Abstract
Topical ketoprofen patches are widely used in the treatment of musculoskeletal pain, but the pharmacokinetics of ketoprofen following topical application remain unclear. This open-label, single-dose pharmacokinetic study was designed to determine the concentrations of ketoprofen in the semitendinosus muscle/tendon and plasma after topical application or oral administration to patients scheduled for anterior cruciate ligament reconstruction. Two ketoprofen patches (20 mg each) were applied over the semitendinosus muscle/tendon for 1, 6, 14, or 20 h before surgery in 21 patients, while one sustained-release 150 mg ketoprofen capsule was administered to six patients 14 h before surgery. Ten untreated patients served as the control group. The main outcome measures were the semitendinosus muscle/tendon and plasma concentrations of ketoprofen at 1, 6, 14, and 20 h. Ketoprofen was detected in the semitendinosus muscle/tendon from about 1 h after topical application. The peak concentration was reached at 6 h, and it decreased gradually until 20 h, although the concentration at 20 h was still higher than that at 1 h. Unlike the tissue concentration, the plasma concentration of ketoprofen increased gradually after topical application. At 14 h, there was no significant difference of the tissue concentration between the topical and oral groups, although the plasma concentration was about 17-fold higher in the oral group than in the topical group. In conclusion, following topical application in a patch, ketoprofen shows rapid and sustained delivery to the underlying tissues without a significant increase of the plasma drug concentration.
Key words: ketoprofen, patch, semitendinosus muscle, tissue concentration, topical application
INTRODUCTION
Ketoprofen alleviates pain in patients suffering from inflammatory musculoskeletal disorders, such as osteoarthritis or rheumatoid arthritis, and is also useful for traumatic pain in patients with acute low back injury or soft-tissue injury (1–3). As with other nonsteroidal anti-inflammatory drugs, oral or intrarectal administration of ketoprofen may have adverse effects on the gastrointestinal tract.
A patch formulation of ketoprofen has been developed to reduce the risk of systemic adverse effects, and it was reported to effectively alleviate pain in patients with tendinitis (4) and ankle sprain (5). The concentration of ketoprofen after application of the patch formulation has been measured in intra-articular tissues and in the tendon sheaths at the wrist (6).
The ratio of ketoprofen levels in target tissues to plasma after topical application or oral administration has also been compared (7,8). Rolf et al. applied ketoprofen plasters once daily for 5 days or administered a single oral dose of quick-release ketoprofen to patients who underwent surgery for Achilles or patellar tendinopathy. Rolf et al. found that the ketoprofen concentration was high in the subcutaneous tissue after multiple topical applications. However, the frequency of oral administration and topical application differed, and the effect of the route of administration could not be discussed (7). Rolf et al. also demonstrated that ketoprofen was detectable in the intra-articular tissues of patients suffering from knee disorders after a single topical application or oral dose of the drug (8). However, it should be noted that the volume of synovial fluid will vary depending on the severity of knee disorders, and this may influence the ketoprofen concentration in intra-articular tissues.
In the present study, the ketoprofen concentration in the tissues (muscle and tendon) of the thigh was measured after application of ketoprofen patches or oral administration of sustained-release ketoprofen capsules. The objective was to compare the tissue levels of ketoprofen after topical and oral administration. Blood was also collected simultaneously to determine the ratio of the tissue concentration to the plasma drug concentration.
MATERIALS AND METHODS
Subjects and Ethical Considerations
This study was conducted in accordance with the declaration of Helsinki and was approved by the Institutional Review Board of Tokyo Medical and Dental University. Written informed consent was obtained from each subject. Thirty-seven patients (20 women and 17 men) with a mean age of 25 years (range 15 to 51 years), who were scheduled to undergo anterior cruciate ligament reconstruction, were enrolled. Subjects were instructed not to take other nonsteroidal anti-inflammatory drugs unless it was essential during the week prior to surgery.
