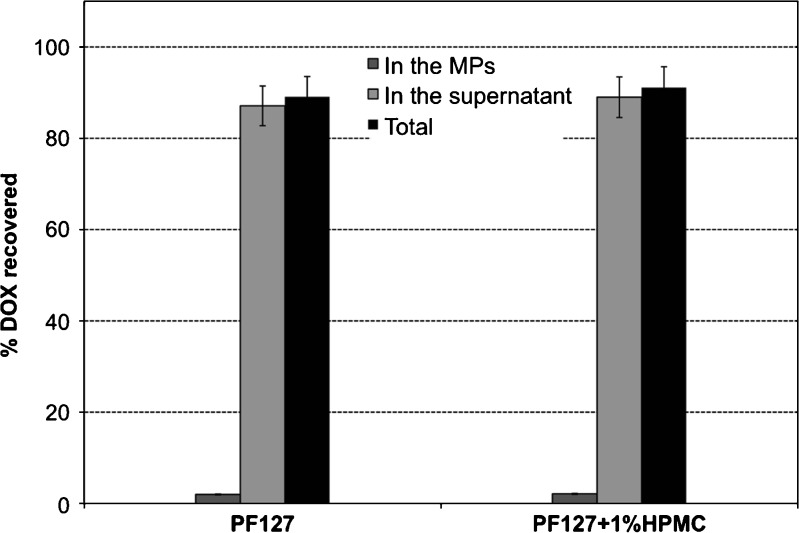
The mass balance study at day 6 showed that almost all the drug was recovered and just a negligible amount remained into the MPs (Fig. 9).
Fig. 9.
DOX mass balance after day 6 of release in ALG–DOX hydrogel MPs embedded into PF127 thermogels in 0.05 M phosphate buffer, pH 5, at 37°C
Release Mechanism
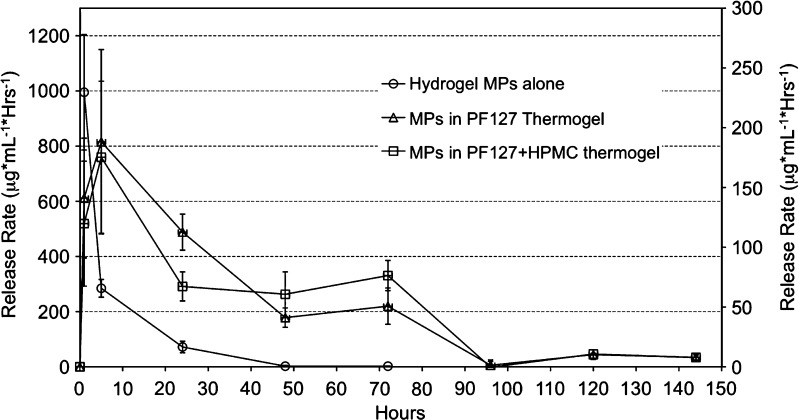
Out of the three models employed to fit release data, the Weibull function resulted the best model over the entire data range according to the reduced χ2 and adjusted r2 values reported in Table IV. This prevalence was confirmed also by the AIC terms, which always were smaller for the Weibull model compared to Peppas’ and Higuchi’s (Table IV). Such evidences suggested using the Weibull function to describe DOX release behavior according to the established link of the Weibull’s b parameter to the release mechanism as described in the literature (26,27,37). In this regard, the b coefficient increased from 0.52 for the MPs alone to 0.77 and 0.99 when the MPs were embedded into thermogels without and with 1% HPMC, respectively (Table V). Such values agreed with Fickian diffusion (case I transport) plus case II transport combined mechanism from MPs in PF127 thermogels (0.75 < b < 1) (26,27,37). On the other hand, DOX release from the MPs alone is closer to a Fickian diffusion mechanism in a disordered fractal space (b < 0.69) (26,27). Such mechanisms correlate to the release from swelling controlled systems in which polymer relaxation governs matrix permeation to the solvent and the subsequent solubilization and diffusion of the drug being released (27,38). Case II transport is less prevalent when MPs alone are being concerned, and the prevalence of Fickian diffusion is explained by the reduced size of MPs which determines a fast hydration and swelling of the polymer matrix. Therefore, in this case, a first-order kinetics characterizes DOX release. As a support, the release rate profiles of the MPs alone and in the thermogels indicated a typical first-order kinetics for DOX liberation from the MPs, which shifts toward a zero-order kinetics from within the thermogels (Fig. 10). In fact, when the MPs are dispersed within the thermogel, the case II transport becomes predominant, at least initially, over the case I transport due to the presence of a thick layer of PF127 polymer matrix. Moreover, the addition of HPMC to the thermogel thickened further the PF127 matrix, shifting even more the mechanism toward a case II transport (b moves from 0.77 to 0.99).
Table IV.
