Abstract
The discovery of the HD (Huntington’s disease) gene in 1993 led to the creation of genetic mouse models of the disease and opened the doors for mechanistic studies. In particular, the early changes and progression of the disease could be followed and examined systematically. The present review focuses on the contribution of these genetic mouse models to the understanding of functional changes in neurons as the HD phenotype progresses, and concentrates on two brain areas: the striatum, the site of most conspicuous pathology in HD, and the cortex, a site that is becoming increasingly important in understanding the widespread behavioural abnormalities. Mounting evidence points to synaptic abnormalities in communication between the cortex and striatum and cell–cell interactions as major determinants of HD symptoms, even in the absence of severe neuronal degeneration and death.
Keywords: excitatory amino acid, Huntington’s disease, mouse model, neurodegeneration, striatum, synaptic activity
Abbreviations: AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; BAC, bacterial artificial chromosome; BDNF, brain-derived neurotrophic factor; DA, dopamine; enk, enkephalin; EPSC, excitatory postsynaptic current; GABA, γ-aminobutyric acid; HD, Huntington’s disease; htt, huntingtin; IPSC, inhibitory postsynaptic current; IR-DIC, infrared differential interference contrast; MSSN, medium-sized spiny projection neuron; NII, neuronal intranuclear inclusion; NMDA, N-methyl-d-aspartate; WT, wild-type; YAC, yeast artificial chromosome
WHAT IS HUNTINGTON’S DISEASE?
HD (Huntington’s disease) is a progressive, debilitating, and fatal neurological disorder. Its principal symptoms include chorea (uncontrollable dance-like movements), cognitive disturbances, depression and other psychiatric changes (Harper, 1996). HD is inherited in an autosomal dominant fashion. The mutated gene is located on the short arm of chromosome 4 and contains an expansion in the normal number of CAG (glutamine) repeats, generally >40 (The Huntington’s Disease Collaborative Research Group, 1993). It is believed that the severity of symptoms is directly correlated with the number of CAG repeats (Harper and Jones, 2002). HD is typically a late-onset disease, although juvenile variants occur, generally when more CAG repeats are present. In young children with HD, the symptoms also include epileptic seizures (van Dijk et al., 1986; Rasmussen et al., 2000; Gambardella et al., 2001; Seneca et al., 2004). The protein encoded by the HD gene, htt (huntingtin), is normally a cytoplasmic protein closely associated with vesicle membranes and microtubules. Although its normal function is not completely understood, considerable evidence suggests it has a role in vesicle trafficking, exocytosis and endocytosis (DiFiglia et al., 1995; Caviston and Holzbaur, 2009).
Neuropathologically, HD is primarily characterized by loss of MSSNs (medium-sized spiny projection neurons) in the striatum (caudate nucleus and putamen) and pyramidal neurons in the cerebral cortex (Vonsattel et al., 1985). Striatal and cortical interneurons are relatively spared (Vonsattel and DiFiglia, 1998). Cell loss leads to substantial atrophy of the striatum, enlarged lateral ventricles, and thinning of the cortical mantle (Rosas et al., 2005). Within the striatum, MSSNs that project to the external globus pallidus (indirect pathway) are the most vulnerable (Reiner et al., 1988; Albin et al., 1992). These neurons express enk (enkephalin) and DA (dopamine) D2 receptors. In contrast, MSSNs that project to the substantia nigra pars reticulata (direct pathway), express SP (substance P) and DA D1 receptors (Gerfen et al., 1990), and become affected later in the course of the disorder.
Another pathological landmark of HD is the presence of aggregated forms of mutant htt in neurons. These aggregates comprise intranuclear [NII (neuronal intranuclear inclusion)] and cytoplasmic inclusions, as well as microaggregates. The role of inclusions in cell death is controversial as there is evidence for both deleterious and protective effects (Ho et al., 2001; Arrasate et al., 2004; Reiner et al., 2007). Alterations in the hypothalamus and the endocrine system (e.g. loss of orexin-containing cells and increased cortisol levels), also play an important role in the manifestation of HD symptoms (Petersen et al., 2005; Bjorkqvist et al., 2006; Aziz et al., 2009; Petersen et al., 2009). Dysfunction in these systems lead to sleep and metabolic disturbances. Finally, widespread pathology in peripheral tissues including skeletal muscle, heart, bone and testis, are beginning to be recognized. These changes are independent of brain dysfunction and appear to be related to the expression of mutant htt in peripheral tissues (van der Burg et al., 2009).
EARLY ANIMAL MODELS OF HD
In the late 1970s, several lines of investigation converged to generate animal models of HD (Coyle, 1979). First, glutamate was shown to be the main excitatory amino acid in the brain [reviewed in (Watkins and Jane, 2006)]. Secondly, glutamate also was shown to be the transmitter of the corticostriatal projection providing the major excitatory input to the striatum (McGeer et al., 1977; Fonnum et al., 1981). Finally, specific glutamate analogues were shown to produce selective, axon-sparing lesions in the brain (Coyle et al., 1978). As striatal cell loss is the primary neuropathological landmark in HD, one of the first rodent models used the excitotoxin kainic acid to selectively destroy striatal MSSNs (Coyle and Schwarcz, 1976; Schwarcz and Coyle, 1977). Later, quinolinic acid [a selective NMDA (N-methyl-d-aspartate) receptor agonist] was used as it better replicated the neuropathology seen in HD (Schwarcz et al., 1983). Thus the roles of glutamate transmission and postsynaptic NMDA receptors became important and generated the core of the excitotoxicity hypothesis of HD which posits that striatal neurons degenerate in HD because of enhanced glutamate neurotransmission.
As deficits in energy metabolism also occur in HD, other models that targeted mitochondrial function were developed. Inhibition of complex II with 3-nitropropionic acid became a useful model of HD because it produced lesions in the striatum that were similar to the cell loss in HD (Beal et al., 1993; Damiano et al., 2009). The major advantage of both the excitotoxic and the 3-nitropropionic acid models was that the neuropathology was replicated in non-human primates (Brouillet et al., 1995).
Although toxic models were instrumental in the understanding of some of the mechanisms involved in cell death (DiFiglia, 1990), they were limited because it was not possible to study the progression of the disease or to replicate the widespread neuropathology observed in the human condition. This fact is important as, although it was generally believed that the progression of symptoms in HD was due to neurodegeneration of MSSNs, studies in genetic animal models have demonstrated that severe neuronal dysfunction precedes degeneration and is probably the major cause of many symptoms (Levine et al., 2004). The discovery of the HD gene in 1993 led to the creation of genetic models of the disease and opened the doors for mechanistic studies.
