Abstract
The present study was designed to improve the bioavailability of forskolin by the influence of precorneal residence time and dissolution characteristics. Nanosizing is an advanced approach to overcome the issue of poor aqueous solubility of active pharmaceutical ingredients. Forskolin nanocrystals have been successfully manufactured and stabilized by poloxamer 407. These nanocrystals have been characterized in terms of particle size by scanning electron microscopy and dynamic light scattering. By formulating Noveon AA-1 polycarbophil/poloxamer 407 platforms, at specific concentrations, it was possible to obtain a pH and thermoreversible gel with a pHgel/Tgel close to eye pH/temperature. The addition of forskolin nanocrystals did not alter the gelation properties of Noveon AA-1 polycarbophil/poloxamer 407 and nanocrystal properties of forskolin. The formulation was stable over a period of 6 months at room temperature. In vitro release experiments indicated that the optimized platform was able to prolong and control forskolin release for more than 5 h. The in vivo studies on dexamethasone-induced glaucomatous rabbits indicated that the intraocular pressure lowering efficacy for nanosuspension/hydrogel systems was 31% and lasted for 12 h, which is significantly better than the effect of traditional eye suspension (18%, 4–6 h). Hence, our investigations successfully prove that the pH and thermoreversible polymeric in situ gel-forming nanosuspension with ability of controlled drug release exhibits a greater potential for glaucoma therapy.
Key words: forskolin, glaucoma, ocular drug delivery, nanocrystal, sol–gel techniques
INTRODUCTION
Glaucoma is the second most common cause of blindness worldwide, after cataract (1), and there will be 60.5 million people with open angle glaucoma (OAG) and angle closure glaucoma in 2010, increasing to 79.6 million by 2020, 74% of whom will have OAG (2). Glaucoma is usually, but not always, associated with elevated intraocular pressure (IOP) in eye which leads to the damage of eye (optic) nerve. Forskolin (7beta-acetoxy-8, 13-epoxy-1α, 6β, 9α-trihydroxy-labd-14-en-11-one) is a diterpenoid isolated from plant Coleus forskohlii (Lamiaceae). It is a lipid-soluble compound that can penetrate cell membranes and stimulates enzyme adenylate cyclase which, in turn, stimulates ciliary epithelium to activate cyclic adenosine monophosphate, which decreases IOP by reducing aqueous humor inflow (3,4). The topical application of forskolin is capable of reducing IOP in rabbits, monkeys, and humans (5–7).
The number of drugs and new chemical entities with potential for pharmaceutical use is steadily increasing. In most cases, low solubility is generally associated with low ocular bioavailability (8–12). Therefore, it is of high importance to come up with innovative formulation technologies to make these poorly soluble molecules bioavailable to avoid loosing interesting drug candidates. Nanosizing (drug nanocrystals) is an advanced approach to overcome the issue of poor aqueous solubility of active pharmaceutical ingredients. Due to reduction in size, surface area and saturation solubility increases and leads to an increase in dissolution velocity which means that nanocrystals are a formulation solution for classes II and IV drugs of the biopharmaceutical classification system.
A drop of an ophthalmic solution, irrespective of the instilled volume, often eliminates rapidly within 5 to 6 min after administration, and only a small amount (1–3%) of it actually reaches the intraocular tissue. Thus, it cannot provide and maintain an adequate concentration of drug in the precorneal area. More than 75% of applied ophthalmic solution is lost via nasolachrymal drainage and absorbed systemically via conjunctiva which reduces ocular drug availability. To increase ocular bioavailability and duration of drug action, various ophthalmic vehicles, such as viscous solutions, suspensions, ointments, aqueous gels, and polymeric inserts, have been investigated for topical application to the eye (13,14).
From the point of view of patient compliance, a liquid dosage form that can sustain drug release and remain in contact with the cornea of the eye for extended periods of time is ideal. If the precorneal residence time of a drug could be improved from few minutes to, say a few hours, then improved local bioavailability, reduced dose concentrations, dosing frequency, and improved patient acceptability may be achieved. Such properties can be achieved by delivery systems based on in situ gel-forming solutions which consist of phase transition systems that are instilled in a liquid form and shift to the gel or solid phase once in the cul-de-sac of the eye. Several in situ gelling systems (ISGS) have been developed to prolong the precorneal residence time of a drug and improve ocular bioavailability. Depending on the method employed to produce the sol to gel phase transition on the ocular surface, the following three types of systems have been used: pH-triggered systems (15,16), temperature-dependent systems (17–19), and ion-activated systems (20–22).
The aim of the present work was to formulate forskolin (class II or IV drug) as mucoadhesive in situ gel-forming nanosuspension, i.e., combining the properties of mucoadhesive drug delivery systems, in this case hydrogels, with nanosuspension to overcome the problem of poor bioavailability caused by poor aqueous solubility and rapid nasolachrymal drainage. The high-performance disperser was evaluated for preparation of forskolin nanosuspension preparation for ophthalmic administration. A physical admixture of two different polymers (Noveon® AA-1 polycarbophil/poloxamer 407) was used to form an aqueous composition which undergo gelation when instilled into the cul-de-sac of the eye in response to simultaneous variations in pH and temperature and provide sustained release of the drug during the treatment of glaucoma. Noveon® AA-1 polycarbophil, USP is a high molecular weight polyacrylic acid polymer crosslinked with divinyl glycol and exhibits a definite sol to gel transition in aqueous solutions as the pH is raised above its pKa of about 6.0 ± 0.5. It provides excellent bioadhesive properties and has been used extensively to enhance the delivery of active ingredients to various mucous membranes. It is the recognized industry standard for bioadhesion and can be used for the formulation of buccal, vaginal, nasal, ophthalmic, and rectal bioadhesive products (23).
MATERIALS AND METHODS
Materials
Forskolin (95%, 98%) was gifted by Sami Laboratories, Bangalore, India. Noveon® AA-1 polycarbophil was gifted by Lubrizol Advanced Materials, Mumbai, India. Pluronic F-127 (poloxamer 407) and methylated β-cyclodextrin were obtained from Sigma Aldrich, India. Benzalkonium chloride (BKC; 50%), ethanol (99.9%), methanol, sodium bicarbonate (NaHCO3), and capillary tubes were obtained from S.D. Fine Chemicals, India. Fluid thioglycolate media (FTM) and soybean casein digest medium (SCDM) were obtained from Hi Media, India. Mannitol and calcium chloride dehydrate (CaCl2.2H2O) were obtained from Qualigens Fine Chem, India. Sodium chloride was obtained from Ranbaxy, India. Xylocaine (4%, w/v) topical solution was obtained from Astra Zeneca Pharma Ltd., India. Dexamethasone injection (4 mg/ml) was obtained from Wockhardt Ltd., India. Sterile 10-ml eye drops bottles were gifted by Vijay Bakelite Trading Co., Mumbai, India. Syringe membrane filters were gifted by Mdi, Ambala, India.
Preparation of Forskolin Nanocrystals
Forskolin nanocrystals were prepared using a wet crushing technique. The block copolymer poloxamer 407 was selected as the stabilizer for the formulation after a screening study. The drug powder in a concentration of 10% (w/v) was dispersed in a 1–5% (w/w) aqueous surfactant solution (poloxamer 407) by high-speed stirring for 3 min. The starting size of each drug presuspension was determined using an optical microscope (Shimadzu, Japan). The 10-ml “macro”-suspension was transferred to a 50-ml beaker followed by wet crushing of forskolin crystals using a high-performance disperser T 25 digital ULTRA-TURRAX® in an ice bath. Applied speed ranged from about 15,000 to 20,000 rpm for up to ten cycles of 10 min each. Sterilization of containers was done by dry heat sterilization in hot air oven at 160°C for 2 h. Sterilization of drug (98% forskolin) was done by filtration of 0.51% (w/v) forskolin solution in ethanol (99.9%, v/v) through sterile syringe membrane filter (0.22 µm) under laminar flow in aseptic room followed by aseptic crystallization.