Fitting Results of Higuchi, Peppas, and Weibull Models to DOX Release Data from the MPs Alone and Within Thermogels
| Formulations | Higuchi | Peppas | Weibull | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Reduced χ 2a | Adjusted r2 | AIC | Reduced χ2a | Adjusted r2 | AIC | Reduced χ2a | Adjusted r2 | AIC | |
| ALG-DOX MPsb | 0.0454 | 0.67794 | −15.73 | 0.00916 | 0.93498 | −21.21 | 0.00206 | 0.98538 | −31.65 |
| ALG-DOX MPs in PF127 thermogelc | 0.00481 | 0.96385 | −43.09 | 0.00475 | 0.96431 | −39.61 | 0.000932 | 0.99254 | −53.69 |
| ALG-DOX MPs in PF127-HPMC thermogelc | 0.00490 | 0.96579 | −42.93 | 0.00547 | 0.96180 | −38.34 | 0.00213 | 0.98515 | −46.84 |
a χ 2/DoF as obtained by the Levenberg–Marquardt method
b n = 7
c n = 9
Table V.
Calculated a and b Coefficients of the Weibull Function Resulting from the Fitting of Table IV
| Preparation | Weibull | |
|---|---|---|
| a ± (SE) | b ± (SE) | |
| ALG-DOX MPsa | 0.34 ± 0.05 | 0.52 ± 0.06 |
| ALG-DOX MPs in PF127 thermogelb | 0.051 ± 0.011 | 0.77 ± 0.05 |
| ALG-DOX MPs in PF127-HPMC thermogelb | 0.019 ± 0.008 | 0.99 ± 0.11 |
a n = 7
b n = 9
Fig. 10.
Rate of DOX release from ALG–DOX hydrogel MPs alone and embedded into PF127 thermogels in 0.05 M phosphate buffer, pH 5, at 37°C
CONCLUSION
The preformulation and formulation efforts performed suggest that sustained delivery of DOX can be achieved by the thermogel/MPs strategy. Embedding of the ALG-DOX hydrogel MPs into the PF127 thermogel provided the desired DOX sustained release over a period of almost a week without modifying thermogel properties. The best behavior was obtained with PF127 in 1%HPMC from which DOX is released following a case I and case II transport combined mechanism. As previously underlined, less extreme sink conditions, likely to occur in vivo, may produce an even more sustained DOX release. The results suggest that improvement of release might be achieved by employing a more suitable ALG form and/or optimizing the preparation conditions. Although studies on injectability are crucial to this point, it can be inferred that this system may represent a potentially valid DOX delivery formulation to be employed in the DFNs treatment.
References
- 1.Sweet RL, Schachter J, Landers DV, Ohm-Smith M, Robbie MO. Treatment of hospitalized patients with acute pelvic inflammatory disease: comparison of cefotetan plus doxycycline and cefoxitin plus doxycycline. Am J Obstet Gynecol. 1988;158(3 Pt 2):736–41. doi: 10.1016/s0002-9378(16)44537-0. [DOI] [PubMed] [Google Scholar]
- 2.Määttä M, Kari O, Tervahartiala T, Peltonen S, Kari M, Saari M, Sorsa T. Tear fluid levels of MMP-8 are elevated in ocular rosacea—treatment effect of oral doxycycline. Graefes Arch Clin Exp Ophthalmol. 2006;244(8):957–62. doi: 10.1007/s00417-005-0212-3. [DOI] [PubMed] [Google Scholar]
- 3.Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother. 2006;50(9):3124–31. doi: 10.1128/AAC.00394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita M, Nakanishi Y. Two different functions of doxycycline which is both an antimicrobial agent and an immune modulator. Anti-Inf Agents Med Chem. 2007;6(4):222–7. [Google Scholar]
- 5.Antibiotics could help slow MS. BBC News (11 December 2007).
- 6.Saraiva IH, Jones RN, Erwin M, Sader HS. Evaluation of antimicrobial sensitivity of 87 clinical isolates of vancomycin-resistant enterococci. Rev Assoc Med Bras. 1997;43(3):217–22. doi: 10.1590/S0104-42301997000300009. [DOI] [PubMed] [Google Scholar]
- 7.Burrows PE, Mitri RK, Alomari A, Padua HM, Lord DJ, Sylvia MB, Fishman SJ, Mulliken JB. Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphatic Research and Biology. 2008;6(3–4):209–16. doi: 10.1089/lrb.2008.1004. [DOI] [PubMed] [Google Scholar]
- 8.Gericke KR. Doxycycline as a sclerosing agent. Ann Pharmacother. 1992;26:648–9. [PubMed] [Google Scholar]
- 9.Chen R, Rubenstein AE, Shen X, Stewart S, Yu JC. Nexgenix Pharmaceuticals, L.L.C., USA. PCT Int. Appl. 2006;36.
- 10.Chen R, Rubenstein AE, Shen X. Nexgenix Pharmaceuticals, Llc., USA. PCT Int. Appl. 2008;39.