Study Design and Sampling
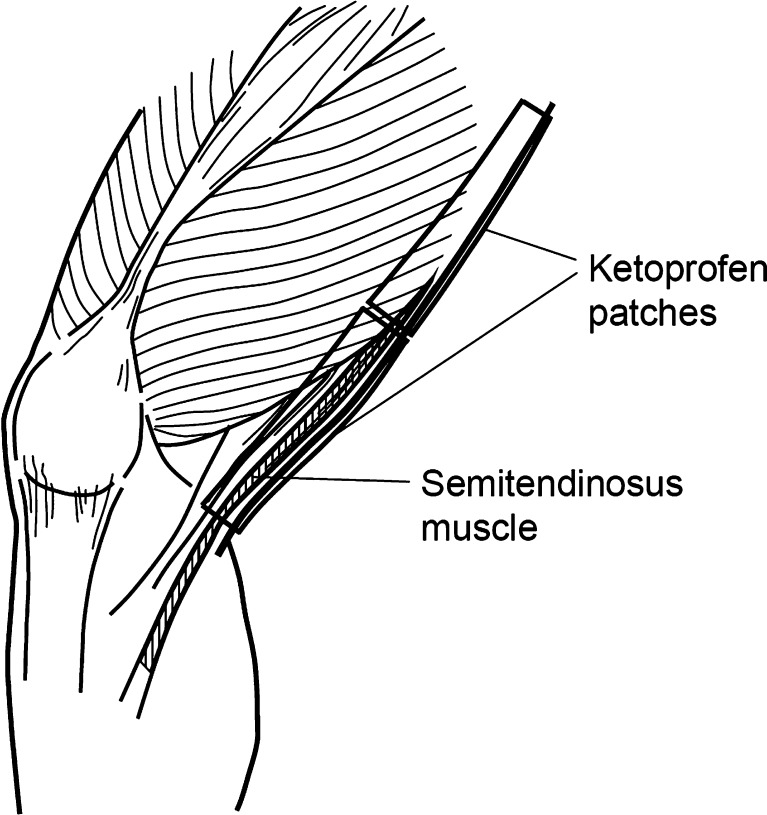
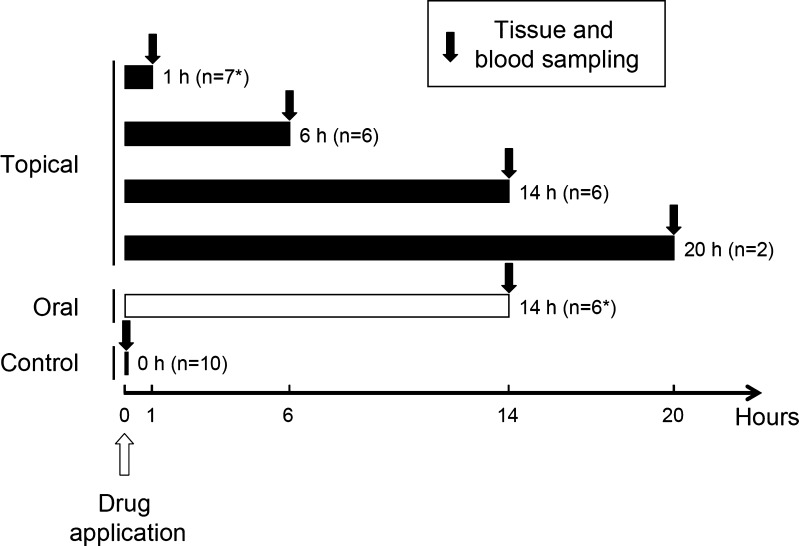
Ketoprofen patches were applied topically in 21 patients, and the drug was administered orally to six patients. Another 10 patients served as the control group and were not given any drug. The ketoprofen patches used in this study measured 7 × 10 cm and contained 20 mg of ketoprofen. In the topical group, two patches were applied to the skin of the thigh over the semitendinosus muscle (Fig. 1) for 1, 6, 14, or 20 h, being removed just before surgery. In the oral group, a single sustained-release capsule containing 150 mg of ketoprofen was administered after dinner at 14 h before surgery (Fig. 2).
Fig. 1.
Application sites of the ketoprofen patches
Fig. 2.