GENETIC MOUSE MODELS
Genetic mouse models of HD permit examination of the progression of the disease in detail. The first mouse model of HD was created in the laboratory of Gillian Bates (Mangiarini et al., 1996). Since then, numerous genetic mouse models have been generated. These models include transgenic (with truncated or full-length human mutant genes), knock-in and conditional models. We would like to point out that the models we describe in this review do not represent an exhaustive or complete list. Only those models deemed relevant for our major points are included. We apologize in advance for not citing other important models that also have added to our understanding of HD mechanisms.
Fragment models
The R6 line of transgenic mice (Mangiarini et al., 1996) remains one of the most widely used models, not only because it was the first genetic mouse model generated, but because it offers many advantages. In particular, R6/2 mice (with approx. 150 CAG repeats) manifest a very aggressive, rapidly progressing form of HD, similar to the juvenile form in humans. R6/2 mice display overt behavioural symptoms as early as 4–5 weeks of age, show hindlimb clasping and weight loss and die at approx. 15 weeks. Alterations include the formation of NIIs (Davies et al., 1997), which were later also shown to be present in human HD brains (DiFiglia et al., 1997). NIIs can be observed very early, in the presymptomatic stage (Morton et al., 2000). There are also changes in neurotransmitter receptor expression (Cha et al., 1998; Ariano et al., 2002) and altered signalling mechanisms (Bibb et al., 2000; Luthi-Carter et al., 2000; Menalled et al., 2000). Many of these alterations are correlated with motor (Carter et al., 1999) and learning impairments on a number of cognitive tasks (Lione et al., 1999), as well as deficits in synaptic plasticity at hippocampal CA1 (cornu ammonis 1) synapses before an overt phenotype appears (Murphy et al., 2000).
Another line, the R6/1 (with approx. 110 CAG repeats and decreased mutant htt expression compared with the R6/2), presents with similar phenotypic alterations as the R6/2, but in a more protracted form (Mangiarini et al., 1996). Weight loss and clasping can be observed at 19–23 weeks and become more pronounced with age. In addition, aberrant synaptic plasticity, which occurs prior to the formation of nuclear aggregates, has been described in these mice (Cummings et al., 2006; Milnerwood et al., 2006).
FULL-LENGTH MODELS
The most widely studied full-length model uses YAC (yeast artificial chromosomes) expressing normal (YAC18) and mutant (YAC46 and YAC72, and YAC128) htt (Hodgson et al., 1999; Slow et al., 2003). YAC72 mice display abnormal behaviour around 7 months of age, as well as selective degeneration of MSSNs in the lateral striatum by 12 months. Subsequent experiments in these mice showed that the formation of aggregates may not be essential to initiate neuronal death (Hodgson et al., 1999). An increase in CAG repeat length led to a more severe phenotype, arguing in favour of a gene–dose effect. YAC128 mice display alterations similar to YAC72 mice, but these alterations are more severe and occur earlier (Slow et al., 2003). YAC128 mice exhibit increased open-field activity at approx. 3 months, followed by rotarod abnormalities at 6 months. By 12 months, open-field activity is diminished significantly compared with controls. In addition, atrophy and neuronal loss (approx. 10%) occur in the striatum and cortex of YAC128 mice (Van Raamsdonk et al., 2005).
A recently described full-length model of HD uses a BAC (bacterial artificial chromosome)-expressing mutant htt with 97 glutamine repeats (Gray et al., 2008b). These mice exhibit progressive motor deficits, neuronal synaptic dysfunction and late-onset selective neuropathology in both the striatum and cortex. Interestingly, studies in BACHD mice revealed that the pathogenic process can occur without early and diffuse nuclear accumulation of aggregated mutant htt (Gray et al., 2008b). BAC models also have been instrumental in demonstrating an important role of cell–cell interactions, compared with cell autonomous processes, in HD pathogenesis (Gu et al., 2005, 2007; Gray et al., 2008a). However, cell autonomous processes also occur. In a different HD model (N171-98Q), striatal expression of mutant htt was sufficient to produce NIIs and motor impairment, which the investigators attributed to cell-autonomous transcriptional dysregulation (Brown et al., 2008).
KNOCK-IN MODELS
Knock-in models also contributed to our understanding of HD. The major advantage of these models is that they express full-length htt in its native genomic context. Several models that differ mainly in the number of CAG repeats (from 48 to 200) have been generated (White et al., 1997; Levine et al., 1999; Shelbourne et al., 1999; Wheeler et al., 2000; Lin et al., 2001; Heng et al., 2007). Although overt behavioural changes are often subtle in knock-in mice, sensitive and careful testing demonstrates abnormalities as early as 1–2 months of age (Menalled et al., 2002, 2003). Furthermore, a consistent feature of knock-in mice is the presence of nuclear staining and microaggregates at 2–6 months, which is relatively early in the course of the disease. By contrast, NIIs are observed only in older mice (10–18 months) (Menalled et al., 2002), and loss of MSSNs occurs at approx. 2 years (Hickey et al., 2008). Two additional knock-in models, the HdhQ150 and HdhQ200, display a behavioural phenotype that is CAG-length dependent, with motor abnormalities appearing at 50 or 100 weeks of age and reduced DA receptors in heterozygotes (Heng et al., 2007, 2009). The HdhQ200 mice also show regional-selective pathology in the striatum and cortex, including NIIs and gliosis, and display age-dependent weight loss beginning at approx. 70 weeks (Heng et al., 2009).
CONDITIONAL MODELS
An important step in the understanding of HD pathology was the creation of conditional mouse models of the disease. Taking advantage of the tetracycline-regulatable system, mutated htt can be ‘turned off’ after being expressed in the brain (Yamamoto et al., 2000). One contribution of this model was the demonstration of a close relationship between the HD phenotype and the presence of NIIs. Interrupting the expression of mutant htt led to inclusion clearance and a reversal of motor symptoms. Thus a continuous influx of mutant htt seems to be required to support inclusions and symptoms. Autophagy, via acetylation, appears to play a critical role in this effect (Jeong et al., 2009).
USING MOUSE MODELS TO UNDERSTAND MECHANISMS
As pointed out above, HD mouse models provide a way to follow the progression of the disorder especially before overt symptoms are present. Since each model is unique, similar phenotypic changes in different models provide a way to validate alterations and to make sure such changes are not due to idiosyncratic effects of variables relating to a particular mouse model. However, no mouse model recapitulates the human condition in its entirety, nor displays the degree of neurodegeneration that occurs in humans (Levine et al., 2004). Regardless, each model provides relevant information for the understanding of HD mechanisms.
