Abstract
Objective: To compare risk of myocardial infarction associated with smoking in men and women, taking into consideration differences in smoking behaviour and a number of potential confounding variables.
Design: Prospective cohort study with follow up of myocardial infarction.
Setting: Pooled data from three population studies conducted in Copenhagen.
Subjects: 11 472 women and 13 191 men followed for a mean of 12.3 years.
Main outcome measures: First admission to hospital or death caused by myocardial infarction.
Results: 1251 men and 512 women had a myocardial infarction during follow up. Compared with non-smokers, female current smokers had a relative risk of myocardial infarction of 2.24 (range 1.85-2.71) and male smokers 1.43 (1.26-1.62); ratio 1.57 (1.25-1.97). Relative risk of myocardial infarction increased with tobacco consumption in both men and women and was higher in inhalers than in non-inhalers. The risks associated with smoking, measured by both current and accumulated tobacco exposure, were consistently higher in women than in men and did not depend on age. This sex difference was not affected by adjustment for arterial blood pressure, total and high density lipoprotein cholesterol concentrations, triglyceride concentrations, diabetes, body mass index, height, alcohol intake, physical activity, and level of education.
Conclusion: Women may be more sensitive than men to some of the harmful effects of smoking. Interactions between components of smoke and hormonal factors that may be involved in development of ischaemic heart disease should be examined further.
Introduction
Ischaemic heart disease is responsible for about 40% of deaths in Western countries, with smoking as a major modifiable risk factor.1 The steep rise in the worldwide prevalence of smoking among women is expected to continue in the near future. At the start of the smoking epidemic, female smokers were few and differed extensively from male smokers in factors such as age of starting smoking, amount smoked, and inhalation habits, and the risk associated with smoking in women may have been underestimated.2–4 Within the past two or three decades male and female smoking habits have become similar, and a more fair comparison of the risk associated with smoking in both sexes based on recent prospective population studies is now possible. From a public health point of view, as well as a clinical point of view, it is important to recognise sex differences in risk associated with smoking and elucidate possible mechanisms by which these differences could act.
We recently found that the relative mortality from vascular disease was higher in female smokers than in male smokers.5 Consequently, we aimed to examine sex differences in smoking related risk of myocardial infarction while simultaneously including multiple cardiovascular risk factors in a prospective population study conducted in Copenhagen.
Methods
This study is based on data from three longitudinal population studies: the Copenhagen city heart study, with 15 789 subjects from central Copenhagen; the Glostrup population studies, with 6341 subjects from Copenhagen suburbs; and the Copenhagen male study, which sampled 3355 subjects from 14 large workplaces. All datasets with sufficient information on cardiovascular risk factors were included. The study population is outlined in table 1. Overall response rate at first examination was 77% (range 69-88%).
Table 1.
Overview of study population
| Cohort of origin* | No of women | No of men | Year of examination | Age at examination | No of myocardial infarctions |
|---|---|---|---|---|---|
| Copenhagen city heart study | 8 395 | 7 033 | 1976/81 | 20-93 | 1368 |
| Glostrup population studies | 547 | 503 | 1976 | 40 | 30 |
| 1 828 | 1 889 | 1982 | 30-60 | 119 | |
| 702 | 695 | 1987 | 30-60 | 25 | |
| Copenhagen male study | — | 3 071 | 1985 | 45-64 | 221 |
| Total | 11 472 | 13 191 | 1976-87 | 20-93 | 1763 |
Subjects with myocardial infarction before enrolment (n=627), double participants (n=191), and subjects lost to follow up (n=1) were excluded.
Cardiovascular risk factors were assessed by a self administered questionnaire and various laboratory tests. Tobacco consumption was studied in six categories: never smokers; ex-smokers; non-inhaling current smokers; and inhaling current smokers of 1-14, 15-24, and ⩾25 g tobacco per day. Type of tobacco (cigarette, cheroot, cigar, pipe, or mixed) was recorded for current smokers, as were years of smoking for both current and former smokers. Current tobacco consumption was calculated by equating a cigarette to 1 g tobacco, a cheroot to 3 g tobacco, and a cigar to 5 g tobacco. Pack years in current smokers was calculated as years of smoking multiplied by packs (of 20 cigarettes) currently consumed.