Preparation of In Situ Gel-Forming Solution, Suspension, and Gels
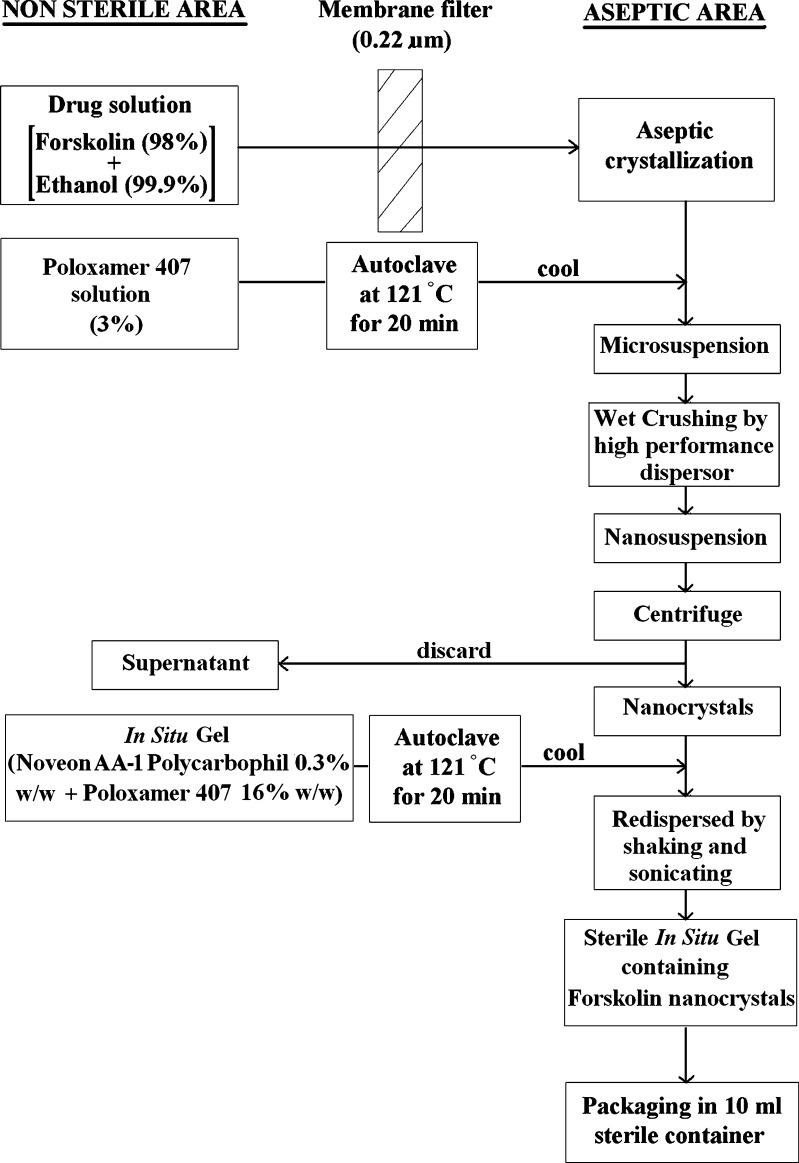
Poloxamer 407 solution (30.0%, w/w) and Noveon® AA-1 polycarbophil solution (1.0%, w/w) were prepared by gradually dispersing the required amount of polymer in 0.01% BKC solution (precold to <10°C) with continuous stirring for 1 h. The Noveon® AA-1 polycarbophil solution and partially dissolved poloxamer 407 solution were stored in the refrigerator until the entire polymer was completely dissolved (approximately 24 h). The in situ gel-forming solutions were prepared by mixing weighed quantities of poloxamer 407 solutions (30.0%, w/w) with the Noveon® AA-1 polycarbophil solutions (1.0%, w/w) in glass vials. The pH of the samples was then adjusted to 4.4 ± 0.1 by the addition of measured volumes of a 0.5-M sodium hydroxide solution. After the polymer solutions were mixed, 0.01% BKC solution was added to obtain a final polymer concentration. Mannitol was added into the formulations as the osmotic agent. The vials were tightly capped, and the samples were allowed to equilibrate for 24 h in refrigerator prior to the experiments. For preparation of 1% (w/v) forskolin in situ gel-forming suspension, the desired amounts of forskolin nanocrystals were dispersed to the poloxamer 407/Noveon® AA-1 polycarbophil solutions with continuous stirring until thoroughly mixed. All the sample solutions were adjusted to pH 4.4 ± 0.1 or 7.4 ± 0.1 by 0.5-M sodium hydroxide solution and then stored in the refrigerator prior to the evaluation of their rheological properties. Sterilization of 0.01% BKC and poloxamer 407/Noveon® AA-1 polycarbophil solutions was done by autoclaving at 121°C for 20 min. The final optimized formulations were formulated in aseptic conditions under laminar flow. The flow diagram is shown in Fig. 1.
Fig. 1.
Formulation and sterilization flow chart
Characterization of Nanoparticle Morphology and Size
The surface morphology of the particles, nature of aggregation, and size of the nanocrystals were studied by scanning electron microscopy (SEM). A drop of particle suspension was deposited on a silicon wafer, dried, and sputtered for 20 s with gold and, finally, analyzed by SEM (Sirion, FEI, The Netherlands).
Crystal size was evaluated by dynamic light scattering (DLS; Brookhaven Instruments Corporation, Holtsville, NY, USA) technique. The nanocrystal samples for particle size analysis were added to the small sample dispersion unit containing water as a dispersant. A refractive index of 1.5 was used for measurements, and the laser obscuration range was maintained between 10% and 20%. Three observations were recorded for each sample. The diameters reported were calculated using volume distribution.
Thermal Analysis Using Differential Scanning Calorimetry
Thermal behavior of the materials was determined using differential scanning calorimetry (DSC 822, Differential Scanning Calorimeter, Mettler Toledo, Columbus, OH, USA). Approximately 5 mg of forskolin, forskolin nanocrystals, poloxamer 407, Noveon® AA-1 polycarbophil, and their mixtures were accurately weighed into aluminum pans and crimped by aluminum caps with a pinhole. Forskolin nanocrystals were separated from formulation by centrifugation followed by drying at 40°C. The samples were initially kept at room temperature 25°C and then heated at a rate of 10°C/min up to 260°C under nitrogen purge. Data analysis was performed using STARe software (Mettler Toledo).
Fourier Transform Infrared Spectrum Analysis
Fourier transform infrared (FTIR) spectra were measured using an optical bench (Shimadzu FTIR 8400S, Japan) to determine the possible interactions between forskolin, poloxamer 407, and Noveon® AA-1 polycarbophil. The drug, individual polymer (poloxamer 407 and Noveon® AA-1 polycarbophil), and 1:1 physical mixture of drug and polymer (each 10 mg) were prepared and mixed with 400 mg of potassium bromide. About 100 mg of this mixture was compressed to form a transparent pellet using a hydraulic press at 15 tonnes pressure. It was scanned from 4,000 to 400 cm−1 in a Shimadzu FTIR 8400S spectrophotometer. The IR spectrum of the physical mixture was compared with those of pure drug and polymers, and matching was done to detect any appearance or disappearance of peaks. Two hundred fifty-six scans at 4 cm−1 resolution were used to obtain each spectrum.
Estimation of Drug Content
The bottle containing the formulations was shaken for 2 to 3 min, and 1 ml of the formulation was dropped in a 10-ml volumetric flask and final volume made up with methanol. The amount of forskolin was analyzed by high-performance liquid chromatography (HPLC) at 220 nm using a octadecylsilate column. Mobile phase was a mixture of acetonitrile and water (45:55) and the flow rate was 1.8 ml/min. Each experiment was performed in triplicate.
Osmolality Measurements
The osmolality of the samples was measured using the freezing point osmometer (osmomat 030, Gonotec, Germany). A 50-µl sample was conveyed to the sample chamber. All measurements were done in triplicate.
Rheological Studies
The rheological studies of the formulations were carried out using rheometer (Brookfield model R/S CPS, USA). The pH of the solutions was raised from 4.4 to 7.4 by neutralizing with 0.5 M NaOH, and simultaneously, the temperature of the solution was increased from 21°C to 37°C. The viscosity of the samples was recorded before and after gelling. Each experiment was performed in triplicate.
In Vitro Release Studies
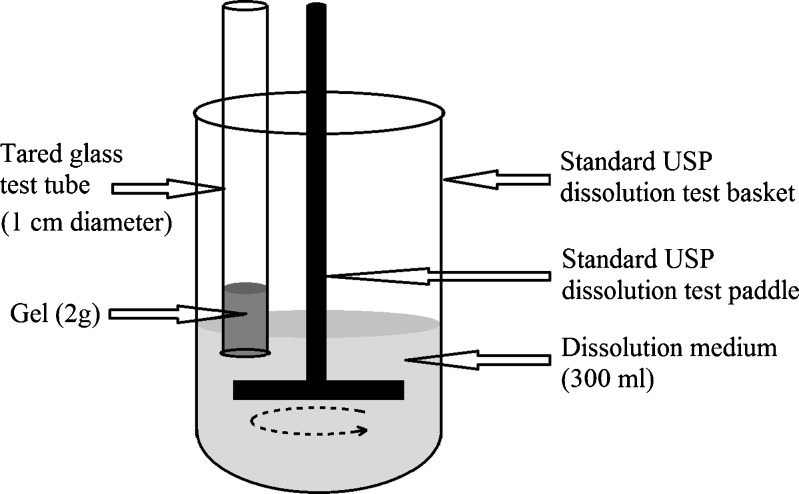
The in vitro release of forskolin from the formulation was studied through membraneless model (24,25) using a modified USP XXIII dissolution testing apparatus (TDT-08L, Electrolab, India; Fig. 2). Freshly prepared simulated tear fluid (STF; pH 7.4, ionic strength of 0.188) with 2% (w/v) methylated β-cyclodextrin was used as the dissolution medium. Methylated β-cyclodextrin was used to solubilize forskolin and provide its sink condition. STF was made using NaCl 0.67 g, NaHCO3 0.20 g, CaCl2 2H2O 0.008 g, and distilled deionized water to 100 g (26). The cold formulations (2 g) were neutralized and then transferred into tared glass test tube (open at both ends and of 1 cm diameter) and placed in oven at 34 ± 0.1°C. Care was taken that the gel contained no air bubbles and the surface was smooth. These formulations gelled upon equilibration at the experimental temperature. The difference in weight of the vial with and without the formulation gave the initial weight of the formulation. The vial was attached to the metallic driveshaft and suspended in 300 ml of dissolution medium maintained at 34 ± 0.1°C so that the tared surface just touched the receptor medium surface. The paddle rotating speed was set at 25 rpm. At selected time intervals, 5 ml of solution was withdrawn from the cell and replaced with fresh dissolution medium. Forskolin was quantified by HPLC using a octadecylsilate column and a mobile phase composed of acetonitrile/water (45:55, v/v). The flow rate and UV wavelength were 1.8 ml min−1 and 220 nm, respectively. To prove the analytical validation of release studies, linearity, and range, accuracy and precision tests were carried out. For linearity and range, correlation coefficient (r) was found to be greater than 0.99 for concentration ranges 10–50 µg/ml. Each experiment was performed in triplicate.