It has been argued that knock-in models are more faithful in terms of genetic context and in recapitulating the late onset, slow natural progression and neuropathology of HD (Heng et al., 2008). However, in these models the symptoms are generally mild and normal aging may be a confounding factor. The R6/2 model has been criticized for the rapid onset and aggressive development of the phenotype, as well as early and widespread presence of NIIs. This has raised questions about its validity for drug screening (Heng et al., 2008), even though it has been standardly used in many screening experiments (Gil and Rego, 2009), and whether or not it recapitulates adult-onset HD since many of the phenotypic properties of the model are similar to juvenile HD (Cummings et al., 2009). The conclusion from a careful and systematic comparison of fragment and full-length knock-in models of HD was that, when strain background and CAG repeat length are controlled for, fragment and knock-in models develop comparable phenotypes (Woodman et al., 2007). Full-length models, such as the BACHD and the YAC, display a more robust phenotype than knock-in models, but they also have shortcomings. In particular, these mice tend to be heavier than their WT (wild-type) littermates. In human HD and in other genetic mouse models, weight loss is the common denominator. The increased body weight in these models has been associated with overexpression of htt (Van Raamsdonk et al., 2006). Interestingly, in contrast with R6/2 and some of the knock-in mice where there are considerable alterations in receptor expression, alterations in YAC128 mice appear limited to glutamate receptors, as DA, GABA (γ-aminobutyric acid) and adenosine receptor binding remained unchanged (Benn et al., 2007).
NEURONAL MORPHOLOGICAL AND FUNCTIONAL ALTERATIONS
As in the human disease, most mouse models of HD display significant reductions in brain volume. However, in mice this is probably not the result of a decrease in the number of neurons. Instead, reduced somatic size and loss or thinning of dendrites and spines are the main contributors (Klapstein et al., 2001). The lack of cell loss should not be surprising and could be related to the resiliency of neurons in mouse compared with the human brain. For example, most genetic mouse models of Parkinson’s disease have been unable to reproduce significant loss of DA cells even though functional changes occur (Goldberg et al., 2003; Itier et al., 2003; Kitada et al., 2009; Watson et al., 2009; Wu et al., 2009). Similarly, it is probable that the majority of symptoms in mice can be attributed to functional rather than structural alterations. In particular, changes in voltage- and ligand-gated ion-channel function and alterations in synaptic activity occur and become more pronounced with disease progression (Cepeda et al., 2007). At this point it remains unknown whether morphological changes are produced by initial functional alterations or if these alterations are the consequence of gene-induced structural abnormalities.
NMDA RECEPTOR-MEDIATED RESPONSES IN HD MOUSE MODELS
As pointed out above, the excitotoxicity hypothesis, based on the acute toxicity models, was the initial underlying hypothesis used to explain neurodegeneration in HD. Accordingly, increased glutamate release and sensitivity of NMDA receptors caused striatal cell death. Thus initial studies in mouse models were aimed at verifying or refuting this hypothesis. The two most vulnerable areas in HD are the striatum, especially the MSSNs, and the cerebral cortex. The R6/2 line has been particularly useful to verify the excitotoxicity hypothesis. The rapid progression of the disease allows electrophysiological studies in brain slices which require visualization of individual neurons with infrared videomicroscopy and IR-DIC (infrared differential interference contrast) optics, a technique better suited for younger animals. Our laboratory performed one of the first studies examining NMDA receptor function in genetic mouse models of HD. Long-lasting bath application of NMDA induces cell swelling, an index of excitotoxicity that can be visualized using IR-DIC microscopy (Dodt et al., 1993; Colwell and Levine, 1996). In striatal slices from symptomatic R6/2 mice and CAG94 (but not CAG72) knock-in mice, NMDA-induced cell swelling was enhanced compared with slices from control mice (Levine et al., 1999), thus providing evidence for the excitotoxicity hypothesis. In contrast, in vivo studies from another laboratory using R6/1 mice showed that injections of quinolinic acid produced less damage in HD mice (Hansson et al., 1999). Initially, these differences were difficult to explain. However, it is well-known that, in vivo, excitotoxic lesions require the integrity of the glutamatergic corticostriatal pathway (McGeer et al., 1978). Subsequently we showed that the corticostriatal connection is compromised in the R6 mice as well as in other mouse models which then reduces the excitotoxic response in these models. Interestingly, in other models [e.g. the CAG100 (Laforet et al., 2001)], in vivo neuroprotection to quinolinic acid did not occur (Petersen et al., 2002) and in the YAC72 model neurotoxicity was enhanced (Zeron et al., 2002). Increased NMDA receptor function was also verified using electrophysiological recordings and calcium imaging showing that a population of MSSNs had increased responses to NMDA (Cepeda et al., 2001). Similar findings have been obtained in YAC72 (Hodgson et al., 1999) and 128 (Graham et al., 2009) mice.
Another significant alteration in striatal NMDA receptor function is the early and persistent reduction of Mg2+ sensitivity (Table 1). When the cell membrane is hyperpolarized or at normal resting membrane potential (approx. −70 mV), NMDA receptor channels are blocked by Mg2+ (Nowak et al., 1984). The channel activates upon membrane depolarization or in conditions of reduced Mg2+ block. In HD, it appears that NMDA receptors of MSSNs are potentially activated at more hyperpolarized potentials, which could be deleterious for the cell. Increased NMDA currents due to reduced Mg2+ sensitivity are observed in MSSNs of symptomatic R6/2 mice (Cepeda et al., 2001) and in a subset of neurons in young mice (Starling et al., 2005). In addition, this alteration appears to be intrinsic to MSSNs, i.e. cell autonomous, as it occurs even after inactivation of mutant htt in the cortex (Gu et al., 2007). Surprisingly, cortical pyramidal neurons of R6/2 mice at similar ages display increased Mg2+ sensitivity, thus leading to reduced NMDA currents (André et al., 2006). This alteration could underlie the cognitive impairments found in HD mice.
Table 1. Intrinsic and synaptic alterations in cortical and striatal neurons in mouse models of HD.
