Abstract
The purpose of this study was to prepare and characterize an ocular effective prolonged-release liposomal hydrogel formulation containing ciprofloxacin. Reverse-phase evaporation was used for preparation of liposomes consisting of soybean phosphatidylcholine (PC) and cholesterol (CH). The effect of PC/CH molar ratio on the percentage drug encapsulation was investigated. The effect of additives such as stearylamine (SA) or dicetyl phosphate (DP) as positive and negative charge inducers, respectively, were studied. Morphology, mean size, encapsulation efficiency, and in vitro release of ciprofloxacin from liposomes were evaluated. For hydrogel preparation, Carbopol 940 was applied. In vitro transcorneal permeation through excised albino rabbit cornea was also determined. Optimal encapsulation efficiency of 73.04 ± 3.06% was obtained from liposomes formulated with PC/CH at molar ratio of 5:3 and by increasing CH content above this limit, the encapsulation decreased. Positively charged liposomes showed superior entrapment efficiency (82.01 ± 0.52) over the negatively charged and the neutral liposomes. Hydrogel containing liposomes with lipid content PC, CH, and SA in molar ratio 5:3:1, respectively, showed the best release and transcorneal permeation with the percentage permeation of 30.6%. These results suggest that the degree of encapsulation of ciprofloxacin into liposomes and prolonged in vitro release depend on composition of the vesicles. In addition, the polymer hydrogel used in preparation ensure steady and prolonged transcorneal permeation. In conclusion, ciprofloxacin liposomal hydrogel is a suitable delivery system for improving the ocular bioavailability of ciprofloxacin.
Key words: ciprofloxacin, hydrogel, liposome, ocular drug delivery system, transcorneal permeation
INTRODUCTION
Drug delivery in ocular therapy has long been a difficult task. Poor bioavailability of drugs from conventional ocular dosage forms is mainly due to the precorneal loss factors, which include tear dynamics, insufficient residence time in the conjunctival sac and non-productive absorption (1–3).
Fluoroquinolones, as a group, have shown excellent activity against the most frequently occurring gram-positive and gram-negative ocular pathogens (4–8). Earlier generation Fluoroquinolones such as ofloxacin have used widely to treat various pathogenic conditions. However, development of resistant strain against these fluroquinolone has been reported (9,10).
Ciprofloxacin, a fluoroquinolone antibacterial agent, is active against a broad spectrum of aerobic Gram-positive and Gram-negative bacteria. Resistance to this drug develops slowly, and minimal toxicity is associated with its use. It is currently the drug of choice as an anti-infective agent for the eye (11,12). Ciprofloxacin is water-soluble drug; its aqueous solubility is 35 mg/ml (13). Efficacy of the marketed ophthalmic fluoroquinolone products, mostly aqueous solutions, is limited by poor ocular bioavailability, compelling the frequent dosing regimen and the concomitant patient compliance (14,15).
In order to improve ophthalmologic bioavailability of drugs and to prolong the therapeutic action, introduction of novel delivery systems, including vesicular drug delivery systems, like liposomes have recently been proposed (16–19). Liposomes have investigated since 1970 as a system for the delivery or targeting of drugs to the specific sites in the body. Some liposomal drug delivery systems exhibited superior pharmacological properties to those observed with conventional formulation (20). Activity of liposomes as a carrier for drugs depends upon various factors such as charge, rigidity, composition of the liposomal membrane, encapsulation efficiency and release rate (21–24). Liposomes can be formulated from a variety of lipid and lipid mixtures with different composition (25), they can be modified in particle size, structure, and surface charge to obtain desirable physicochemical properties to suit particular needs (26,27).
Recently, due to the low viscosity of colloidal suspensions of liposomes that does not allow sufficient retention time of the dosage form at site of action, different hydrogel matrices such as carbopol 940 have been used to increase the viscosity of topical preparation and to increase the retention time of the formulation at site of administration due to good bioadhesive properties. In addition, the liposomal hydrogel ensure a steady and prolonged release of ciprofloxacin from liposomes, and make an intimate contact with corneal and conjunctival surface. The objective of the present study was to prepare and characterize topically effective prolonged-release ophthalmic ciprofloxacin liposomal-based hydrogel formulation. The factors influencing the encapsulation of ciprofloxacin into liposomes were investigated, characterization of the prepared liposomes regarding morphology, particle size, and in vitro drug release was performed, liposomal based hydrogel was prepared, in vitro transcorneal permeation study was carried to investigate the permeation properties of ciprofloxacin from optimized liposomal hydrogel formulation.