Fig. 2.
Modified USP XXIII in vitro dissolution testing apparatus
Sterility Studies
Sterility test was carried out according to USP method with the help of FTM and SCDM (27).
In Vivo Studies
Male New Zealand albino rabbits, ranging in weight from 2.0 to 2.5 kg, were obtained from the animal house of J.S.S. College of Pharmacy, Ooty, Tamil Nadu, India and were treated as prescribed in the publication Guide for the Care and the Use of Laboratory Animals (NIH Publication No. 92–93, revised 1985). The in vivo experimental protocol was approved by the Ethical-Scientific Committee of the J.S.S. College of Pharmacy, and the experiments were carried out under the supervision of a veterinary doctor. The animals were housed singly in standard cages, in a light-controlled room (10:14 h dark/light cycle) at 19 ± 1°C and 50 ± 5% RH, with no restriction of food or water. During experiments, the rabbits were placed in restraining boxes in a way that they could move their heads and eye freely.
Eye Irritation Test
Acute eye irritation/corrosion and ocular irritation potential of each in situ gel or its components (Noveon® AA-1 polycarbophil and poloxamer 407) were tested on three New Zealand albino rabbits. The irritation test was performed according to the Organization for Economic Cooperation and Development test guideline 405 (28). In situ gels (25 μl) were prepared from 0.3% (w/w) Noveon® AA-1 polycarbophil/16% (w/w) poloxamer 407 without drug and placed in the conjunctival sac of the eye of each animal after gently pulling the lower lid away from the eyeball. The lids were then gently held together for about 1 s to limit loss of the material. The other eye, which was untreated, served as a control. The eyes were examined at 1, 24, 48, and 72 h. If there was no evidence of irritation at 72 h, the study was terminated. Extended observations (e.g., at 7 and 21 days) may be necessary if there is persistent corneal involvement or other ocular irritation to determine the progress of the lesions and their reversibility or irreversibility. Experiments were done in triplicate.
Corneal Residence Time Evaluation
Noninvasive method based on tears sampling (29) followed by HPLC analysis was used for the study. Tear samples equivalent to 1 µl were collected from the right eye after application of test delivery system at 0, 0.017, 0.083, 0.5, 1, 2, 3, 4, 5, and 6 h postdosing. Glass capillary tubes having 320 µm internal diameter and 1 µl Premark were placed near the cantus of the eye without applying pressure. Tear fluid was drained into the tubes due to capillary action. Samples equivalent to 1 µl were mixed with 50 µl of methanol and analyzed by HPLC at 220 nm using a octadecylsilate column. Mobile phase was a mixture of acetonitrile and water (45:55), and the flow rate was 1.8 ml/min. Area under the tear fluid concentration–time curve was calculated using the trapezoidal rule 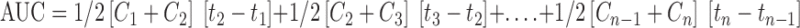 , where C is the concentration of forskolin and t is the time (27).
, where C is the concentration of forskolin and t is the time (27).
Pharmacodynamic Studies
Adult male glaucomatous New Zealand albino rabbits weighing 2.0–2.5 kg were used. To induce ocular hypertension, young New Zealand albino rabbits were treated with bilateral topical instillation of 25 µl of 0.1% (w/v) dexamethasone four times daily for 2 weeks (30,31). The IOP of each eye was monitored weekly. Based on the change of IOP, animals were divided into dexamethasone responders (IOP increase in both eyes at the second week ≥5 mmHg) and nonresponders (IOP increase in either eye <5 mmHg at week 2). Dexamethasone responders group of animals were then treated with topical forskolin in situ gel (0.25 mg; 25 µl of 1% (w/v) suspension) and suspension (0.5 mg; 50 µl of 1% (w/v) suspension) vehicle at the indicated days and IOP measured. IOP was measured using a Schiötz tonometer (32–34), by the same operator, using the same tonometer after instilling a drop of xylocaine (a local anesthetic, 4%) into each eye of rabbits. All the measurements were made three times at each interval, and a mean of these was taken. All measurement periods began during the same hour on each day. For measuring the IOP, rabbits were placed in restraining boxes and eyelids were retracted gently with one hand, without exerting pressure on the eyeball, and tonometer was placed in the horizontal position on the center of the cornea. The position of the pointer was noted, and the tension in millimeters of mercury was determined from the calibration scale. The observations were tabulated and the changes in IOP were calculated. The IOP was measured in both the eyes immediately prior to giving the drug (IOPzero time) and at regular intervals (IOPtime, t) following the treatment. The only restraint was the hands of the investigator lightly laid on the back and shoulders of the rabbits. Rabbits that showed a consistent difference in IOP between the eyes during the baseline measurements or any signs of eye irritation were excluded from the study. Formulations were instilled topically into the upper quadrant of the eye, and the eye was manually blinked three times; one eye received one drop of the formulation and the contralateral eye served as the control. The placebo formulations were used as a control. IOP was measured immediately, prior to giving the drug and at 0, 0.5, 1, 2, 3, 4, 6, 8, 9, 10, 11, and 12 h following the treatment. Each formulation was tested on a group of at least six glaucomatous male rabbits. Each animal was given a washout period of 6 days after every treatment. The change in IOP (∆IOP) was determined according to the following equation:
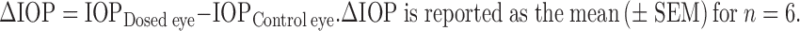 |
During all of the in vivo studies, potential ocular and systemic untoward effects were carefully assessed by trained technicians who were familiar with the general and normal morphology and behavior of the rabbit and were skilled in discerning abnormalities. However, these general observations were performed without the aid of sophisticated equipments.
Stability Studies
Stability studies were carried out on optimized sterile formulation at 25°C ± 2°C/60% RH ± 5% RH over a period of 6 months in previously sterilized plastic (high-density polyethylene) eye drops bottles.
RESULTS AND DISCUSSION
Structure and Particle Size of Forskolin Nanocrystals
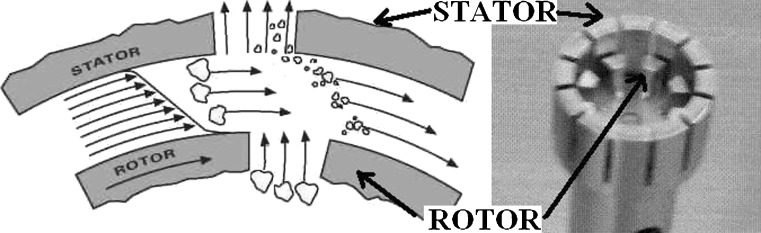
The key to the drug nanoparticle processing is how to compensate the extra free energy of freshly exposed surfaces. In wet comminution process, the degree of compensation depends on the interactions between drugs and stabilizers (35). The stabilizer used in the preparation of nanocrystals should adsorb onto the surfaces of drug nanocrystals and provide steric stabilization effect. The disintegration principle behind wet crushing by the high-performance disperser (T 25 digital ULTRA-TURRAX®) is due to the high rotation speed of the rotor. The medium processed was automatically drawn axially into the dispersion head and then forced radially through the slots in the rotor/stator arrangement (Fig. 3). The high accelerations acting on the material produce extremely strong shear and thrust forces. In addition, high turbulence occurs in the shear gap between rotor and stator, which provides optimum mixing of the suspension. The dispersion effectiveness was heavily dependent on the product of the shear gradient and the time the particles spend in the shear zone. The optimum range for the circumferential velocity of the rotor/stator arrangement was 15–24 m/s. A processing time of a few minutes was usually sufficient to produce the desired fineness. Long processing times bring only insignificant improvements in the obtainable fineness; the energy expended serves merely to increase the temperature of the medium. During process, the sample was kept on ice bath to avoid increase in temperature.
Fig. 3.
Principle behind wet crushing by the high-performance disperser
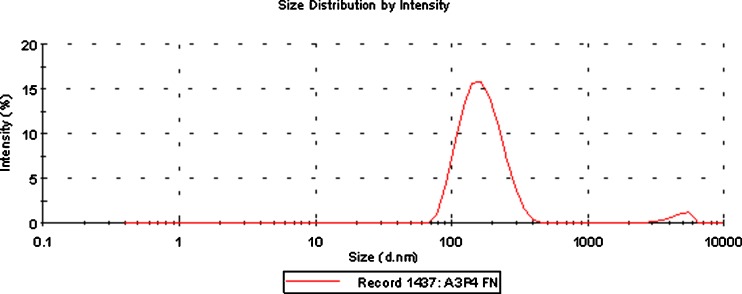
The crystal-size distribution of forskolin was characterized using DLS. Table I summarizes the particle sizes of the forskolin crystals after a wet-crushing process using the high-performance disperser (T 25 digital ULTRA-TURRAX®) with poloxamer 407 which was selected as a stabilizer after a screening study. The results showed that nanosized particles could be achieved for the forskolin using poloxamer 407. We investigated extensively the wet-crushing process variables and conditions such as crushing time, crushing speed (revolutions per minute), and forskolin/poloxamer ratio. Investigations led to the formation of nanosized particles of the forskolin using 3% poloxamer 407. A range of nanocrystals from 45 to 400 nm was successfully achieved, and the suspension of forskolin nanocrystals was stable at room temperature for at least 6 months without agglomeration or crystal growth. Size distribution (Fig. 4) of the forskolin nanocrystals after 6 months was determined with Malvern Zetasizer 3000HS (Malvern, Worcestershire, UK). The average crystal size after 6 months was found to be 164 nm with two peaks [peak 1 = 171 nm (96% intensity and 57.8 nm width) and peak 2 = 4,780 nm (3% intensity, 733 nm width)].