Electrophysiological alterations in cortical and striatal neurons from R6/1, R6/2 and YAC mouse models of HD during early (E) and late (L) stages of the disease. In early HD, pre-symptomatic and early symptomatic (mean age of 6 weeks in R6 models and 9 weeks in most other models), changes in MSSN cell properties include increased membrane input resistance, reduced cell capacitance and a decrease in K+ channel inward rectification. These alterations in cell properties, including the appearance of depolarized resting membrane potentials, are seen also in MSSNs from behaviourally symptomatic mice. In cortical pyramidal neurons from HD mice, similar changes in cell properties are evident, but only in late stages of the disease. Biphasic changes in spontaneous EPSCs are evident in MSSNs from HD mice in which increased activity occurs early, but is followed by reduced activity in overtly symptomatic mice. A similar pattern occurs with IPSC activity in cortical pyramidal neurons from HD mice. NMDA receptor activity (as measured by current amplitude and density) and Mg2+ sensitivity differ between cell types. RMP, resting membrane potential; VG, voltage-gated; VGCC, voltage-gated calcium channels.
| Properties | Cortical pyramidal neurons | MSSNs |
|---|---|---|
| Cell membrane properties | ||
| Membrane capacitance | Reduced cell capacitance (L) (Cummings et al., 2006, 2009) | Reduced cell capacitance (E, L) (Klapstein et al., 2001; Cepeda et al., 2003) |
| Input resistance | Increased membrane input resistance (L) (Cummings et al., 2006, 2009) | Increased membrane input resistance (E, L) (Klapstein et al., 2001; Cepeda et al., 2003) |
| RMP | Depolarized resting membrane potentials (L) (Cummings et al., 2006, 2009) | Depolarized resting membrane potentials (L) (Klapstein et al., 2001) |
| VG channel activity | ||
| VGCCs | Increased VGCC currents (L) (André et al., 2006) | Decreased VGCC currents (E) (Cepeda et al., 2001) |
| K+ channels | Decreased K+ channel inward rectification (E, L) (Ariano et al., 2001; Klapstein et al., 2001) | |
| Synaptic activity | ||
| EPSCs | Increased spontaneous EPSC frequency (L) (Cummings et al., 2009) | Decreased spontaneous EPSC frequency (L) (Cepeda et al., 2003) |
| Increased evoked EPSCs (L)(Cummings et al., 2009) | Large-amplitude spontaneous EPSCs (E) (Cepeda et al., 2003) | |
| Increased evoked EPSCs (E) (Joshi et al., 2009) | ||
| Decreased evoked EPSCs (L) (Joshi et al., 2009) | ||
| IPSCs | Increased spontaneous IPSC frequency (E) (Cummings et al., 2009) | Increased spontaneous IPSC frequency (L) (Cepeda et al., 2004) |
| Decreased spontaneous IPSC frequency (L) (Cummings et al., 2009) | ||
| Action potentials | ||
| Frequency | Increased firing rate (E) (Walker et al., 2008) | Increased firing rate (E) (Rebec et al., 2006; Miller et al., 2008) |
| Correlated Activity | Decreased synchrony between neuronal pairs (E) (Walker et al., 2008) | Decreased correlated firing (E) (Rebec et al., 2006; Miller et al., 2008) |
| Receptors | ||
| NMDA receptors | Decreased NMDA receptor currents (E) (André et al., 2006) | Increased NMDAR currents (E, L) (Cepeda et al., 2001; Starling et al., 2005; Zeron et al., 2002; Graham et al., 2009) |
| Increased NMDAR Mg2+ sensitivity (E) (André et al., 2006) | Decreased NMDAR Mg2+ sensitivity (E, L) (Cepeda et al., 2001; Starling et al., 2005) |
RESTING MEMBRANE POTENTIALS AND IONIC CONDUCTANCE ALTERATIONS
In the striatum, other signs of functional pathology seen in MSSNs of HD mice are increases in cell membrane input resistance and depolarized resting membrane potentials (Levine et al., 1999; Klapstein et al., 2001; see Table 1). Determinants of these biophysical parameters are cell size and K+ conductances, in particular those dependent on leak and inwardly rectifying K+ channels. Cell size is decreased in HD mice (Levine et al., 1999; Klapstein et al., 2001) and K+ conductances are reduced (Ariano et al., 2005). We examined changes in inward rectification in R6/2 mice and found a significant reduction concomitant to the onset of overt behavioural symptoms. In addition, correlative immunofluorescence studies demonstrated decreases in the expression of K+ channel subunit proteins, Kir2.1 and Kir2.3 (Ariano et al., 2005). One of the consequences of increases in membrane input resistance is an enhancement of cell excitability which, coupled with depolarized membrane potentials, could lead to amplification of synaptic inputs and increased action potential firing in MSSNs. Consistent with this idea, in vivo recordings of striatal neurons demonstrated that cell firing is elevated in R/2 transgenic relative to WT mice at 6–9 weeks of age (Rebec et al., 2006). Not only the frequency, but also the burst activity and correlated firing patterns were altered in HD mice (Miller et al., 2008). Thus correlated firing and coincident bursts between pairs of MSSNs were prominent in cells from WT animals, but reduced in R6/2 and knock-in models, suggesting that information processing at both the single-neuron and population level is compromised in the striatum of symptomatic HD mice (Miller et al., 2008). Similar changes were also observed in cortical pyramidal neurons (Walker et al., 2008).
GLUTAMATE AND GABA SYNAPTIC ACTIVITY IN STRIATUM AND CORTEX
In neurons the htt protein distribution is very similar to that of synaptophysin (Wood et al., 1996) and it has been shown to associate with various proteins involved in synaptic function. Mutant htt produces specific impairment of exocytosis and endocytosis, potentially causing abnormal synaptic transmission, thus leading to the proposal that HD is a synaptopathology (Li et al., 2003). Using genetic mouse models we were able to examine the progression of synaptic alterations along the corticostriatal pathway in HD. Since cortical neurons are affected, we also examined synaptic changes in pyramidal neurons. Our results showed that changes in synaptic transmission are time- and region-dependent.
In R6/2 and YAC128 HD mice, alterations in glutamatergic function along the corticostriatal pathway change dynamically in a biphasic manner (Table 1). Although a progressive reduction in spontaneous and evoked glutamatergic synaptic activity, coinciding with the appearance of overt behavioural alterations, is the most noticeable change in R6/2 mice (Klapstein et al., 2001; Cepeda et al., 2003), dysregulation of glutamatergic input occurs early and is manifested by the presence of large-amplitude and complex synaptic events that peak at approx. 5–7 weeks of age (Cepeda et al., 2003). These large events could reflect increased cortical excitability and a possible reduction in presynaptic receptor function, including DA D2, metabotropic glutamate (mGluR2/3) and endocannabinoid CB1 receptors (Cha et al., 1998; Luthi-Carter et al., 2000; Ariano et al., 2002). As striatal neuronal action potential generation is highly dependent on cortical inputs, the presence of large-amplitude synaptic events in the striatum of R6/2 mice at 5–7 weeks, in conjunction with higher membrane input resistance, predicts transiently increased activity along the corticostriatal pathway in a subset of MSSNs.