Arterial blood pressure was measured with the subject in a sedentary position after at least five minutes’ rest. Blood lipids were non-fasting in the Copenhagen city heart study and fasting in the remaining cohorts. Body mass index was calculated as weight (kg) divided by height squared (m2). Educational level was divided into three categories: <8 years of schooling (completed primary school), 8-11 years, and >11 years. Alcohol consumption was classified according to total weekly intake: <1 drink per week, 1-6 drinks, 7-13 drinks, 14-27 drinks, 28-41 drinks, and >41 drinks; one drink contained 9-13 g alcohol. Physical activity in leisure time was classified into three categories as sedentary; moderate activity <4 hours per week; and moderate activity >4 hours per week. Self reported diabetes was defined as an affirmative answer to the question “Do you have diabetes?”
Subjects were followed until 31 December 1993 for fatal and non-fatal myocardial infarction (ICD-8 diagnosis code 410); the information was obtained from the National Board of Health or the National Hospital Discharge Register. Subjects with myocardial infarction before enrollment were excluded; analyses therefore concern first myocardial infarction only.
Statistical analysis
Arterial blood pressure, total and high density lipoprotein cholesterol concentrations, triglyceride concentrations, height, weight, and body mass index were divided into fifths within cohorts, by sex and by 10 year age groups. In this way differences in methods of measurement between the three cohorts were taken into account. Association between risk factors and myocardial infarction was analysed by using Cox proportional hazards regression models with age as underlying timescale and delayed entry accordingly. Relative risks for covariates other than smoking did not differ between men and women, and the final analyses were performed on the pooled data stratified by sex; this assumed the same effect of covariates in men and women but allowed for different baseline hazard in men and women. All covariates were treated as categorical variables as described above, and tests for interaction were done by using the likelihood ratio test. There were no significant interactions between sex and cardiovascular risk factors other than smoking. Both incidence rate and distribution of risk factors differed in the three study groups, but risk estimates did not differ. We therefore report results from the pooled data adjusted for cohort of origin. Incidence rates were based on number of events and person years of observation in 5 year age bands. The Stata statistical package was used for estimation.6
Results
Analyses were based on 11 472 women and 13 191 men (table 1). During follow up, 512 women and 1251 men had myocardial infarctions, of which 104 and 274, respectively, were fatal.
With the exception of alcohol consumption and physical activity, men had a more disadvantageous cardiovascular risk profile (table 2).
Table 2.
Background characteristics and risk factors by sex. Values are mean (SD) unless otherwise indicated
| Characteristic | Women (n=11 472) | Men (n=13 191) | P value |
|---|---|---|---|
| Age (years) | 49.7 (12.4) | 52.5 (12.6) | <0.001 |
| Body mass index (kg/m2) | 24.4 (4.4) | 25.6 (3.6) | <0.001 |
| Systolic blood pressure* (mm Hg) | 130.4 (22.3) | 135.6 (20.5) | <0.001 |
| Diastolic blood pressure* (mm Hg) | 79.2 (12.0) | 83.3 (12.3) | <0.001 |
| Plasma cholesterol* (mmol/l) | 6.16 (1.28) | 5.98 (1.19) | <0.001 |
| Plasma triglyceride* (mmol/l) | 1.37 (0.85) | 1.89 (1.41) | <0.001 |
| High density lipoprotein cholesterol* (mmol/l) | 1.49 (0.46) | 1.26 (0.37) | <0.001 |
| No (%) current smokers | 6461 (56.3) | 8490 (64.5) | <0.001 |
| Daily tobacco consumption†(g) | 13.8 (7.9) | 17.9 (9.8) | <0.001 |
| No (%) with <8 years education | 5071 (44.2) | 6081 (46.1) | 0.003 |
| No (%) physically inactive in leisure time | 2719 (23.7) | 2454 (18.6) | <0.001 |
| No (%) with intake >14 drinks/week | 987 (8.6) | 5514 (41.8) | <0.001 |
| No (%) with self reported diabetes | 149 (1.3) | 303 (2.3) | <0.001 |
In men, based on 10 120 subjects. Subjects from Copenhagen male study excluded because of difference in method of measurement.
Among current smokers.