Table I.
Effect of Time and Poloxamer 407 Concentration on Particle Size Reduction
| Poloxamer 407 concentration (%) | Time (min; 10 cycles) | Size range at 20,000 rpm (nm) | |
|---|---|---|---|
| After 24 h | After 1 week | ||
| 2 | 5 | 527–1,110 | >1 µm |
| 10 | 135–770 | >1 µm | |
| 15 | 102–752 | >1 µm | |
| 3 | 5 | 458–1,010 | 457–1,011 |
| 10 | 45–403 | 46–406 | |
| 15 | 42–377 | 44–378 | |
| 4 | 5 | 386–1,020 | 388–1,018 |
| 10 | 49–531 | 51–534 | |
| 15 | 48–376 | 50–379 | |
Fig. 4.
Size distribution of forskolin nanocrystals in in situ gel after 6 months
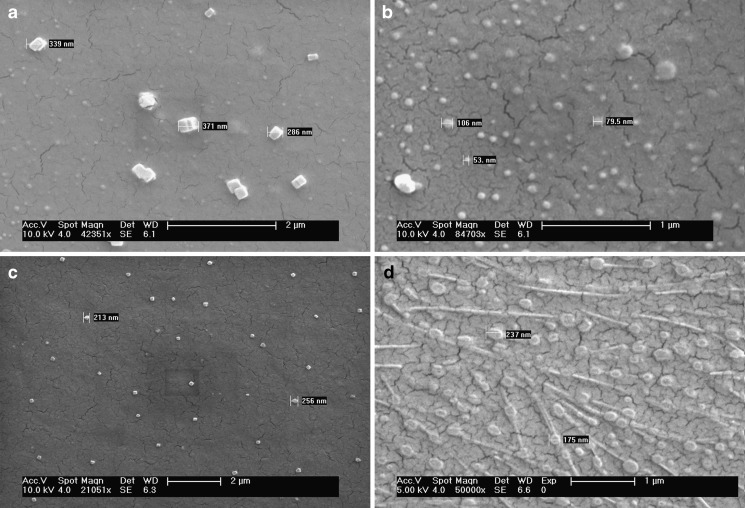
With SEM studies, the forskolin nanoparticles were seen to be distinct, crystalline particles with solid dense structure (Fig. 5). Figure 5d is looking little different from other pictures because of high concentration polymeric gel dried layer. Nanocrystals appeared to be considerably comparable when viewed with SEM to the average particle size obtained with DLS. Depending on the experimental parameters used to prepare the nanoparticles, sizes between 45 and 400 nm were obtained.
Fig. 5.
SEM of forskolin nanocrystals prepared by wet crushing by the high-performance disperser. a, b after 24 h, c after 1 week, d after 6 months
Identification of Nanoparticle Constituents
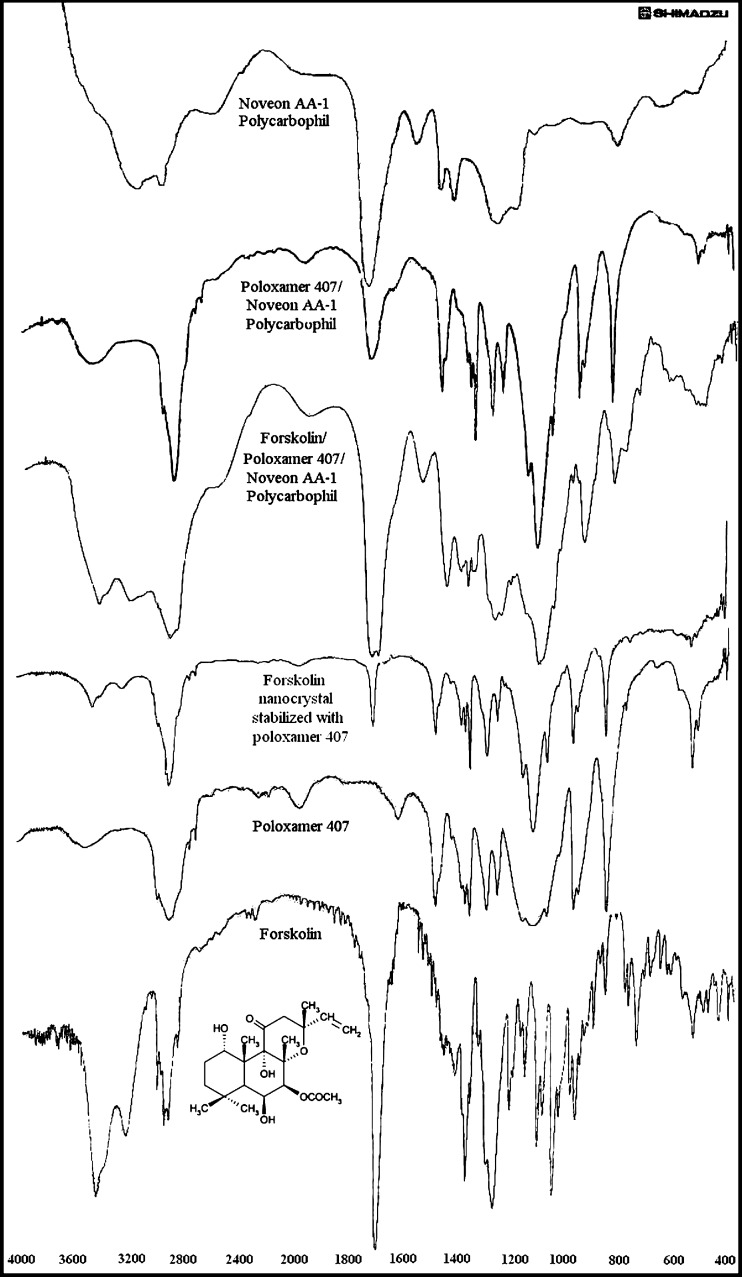
Forskolin, poloxamer 407, and Noveon® AA-1 polycarbophil were analyzed using FTIR spectrophotometer for characteristic absorption bands indicative of their interaction (Fig. 6). The characteristic peaks (per centimeter, KBr) in forskolin were found at 1,273 (C–O), 1,458 (C=C), 1,697 (C=O), 3,011 (Ar C–H), and 3,446 (O–H). The FTIR for poloxamer 407 showed characteristic peaks (per centimeter, KBr) at 1,242 (C–O), 2,887 (C–H), and 3,462 (O–H). Moreover, forskolin nanocrystal stabilized with poloxamer 407 showed characteristic peaks (per centimeter, KBr) at 1,242 (C–O), 1,280 (C–O), 1,467 (C=C), 1,697 (C=O), 2,889 (C–H), 3,010 (Ar C–H), and 3,445 (O–H). FTIR studies were also done for forskolin combination with poloxamer and Noveon® AA-1 polycarbophil which shows characteristic peaks (per centimeter, KBr) at 1,274 (C–O), 1,452 (C=C), 1,701 (C=O), 3,010 (Ar C–H), and 3,443 (O–H). All characteristic peaks observed in individual forskolin and poloxamer 407 are present in forskolin nanocrystal which shows that all groups were present in the prepared nanocrystals. All the characteristic peaks of forskolin are also present in forskolin combination with poloxamer and Noveon® AA-1 polycarbophil. Hence, there was no change in structure of forskolin molecule, and forskolin was compatible with both poloxamer 407 and Noveon® AA-1 polycarbophil.
Fig. 6.