Hyperexcitability in cortical networks was confirmed in the R6/2 and other mouse models. Examination of somatosensory cortical pyramidal neurons in layers II/III in slices from R6/2 mice revealed that spontaneous EPSCs (excitatory postsynaptic currents) occurred at a higher frequency in behaviourally phenotypic mice, whereas spontaneous IPSCs (inhibitory postsynaptic currents) were initially increased in frequency and subsequently decreased at 80–90 days (Cummings et al., 2009). Decreased inhibition in cortical pyramidal neurons, manifested by a reduction in spontaneous IPSCs, was also observed in the BACHD model at 6 months, when motor dysfunction occurs (Spampanato et al., 2008). Furthermore, compared with control animals, R6/2 mice demonstrate increased epileptiform activity in cortical slices and seizure susceptibility in vivo after blockade of GABAA receptors with bicuculline and picrotoxin respectively (Cummings et al., 2009). Interestingly, in contrast with reduced IPSC frequency in the cortex of symptomatic animals, the frequency of inhibitory GABAA receptor-mediated synaptic events is increased in the striatum of R6/2 and other models of HD (Cepeda et al., 2004; Cummings et al., 2007). This observation predicts reduced output of MSSNs to target structures along the direct and indirect pathways. Increased GABA release also could dampen glutamate inputs either by shunting (increase in the membrane conductance) or by activation of GABAB receptors located on corticostriatal terminals.
Recently we examined alterations in glutamate release in the corticostriatal pathway of YAC128 mice at different stages of disease progression (1, 7 and 12 months), using combined optical and electrophysiological methods. Similar to results from R6/2 mice, the results in YAC128 mice demonstrated biphasic age-dependent changes in corticostriatal function. At 1 month, before the behavioural phenotype develops, glutamate release and AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptor-mediated synaptic currents evoked by cortical stimulation were increased. At 7 and 12 months, after the development of the behavioural phenotype, glutamate release and AMPA synaptic currents were significantly reduced (Joshi et al., 2009). These effects were due to combined pre- and post-synaptic alterations.
The susceptibility to excitotoxic stress in YAC128 mice also changes in a biphasic manner (Graham et al., 2009). At 1 month, before phenotypic changes occur, mice display increased sensitivity to NMDA and quinolinic acid. In contrast, at 7–10 months symptomatic mice are resistant to quinolinic acid neurotoxicity. These changes are paralleled by increased NMDA receptor-mediated synaptic currents in slices and increased postsynaptic currents in acutely isolated MSSNs from presymptomatic, followed by reduced currents in symptomatic, YAC128 mice (Graham et al., 2009).
Multiple alterations in corticostriatal synaptic function also depend on the pathogenicity of mutant htt (Milnerwood and Raymond, 2007). Presynaptic dysfunction and a propensity towards synaptic depression in YAC72 and YAC128 compared with YAC18 mice occurs at 1 month. In YAC128 mice, reduced AMPA responses evoked by intrastriatal stimulation were also observed. In contrast, when normalized to evoked AMPA currents, postsynaptic NMDA currents were enhanced in all three pathologic HD YAC variants (Milnerwood and Raymond, 2007).
One of the outcomes of excitatory activity in the corticostriatal pathway is the concomitant release of BDNF (brain-derived neurotrophic factor), a trophic factor essential for postsynaptic neuronal support that is reduced in HD (Zuccato and Cattaneo, 2007). Thus the progressive reduction in glutamate synaptic activity will also reduce the release of this important factor and potentially facilitate cellular dysfunction and subsequent degeneration.
DIFFERENTIAL VULNERABILITY OF MSSNs
One of the enigmas of HD is the particular vulnerability of MSSNs forming the indirect pathway. Decreased enk expression is one of the earliest molecular markers of neuropathology in HD mice (Menalled et al., 2000). What makes these neurons more susceptible to degeneration? The EGFP (enhanced green fluorescent protein) gene has been used as a reporter to identify DA D1 (direct pathway) and D2 (indirect pathway) receptor-expressing striatal cells. Several studies in MSSNs from intact mice have shown that dendritic elaboration and cell capacitance are reduced in D2 cells (Gertler et al., 2008). We have examined and compared electrophysiology in D1 and D2 receptor-expressing MSSNs. D2 receptor-expressing MSSNs are more excitable and appear to be better connected to the cerebral cortex than D1 cells. For example, cortical disinhibition using GABAA receptor blockers leads to the occurrence of membrane depolarizations and bursts in D2 receptor-expressing, but not in D1 receptor-expressing, MSSNs (Cepeda et al., 2008), confirming previous studies showing selective activation of enk-containing neurons using expression of immediate-early gene proteins, Fos and Jun B, after cortical disinhibition (Berretta et al., 1997; Parthasarathy and Graybiel, 1997). The size of corticostriatal glutamatergic boutons synapsing on to D2 receptor-expressing MSSNs is larger than those contacting D1 receptor-expressing MSSNs, as shown by electron microscopy (Reiner et al., 2003). This finding implies that D2 receptor-expressing MSSNs are subjected to increased glutamate release at the synapse. Perhaps these differences in glutamate release make striatal D2 cells more susceptible to degeneration in HD.
MECHANISMS OF NEURONAL DEGENERATION IN HD
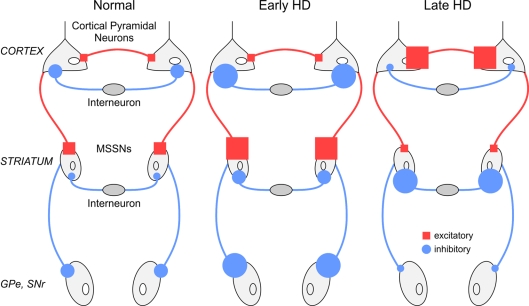
How do the electrophysiological findings reviewed here contribute to our understanding of neuronal dysfunction and degeneration in HD? The findings from studies of mouse models permit the generation of a model of neuronal dysfunction and degeneration that may be applicable to the human disease (Figure 1). The starting point is dysregulation of glutamate release along the corticostriatal pathway and altered modulation by presynaptic receptors, followed by a series of physiological, compensatory mechanisms that attempt to counter the change in glutamate release. Compensatory mechanisms are effective initially but, eventually, they can have deleterious effects. It is worth mentioning that a similar mechanism, i.e. calcium-dependent increase in neurotransmitter release as the root of neuronal degeneration, was also postulated in a Drosophila model of HD (Romero et al., 2008).
Figure 1. Simplified model of early and late changes in excitatory and inhibitory synaptic transmission within the cerebral cortex and striatum of HD mouse models.
MSSNs in the striatum receive excitatory inputs from the cortex and inhibitory inputs mainly from local interneurons. Early in the disease and coinciding with overt symptoms, dysregulation of corticostriatal input produces increased excitation and firing of MSSNs, increasing striatal output to the external segment of the globus pallidus (GPe) and to the substantia nigra pars reticulata (SNr). Late in the disease, in spite of increased cortical excitability, excitatory corticostriatal input is decreased while inhibitory activity is increased, leading to the obliteration of striatal output. In the Figure, neuronal projections coloured in red are excitatory, whereas those in blue are inhibitory.