MATERIALS AND METHODS
Materials
Ciprofloxacin, soybean phosphatidylcholine, and cholesterol were purchased from Sigma Chemical Company (St. Louis, USA). Stearylamine, dicetyl phosphate was obtained from Fluka Chemical Company (Germany), carbopol 940 at BF Goodrich Company (USA), and triethanolamine at Junsei Chemical Company (Japan). All other reagents were commercially available and of analytical grade. Spectra/Pore® dialysis membrane (12,000–14,000 molecular weight cutoff) was purchased from Spectrum Laboratories Inc. (USA).
Methods
Preparation of Liposomes
Ciprofloxacin liposomes were prepared using the reverse-phase evaporation technique (28). The lipid component (300 mg) including PC and CH, either alone or mixed with SA or DP, were accurately weighed into a round-bottom flask and dissolved in chloroform/methanol mixture (1:2 v/v). A thin lipid film formed on the inner side of the flask by evaporating the organic solvents under vacuum using rotary evaporator at 40°C (Buchi R-110 Rotavapor, Switzerland). The lipid film redissolved in 10 ml ether and the drug solution (100 mg) in 10 ml acetone together with 10 ml phosphate buffered saline (PBS, pH 7.4) added. The mixture was then placed on the rotary evaporator to evaporate organic solvent. The liposomes allowed equilibrating at room temperature, and then the liposomal suspension was completed to 10 ml with PBS, which was kept in the refrigerator overnight.
Determination of Ciprofloxacin Entrapment Efficiency in Liposomes
To determine the encapsulation efficiency of liposomes, a known amount of liposomes was centrifuged at 10,000 rpm for 30 min at 4°C (Bechman Coulter Inc, Fullerton, CA). The liposomes separated from the supernatant and sonicated with methanol for 15 min. The concentration of ciprofloxacin in methanol was determined spectrophotometrically at 273 nm in spectrophotometer (UV-1601 PC, Schimadzu, Japan). The entrapment efficiency (EE percent) was calculated as follows
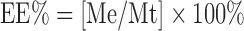 |
1 |
Where Me and Mt represent the encapsulated ciprofloxacin and total ciprofloxacin amount, respectively. EE percent was determined to evaluate the effect of PC/CH molar ratios on EE percent using different molar ratios for PC/CH ( 5:1, 5:2, 5:3, 5:4, and 5:5) with constant added ciprofloxacin content. In addition, EE percent was determined to study effect of adding positive charge inducer (SA) and negative charge inducer (DP) on EE percent of ciprofloxacin in liposomes.
Morphology and Size Analysis of Liposomes
The physical morphology of samples of ciprofloxacin liposomes composed of PC/CH in molar ratio 5:3 were examined using transmission electron microscope (ZEISS, EM 10, Germany). A drop of liposome dispersion sample applied on a 300-mesh collodion copper grid on paraffin and left for 10 min to allow some of the liposome powder to adhere on the collodion. The remaining dispersion was removed, and a drop of 2% aqueous solution of uranyl acetate was applied for 2 min. The remaining solution was then removed, and the sample was air-dried, prior to measurement with a transmission electron microscope. The mean particle size was determined by using laser diffraction particle size analysis (Malvern Instruments Ltd., Malvern, UK).
In Vitro Drug Release Studies
The in vitro release of ciprofloxacin from neutral, positively, and negatively charged liposomes was measured using dialysis method. Dialysis method was applied using a Spectra/Pore® dialysis membrane (12,000–14,000 molecular weight cutoff). This membrane assures the permeation of the drug and at same time retains liposomal forms (27).
An accurately measured amount of ciprofloxacin liposomal suspension equivalent to 3 mg ciprofloxacin suspended in 1 ml PBS (pH 7.4) in a glass cylinder having a length of 8 cm and diameter of 2.5 cm. This cylinder was fitted, before addition of liposomal suspension, with a presoaked dialysis membrane and was suspended in the dissolution flask of the United State Pharmacopeia (USP) dissolution tester (Pharma test, Hainburg, Germany) containing 75 ml PBS (pH 7.4) and maintained at a temperature of 37°C. The glass cylinder adjusted to rotate at a constant speed (75 rpm). At predetermined time intervals (0.5, 1, 1.5, 2, 3, 4, 6, and 8 h), 4 ml of the release medium withdrawn and assayed spectrophotometrically for drug content at 273 nm.