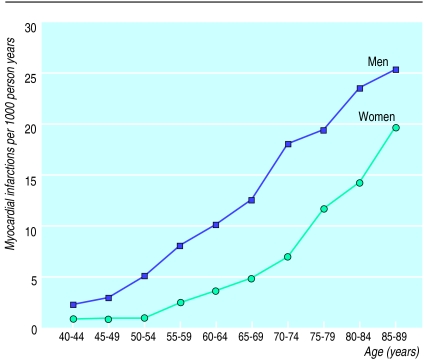
Figure 1 shows age and sex specific incidence rates of myocardial infarction. Men had higher incidence rates than women at all ages but the male-female risk ratio decreased from about 3 in the younger groups to 1.5 in older groups.
Figure 1.
Age specific incidence rate of myocardial infarction in 11 472 women and 13 191 men from Copenhagen
Systolic blood pressure, diastolic blood pressure, total and high density lipoprotein cholesterol concentrations, triglyceride concentrations, body mass index, height, education, alcohol intake, leisure time physical activity, and diabetes were all strongly associated with risk of myocardial infarction, and relative risks were similar in men and women.
Female current smokers had a relative risk of 2.24 (range 1.85-2.71) and male smokers 1.43 (1.26-1.62) relative to non-smokers. This difference was significant (ratio 1.57 (1.25-1.97), P=<0.001) and was not changed after multiple adjustment for other risk factors. Risk in ex-smokers was not increased, but in current smokers there was a clear dose-response relation from a relative risk of 1.70 (1.31-2.21) and 1.26 (0.98-1.61) in female and male non-inhalers, respectively, to a maximum of 3.31 (1.91-5.73) and 2.08 (1.60-2.69) in heavy smokers (table 3). All risk estimates were higher in women than in men and were not affected by adjustment for other major risk factors, as indicated. Similar results were seen after categorisation by pack years: maximum risk was seen in inhaling smokers of more than 30 pack years (3.26 (2.36-4.50) in women, 1.76 (1.41-2.19) in men; ratio 2.13 (1.48-3.07)). There was no interaction between smoking and other risk factors.
Table 3.
Relative risk (95% confidence interval) of myocardial infarction by current tobacco exposure in 11 472 women and 13 191 men. Results from Cox proportional hazards regression analysis
| Smoking status | Unadjusted*
|
Adjusted†
|
|||||
|---|---|---|---|---|---|---|---|
| Women | Men | Ratio | Women | Men | Ratio | ||
| Never smoker | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Ex-smoker | 0.99 (0.71 to 1.38) | 1.14 (0.90 to 1.44) | 0.87 (0.58 to 1.30) | 1.05 (0.74 to 1.50) | 1.11 (0.86 to 1.42) | 0.95 (0.61 to 1.46) | |
| Current smoker: | |||||||
| Non-inhaling | 1.70 (1.31 to 2.21) | 1.26 (0.98 to 1.61) | 1.35 (0.94 to 1.94) | 1.82 (1.39 to 2.41) | 1.37 (1.06 to 1.78) | 1.33 (0.91 to 1.94) | |
| Inhaling: | |||||||
| 1-14 g/day | 2.59 (1.98 to 3.38) | 1.52 (1.19 to 1.95) | 1.70 (1.18 to 2.44) | 2.76 (2.08 to 3.68) | 1.60 (1.24 to 2.07) | 1.72 (1.18 to 2.53) | |
| 15-24 g/day | 2.99 (2.25 to 3.99) | 1.72 (1.37 to 2.17) | 1.74 (1.20 to 2.51) | 3.27 (2.42 to 4.42) | 1.75 (1.37 to 2.23) | 1.87 (1.27 to 2.75) | |
| >24 g/day | 3.31 (1.91 to 5.73) | 2.08 (1.60 to 2.69) | 1.60 (0.87 to 2.93) | 2.82 (1.45 to 5.46) | 2.09 (1.58 to 2.77) | 1.34 (0.66 to 2.75) | |
| Test for interaction‡ | P= 0.002 | P= 0.005 | |||||
Adjusted for age and cohort.
Cox regression model stratified by sex and adjusted for age, cohort of origin, smoking status, sex, systolic blood pressure, diastolic blood pressure, cholesterol, triglyceride, body mass index, education, alcohol, diabetes, physical activity, and height.