The function of normal htt is not well understood, but convergent evidence points to this protein having a role in vesicular trafficking, exocytosis and endocytosis (DiFiglia et al., 1995; Caviston and Holzbaur, 2009). Elongation of the CAG tract leads to alterations in the synaptic machinery producing initial increases in glutamate release reflected by enhanced synaptic responses and large amplitude spontaneous synaptic events. Excess glutamate release causes the collapse of spines on postsynaptic MSSNs, which in turn increases membrane input resistance, reduces K+ conductances, and further amplifies synaptic signals. Dysregulation of glutamate release is compounded by loss of DA D2, CB1, mGluR2/3 and other presynaptic receptors regulating glutamate release at corticostriatal terminals. A compensatory mechanism might be the increase of GABAergic synaptic activity that occurs within the striatum. Increasing GABAergic synaptic transmission could aid in preventing further neuronal damage by reducing glutamate release, by activation of presynaptic GABAB receptors, and/or inducing postsynaptic alterations by shunting excitatory inputs to MSSNs, via GABAA receptors.
The period of increased glutamate release in the striatum will be followed by a period of reduced synaptic input. Another adverse effect of reduced corticostriatal communication is the reduced release of BDNF. Lack of this trophic factor will negatively impact MSSNs. During this time, K+ conductances are also reduced, and the resting membrane potential is depolarized, leading to an increased propensity for MSSNs to produce action potentials. Although these cells will fire more frequently, their firing patterns are now disorganized.
Why should changes occur earlier and/or be more evident in MSSNs of the indirect pathway? One possible reason is that these cells appear to be more connected to cortical pyramidal neurons, and reduced corticostriatal communication will first affect this subpopulation of cells. Enk expression is tightly regulated by cortical inputs (Uhl et al., 1988) and decreased cortical communication will reduce enk expression in these MSSNs.
Reductions in spine density and synaptic markers cause a redistribution of postsynaptic glutamate receptors that is also deleterious in neurons. Synaptic NMDA receptors activate a pro-survival cascade and BDNF transcription. In contrast, extrasynaptic NMDA receptors (rich in NR2B subunits) activate a pro-apoptotic cascade (Papadia and Hardingham, 2007). In the striatum of HD mice, there is evidence of increased extrasynaptic NMDA receptor expression and signalling (Milnerwood et al., 2010). Thus the balance between synaptic and extrasynaptic activity determines neuronal survival (Okamoto et al., 2009; Levine et al., 2010). Interestingly, synaptic activity is required for inclusion formation which, in turn, is believed to be neuroprotective (Okamoto et al., 2009).
FUTURE DIRECTIONS
Many unanswered questions still remain. A major one concerns the role of brain regions other than the striatum and cortex in HD manifestations. In humans with HD there is evidence of significant cell loss within the thalamus (Heinsen et al., 1996) and hypothalamus (Kremer et al., 1990). The thalamus, particularly the centromedian and parafascicular nuclei, is the origin of a second major glutamatergic input to the striatum (Smith et al., 2004). The nature of these inputs is just beginning to be unravelled in slice preparations that preserve both cortico- and thalamo-striatal inputs (Ding et al., 2008; Smeal et al., 2008). It will be important to determine whether this pathway also plays a role in the alterations of excitatory synaptic activity that are observed in HD mouse models.
Another area that has not been extensively investigated is the function of glial cells and glio-neuronal interactions in HD. How are astrocytes and glutamate transporters changed in HD and how do they modulate neuronal degeneration? Initial evidence points to an important role of mutant htt in astrocytes, which might lead to decreased levels of glutamate transporters (Lievens et al., 2001; Shin et al., 2005; Estrada-Sanchez et al., 2009). The contribution of glial cells to disease progression further supports a non-cell autonomous process in HD (Lobsiger and Cleveland, 2007).
CONCLUSIONS
Genetic mouse models of HD have become important tools for examining the natural evolution of the disease and understanding its mechanisms. Convergent evidence supports cell-autonomous and non-cell autonomous processes in neuronal dysfunction and subsequent degeneration. In particular, striatal neuronal dysfunction seems to depend on both intrinsic changes in NMDA receptor sensitivity, as well as altered cortical input. Synaptic alterations occur early, before overt symptoms emerge, and are biphasic in nature, with age-dependent increases followed by decreases in excitatory transmission along the corticostriatal pathway. In terms of therapeutics, structural and functional changes are time- and region-dependent. In consequence, treatments should be tailoured to different stages of the disease and be specific to different brain areas.
Footnotes
Work in the author’s laboratory is supported by the United States Public Health Service (USPHS) [grant number NS41574]; and contracts from CHDI, Incorporated and the Hereditary Disease Foundation.