Preparation and In Vitro Release of Ciprofloxacin from Liposomal Hydrogel
Hydrogel dosage form of ciprofloxacin liposomes were prepared using deionized water as a vehicle and carbopol 940 as gelling agent at concentration of 1.5% (w/w). After complete hydration of the carbopol (150 mg) by 6.5 ml of deionized water, 3.5 ml of ciprofloxacin liposomal suspension composed of PC, Ch, and SA in a molar ratio (5:3:1) were added to prepare 10 gm of hydrogel with a concentration of 0.3% (w/w) of the drug and pH 5.9 ± 0.1. Then the in vitro release of ciprofloxacin from prepared hydrogel measured using the same method as liposomal suspension and compared with 0.3% ciprofloxacin aqueous solution and liposomal suspension.
In Vitro Drug Transcorneal Permeation
The transcorneal experiment performed with a conventional diffusion chamber. Albino rabbits were humanely killed by intravenous injection of excess sodium phenobarbital and the whole eyes enucleated. The method of dissection of cornea was the same as those described previously (29). The corneas of both eyes were excised and then mounted on the diffusion chamber. The receptor compartment was filled with 10 ml of PBS (pH 7.4), while 1 gm of hydrogel containing ciprofloxacin liposomes, or 1 ml of ciprofloxacin solution (composed of pure drug dispersed in distilled water), or 1 ml of ciprofloxacin liposomal suspension were applied to the epithelial side. The temperature in the diffusion chamber maintained at 34°C by thermostatic water bath. The receptor buffer was removed 0.5, 1, 1.5, 2, 3, 4, and 6 h and immediately replaced by previously aerated fresh receptor buffer. The samples analyzed spectrophotometrically for drug content at 273 nm. Results expressed as amount permeated and percent permeation or in vitro ocular availability as follows:
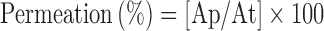 |
2 |
Where Ap and At represent amount of ciprofloxacin permeated in receptor and initial amount of ciprofloxacin in donor, respectively.
RESULTS AND DISCUSSION
Factors Affecting Entrapment Efficiency
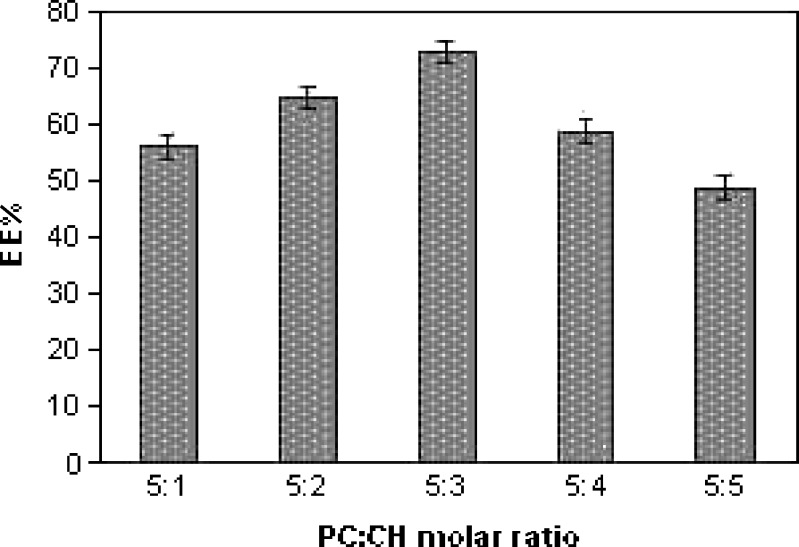
Figure 1 shows the effect of molar ratio of PC/CH on the EE percent of ciprofloxacin in liposomes. As observed, the EE percent increase by increasing the (CH) content until certain limit, above which the EE percent decrease by increasing (CH) concentration. The maximum EE percent of ciprofloxacin into liposomes (73.04 ± 3.06) was obtained from liposomes with PC/CH molar ratio (5:3). The 1-way analysis of variance (ANOVA) showed that a significant difference in EE percent between liposomes with different molar ratio of PC: CH ( p < 0.001). The increase in EE percent caused by increased CH content occurs because of increased cholesterol concentration in lipid bilayer and the rigidity of bilayer increase, resulting in higher stability and reduce permeability of the liposomal membrane (30) and hence greater drug retention (31). Decreasing EE percent with increasing cholesterol ratio above a certain limit may be due to that increasing cholesterol above certain concentration can disrupt the regular linear structure of liposomal membrane (32). Thus, the ratio 5:3 (PC/CH) was the most efficient regarding EE percent.
Fig. 1.