Schedule of the study. In the topical group, two patches were applied at 1 (n = 7), 6 (n = 6), 14 (n = 6), or 20 (n = 2) hours before surgery. In the oral group, ketoprofen was administered at 14 h before surgery (n = 6). The control group did not receive ketoprofen (n = 10). Blood was collected just before surgery started, while semitendinosus muscle/tendon samples were obtained just after starting the operation. The number of blood samples actually obtained was 6 and 5 in the topical 1 h group and the oral group, respectively (asterisk)
The subjects underwent anterior cruciate ligament reconstruction under spinal anesthesia. Semitendinosus muscle/tendon samples were obtained from the dissected semitendinosus tendon, which was harvested with a tendon stripper for use in ligament reconstruction. The muscle and tendon samples used for the present study were discarded by the surgeons after fashioning of the grafts for ligament reconstruction. Blood was collected just before removing the patches, in the operating room at the time of inserting the intravenous catheter. The blood samples were immediately centrifuged, and the plasma thus obtained was transferred into polypropylene tubes. Plasma and tissue samples were immediately stored at −20°C until analysis.
Analytical Procedures
Ketoprofen in an aliquot of plasma (0.25 mL) was acidified and extracted by liquid–liquid extraction with diethyl ether. After evaporation of the organic phase, the residue was dissolved in a methanol/water mixture and transferred to a vial. Tissue specimens were minced, and 50 to 100 mg of minced tissue was homogenized in methanol, after which the homogenate was extracted by the same procedure as that used for plasma. Ketoprofen extracts from the plasma and tissue samples were assayed by high-pressure liquid chromatography with a positive ion spray tandem mass spectrometry detector (2695 separation module, Waters Corp., Milfold, MA, USA) and an API-4000(Applied Biosystems/MDS SCIEX, Foster City, CA, USA). The methods used for determination of ketoprofen in plasma and tissue samples were validated before the start of analysis. The range of quantification for ketoprofen was 2.5 to 500 ng/mL in plasma and 10 to 2,000 ng/g in tissue. The intra-assay precision and accuracy of the methods were assessed by assay of five samples, and respectively ranged from 0.7% to 2.1% and from 3.3% to 10.0% for plasma samples and from 1.0% to 3.1% and from −3.1% to 10.7% for tissue samples. The inter-assay precision and accuracy of the methods were tested over 3 days. These parameters, respectively, ranged from 2.7% to 4.5% and from 3.1% to 10.2% for plasma samples as well as from 1.9% to 6.1% and from −0.9% to 12.5% for tissue samples.
Statistical Methods
The Mann–Whitney U test was used to examine differences of the ketoprofen concentration in plasma and tissue samples between the oral and topical groups. In the topical group, Friedman’s test was used to examine differences between the tissue and plasma levels of ketoprofen at each time. Differences were considered to be statistically significant at p < 0.05 (two-sided). Values below the limit of quantification (BLQ) were defined as 0 for calculation of the mean and standard error.
Pharmacokinetics
In the topical group, the mean maximum plasma and tissue concentration (Cmax), mean time to reach Cmax (Tmax), and mean area under the plasma or tissue concentration vs. time curve until 20 h (AUC0–20) were calculated from the mean ketoprofen concentrations at each time point.
Results
Ketoprofen Levels after Topical or Oral Administration
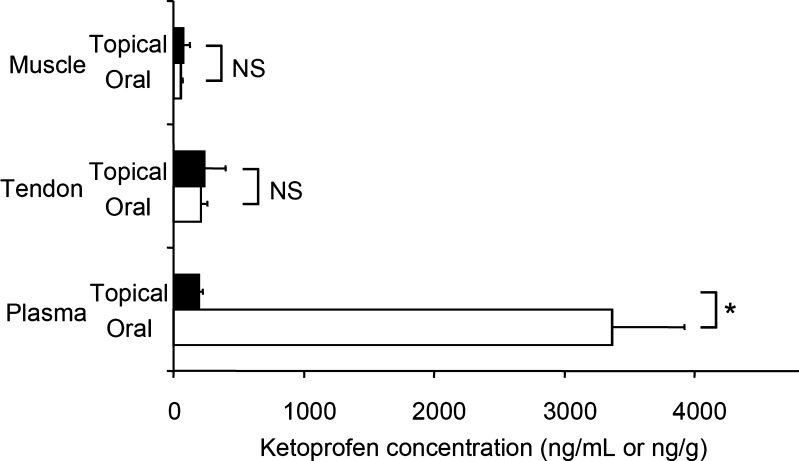
The ketoprofen concentrations in semitendinosus muscle/tendon and plasma were compared between the topical and oral groups at 14 h after administration. The ketoprofen concentration in semitendinosus muscle was 80 ± 47 and 56 ± 12 ng/g in the topical and oral groups, respectively, while the levels in semitendinosus tendon were 235 ± 162 and 211 ± 48 ng/g, respectively. Although both groups showed similar levels in muscle and tendon, the plasma concentration of ketoprofen differed markedly, being 196 ± 27 ng/mL in the topical group and 3,365 ± 559 ng/mL (17-fold higher) in the oral group (p = 0.004, Fig. 3, Table I). The mean tissue/plasma concentration ratio of ketoprofen was higher after topical application than after oral administration (Table II).