Likelihood ratio test for interaction between smoking and sex.
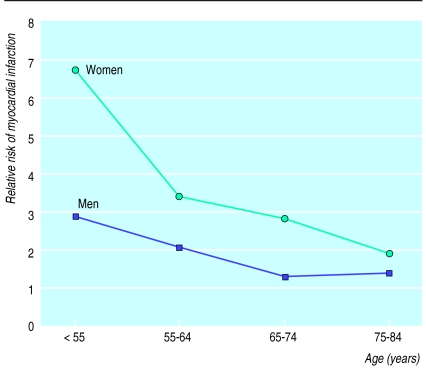
Figure 2 shows relative risk for inhaling current smokers versus never smokers by age in men and women up to age 85. The risk of myocardial infarction decreased with age but was higher in women at all ages. The interaction term between smoking and sex did not differ in the four 10 year intervals (P=0.73) and when the age dependence of the risk associated with smoking was adjusted for, the overall ratio between female and male relative risk was 2.01 (1.39 to 2.90).
Figure 2.
Relative risk of myocardial infarction for inhaling current smokers compared with never smokers
Discussion
In this prospective study of almost 25 000 subjects the main result was that relative risk of myocardial infarction in female smokers exceeded that of male smokers by more than 50%. This difference was not affected by multiple adjustment for major cardiovascular risk factors.
Myocardial infarction
Our end point was defined as ICD-8 code 410, ascertained from the Hospital Discharge Register and from registration of cause of death with the National Board of Health, and included both fatal and non-fatal myocardial infarction. Some infarctions did not lead to hospital admission or may have been coded differently, for instance as codes 411 (other acute ischaemic heart disease), 427 (symptomatic heart disease), and 795 (sudden death). However, only differential misclassification (related to both sex and smoking status) will bias results, and there is no reason to suspect this.
Incidence rates of myocardial infarction in men were similar to those in other studies but the rates in women were higher. This is consistent with reports showing that mortality in middle aged Danish women is among the highest in western Europe and is at least partly caused by the high prevalence of smoking.7 Consistent with the observation that ex-smokers reduce their excess risk of myocardial infarction by as much as 50% within the first year after quitting,1 we found that myocardial infarction was more strongly associated with current exposure than with accumulated exposure to tobacco. This is in agreement with findings that the short term effects of components in tobacco smoke—on the haemodynamic system, for example—are more important than the chronic exposure in development of coronary thrombosis.
Comparison by sex
In a previous study we showed that female smokers have about a 50% higher relative risk of dying from vascular disease.5 The present study confirmed this sex difference and found that the difference is not affected by adjustment for other cardiovascular risk factors.
Our analyses were based on baseline smoking status. If substantially more men than women gave up smoking during follow up, this could explain some of the observed difference. In 11 094 subjects in the Copenhagen city heart study who were re-examined after 5 years, 12.8% of men and 11.6% of women who smoked at baseline had given up smoking. As quitting rates were similar, this is not likely to have affected results.
A few studies have addressed the issue of sex difference in effect of smoking on ischaemic heart disease.4,8–12 In a large prospective Norwegian study that included 11 843 subjects, relative risk of myocardial infarction was 3.3 in female current smokers and 1.9 in male current smokers after adjustment for total and high density lipoprotein cholesterol concentrations, triglyceride concentrations, body mass index, and systolic blood pressure.9 This study also showed that relative risks associated with other risk factors were similar in both sexes. In the West of Scotland study, in which 4696 women and 5714 men were followed for death from ischaemic heart disease, relative risk was 1.9 in women and 1.6 in men after adjustment for major risk factors.10 In a Swedish study that included 10 945 twins, relative risk was 1.6 in female smokers and 1.4 in male smokers.11 However, the Framingham study found no significant relation between smoking and ischaemic heart disease in women,4 and in a study of death from ischaemic heart disease by LaCroix et al, which included 7178 subjects aged over 65, relative risks were 1.6 in female smokers and 2.0 in male smokers, with no adjustment for other risk factors.12 In these and in other important studies of coronary mortality2,3,13–15 an apparent lack of difference between the sexes in risk associated with smoking may be due to the extensive differences in smoking habits between male and female smokers from older birth cohorts.