Effect of molar ratio of PC/CH on ciprofloxacin entrapment efficiency (EE percent; mean±SD, n = 3)
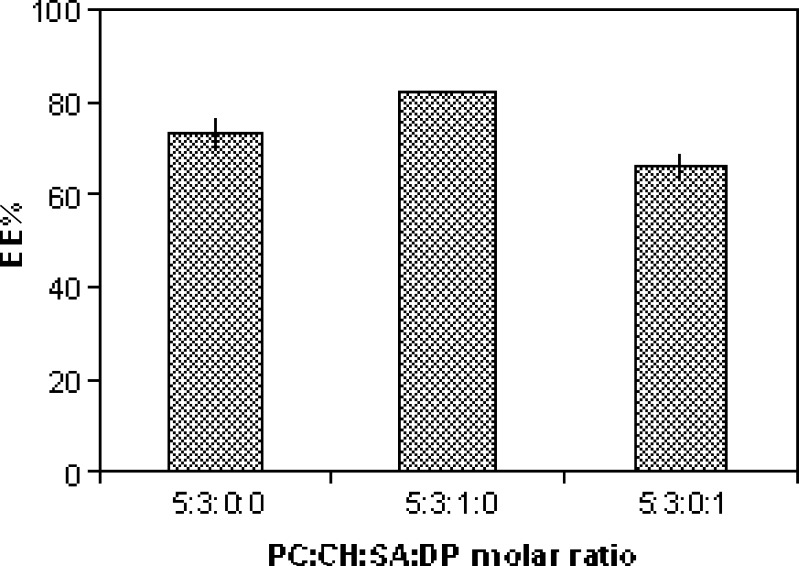
The effect of charge inducing agents on EE percent of ciprofloxacin in liposomes contain PC/CH in molar ratio 5:3 is shown in Fig. 2. The positively charged liposomes contain PC/CH/SA in molar ratio 5:3:1exhibited the highest EE percent (82.01 ± 0.52), followed by neutral ones contain PC/CH in molar ratio 5:3 (73.04 ± 3.66), and then by negatively charged ones containing PC/CH/DP in molar ratio 5:3:1 (65.52 ± 1.57). This order of EE percent would result because ciprofloxacin this could be due to that ciprofloxacin is a zwitterion, which has 2 pka (approximately 6 and 8.8) and isoelectric point at pH 7.2. So at pH 7.4, ciprofloxacin presents in a form of ionized negative charge carboxylic group, so an electrostatic attraction would occur between the drug and positively charged SA, this attraction leads to increasing EE% when compared with negatively charged liposome. On the other hand, electrostatic repulsion would occur between drug and negatively charged (DP) lead to decrease EE percent less than that for neutral liposomes. Similar results also obtained during the incorporation of charge inducing agent into azathioprine liposomes (33), indomethacin liposomes (34), and acetazolamide liposomes (35).
Fig. 2.
Effect of charge on ciprofloxacin entrapment efficiency (EE percent; mean±SD, n = 3)
Morphology and Size Analysis of Liposomes
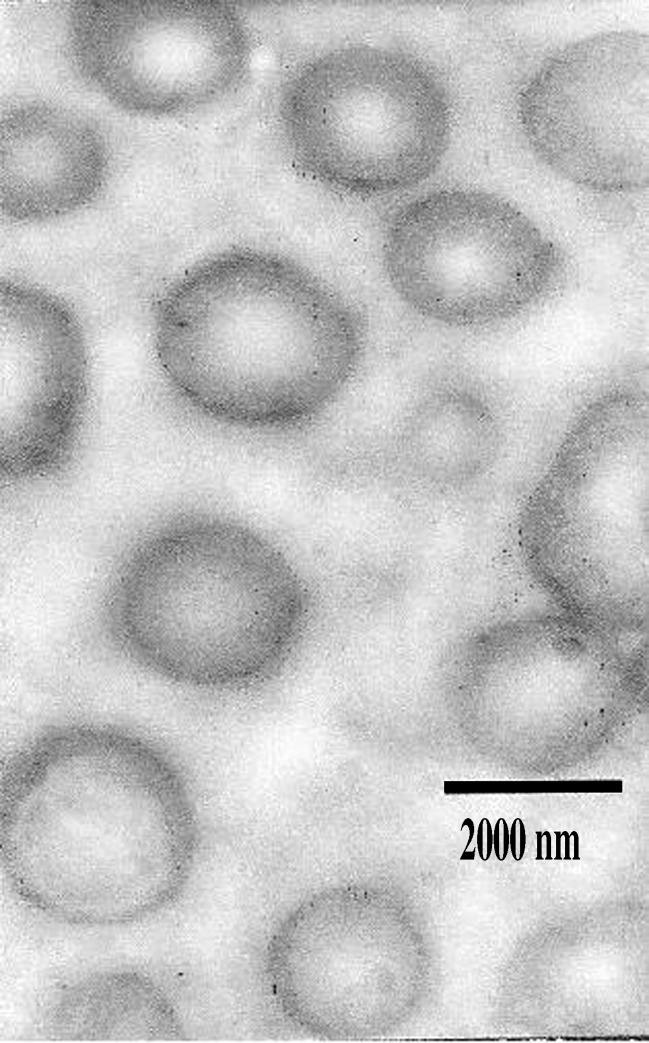
Figure 3 shows the ciprofloxacin liposomes prepared by reverse-phase evaporation method by molar ratio 5:3 (PC/CH); it revealed the presence of homogenous unilamellar vesicles with one phospholipid bilayer. As observed, they are well-identified spheres with large internal aqueous space; they exist in disperse and aggregate collections.