REFERENCES
- Albin RL, Reiner A, Anderson KD, Dure 4th LS, Handelin B, Balfour R, Whetsell WO, Jr, Penney JB, Young AB. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington’s disease. Ann Neurol. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- André VM, Cepeda C, Venegas A, Gomez Y, Levine MS. Altered cortical glutamate receptor function in the R6/2 model of Huntington’s disease. J Neurophysiol. 2006;95:2108–2119. doi: 10.1152/jn.01118.2005. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Aronin N, Difiglia M, Tagle DA, Sibley DR, Leavitt BR, Hayden MR, Levine MS. Striatal neurochemical changes in transgenic models of Huntington’s disease. J Neurosci Res. 2002;68:716–729. doi: 10.1002/jnr.10272. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington’s disease transgenic mice. J Neurophysiol. 2005;93:2565–2574. doi: 10.1152/jn.00791.2004. [DOI] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- Aziz NA, Pijl H, Frolich M, van der Graaf AW, Roelfsema F, Roos RA. Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J Clin Endocrinol Metab. 2009;94:1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn CL, Slow EJ, Farrell LA, Graham R, Deng Y, Hayden MR, Cha JH. Glutamate receptor abnormalities in the YAC128 transgenic mouse model of Huntington’s disease. Neuroscience. 2007;147:354–372. doi: 10.1016/j.neuroscience.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, Nairn AC, Messer A, Greengard P. Severe deficiences in dopamine signaling in presymptomatic Huntington’s disease mice. Proc Natl Acad Sci USA. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkqvist M, Petersen A, Bacos K, Isaacs J, Norlen P, Gil J, Popovic N, Sundler F, Bates GP, Tabrizi SJ, Brundin P, Mulder H. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington’s disease. Hum Mol Genet. 2006;15:1713–1721. doi: 10.1093/hmg/ddl094. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, Beal MF. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci USA. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TB, Bogush AI, Ehrlich ME. Neocortical expression of mutant huntingtin is not required for alterations in striatal gene expression or motor dysfunction in a transgenic mouse. Hum Mol Genet. 2008;17:3095–3104. doi: 10.1093/hmg/ddn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Ariano MA, Calvert CR, Flores-Hernandez J, Chandler SH, Leavitt BR, Hayden MR, Levine MS. NMDA receptor function in mouse models of Huntington disease. J Neurosci Res. 2001;66:525–539. doi: 10.1002/jnr.1244. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Calvert CR, Hernandez-Echeagaray E, Nguyen OK, Jocoy E, Christian LJ, Ariano MA, Levine MS. Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington’s disease. J Neurosci. 2003;23:961–969. doi: 10.1523/JNEUROSCI.23-03-00961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, André VM, Ariano MA, Levine MS. Increased GABAergic function in mouse models of Huntington’s disease: reversal by BDNF. J Neurosci Res. 2004;78:855–867. doi: 10.1002/jnr.20344. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Prog Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Cha JH, Kosinski CM, Kerner JA, Alsdorf SA, Mangiarini L, Davies SW, Penney JB, Bates GP, Young AB. Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human huntington disease gene. Proc Natl Acad Sci USA. 1998;95:6480–6485. doi: 10.1073/pnas.95.11.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Levine MS. Glutamate receptor-induced toxicity in neostriatal cells. Brain Res. 1996;724:205–212. doi: 10.1016/0006-8993(96)00323-x. [DOI] [PubMed] [Google Scholar]
- Coyle JT. An animal model for Huntington’s disease. Biol Psychiatry. 1979;14:251–276. [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington’s chorea. Nature. 1976;263:244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME, Kuhar MJ. In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J Comp Neurol. 1978;180:301–323. doi: 10.1002/cne.901800208. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Milnerwood AJ, Dallerac GM, Waights V, Brown JY, Vatsavayai SC, Hirst MC, Murphy KPSJ. Aberrant cortical synaptic plasticity and dopaminergic dysfunction in a mouse model of Huntington's Disease. Hum Mol Genet. 2006;15:2856–2868. doi: 10.1093/hmg/ddl224. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Cepeda C, Leavitt BR, Hayden MR, Zeitlin SO, Levine MS. Altered striatal glutamatergic and GABAergic neurotransmission are consistent across genetic mouse models of Huntington’s disease. Abstract Viewer/Itinerary Planner San Diego, CA: Society for Neuroscience Program No. 372.5. 2007 [Google Scholar]
- Cummings DM, Andre VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington’s disease. J Neurosci. 2009;29:10371–10386. doi: 10.1523/JNEUROSCI.1592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano M, Galvan L, Deglon N, Brouillet E. Mitochondria in Huntington’s disease. Biochim Biophys Acta. 2009;1802:52–61. doi: 10.1016/j.bbadis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington’s disease. Trends Neurosci. 1990;13:286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel JP, Carraway R, Reeves SA. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Hager G, Zieglgansberger W. Direct observation of neurotoxicity in brain slices with infrared videomicroscopy. J Neurosci Methods. 1993;50:165–171. doi: 10.1016/0165-0270(93)90005-c. [DOI] [PubMed] [Google Scholar]
- Estrada-Sanchez AM, Montiel T, Segovia J, Massieu L. Glutamate toxicity in the striatum of the R6/2 Huntington’s disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol Dis. 2009;34:78–86. doi: 10.1016/j.nbd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Storm-Mathisen J, Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6:863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Gambardella A, Muglia M, Labate A, Magariello A, Gabriele AL, Mazzei R, Pirritano D, Conforti FL, Patitucci A, Valentino P, Zappia M, Quattrone A. Juvenile Huntington’s disease presenting as progressive myoclonic epilepsy. Neurology. 2001;57:708–711. doi: 10.1212/wnl.57.4.708. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil JM, Rego AC. The R6 lines of transgenic mice: a model for screening new therapies for Huntington’s disease. Brain Res Rev. 2009;59:410–431. doi: 10.1016/j.brainresrev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Graham RK, Pouladi MA, Joshi P, Lu G, Deng Y, Wu NP, Figueroa BE, Metzler M, Andre VM, Slow EJ, Raymond L, Friedlander R, Levine MS, Leavitt BR, Hayden MR. Differential susceptibility to excitotoxic stress in YAC128 mouse models of Huntington disease between initiation and progression of disease. J Neurosci. 2009;29:2193–2204. doi: 10.1523/JNEUROSCI.5473-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Gu X, Shiraski D, Cepeda C, Yamazaki I, Levine MS, Yang X. Cortical control of striatal pathogenesis in the Cre/LoxP conditional BAC transgenic mouse model of Huntington’s Disease (BACHD). Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience Program No. 114.6. 2008a [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008b;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S, Li XJ, Mody I, Heintz N, Yang XW. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Gu X, Andre VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington’s disease. Mol Neurodegener. 2007;2:8. doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Petersen A, Leist M, Nicotera P, Castilho RF, Brundin P. Transgenic mice expressing a Huntington’s disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity. Proc Natl Acad Sci USA. 1999;96:8727–8732. doi: 10.1073/pnas.96.15.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper PS. New genes for old diseases: the molecular basis of myotonic dystrophy and Huntington’s disease. The Lumleian Lecture 1995. J R Coll Physicians Lond. 1996;30:221–231. [PMC free article] [PubMed] [Google Scholar]