FTIR spectra
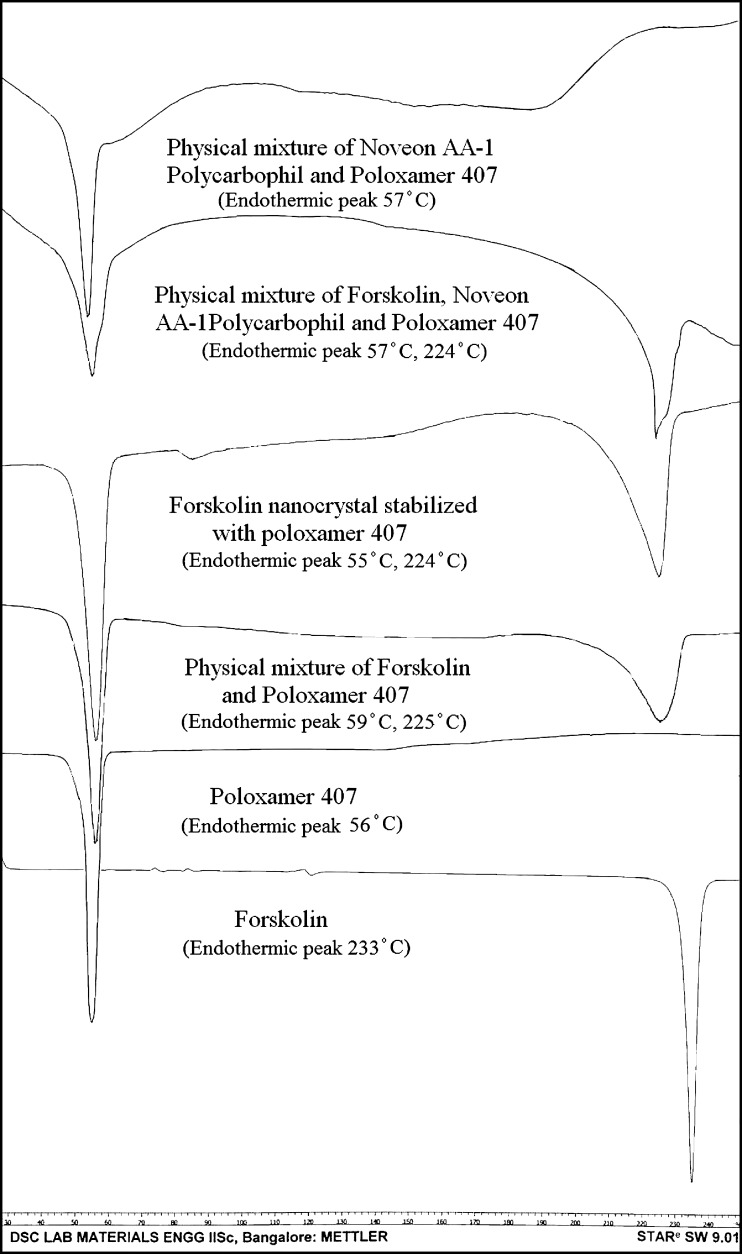
DSC thermograms of the forskolin, poloxamer 407, forskolin nanocrystal stabilized with poloxamer 407, physical mixture of forskolin and poloxamer 407, physical mixture of poloxamer 407 and Noveon® AA-1 polycarbophil, and physical mixture of forskolin, Noveon® AA-1 polycarbophil, and poloxamer 407 showed characteristic endothermic peaks as shown in Fig. 7. Forskolin nanocrystals were lyophilized using a freeze dryer to obtain dried nanocrystals. The characteristic peak for forskolin was found to be reduced in intensity and shifted to 224°C from 233°C probably because of encapsulation in poloxamer 407.
Fig. 7.
DSC thermograms
Optimum Concentration of Vehicles
For the in situ gelling system that gels with an increase in pH and temperature, the sol–gel transition pH and temperature have to be significantly lower than the pH (7.4) and temperature (35°C) in the eye. Aqueous solutions containing Noveon® AA-1 polycarbophil and poloxamer 407 were prepared to evaluate the compositions suitable for ISGS. An ideal ISGS delivery system should be a free flowing liquid with low viscosity which can be conveniently dropped as a solution into the conjunctival sac, where they undergo a transition into a gel at physiological condition and thus increase the precorneal residence time of the delivery system and thereby enhance ocular bioavailability of the drug. The use of Noveon® AA-1 polycarbophil in in situ gelling system is substantiated by transformation into stiff gels when the pH is raised. However, the concentration (0.3–0.5%, w/w) of Noveon® AA-1 polycarbophil required to form stiff gels results in highly acidic solution which are not easily neutralized by the buffering action of the tear fluid. A reduction in Noveon® AA-1 polycarbophil concentration without compromising the gelling capacity and rheological properties of the delivery system may be achieved by the addition of thermoreversible gelling polymer which is basic in nature. From the preparation procedures of poloxamer 407 solutions, it was found that the phase transition temperature increases with decreasing concentration (18–22%, w/w), and all preparations had a transition temperature below 35°C. At high concentrations, the preparations were gel already at room temperature. However, poloxamer 407 in combination with Noveon® AA-1 polycarbophil shows phase transition at lower concentrations (15–17%, w/w). Table II shows the gelling capacity, viscosity, and osmolality of formulations A2P2–A4P5. A concentration of 0.3% (w/w) Noveon® AA-1 polycarbophil and 16% (w/w) poloxamer 407 was selected as it had satisfactory attributes of viscosity and gelling capacity. The osmolality of the formulations were adjusted in range from 280 to 360 mOsm/kg by the addition of mannitol, if required. The forskolin nanocrystals (equivalent to 1.10 g) were centrifuged and redispersed by shaking and sonicating in a sufficient quantity (100 ml) of formulation. BKC (0.01%, w/v) was incorporated as a preservative which also acts as corneal permeation enhancer for forskolin. The resultant formulation was coded A3P4FN.
Table II.
Combination of Noveon® AA-1 Polycarbophil and Poloxamer 407 Studies
| Batch code | Concentration (%, w/w) | Gelling capacity | Viscosity (cP) At pH 7.4 and 34°C (20 rpm) | Osmolality (mOsm/kg) | ||
|---|---|---|---|---|---|---|
| Noveon® AA-1 polycarbophil | Poloxamer 407 | Mannitol | ||||
| A2P1 | 0.2 | 13 | 2.5 | − | 342.5 ± 16 | 297 ± 2 |
| A2P2 | 0.2 | 14 | 2.0 | − | 662.3 ± 32 | 295 ± 1 |
| A2P3 | 0.2 | 15 | 1.5 | − | 784.0 ± 38 | 292 ± 1 |
| A2P4 | 0.2 | 16 | 0.5 | ++ | 2,307.7 ± 96 | 287 ± 2 |
| A2P5 | 0.2 | 17 | – | +++ | 8,844.5 ± 116 | 310 ± 2 |
| A3P2 | 0.3 | 14 | 2.0 | ++ | 2,502.5 ± 104 | 296 ± 1 |
| A3P3 | 0.3 | 15 | 1.5 | +++ | 3,095.5 ± 101 | 293 ± 1 |
| A3P4 | 0.3 | 16 | 0.5 | +++ | 6,863.5 ± 102 | 288 ± 1 |
| A3P5 | 0.3 | 17 | – | +++ | 12,662 ± 118 | 311 ± 2 |
| A4P2 | 0.4 | 14 | 2.0 | +++ | 2,974.5 ± 104 | 297 ± 1 |
| A4P3 | 0.4 | 15 | 1.5 | +++ | 3,682 ± 98 | 294 ± 1 |
| A4P4 | 0.4 | 16 | 0.5 | +++ | 9,436.9 ± 121 | 289 ± 2 |
| A4P5 | 0.4 | 17 | – | +++ | 15,657 ± 126 | 312 ± 2 |
| PL18 | – | 18 | – | +++ | 6,624 ± 108 | 355 ± 1 |
| AA03 | 0.3 | – | 5.0 | ++ | 2,426 ± 114 | 275 ± 1 |
SEM ± SD, n = 3
− no gelation, ++ gelation immediate, remains for few hours, +++ gelation immediate, remains for extended period
Rheological Studies
The rheological behaviors of various polymer solutions were investigated as functions of temperature and pH. All measurements were performed in triplicate with good reproducibility. For all the polymer systems studied, the shear stress and viscosity at pH 7.4 and 34°C were higher than those at pH 4.4 and 25°C, some of which transform into gels with high viscosity. The formulations were shear thinning and an increase in shear stress was observed with increase in shear rate. At low shear rates, the high viscosity of gel will aid in maintaining contact between the corneal surface and the delivery system. The shear thinning nature of the gel in response to blinking will allow distribution over the surface of the eye.
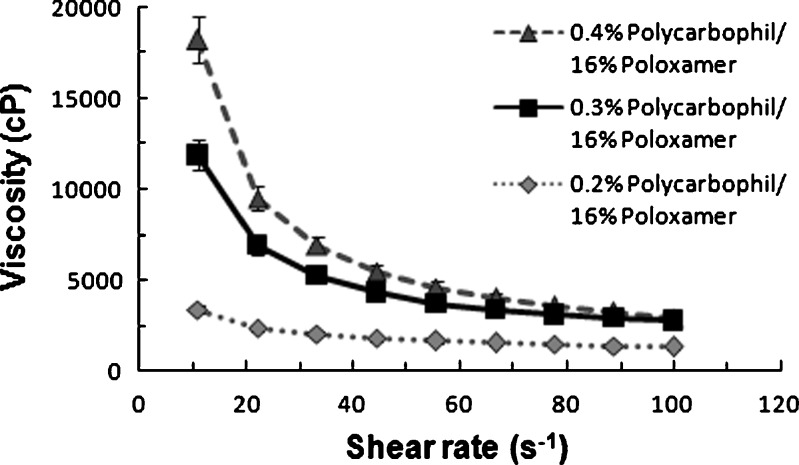
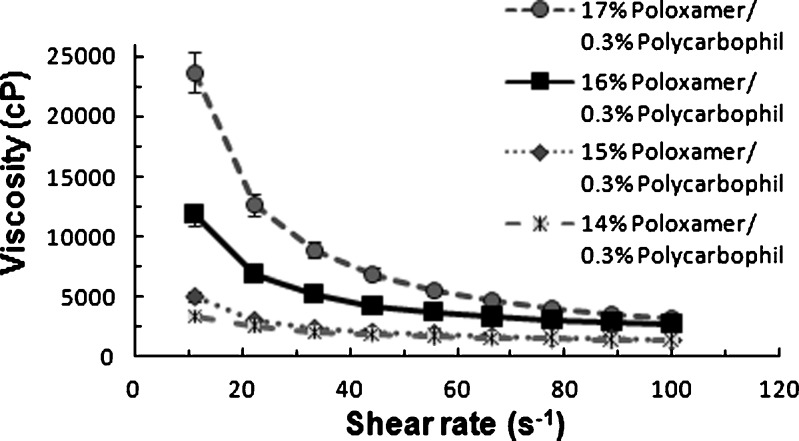
Figure 8 illustrates the representative profiles of viscosity versus shear rate of in situ gel and gels containing different Noveon® AA-1 polycarbophil concentrations (0.2–0.4%, w/w). The poloxamer 407 concentration in the formulations was 16% (w/w). The study helped identify the minimum Noveon® AA-1 polycarbophil concentration in combination with poloxamer 407 required for stiff gel formation at 7.4 pH/34°C. The lower the concentration of Noveon® AA-1 polycarbophil in the gel forming solutions, the lower is its buffering capacity and the faster is the gel is formed in eye. Although phase transition does occur when the Noveon® AA-1 polycarbophil concentration is 0.2% (w/w), the gel formed has a much lower viscosity than those containing the higher concentration of 0.3% or 0.4% (w/w) which indicate that the gels formed at higher concentration are stronger.