Fig. 3.
Transmission electron microphotograph of ciprofloxacin reverse-phase evaporation liposomes composed of PC/CH (5:3) molar ratio. Magnification ×10,000
The results of particle size analysis were 1,850, 1,720, and 1,630 nm for positive, negative, and neutral liposomes, respectively. These orders would result because in positively charged liposomes, many factors lead to increase in size. First, the inclusion of charge leads to increase spacing between the adjacent bilayer (36); second, the inclusion of large amount of ciprofloxacin as mentioned in EE percent determination; third, those positively charged electrostatically attracts the drug, which pushes phospholipid head group apart, leading to increasing the particle diameters (37). The particle diameter of negatively charged liposomes are also larger than neutral ones; this is due to the fact that inclusion of charge leads to increase spacing between adjacent bilayer.
In Vitro Drug Release Study
Figure 4 shows the release profile of ciprofloxacin from neutral, positively, and negatively charged liposomes. From all release profile, it is obvious that the negatively charged liposomes show the highest rate and extent of drug release followed by neutral and finally, the positively charged ones that give more prolonged and controlled release. This order may be due to electrostatic attraction forces that may exist between anion drug and amino moiety of (SA). Electrostatic repulsion may occur between the drug and negatively charged liposomes resulting in a higher rate of drug release. 1-way ANOVA reveals that the difference is not significant at (p < 0.01). Due to the non-significant difference in the in vitro release and due to higher EE percent of positively charged liposomes as mentioned before, a positively charged liposomes with lipid content PC, CH, and SA in molar ratio (5:3:1) was chosen for preparation of the liposomal hydrogel, in order to prepare topically effective prolonged-release ophthalmic ciprofloxacin formulation.
Fig. 4.
Effect of charge on release profile of ciprofloxacin from liposomes (mean±SD, n = 3)
In Vitro Drug Release From Liposomal Hydrogel
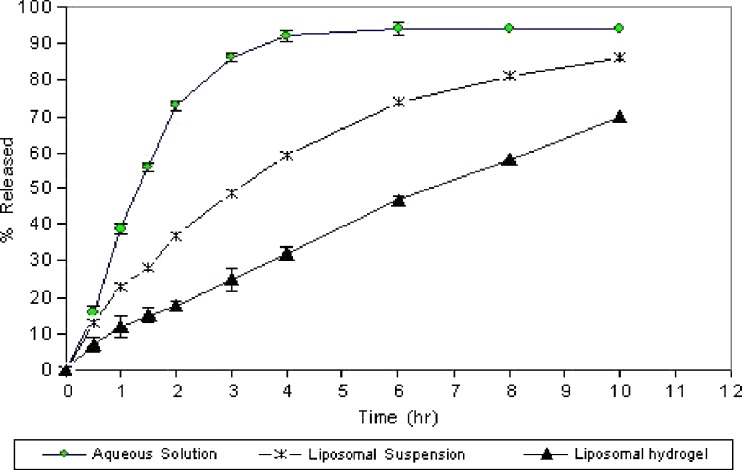
Figure 5 shows the release profile of ciprofloxacin from 0.3% aqueous solution, suspension of ciprofloxacin liposomes with lipid content PC, CH, and SA in (5:3:1) molar ratio (uncoated), and liposomes dispersed in 1.5% (w/w) carbopol gel (coated). It is clear that the encapsulation of ciprofloxacin within liposome extends the time require for ciprofloxacin release. The time required for 75% ciprofloxacin release is 6 h in comparison with 2 h for 75% release from 0.3% aqueous solution. This result can explained by the presence of cholesterol in bilayer above the phospholipids, which modulates fluidity by restricting the movement of ciprofloxacin, reducing bilayer permeability, and decreasing the efflux of the encapsulated drug, resulting in prolonged drug retention (38,39). It is also clear that ciprofloxacin release was retarded and more extended by the presence of the carbopol coat, about 10 h required for 75% release of ciprofloxacin. This may due to the higher viscosity of polymeric hydrogel, which provide an extra barrier for ciprofloxacin release (40).