Our results are based on a multiplicative model, which is the most widely used model in cardiovascular epidemiology. That cardiovascular risk factors should act multiplicatively is biologically plausible, given what we know of the pathogenesis of myocardial infarction. If effects of cardiovascular risk factors are additive, relative risks will vary between men and women simply because risks vary at baseline. In this and other studies, however, relative risks did not vary for the other cardiovascular risk factors examined,9 and the sex difference in effect of tobacco thus cannot simply be put down to differing baseline rates. Although relative risks are higher in women, suggesting differences in mechanism of action of tobacco in men and women, smoking may well cause more cases of myocardial infarction among men. In our study population, the difference in risk was higher in men to age 65 and in women after age 65.
A possible cause of the sex difference is an interaction of some hormonal factors with components of the inhaled smoke. There is growing epidemiological evidence that women who smoke are relatively deficient in oestrogen: they have an earlier menopause, decreased risk of cancer of the endometrium, greater likelihood of osteoporosis and of osteoporotic fractures, and reduced incidence of a number of “minor” disorders such as uterine fibroids.16 Possible biological mechanisms have been suggested.16–18 Oestrogen deficiency, on the other hand, is associated with cardiovascular disease: rates of ischaemic heart disease increase sharply in women after menopause; young women with bilateral oophorectomy have an increased risk of ischaemic heart disease; and accumulating epidemiological data show that women who use hormone replacement therapy after menopause have lower rates of ischaemic heart disease.19,20 This mechanism may be by lowering low density lipoprotein and fibrinogen, increasing high density lipoprotein cholesterol,21,22 increasing blood flow, and reducing artherosclerosis.19,23 Thus it is possible that tobacco smoke, in addition to the (partly unknown) mechanisms by which it increases risk of ischaemic heart disease in both men and women, interacts with sex hormones and thus increases risk of ischaemic heart disease relatively more in female smokers than in men. In support of this, Criqui et al found that hormone replacement therapy was protective only in smokers,24 and in a study based on the Copenhagen city heart study Lindenstrøm et al found that hormone replacement therapy reduced the risk of stroke only in smokers,25 results which have yet to be confirmed in other studies. Because of insufficient information on hormone replacement therapy we could not examine this.
Conclusion
Female smokers have a higher relative risk of myocardial infarction than male smokers, even after adjustment for major cardiovascular risk factors. This raises the question of whether tobacco smoke may be more harmful to women with regard to ischaemic heart disease, possibly because of constituents of tobacco smoke exerting anti-oestrogenic effects. Results from large ongoing clinical trials of hormonal replacement therapy may be able to elucidate this.
Acknowledgments
The Copenhagen Center for Prospective Population Studies (T I A Sørensen, G Jensen, H O Hein, T Jørgensen, N Keiding, J Vestbo, M Grøenbak) consists of the Glostrup population studies (T Jørgensen, H Ibsen, K Borch-Johnsen, P Thorvaldsen, T Thomsen), the Copenhagen male study (H O Hein, F Gyntelberg, P Suadicani) and the Copenhagen city heart study (G Jensen, P Schnohr, M Appleyard, P Lange, B Nordestgaard, M Grønbæk).
Footnotes
Funding: Grants from the Danish Ministry of Health, the Health Insurance Fund, the Danish Heart Foundation, and the Danish Medical Research Council (12-1661-1).
Conflict of interest: None.
References
- 1.Department af Health and Human Services. Epidemiology. In: National Heart, Lung, and Blood Institute. Report of the Task Force on Research in Epidemiology and Prevention of Cardiovascular Diseases. Rockville, MD: Public Health Service, National Institutes of Health, 1994:19-72.
- 2.Doll R, Gray R, Hafner B, Peto R. Mortality in relation to smoking: 22 years’ observations on female British doctors. BMJ. 1980;280:967–971. doi: 10.1136/bmj.280.6219.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seltzer CC. Framingham study data and “established wisdom” about cigarette smoking and coronary heart disease. J Clin Epidemiol. 1988;42:743–750. doi: 10.1016/0895-4356(89)90070-x. [DOI] [PubMed] [Google Scholar]
- 5.Prescott E, Osler M, Andersen PK, Hein HO, Borch-Johnsen K, Lange P, et al. Mortality in women and men in relation to smoking: results from the Copenhagen center for prospective population studies. Int J Epidemiol (in press). [DOI] [PubMed]
- 6.StataCorp. Stata statistical software: release 5.0. College Station, TX: Stata Corporation, 1997.