Fig. 8.
Viscosity versus shear rate flow curves of different Noveon AA-1 polycarbophil concentrations in combination with poloxamer 407 at 7.4 pH and 34°C
Studies on viscosity versus shear rate of ISGS and gels containing different poloxamer 407 concentrations (14–17%, w/w) in combination with 0.3% (w/w) Noveon® AA-1 polycarbophil are illustrated in Fig. 9. The concentration dependency of viscosity was gradual and as concentration was increased, the systems underwent a sol–gel transition and their viscosity was increased proportionally. The concentration of poloxamer 407 with 0.3% (w/w) Noveon® AA-1 polycarbophil to form stiff gels above 17% (w/w) results in a viscous preparation at room temperature which are not able to redisperse the suspended forskolin nanocrystals easily. All preparations behave as gels at physiological condition, but the lowest concentration had a small frequency dependence on the viscosity modulus, probably due to the fact that the sol–gel transition is not completed at 35°C.
Fig. 9.
Viscosity versus shear rate flow curves of different poloxamer 407 concentrations in combination with Noveon AA-1 polycarbophil at 7.4 pH and 34°C
Microbial contamination of ophthalmic preparation must be avoided, and of the various sterilization techniques available, autoclaving is the method of choice. It was therefore necessary to examine the effect of autoclaving on the mechanical properties of the polymer solution. The viscosity profiles of the in situ gel before and after autoclaving were studied, and results showed that autoclaving did not affect the viscometric properties of the in situ gel and the pH remains unchanged.
The viscosities of ISGS decreased between 10–15-fold by addition of isotonic agent 0.9% NaCl. In the eye, the gel will be exposed to tear fluid containing cations which may decrease the viscosity of Noveon® AA-1 polycarbophil, but at the same time, poloxamer 407 will increase viscosity (36) and balance the formulation viscosity. Therefore, to avoid sodium ions in gel, mannitol is selected as an osmotic agent in the formulation. There was no effect of mannitol on ISGS viscosity. Forskolin is an uncharged drug and studies show that its nanocrystals did not affect the viscometric properties of in situ gel.
Evaluation of Formulation
The phase transition temperature at formulation pH, viscosity at 20 rpm (20°C/formulation pH and 34°C/7.4 pH), drug content, and pH of the formulations were found to be satisfactory (Table III). The outer surface of the eye can well tolerate preparations with pH values between 4 to 9; however, products outside the pH range may cause irritation (37,38). Hence, long-term use of developed formulation of this low pH may not be problematic. Formulations containing 1% (w/v) forskolin nanocrystals were abbreviated as FN with batch code. The formulations were liquid at room temperature 20°C and underwent rapid transition into the gel phase at the eye temperature/pH (34°C/7.4).
Table III.
Evaluation of Formulations
| Formulation | Phase transition (sol–gel) temperature (°C) at formulation pH | Viscosity (cP) at 20 rpm | Drug content (mg/ml) | Formulation pH | |
|---|---|---|---|---|---|
| At 20°C/formulation pH | At 34°C/pH 7.4 | ||||
| PL18FN | 32 ± 0.5 | 25 ± 3 | 6,618 ± 106 | 10.08 ± 0.12 | 7.44 ± 0.1 |
| AA03FN | – | 18 ± 3 | 2,423 ± 112 | 9.98 ± 0.10 | 4.44 ± 0.1 |
| A3P4FN | 31 ± 0.5 | 26 ± 2 | 6,863 ± 106 | 10.06 ± 0.16 | 4.42 ± 0.2 |
| A2P4FN | 30 ± 0.5 | 24 ± 2 | 2,307 ± 119 | 10.02 ± 0.15 | 4.51 ± 0.1 |
| A4P4FN | 31 ± 0.5 | 26 ± 3 | 9,436 ± 124 | 9.98 ± 0.12 | 4.37 ± 0.2 |
| A3P3FN | 32 ± 0.5 | 20 ± 2 | 3,095 ± 104 | 9.92 ± 0.15 | 4.43 ± 0.2 |
| A3P5FN | 28 ± 0.5 | 51 ± 4 | 12,662 ± 125 | 9.90 ± 0.11 | 4.52 ± 0.2 |
| 1% forskolin suspension | – | – | – | 9.88 ± 0.18 | 7.45 ± 0.1 |
Each value given in the table was calculated from n = 3 parallels and was given by the mean ± SD
In Vitro Release Studies
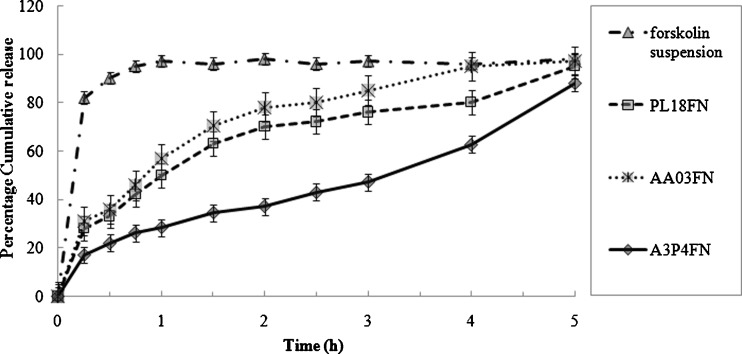
The cumulative percent of forskolin released versus time profiles for Noveon® AA-1 polycarbophil/poloxamer 407 in situ gel and suspension containing 1% (w/w) forskolin nanocrystals are shown in Fig. 10. All whole forskolin was released from the reference suspension almost instantaneously after the start of release experiment, indicating that sink conditions were appropriately maintained in the apparatus. In case of 0.3% (w/w) Noveon® AA-1 polycarbophil in situ gel containing forskolin nanocrystals (AA03FN), the drug released was about 50% to the medium after 1 h and then the drug released gradually afterward. Approximately 95% of forskolin was released from the Noveon® AA-1 polycarbophil in situ gel after 5 h. The forskolin containing 18% (w/w) poloxamer 407 in situ gel containing forskolin nanocrystals (PL18FN) had almost similar release trend as the Noveon® AA-1 polycarbophil in situ gel. Nearly 56% of forskolin was released in 1 h from poloxamer 407 in situ gel followed by 96% released after 5 h. For the 0.3% (w/w) Noveon® AA-1 polycarbophil/16% (w/w) poloxamer 407 in situ gel containing forskolin nanocrystals (A3P4FN), significant lower drug release rates were observed. There was only 28% forskolin released in the first hours, approximately 88% released after 5 h, and the release profile was still climbing thereafter. These results indicate that the formulation A3P4FN has a better ability to retain drug than the individual polymer in situ gel and can be used as an ophthalmic sustained release drug delivery system. The drug release from gel-forming solution may occur partly via diffusion from the gels and partly due to simultaneous dissolution of the gels into the surrounding media. The in vitro drug release conditions may be very different from those likely to be encountered in the eye. However, the in vitro results clearly showed that the gels have the ability to retain the drug for prolonged period and that premature drug release will not occur. In the cul-de-sac, the gel may probably undergo faster dissolution due to the shearing action of the eyelids and eyeball movements.
Fig. 10.
In vitro release of forskolin nanocrystals from temperature and pH-triggered gel-forming solution systems (average of three experiments)
Sterility Testing
Sterility is one of the most vital requirements for an ophthalmic preparation to determine the absence of viable microorganisms that may harm the eye of the patient. FTM and SCDM were used for determining the presence/absence of aerobic/anaerobic bacteria and fungus. Microbial growth was observed in FTM and SCDM media which were inoculated with positive control on the second day. No growth was observed in sterilized ophthalmic in situ gels. Based on these observations, it was concluded that moist heat sterilization of polymeric solutions and drug sterilization by filtration followed by aseptic crystallization could be done to achieve the sterility.
In Vivo Studies
Eye Irritation Test
The possibility of eye irritation due to the in situ gel administration was evaluated in rabbits. Three veterinarians independently graded the rabbits for ocular lesions, and no symptoms of ocular irritation such as redness, tearing, inflammation, or swelling were observed after in situ gel administration. Furthermore, fluorescein staining did not indicate corneal or conjunctival epithelial defects. Thus, the developed ocular drug delivery systems are apparently free from any ocular irritation potential and can be safely administered to humans.
Corneal Residence Evaluation
Poor ocular bioavailability of conventional ophthalmic dosage forms is attributed to certain precorneal constraints, namely solution drainage, lacrimation, tear dilution, tear turnover (about 16%), and conjunctival absorption. Binding of drug to protein also contributes to the loss of drug through the precorneal parallel elimination loss pathway. The tears contain both bound and free drug to overcome the limitations of conventional therapy and achieve optimum efficacy. Precorneal residence time of ocular drugs is being assessed by certain invasive techniques (39) and noninvasive techniques (40). These approaches, however, require isolation of ocular tissues or the use of radioisotopes. The precorneal residence of forskolin after application of equivalent doses containing ophthalmic in situ gels in rabbit eyes is given in Table IV. There was a significant improvement in precorneal residence of forskolin after application of the formulated delivery systems. Measurable tear fluid levels were noted from 0.5 h onward.