Fig. 5.
In vitro release profile of ciprofloxacin from liposomal hydrogel and liposomal suspension in comparison to its solution (mean±SD, n = 3)
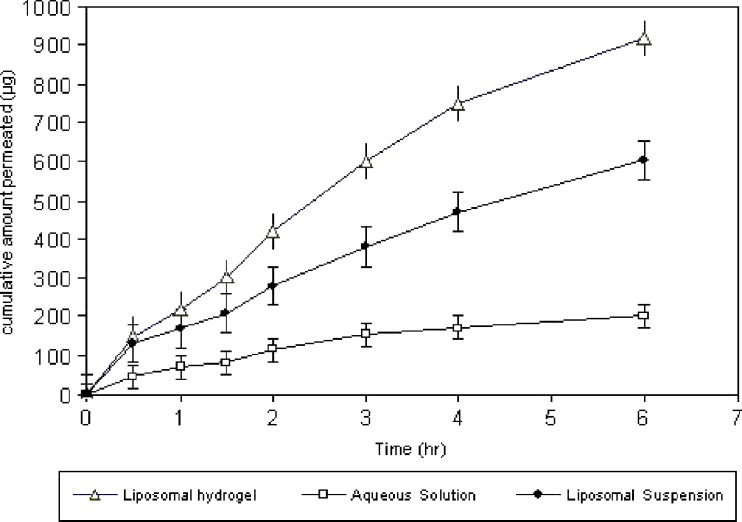
In Vitro Drug Transcorneal Permeation
Transcorneal permeation profile of ciprofloxacin from its 0.3% aqueous solution, liposomal suspension, and 0.3% liposomal hydrogel are represented in Fig. 6. The extent of drug permeation after 6 h from liposomal hydrogel and aqueous solution was significantly different. The cumulative amount of ciprofloxacin permeated after 6 h was 918 ± 25.11 µg, and percentage permeated was 30.6% from liposomal hydrogel, while the cumulative amount permeated from liposomal suspension was 614 ± 14.2, and percent permeated was 20.4%. However, the cumulative amount permeated from aqueous solution was 201 ± 8.7 µg only, and the percent permeated was 6.7%; this means that liposomal suspension show 3-folds higher permeation percent than aqueous solution. This may explained by the fact that an electrostatic attraction force occur between positively charged liposomes and negatively charge corneal membrane. We speculate then that liposomes, adsorbed to the corneal surface, transfer their membrane-associated drug directly to the corneal epithelial cell membranes, thereby facilitating drug transport across the cornea (41,42). Other mechanisms such as endocytosis of liposomes or fusion of liposome membrane with the plasmalemma are also involved in enhanced transport (43). While liposomal hydrogel show 5-folds higher permeation than aqueous solution, this may explained by the fact that mucoadhesive properties of hydrogel base ensures an intimate contact between liposomes and corneal membrane which prolong the retention of the formulation at the site of administration, which is beneficial for enhancing permeation (44,45).
Fig. 6.
Transcorneal permeation profile of ciprofloxacin from liposomal hydrogel and liposomal suspension in comparison to its solution (mean±SD, n = 3)
CONCLUSION
Based on these studies, it can be concluded that formulation of ciprofloxacin as liposomal hydrogel will provide the maximum in vitro ocular availability through albino rabbit cornea. Addition of cholesterol in liposomes increases ciprofloxacin encapsulation efficiency until certain concentration. Addition of stearylamine as positive charge inducer enhances encapsulation efficiency. Dispersion of liposomes in hydrogel matrix ensures a steady, prolonged release, and higher transcorneal permeation, which improve ocular bioavailability about five times more than aqueous solution.