Fig. 3.
Ketoprofen concentrations in muscle, tendon, and plasma at 14 h after topical application or oral administration. Values are the mean plus standard error. *p < 0.05 (Mann–Whitney U test, two-sided). NS not significant
Table I.
Mean (SE) Concentrations of Ketoprofen in Muscle, Tendon, and Plasma after Single Topical or Oral Doses
| Tissues | Topical | Oral | |||
|---|---|---|---|---|---|
| 1 h (n = 7a) | 6 h (n = 6) | 14 h (n = 6) | 20 h (n = 2) | 14 h (n = 6a) | |
| Muscle (ng/g) | 56 (27) | 257 (147) | 80 (47) | 63c | 56 (12) |
| Tendon (ng/g) | 118 (57) | 377 (170) | 235 (162) | 210c | 211 (48) |
| Plasma (ng/mL) | 3b (3)b | 132 (32) | 196 (27) | 138c | 3,365 (559) |
| p value* | 0.004 | 0.513 | 0.070 | –c | |
The ketoprofen concentration was BLQ in all samples from the control group (n = 10)
SE standard error, BLQ below the limit of quantification
aThe number of blood samples actually obtained was 6 and 5 in the topical 1 h group and the oral group, respectively
bOf the six patients, four patients had BLQ values, which were defined as 0 for calculation of the mean and standard error
cSE and p value were not calculated due to n = 2
*p < 0.05 (Friedman’s test, two-sided)
Table II.
Mean (SE) Tissue/Plasma Concentration Ratios for Ketoprofen
| T/P ratio | Topical | Oral | ||
|---|---|---|---|---|
| 6 h (n = 6) | 14 h (n = 6) | 20 h (n = 2) | 14 h (n = 5a) | |
| Muscle/Plasma | 3.51 (1.99) | 0.38 (0.20) | 0.47b | 0.021 (0.004) |
| Tendon/Plasma | 4.40 (1.94) | 1.13 (0.70) | 1.58b | 0.074 (0.004) |
The T/P ratio was not calculated in the topical 1 h group because the plasma ketoprofen concentration was BLQ in some patients
SE standard error, T/P ratio tissue/plasma concentration ratio, BLQ below the limit of quantification
aThere were six patients in the oral group, but only five blood samples were obtained
bSE was not calculated due to n = 2
Pharmacokinetics of Ketoprofen after Topical Application
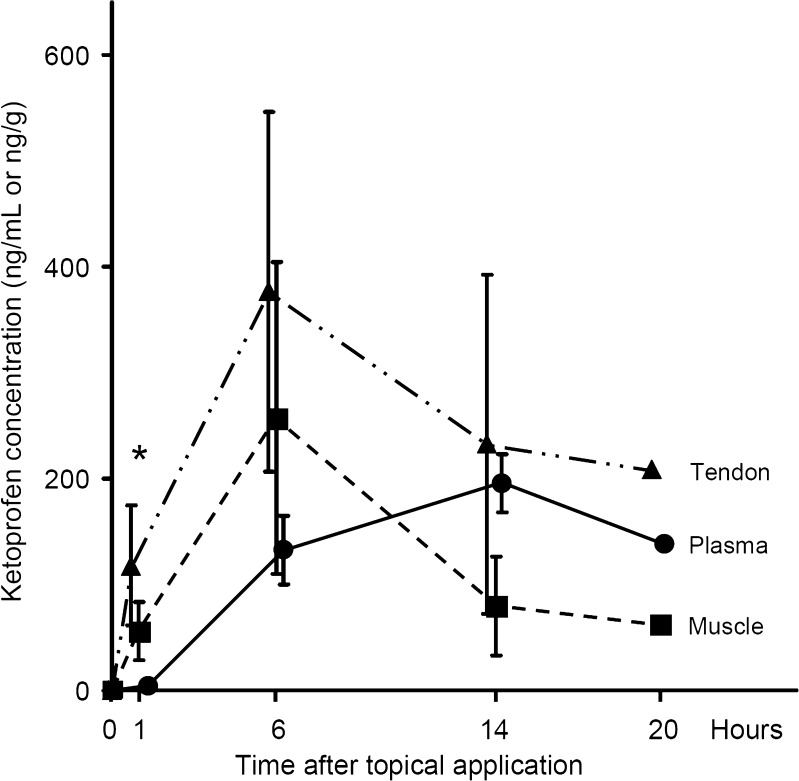
Tissue and plasma ketoprofen concentrations were also investigated at various times after topical application. In plasma, ketoprofen was hardly detected at 1 h, after which the concentration increased gradually to reach its maximum value of 196 ± 27 ng/mL at 14 h. In semitendinosus muscle, the concentration reached 56 ± 27 ng/g at 1 h, increased to its maximum value of 257 ± 147 ng/g at 6 h, and decreased again at 14 h. In semitendinosus tendon, ketoprofen showed a similar profile to that observed in muscle, but the actual concentrations were 1.5 to three times higher. The maximum concentration in tendon tissue was 377 ± 170 ng/g at 6 h after patch application. At 1 h after application, the ketoprofen concentrations in muscle and tendon were similar to those found at 14 h after oral administration. Statistical analysis revealed a significant difference between the tissue and plasma ketoprofen concentrations at 1 h after topical application (p = 0.004, Fig. 4, Table I). The pharmacokinetic parameters of ketoprofen after topical application are shown in Table III.