Table IV.
Tear Fluid Concentration of Forskolin After Administration of Formulation
| Time (h) | Forskolin concentration in tear fluid (µg/µl) | |||
|---|---|---|---|---|
| Forskolin suspension | PL18FN | AA03FN | A3P4FN | |
| 0 | 0 | 0 | 0 | 0 |
| 0.017 | 7.754 ± 0.18 | 3.112 ± 0.26 | 2.935 ± 0.22 | 2.112 ± 0.24 |
| 0.083 | 0.522 ± 0.05 | 3.035 ± 0.12 | 2.435 ± 0.25 | 2.012 ± 0.18 |
| 0.5 | – | 2.567 ± 0.15 | 2.102 ± 0.18 | 1.829 ± 0.16 |
| 1 | – | 2.324 ± 0.14 | 2.042 ± 0.24 | 1.551 ± 0.25 |
| 2 | – | 2.423 ± 0.16 | 1.853 ± 0.12 | 1.531 ± 0.21 |
| 3 | – | 0.172 ± 0.08 | 1.582 ± 0.14 | 1.411 ± 0.15 |
| 4 | – | – | 0.056 ± 0.05 | 1.513 ± 0.14 |
| 5 | – | – | – | 1.603 ± 0.22 |
| 6 | – | – | – | 0.421 ± 0.04 |
| 7 | – | – | – | – |
| AUC0–6 (µg h/µl) | 0.339 | 6.243 | 6.666 | 8.844 |
Each value given in the table was calculated from n = 6 parallels and was given by the mean ± SD
In the case of the prepared ophthalmic in situ gels, the levels of forskolin were maintained for 3 h for the PL18FN, 4 h for the AA03FN, and 6 h for the A3P4FN, but forskolin was missing in case of control 1% (w/v) forskolin suspension at 0.5 h which may be because forskolin was rapidly washed away from the surface of the eye due to the tear.
The areas under tear fluid drug concentration–time curve for the formulated ophthalmic in situ gel were studied to determine the effectiveness of the ophthalmic in situ gels for reducing the intraocular pressure. The AUC for the ophthalmic in situ gels was found to be more in A3P4FN then individual polymers.
The results obtained from precorneal residence time evaluation were well correlated and comparable with in vitro release studies. Hence, formulation behaves in the same manner in selected in vitro dissolution and in vivo dissolution.
Pharmacodynamic Evaluation
Approximately 60% of the young New Zealand albino rabbits treated chronically with a dexamethasone, a potent glucocorticoid developed ocular hypertension associated with a change of glycosaminoglycans content in the trabecular meshwork (41). Among ten rabbits treated with topical doses of dexamethasone (0.1%, w/v) four times daily for 2 weeks, six developed ocular hypertension, as defined by an increase of IOP of 5 mmHg or more in both eyes. In these steroid responders, the IOP at the end of the second week of dexamethasone treatment reached 24.4 ± 1.1 mmHg (mean ± SEM, n = 6), significantly (P < 0.01) higher than that of nonresponders (19.8 ± 1.7 mmHg, n = 4). The IOP of the responders and nonresponders stayed relatively constant from weeks 2 to 4.
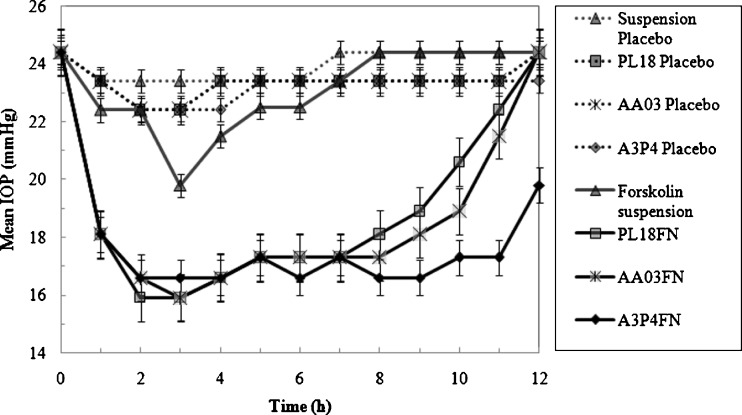
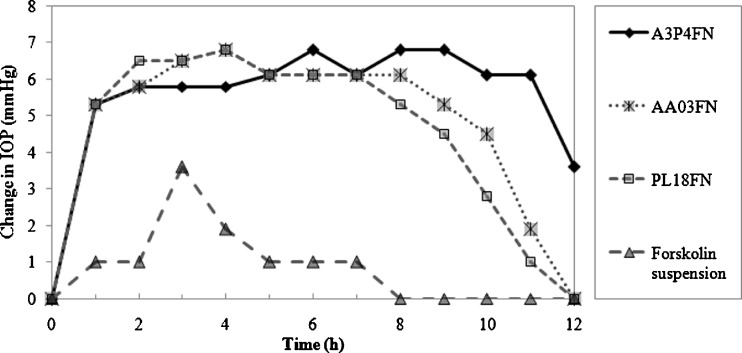
The drug was tested in dexamethasone-induced ocular hypertensive rabbits. In the dexamethasone responder rabbits, topical ocular administration of forskolin in situ gel (0.25 mg; 25 µl of 1% (w/v) suspension) lowered the IOP (Fig. 11). Administration of forskolin suspension (0.5 mg; 50 µl of 1% (w/v) suspension) had a small but significant effect on the IOP. A statistically significant (P < 0.01) reduction was observed in A3P4FN containing forskolin nanocrystals at 1 to 12 h after drug treatment. The IOP was decreased to 16.6 ± 0.7 mmHg (n = 6), equivalent to a 31.96 ± 2.86% reduction, while in PL18FN and AA03FN, the reduction were observed for 1–11 h. Thus, in situ gels were showing a long and a higher response, indicative of a sustained and better absorption of the drug. ∆IOP is reported as the mean (± SEM) for n = 6 (Figs. 11 and 12). An experiment was also performed where only the vehicles were instilled into the treated eye, keeping the other eye as control. No significant change in IOP was found between the control and the treated eye, indicating the absence of any vehicle effect. Moreover, the contralateral eyes in all the experiments showed no significant drop in the IOP. Interestingly, when forskolin in situ gel was administered to the same dexamethasone responder animals 13 days later for three consecutive days, it still generated comparable ocular hypotensive effects, suggesting that acute desensitization did not occur. Again, ocular or systemic side effects of forskolin were not detected.
Fig. 11.
Mean IOP values (millimeters of mercury) after treatment with formulations
Fig. 12.
Change in IOP values (millimeters of mercury) after treatment with formulations
After treatment, corneoscleral angles of three dexamethasone responder rabbits were randomly selected for morphologic evaluation with light microscopy. Treatment with vehicle or forskolin did not produce any noticeable morphologic abnormalities in the trabecular meshwork, aqueous plexus, iris, and cornea.
Stability Studies
It was observed that there was no significant change in terms of physical appearance, drug particle size, drug content, and gelling properties after 6 months of storage at given temperature. It was also observed that the sterility of the preparations was retained throughout the aging period. Similarly, no significant change in drug release was observed. Based on these observations, it was concluded that the developed formulations of forskolin were chemically, physically, and microbiologically stable for 6 months. However, further studies at different temperature and humidity conditions are needed to establish their shelf life.
CONCLUSIONS
New pH and thermoreversible in situ gel formulations were prepared using Noveon® AA-1 polycarbophil/poloxamer 407 with the nanosize forskolin crystals which can be effectively produced with the wet crushing method by the high-performance disperser. The prepared formulations were successfully investigated on dexamethasone-induced glaucomatous rabbits for its intraocular hypotensive effect. The particle size obtained was suitable for ophthalmic administration. To overcome the drug particle growth, the surface of crystal was stabilized by poloxamer 407. High-performance disperser was shown to be a simple and adequate technique for drug particle size reduction and did not seem to alter the crystalline state of the drug, which should be highly relevant when considering drug stability during the storage over a period of 6 months. The formulation was liquid at room temperature 20 ± 10°C which underwent rapid gelation upon raising the eye pH (7.4) and temperature (35°C). The in vitro release results indicated that the developed forskolin in situ gel was able to prolong and control forskolin release for more than 5 h, thus maintaining concentrations for a longer duration. The formulation was therapeutically efficacious capable of reducing IOP for 12 h. In situ gel containing forskolin nanocrystals may produce added value by allowing a reduction in either the dose or its frequency of administration. It is not possible to guess whether this subtle but significant IOP-reducing effect of topically applied forskolin on the IOP of rabbits has any clinical significance for humans or not. Moreover, if the study is extended to humans suffering from glaucoma, we may get promising results. Some workers have reported better results with viscous vehicles in the human eye, in comparison to those obtained with rabbits (42). The developed formulation is a viable alternative to conventional eye suspension by virtue of its ability to enhance bioavailability by the formulation of nanosuspension. Main advantages of nanosuspensions are their increase of saturation solubility and dissolution velocity, improving the bioavailability of drugs. To further prolong the precorneal residence time of the forskolin nanosuspension in the eye, the nanosuspension was formulated with hydrogels made from thermoreversible and pH-triggered mucoadhesive polymers. The developed in situ gel-forming system will have good patient acceptance because it is easy to instill into eye and gradually erodes by dissolution of the gel, obviating the need for removal.