- 11.Theos A, Korf BR. Pathophysiology of neurofibromatosis type 1. Ann Intern Med. 2006;144:842–9. doi: 10.7326/0003-4819-144-11-200606060-00010. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki T, Yomota C, Okada S. Development and release characterization of hyaluronan–doxycycline gels based on metal coordination. J Control Release. 2001;76:337–47. doi: 10.1016/S0168-3659(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 13.Bhattarai N, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermoreversible hydrogel. Macromol Biosci. 2005;5:107–11. doi: 10.1002/mabi.200400140. [DOI] [PubMed] [Google Scholar]
- 14.Satish CS, Satish KP, Shivakumar HG. Hydrogels as controlled drug delivery systems: synthesis, crosslinking, water and drug transport synthesis, crosslinking, water and drug transport synthesis, crosslinking, water and drug transport mechanism. Ind J Pharm Sci. 2006;68(2):133–40. doi: 10.4103/0250-474X.25706. [DOI] [Google Scholar]
- 15.Schacht E, Toncheva V, Vandertaelen K, Heller J. Polyacetal and poly(ortho ester)–poly(ethylene glycol) graft copolymer thermogels: preparation, hydrolysis and FITC–BSA release studies. J Control Release. 2006;116:219–25. doi: 10.1016/j.jconrel.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Escobar-Chávez JJ, López-Cervantes M, Naïk A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermoreversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharmaceut Sci. 2006;9(3):339–58. [PubMed] [Google Scholar]
- 17.Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–28. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 18.Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58:445–55. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80:9–28. doi: 10.1016/S0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 20.Jeong B, Gutowska A. Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002;20:305–11. doi: 10.1016/S0167-7799(02)01962-5. [DOI] [PubMed] [Google Scholar]
- 21.El-KamelInt AH. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. J Pharm. 2002;241(1):47–55. doi: 10.1016/s0378-5173(02)00234-x. [DOI] [PubMed] [Google Scholar]
- 22.Barichello JM, Morishita M, Takayama K, Nagai T. Absorption of insulin from Pluronic F-127 gels following subcutaneous administration in rats. Int J Pharm. 1999;184(2):189–98. doi: 10.1016/S0378-5173(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 23.Alsarra IA, Niazy EM, Al-Sayed YM, Al-Khamis KI, El-Awad Ibrahim M. High performance liquid chromatographic method for determination of doxycycline in human plasma and its application in pharmacokinetic studies. Saudi Pharm J. 2005;13(1):42–7. [Google Scholar]
- 24.Liu Y, Lu WL, Wang JC, Zhang X, Zhang H, Wang XQ, Zhou TY, Zhang Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: in vitro and in vivo characterization. J Control Release. 2007;117:387–95. doi: 10.1016/j.jconrel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in-vitro evaluation of sustained release Poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release. 2002;85:73–81. doi: 10.1016/S0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspensions. J Pharm Sci. 1961;50:874–5. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 27.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 28.Kosmidis K, Argyrakis P, Macheras P. A reappraisal of drug release laws using Monte Carlo simulations: the prevalence of the Weibull function. Pharm Res. 2003;20:988–95. doi: 10.1023/A:1024497920145. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309:44–50. doi: 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Levenberg K. A method for the solution of certain non-linear problems in least squares. Q J Mech Appl Math. 1944;II(2):164–8. [Google Scholar]
- 31.Marquardt M. An algorithm for least-squares estimation of non-linear parameters. J Soc Ind Appl Math. 1963;11(2):431–44. doi: 10.1137/0111030. [DOI] [Google Scholar]
- 32.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–23. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 33.Florence AT, Attwood D. Physicochemical principles of pharmacy, 4th ed. Chapter 3: physicochemical properties of drugs in solution. London: Pharmaceutical Press; 2006. p. 78. [Google Scholar]
- 34.Duarte HA, Carvalho S, Paniago EB, Simas AM. Importance of tautomers in the chemical behavior of tetracyclines. J Pharm Sci. 1999;88(1):111–20. doi: 10.1021/js980181r. [DOI] [PubMed] [Google Scholar]
- 35.Prescott JF, Baggot JD, Walker RD. Antimicrobial therapy in veterinary medicine, 3rd ed. Chapter 4: Principles of antimicrobial drug bioavailability and disposition. Ames: Iowa State University Press; 2000. p. 67. [Google Scholar]
- 36.Washington N, Washington C, Wilson CG. Transdermal drug delivery. In: Wilson CG, Washington N, editors. Physiological pharmaceutics: biological barriers to drug absorption. 2. London: Taylor & Francis; 2001. pp. 179–98. [Google Scholar]
- 37.Giovagnoli S, Blasi P, Ricci M, Schoubben A, Perioli L, Rossi C. Physicochemical characterization and release mechanism of a novel prednisone biodegradable microsphere formulation. J Pharm Sci. 2008;97(1):303–17. doi: 10.1002/jps.21073. [DOI] [PubMed] [Google Scholar]
- 38.Enscore DJ, Hopfenberg HB, Stannett VT. Effect of particle size on the mechanism controlling n-hexane sorption in glassy polystyrene microspheres. Polymer. 1977;18:793–800. doi: 10.1016/0032-3861(77)90183-5. [DOI] [Google Scholar]