Fig. 4.
Ketoprofen concentrations in muscle, tendon, and plasma at 0, 1, 6, 14, and 20 h after topical application. Values are the mean ± standard error. *p < 0.05 (Friedman’s test, two-sided)
Table III.
Mean Tissue and Plasma Pharmacokinetic Parameters of Ketoprofen after Topical Application
| Cmax (ng/g or ng/mL) | Tmax (h) | AUC0-20 (ng·h/g or ng·h/mL) | |
|---|---|---|---|
| Muscle | 257 | 6 | 2,588 |
| Tendon | 377 | 6 | 5,080 |
| Plasma | 196 | 14 | 2,653 |
Discussion
The 10 patients in the control group were not given ketoprofen. Blood, muscle, and tendon samples were collected and were analyzed for ketoprofen in the same way as in the topical and oral groups. As a result, ketoprofen was not detected in the control group. The values obtained in the control group were used as those for time 0 in Fig. 4.
In the oral group, tissue and blood samples were only obtained at 14 h and not at 1, 6, or 20 h after oral administration. Multiple tissue sampling similar to that done in the topical group would have provided more detailed data. In this study, all patients were hospitalized 1 day before the operation and were not allowed food or water after dinner time. Surgery was started in the morning or afternoon on the next day. If patients had taken oral ketoprofen on the day of surgery, data could have been obtained at 1 and 6 h after oral administration. However, taking oral ketoprofen on an empty stomach should be avoided for safety reasons. It was thought that data from 20 h would not be so useful without data from 1 or 6 h, so only data from 14 h were obtained in the oral group.
As a result, the plasma concentration of ketoprofen measured at 14 h after oral administration was similar to the maximum concentration (3.87 ± 0.54 µg/mL) reported by Christophidis et al. (9). Accordingly, this was considered to be the time at which ketoprofen reached an effective concentration in both the plasma and tissues.
At 14 h after administration, the ketoprofen concentrations in semitendinosus muscle and tendon were similar in the topical and oral groups. However, the plasma concentration at 14 h after topical application of ketoprofen was only 1/17 of that after oral administration. In other words, the target tissue concentration of ketoprofen was similar with both methods of administration at this time, but the plasma concentration was much lower after topical application.