Acknowledgments
Mr. Saurabh Gupta would like to thank the Indian Council of Medical Research (ICMR), New Delhi, India for the Senior Research Fellowship awarded. The authors are also grateful to Sami Labs (India), Lubrizol Advanced Materials (India), Vijay Bakelite Trading Co. (India), and Mdi (India) for the generous gifts of forskolin (95%, 98%), Noveon® AA-1 polycarbophil, sterile 10 ml eye drops bottles, and syringe membrane filters, respectively. The authors also acknowledge the help of Dr. K. Elango (Principal), Mr. A. Shanish Antony (Department of Pharmacology), Mr. R. Rajesh Kumar (Department of Pharmaceutical Biotechnology), and Mr. Krishnaraj (TIFAC CORE) of J.S.S. College of Pharmacy (Ooty). The authors also acknowledge the help of Mr. Dinesh Shinde (Formulation Research and Development, Cipla Ltd., Mumbai) and Dr. G.P. Hemant Kumar, Ophthalmologist, Ooty for their kind help in project work.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein NL, Sears ML. Aqueous flow in human eyes is reduced by forskolin a potent adenylate cyclase activator. Exp Eye Res. 1984;39:745–9. doi: 10.1016/0014-4835(84)90073-3. [DOI] [PubMed] [Google Scholar]
- 4.Smith BR, Gaster RN. Forskolin a potent adenylate cyclase activator lowers rabbit IOP. Arch Ophthalmol. 1984;102:146–8. doi: 10.1001/archopht.1984.01040030124051. [DOI] [PubMed] [Google Scholar]
- 5.Caprioli J, Sears M. Forskolin lowers intraocular pressure in rabbits, monkeys, and man. Lancet. 1983;30:958–60. doi: 10.1016/S0140-6736(83)92084-6. [DOI] [PubMed] [Google Scholar]
- 6.Seto C, Eguchi S, Araie M, Matsumoto S, Takase M. Acute effect of topical forskolin on aqueous humor dynamics in man. Jpn J Ophthalmol. 1986;30:238–44. [PubMed] [Google Scholar]
- 7.Caprioli J, Sears M. Combined effect of forskolin and acetazolamide on IOP and aqueous flow in rabbit eye. Exp Eye Res. 1984;39:47–50. doi: 10.1016/0014-4835(84)90113-1. [DOI] [PubMed] [Google Scholar]
- 8.Shah NH, Phuapradit W, Zhang YE, Sandhu H, Zhang L, Malick AW. In: Approaches for improving bioavailability of poorly soluble drugs. 3. Augsburger LA, Hoag SW, editors. London: Informa Healthcare; 2008. p. 62. [Google Scholar]
- 9.Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20. doi: 10.1016/S0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 10.Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60:1281–302. doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- 11.Pozharitskaya ON, Vainshtein VA, Strelkova LF, Kalinina NA. Study of mechanism of dissolution of nifedipine from solid dispersion system with Q10 polyethylene glycol. Farmatsiia-Moskva. 1999;2:18–20. [Google Scholar]
- 12.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 13.Gaudana R, Jwala J, Boddu SHS, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197–216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee VHL. Review: new directions in the optimization of ocular drug delivery. J Ocul Pharmacol. 1990;6:157–64. doi: 10.1089/jop.1990.6.157. [DOI] [PubMed] [Google Scholar]
- 15.Gurny R. Preliminary study of prolonged acting drug delivery system for the treatment of glaucoma. Pharm Acta Helv. 1981;56:130–2. [PubMed] [Google Scholar]
- 16.Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH-triggered in situ gelling system. J Control Release. 2001;73:205–11. doi: 10.1016/S0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 17.Miller SC, Donovan MD. Effect of poloxamer 407 gel in the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm. 1982;12:147–52. doi: 10.1016/0378-5173(82)90114-4. [DOI] [Google Scholar]
- 18.Vadnere M, Amidon G, Lendenbaum S, Haslam JL. Thermodynamic studies on the gel–sol transition of some pluronic polyols. Int J Pharm. 1984;22:207–18. doi: 10.1016/0378-5173(84)90022-X. [DOI] [Google Scholar]
- 19.Spancake CW, Mitra AK, Klidsig DO. Kinetics of aspirin hydrolysis in aqueous solutions and gels of poloxamines (tetronic 1508) influence of micro-environment. Int J Pharm. 1989;57:163–8. doi: 10.1016/0378-5173(89)90305-0. [DOI] [Google Scholar]
- 20.Rozier A, Manuel C, Groove J, Plazonet B. Gelrite: a novel ion activated in situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int J Pharm. 1989;57:163–8. doi: 10.1016/0378-5173(89)90305-0. [DOI] [Google Scholar]
- 21.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12–7. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, Lobel E, Trevgoda A, Peled Y. A novel in situ forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Release. 1997;44:201–8. doi: 10.1016/S0168-3659(96)01523-4. [DOI] [Google Scholar]
- 23.The Lubrizol Corporation. Wickliffe, Ohio, USA: Pharmaceutical ingredients. Online. 2008. http://www.lubrizol.com/pharmaceutical/products/Noveon AA-1Polycarbophil.html.
- 24.El-Kamel AH. In vitro and in vivo evaluation of pluronic F127 based ocular delivery system for timolol maleate. Int J Pharm. 2002;241:47–55. doi: 10.1016/S0378-5173(02)00234-X. [DOI] [PubMed] [Google Scholar]
- 25.Desai SD, Blanchard J. In vitro evaluation of pluronic F127 based controlled release ocular delivery systems for pilocarpine. J Pharm Sci. 1998;87:226–30. doi: 10.1021/js970090e. [DOI] [PubMed] [Google Scholar]
- 26.Lin HR, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release. 2000;69:379–88. doi: 10.1016/S0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Samanta MK. Design and evaluation of thermoreversible in situ gelling system of forskolin for the treatment of glaucoma. Pharm Dev Tech 2009. doi:10.1080/10837450903262033. [DOI] [PubMed]
- 28.Staff OECD . OECD guidelines for the testing of chemicals. Fourteenth addendum. Paris: OECD; 2002. [Google Scholar]
- 29.Small D, Hevy J, Tang-Liu D. Comparison of tear sampling techniques for pharmacokinetic analysis: ofloxacin concentrations in rabbit tears after sampling with schirmer tear strips, capillary tubes or surgical sponges. J Ocul Pharmcol Ther. 2000;16:439–46. doi: 10.1089/jop.2000.16.439. [DOI] [PubMed] [Google Scholar]
- 30.Pang IH, Moll H, Mclaughlin MA, Knepper PA, Santis LD, Epstein DL, et al. Ocular hypotensive and aqueous outflow-enhancing effects of AL-3037A (sodium ferri ethylenediaminetetraacetate) Exp Eye Res. 2001;73:815–25. doi: 10.1006/exer.2001.1087. [DOI] [PubMed] [Google Scholar]
- 31.Knepper PA, Collins JA, Frederick R. Effects of dexamethasone, progesterone and testosterone on IOP and GAGs in the rabbit eye. Invest Ophthalmol Vis Sci. 1985;26:1093–100. [PubMed] [Google Scholar]
- 32.Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm. 2000;199:119–27. doi: 10.1016/S0378-5173(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 33.Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024. [Google Scholar]
- 34.Grant WM. Tonographic method for measuring the facility and rate of aqueous flow in human eye. Arch Ophthalmol. 1950;44:204–14. doi: 10.1001/archopht.1950.00910020209003. [DOI] [PubMed] [Google Scholar]
- 35.Lee J. Drug nano and microparticles processed into solid dosage forms: physical properties. J Pharm Sci. 2003;92(10):2057–68. doi: 10.1002/jps.10471. [DOI] [PubMed] [Google Scholar]
- 36.Miller SC, Drabik BR. Rheological properties of poloxamer vehicles. Int J Pharm. 1984;18:269–76. doi: 10.1016/0378-5173(84)90142-X. [DOI] [Google Scholar]
- 37.Lamberts DW, Potter DE. Clinical ophthalmic pharmacology. UK: Little Brown; 1987. [Google Scholar]
- 38.Gupta SK, Uppal RK, Agnihotri S, Jain N. Need for monitoring strict quality control measures in ophthalmic preparations. East Pharmacist. 1986; 43–8.
- 39.Lee VHL. Precorneal, corneal and postcorneal factors. In: Mitra AK, editor. Ophthalmic drug delivery systems. New York: Marcel Dekker; 1993. pp. 59–81. [Google Scholar]
- 40.Greaves JL, Wilson CG, Galloway NR. A comparison of the precorneal residence of an artificial tear preparation in patients with keratoconjunctivitis sicca and in normal volunteer subjects using gamma scintigraphy. Acta Ophthalmol. 1991;69:432–6. doi: 10.1111/j.1755-3768.1991.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 41.Knepper PA, Breen M, Weinstein HG, Blacik JL. Intraocular pressure and glycosaminoglycan distribution in the rabbit eye, effect of age and dexamethasone. Exp Eye Res. 1978;27:567–75. doi: 10.1016/0014-4835(78)90141-0. [DOI] [PubMed] [Google Scholar]
- 42.Saettone MF, Chetoni P, Cerbai R, Mazzanti G, Braghiroli L. Evaluation of ocular permeation enhancer: in vitro effects on corneal transport of four b-blockers, and in vitro in vivo toxic activity. Int J Pharm. 1986;142:103–13. doi: 10.1016/0378-5173(96)04663-7. [DOI] [Google Scholar]