References
- 1.Le Bourlais CL, Acar L, Zia H, Sado PA, Needham T, et al. Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res. 1998;17:35–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 2.Sultana J, Jain R, Agil M, Ali A. Review of ocular drug delivery. Curr Drug Deliv. 2006;3:207–217. doi: 10.2174/156720106776359186. [DOI] [PubMed] [Google Scholar]
- 3.Budai L, Hajdu M, Budai M, Grof P, Beni S, et al. Gels and liposomes in optimized ocular drug delivery: studies on ciprofloxacin formulations. Int J Pharm. 2007;343:34–40. doi: 10.1016/j.ijpharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Cochereau-Massin I, Bauchet J, Marrakchi-Benjaafar S, Saleh-Mghir A, Faurisson F, et al. Efficacy and ocular penetration of sparfloxacin in experimental streptococcal endophthalmitis. Antimicrob Agents Chemother. 1993;37:633–636. doi: 10.1128/aac.37.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adenis JP, Colin J, Verin P, Saint-Blancat P, Malet F. Ciprofloxacin ophthalmic solution versus rifamycin ophthalmic solution for the treatment of conjunctivitis and blepharitis. Eur J Ophthalmol. 1995;5:82–87. doi: 10.1177/112067219500500203. [DOI] [PubMed] [Google Scholar]
- 6.Diamond JP, White L, Leeming JP, Bing Hoh H, Easty DL. Topical 0.3% ciprofloxacin, norfloxacin, and ofloxacin in treatment of bacterial keratitis: a new method for comparative evaluation of ocular drug penetration. Br J Ophthalmol. 1995;79:606–609. doi: 10.1136/bjo.79.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross RD, Hoffman RO, Lindsay RN. A comparison of ciprofloxacin and tobramycin in bacterial conjunctivitis in children. Clin Pediatr (Phila) 1997;36:435–444. doi: 10.1177/000992289703600801. [DOI] [PubMed] [Google Scholar]
- 8.Jauch A, Fsadni M, Gamba G. Meta-analysis of six clinical phase III studies comparing lomefloxacin 0.3% eye drops twice daily to five standard antibiotics in patients with acute bacterial conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 1999;237:705–713. doi: 10.1007/s004170050300. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry NA, Flynn HW, Murray TG, Tabandeh H, Mello MO, et al. Emerging ciprofloxacin resistant Pseudomonas aeruginosa. Am J Ophthalmol. 1999;128:509–510. doi: 10.1016/S0002-9394(99)00196-8. [DOI] [PubMed] [Google Scholar]
- 10.Garg P, Sharma S, Rao GN. Ciprofloxacin resistant Pseudomonas keratitis. Ophthalmology. 1999;106:1319–1323. doi: 10.1016/S0161-6420(99)00717-4. [DOI] [PubMed] [Google Scholar]
- 11.Appelbaum PC, Hunter PA. The fluoroquinolone antibacterials: past, present, and future perspectives. Int J Antimicrob Agents. 2000;16:5–15. doi: 10.1016/S0924-8579(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 12.Campoli-Richards DM, Monk JP, Price A. Ciprofloxacin. A review of its antibiobacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35:373–447. doi: 10.2165/00003495-198835040-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kalam MA, Sultana A, Samad A, Ali A, Aqil M, et al. Gelrite-based in vitro gelation ophthalmic drug delivery system of ciprofloxacin. J Disp Sci Tech. 2008;29:89–96. doi: 10.1080/01932690701688482. [DOI] [Google Scholar]
- 14.Lin HH, Ko S-M, Hsu LR, Tsai YH. The preparation of norfloxacinloaded liposomes and their in vitro evaluation in pig's eye. J Pharm Pharmacol. 1996;48:801–805. doi: 10.1111/j.2042-7158.1996.tb03977.x. [DOI] [PubMed] [Google Scholar]
- 15.Wiechens B, Neumann D, Grammer JB, Pleyer U, Hedderich J, Duncker GI. Retinal toxicity of liposome-incorporated and free ofloxacin after intravitreal injection in rabbit eyes. Int Ophthalmol. 1998–99;22:133–143. doi: 10.1023/A:1006137100444. [DOI] [PubMed] [Google Scholar]
- 16.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 17.Gregoriadis G, Florence AT. Liposomes in drug delivery: clinical, diagnostic and ophthalmic potential. Drugs. 1993;45:15–28. doi: 10.2165/00003495-199345010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Farkas E, Schubert R, Zelk’o R. Effect of cholesterol on the characteristics of vesicular gels containing chlorhexidine. Int J Pharm. 2004;278:63–70. doi: 10.1016/j.ijpharm.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Foradada M, Estelrich J. Encapsulation of thioguanine in liposomes. Int J Pharm. 1995;124:261–269. doi: 10.1016/0378-5173(95)00097-3. [DOI] [Google Scholar]
- 21.Choudhari KB, Labhasetwar V, Dorle AK. Liposomes as a carrier for oral administration of insulin: effect of formulation factors. J Microencapsul. 1994;11:319–325. doi: 10.3109/02652049409040461. [DOI] [PubMed] [Google Scholar]