At 1 h after topical application, the ketoprofen concentration in semitendinosus muscle and tendon was 56 ± 27 and 118 ± 57 ng/g, respectively, and these values were similar to those found at 14 h after oral administration. If the ketoprofen concentrations in muscle and tendon at 14 h after oral administration were adequate to alleviate pain, the concentrations obtained with the ketoprofen patch would be effective for local pain from 1 h after application.
The concentrations of ketoprofen in semitendinosus muscle and tendon reached a peak at 6 h after topical application and then gradually decreased. On the other hand, the maximum plasma level of ketoprofen was detected at 14 h. This finding suggests that ketoprofen is absorbed directly by the tissues under the skin after transdermal delivery, a mechanism that is supported by the tissue to plasma concentration ratio. At 14 h, the ratio of the ketoprofen levels in muscle to plasma was 0.021 and 0.38 after oral administration and topical application, respectively. Moreover, the tendon to plasma ratio was 0.074 and 1.13 after oral administration and topical application, respectively. Thus, the tissue-to-plasma concentration ratios were much higher after topical application than after oral administration.
Conclusion
Topical application delivered ketoprofen to the target tissues at a similar concentration to that observed after oral administration without a high plasma concentration. The ketoprofen patch rapidly achieved effective tissue levels, which were maintained for at least 20 h.
Acknowledgements
The authors declare that the study drug was supplied to us, and drug concentrations were analyzed by Hisamitsu Pharmaceutical Co., Inc. (Saga, Japan), the manufacturer of ketoprofen patches.
References
- 1.Veys EM. 20 years’ experience with ketoprofen. Scand J Rheumatol. 1991;90:1–44. doi: 10.3109/03009749109097250. [DOI] [PubMed] [Google Scholar]
- 2.Patel RK, Leswell PF. Comparison of ketoprofen, piroxicam, and diclofenac gels in the treatment of acute soft-tissue injury in general practice. General Practice Study Group. Clin Ther. 1996;18:497–507. doi: 10.1016/S0149-2918(96)80031-2. [DOI] [PubMed] [Google Scholar]
- 3.Jokhio IA, Siddiqui KA, Waraich T, Abbas M, Ali A. Study of efficacy and tolerance of ketoprofen and diclofenac sodium in the treatment of acute rheumatic and traumatic conditions. J Pak Med Assoc. 1998;48:373–376. [PubMed] [Google Scholar]
- 4.Mazières B, Rouanet S, Guillon Y, Scarsi C, Reiner V. Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study. J Rheumatol. 2005;32:1563–1570. [PubMed] [Google Scholar]
- 5.Mazières B, Rouanet S, Velicy J, Scarsi C, Reiner V. Topical ketoprofen patch (100 mg) for the treatment of ankle sprain: a randomized, double-blind, placebo-controlled study. Am J Sports Med. 2005;33:515–523. doi: 10.1177/0363546504268135. [DOI] [PubMed] [Google Scholar]
- 6.Osterwalder A, Reiner V, Reiner G, Lualdi P. Tissue absorption and distribution of ketoprofen after patch application in subjects undergoing knee arthroscopy or endoscopic carpal ligament release. Arzneimittelforschung. 2002;52:822–827. doi: 10.1055/s-0031-1299974. [DOI] [PubMed] [Google Scholar]
- 7.Rolf C, Movin T, Engstrom B, Jacobs LD, Beauchard C, Le Liboux A. An open, randomized study of ketoprofen in patients in surgery for Achilles or patellar tendinopathy. J Rheumatol. 1997;24:1595–1598. [PubMed] [Google Scholar]
- 8.Rolf C, Engström B, Beauchard C, Jacobs LD, Le Liboux A. Intra-articular absorption and distribution of ketoprofen after topical plaster application and oral intake in 100 patients undergoing knee arthroscopy. Rheumatology. 1999;38:564–567. doi: 10.1093/rheumatology/38.6.564. [DOI] [PubMed] [Google Scholar]
- 9.Christophidis N, Rotstein A, Louis WJ. Acute and chronic pharmacokinetic studies of slow release ketoprofen (Oruvail) in rheumatoid arthritis. Clin Exp Pharmacol Physiol. 1986;13:555–561. doi: 10.1111/j.1440-1681.1986.tb00938.x. [DOI] [PubMed] [Google Scholar]