- 7.Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr . Mortality from smoking in developed countries, 1950-2000: indirect estimates from national vital statistics. Oxford: Oxford University Press; 1994. [Google Scholar]
- 8.Nyboe J, Jensen G, Appleyard M, Schnohr P. Smoking and the risk of first acute myocardial infarction. Am Heart J. 1991;122:438–447. doi: 10.1016/0002-8703(91)90997-v. [DOI] [PubMed] [Google Scholar]
- 9.Njolstad I, Arnesen E, Lund Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark study. Circulation. 1996;93:450–456. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 10.Janghorbani M, Hedley AJ, Jones RB, Zhianpour M, Gilmour WH. Gender differential in all-cause and cardiovascular disease mortality. Int J Epidemiol. 1993;22:1056–1063. doi: 10.1093/ije/22.6.1056. [DOI] [PubMed] [Google Scholar]
- 11.Floderus B, Cederlöf R, Friberg L. Smoking and mortality: a 21-year follow-up based on the Swedish twin registry. Int J Epidemiol. 1988;17:332–340. doi: 10.1093/ije/17.2.332. [DOI] [PubMed] [Google Scholar]
- 12.LaCroix AZ, Lang J, Scherr PA, Wallace RB, Cornoni-Huntley J, Berkman L, et al. Smoking and mortality among older men and women in three communities. N Engl J Med. 1991;324:1619–1625. doi: 10.1056/NEJM199106063242303. [DOI] [PubMed] [Google Scholar]
- 13.Davis MA, Neuhaus JM, Moritz DJ, Lein D, Barchlay JD, Murphy SP. Health behaviors and survival among middle-aged and older men and women in the NHANES I epidemiologic follow-up study. Prev Med. 1994;23:369–376. doi: 10.1006/pmed.1994.1051. [DOI] [PubMed] [Google Scholar]
- 14.Tverdal A, Thelle D, Stensvold I, Leren P, Bjartveit K. Mortality in relation to smoking history: 13 years’ follow-up of 68,000 Norwegian men and women 35-49 years. J Clin Epidemiol. 1993;46:475–487. doi: 10.1016/0895-4356(93)90025-v. [DOI] [PubMed] [Google Scholar]
- 15.Thun MJ, Day Lally CA, Calle EE, Flanders WD, Heath CW., Jr Excess mortality among cigarette smokers: changes in a 20-year interval [with comments] Am J Public Health. 1995;85:1223–1230. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron JA, Vecchia CL, Levi F. The antiestrogenic effect of cigarette smoke in women. Am J Obstet Gynecol. 1990;162:502–514. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- 17.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 18.Michnovicz JJ, Hershcopf RJ, Haley NJ, Bradlow HL, Fishman J. Cigarette smoking alters hepatic estrogen metabolism in men: implication for atherosclerosis. Metabolism. 1989;38:537–541. doi: 10.1016/0026-0495(89)90213-8. [DOI] [PubMed] [Google Scholar]
- 19.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis. 1995;38:199–210. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 20.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study [with comments] N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 21.Miller VT, Muesing RA, LaRosa JC. Effects of conjugated equine estrogen with and without three different progestogens on lipoproteins, high-density lipoprotein subfractions, and apolipoprotein A-4. Obstet Gynecol. 1991;77:235–240. doi: 10.1097/00006250-199102000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 23.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 24.Criqui MH, Suarez L, Barrett Connor E, McPhillips J, Wingard DL, Garland C. Postmenopausal estrogen use and mortality. Results from a prospective study in a defined, homogeneous community. Am J Epidemiol. 1988;128:606–614. doi: 10.1093/oxfordjournals.aje.a115008. [DOI] [PubMed] [Google Scholar]
- 25.Lindenstrøm E, Boysen G, Nyboe J. Lifestyle factors and risk of cerebrovascular disease in women. Stroke. 1993;24:1468–1472. doi: 10.1161/01.str.24.10.1468. [DOI] [PubMed] [Google Scholar]