- 22.Galovic Rengel R, Barisic K, Pavelic Z, Zanic Grubisic T, Cepelak I, et al. High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur J Pharm Sci. 2002;15:441–448. doi: 10.1016/S0928-0987(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 23.Karathanasis E, Ayyagari AL, Bhavane R, Bellamkonda RV, Annapragada AV. Preparation of in vivo cleavable agglomerated liposomes suitable for modulated pulmonary drug delivery. J Contr Release. 2005;103:159–175. doi: 10.1016/j.jconrel.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Nii T, Ishii F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm. 2005;298:198–205. doi: 10.1016/j.ijpharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Aranda FJ, Teruel JA, Ortiz A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim Biophys Acta. 2005;1713:51–56. doi: 10.1016/j.bbamem.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Law SL, Hung HY. Properties of acyclovir-containing liposomes for potential ocular delivery. Int J Pharm. 1998;161:253–259. doi: 10.1016/S0378-5173(97)00362-1. [DOI] [Google Scholar]
- 27.El-Samaligy MS, Nagia NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308:140–148. doi: 10.1016/j.ijpharm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Szoka F, Papahodjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA. 1978;75:4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra M, Majumdar DK. In vitro transcorneal permeation of ketorolac tromethamine from buffered and unbuffered aqueous ocular drops. Indian J Exp Biol. 1997;35:941–947. [PubMed] [Google Scholar]
- 30.du Plessis J, Ramachandran C, Weiner N, Muller DG. The influence of lipid composition and lamellarity of liposomes on the physical stability of liposomes upon storage. Int J Pharm. 1996;127:273–278. doi: 10.1016/0378-5173(95)04281-4. [DOI] [Google Scholar]
- 31.Perugini P, Pavanetto F. Liposomes containing boronophenylalanine for boron neutron capture therapy. J Microencapsul. 1998;15:473–483. doi: 10.3109/02652049809006874. [DOI] [PubMed] [Google Scholar]
- 32.New RRC. Liposomes: a practical approach. Oxford: Oxford University Press; 1990. [Google Scholar]
- 33.Gulati M, Grover M, Singh M, Singh S. Study of azathioprine encapsulation into liposomes. J Microencapsul. 1998;15:485–494. doi: 10.3109/02652049809006875. [DOI] [PubMed] [Google Scholar]
- 34.Srinath P, Vyas SP, Prakash VD. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev Ind Pharm. 2000;26:313–321. doi: 10.1081/DDC-100100359. [DOI] [PubMed] [Google Scholar]
- 35.Rania MH, Samar M, Nahed DM, Ahmed SG. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharm Sci Tech. 2007;8(1):1–12. doi: 10.1208/pt0802027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagarsenker MS, Londhe VY, Nadkarni GD. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int J Pharm. 1999;190:63–71. doi: 10.1016/S0378-5173(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 37.Gruner SM. Materials properties of liposomal bilayers. In: Ostro MJ, editor. Liposomes from biophysics to therapeutics. New York: Marcel Dekker; 1997. pp. 1–38. [Google Scholar]
- 38.Nagarsenker MS, Londhe VY. Preparation and evaluation of a liposomal formulation of sodium cromoglicate. Int J Pharm. 2003;251:49–56. doi: 10.1016/S0378-5173(02)00583-5. [DOI] [PubMed] [Google Scholar]
- 39.Peschka R, Dennehy C, Szoka FCJ. A simple in vitro model to study the release kinetics of liposome encapsulated material. J Control Release. 1998;56:41–51. doi: 10.1016/S0168-3659(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 40.Durrani AM, Davies NM, Thomas M, Kellaway IW. Pilocarpine bioavailability from a mucoadhesive liposomal ophthalmic drug delivery system. Int J Pharm. 1992;88:409–415. doi: 10.1016/0378-5173(92)90340-8. [DOI] [Google Scholar]
- 41.Singh K, Mezei M. Liposomal ophthalmic drug delivery system. I. Triamcinolone acetonide. Int J Pharm. 1983;16:339–344. doi: 10.1016/0378-5173(83)90152-7. [DOI] [Google Scholar]
- 42.Lee VH, Urrea PT, Smith RE, Schazlin D. Therapeutic review: ocular drug bioavailability from topically applied liposomes. Surv Ophthalmol. 1985;29:335–348. doi: 10.1016/0039-6257(85)90109-2. [DOI] [PubMed] [Google Scholar]
- 43.Schaeffer HE, Krohn DL. Liposomes in topical drug delivery. Invest Ophthalmol Vis Sci. 1982;22:220–227. [PubMed] [Google Scholar]
- 44.Deepika A, Indu PK. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290:155–159. doi: 10.1016/j.ijpharm.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353–369. doi: 10.1081/DDC-120002997. [DOI] [PubMed] [Google Scholar]