- Harper PS, Jones L. Huntington’s disease: Genetic and molecular studies. In: Bates GP, Harper PS, Jones L, editors. In: Huntington’s Disease, Third Edition. Oxford: Oxford University Press; 2002. pp. 113–158. [Google Scholar]
- Heinsen H, Rub U, Gangnus D, Jungkunz G, Bauer M, Ulmar G, Bethke B, Schuler M, Bocker F, Eisenmenger W, Gotz M, Strik M. Nerve cell loss in the thalamic centromedian-parafascicular complex in patients with Huntington’s disease. Acta Neuropathol. 1996;91:161–168. doi: 10.1007/s004010050408. [DOI] [PubMed] [Google Scholar]
- Heng MY, Tallaksen-Greene SJ, Detloff PJ, Albin RL. Longitudinal evaluation of the Hdh(CAG)150 knock-in murine model of Huntington’s disease. J Neurosci. 2007;27:8989–8998. doi: 10.1523/JNEUROSCI.1830-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Detloff PJ, Albin RL. Rodent genetic models of Huntington disease. Neurobiol Dis. 2008;32:1–9. doi: 10.1016/j.nbd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Heng MY, Duong K, Albin R, Hunter J, Osmand A, Paulson H, Detloff PN. Early autophagic response in a novel knock-in model in Huntington disease. Abstract Viewer/Itinerary Planner Chicago, IL: Society for Neuroscience Program No 240.22. 2009 [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levíne MS, Chesselet MF. Extensive early motor and non-motor behavioural deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience. 2008;157:280–295. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LW, Carmichael J, Swartz J, Wyttenbach A, Rankin J, Rubinsztein DC. The molecular biology of Huntington’s disease. Psychol Med. 2001;31:3–14. doi: 10.1017/s0033291799002871. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de Yebenes J. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Jeong H, Then F, Melia TJ, Jr, Mazzulli JR, Cui L, Savas JN, Voisine C, Paganetti P, Tanese N, Hart AC, Yamamoto A, Krainc D. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72. doi: 10.1016/j.cell.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, Andre VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci. 2009;29:2414–2427. doi: 10.1523/JNEUROSCI.5687-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington’s disease transgenic mice. J Neurophysiol. 2001;86:2667–2677. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- Kremer HP, Roos RA, Dingjan G, Marani E, Bots GT. Atrophy of the hypothalamic lateral tuberal nucleus in Huntington’s disease. J Neuropathol Exp Neurol. 1990;49:371–382. doi: 10.1097/00005072-199007000-00002. [DOI] [PubMed] [Google Scholar]
- Laforet GA, Sapp E, Chase K, McIntyre C, Boyce FM, Campbell M, Cadigan BA, Warzecki L, Tagle DA, Reddy PH, Cepeda C, Calvert CR, Jokel ES, Klapstein GJ, Ariano MA, Levine MS, DiFiglia M, Aronin N. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington’s disease. J Neurosci. 2001;21:9112–9123. doi: 10.1523/JNEUROSCI.21-23-09112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Klapstein GJ, Koppel A, Gruen E, Cepeda C, Vargas ME, Jokel ES, Carpenter EM, Zanjani H, Hurst RS, Efstratiadis A, Zeitlin S, Chesselet MF. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington’s disease. J Neurosci Res. 1999;58:515–532. [PubMed] [Google Scholar]
- Levine MS, Cepeda C, Hickey MA, Fleming SM, Chesselet MF. Genetic mouse models of Huntington’s and Parkinson’s diseases: illuminating but imperfect. Trends Neurosci. 2004;27:691–697. doi: 10.1016/j.tins.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Levine MS, Cepeda C, Andre VM. Location, location, location: contrasting roles of synaptic and extrasynaptic NMDA receptors in Huntington’s disease. Neuron. 2010;65:145–147. doi: 10.1016/j.neuron.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Plomann M, Brundin P. Huntington’s disease: a synaptopathy? Trends Mol Med. 2003;9:414–420. doi: 10.1016/j.molmed.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tallaksen-Greene S, Chien WM, Cearley JA, Jackson WS, Crouse AB, Ren S, Li XJ, Albin RL, Detloff PJ. Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Hum Mol Genet. 2001;10:137–144. doi: 10.1093/hmg/10.2.137. [DOI] [PubMed] [Google Scholar]
- Lione LA, Carter RJ, Hunt MJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington’s disease mutation. J Neurosci. 1999;19:10428–10437. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi-Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, Ross CA, Borchelt DR, Tapscott SJ, Young AB, Cha JH, Olson JM. Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Hum Mol Genet. 2000;9:1259–1271. doi: 10.1093/hmg/9.9.1259. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Scherer U, Singh K. A glutamatergic corticostriatal path? Brain Res. 1977;128:369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL, Singh K. Kainate-induced degeneration of neostriatal neurons: dependency upon corticostriatal tract. Brain Res. 1978;139:381–383. doi: 10.1016/0006-8993(78)90941-1. [DOI] [PubMed] [Google Scholar]
- Menalled L, Zanjani H, MacKenzie L, Koppel A, Carpenter E, Zeitlin S, Chesselet MF. Decrease in striatal enkephalin mRNA in mouse models of Huntington’s disease. Exp Neurol. 2000;162:328–342. doi: 10.1006/exnr.1999.7327. [DOI] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Wu Y, Olivieri M, Li XJ, Li H, Zeitlin S, Chesselet MF. Early motor dysfunction and striosomal distribution of huntingtin microaggregates in Huntington’s disease knock-in mice. J Neurosci. 2002;22:8266–8276. doi: 10.1523/JNEUROSCI.22-18-08266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington’s disease with 140 CAG repeats. J Comp Neurol. 2003;465:11–26. doi: 10.1002/cne.10776. [DOI] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington’s disease. J Neurophysiol. 2008;100:2205–2216. doi: 10.1152/jn.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Corticostriatal synaptic function in mouse models of Huntington’s disease: early effects of huntingtin repeat length and protein load. J Physiol. 2007;585:817–831. doi: 10.1113/jphysiol.2007.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Cummings DM, Dallerac GM, Brown JY, Vatsavayai SC, Hirst MC, Rezaie P, Murphy KP. Early development of aberrant synaptic plasticity in a mouse model of Huntington’s disease. Hum Mol Genet. 2006;15:1690–1703. doi: 10.1093/hmg/ddl092. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Lagan MA, Skepper JN, Dunnett SB. Progressive formation of inclusions in the striatum and hippocampus of mice transgenic for the human Huntington’s disease mutation. J Neurocytol. 2000;29:679–702. doi: 10.1023/a:1010887421592. [DOI] [PubMed] [Google Scholar]
- Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington’s disease mutation. J Neurosci. 2000;20:5115–5123. doi: 10.1523/JNEUROSCI.20-13-05115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Hult S, Kirik D. Huntington’s disease: new perspectives based on neuroendocrine changes in rodent models. Neurodegener Dis. 2009;6:154–164. doi: 10.1159/000225377. [DOI] [PubMed] [Google Scholar]
- Petersen A, Chase K, Puschban Z, DiFiglia M, Brundin P, Aronin N. Maintenance of susceptibility to neurodegeneration following intrastriatal injections of quinolinic acid in a new transgenic mouse model of Huntington’s disease. Exp Neurol. 2002;175:297–300. doi: 10.1006/exnr.2002.7885. [DOI] [PubMed] [Google Scholar]
- Petersen A, Gil J, Maat-Schieman ML, Bjorkqvist M, Tanila H, Araujo IM, Smith R, Popovic N, Wierup N, Norlen P, Li JY, Roos RA, Sundler F, Mulder H, Brundin P. Orexin loss in Huntington’s disease. Hum Mol Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Macias R, Yescas P, Ochoa A, Davila G, Alonso E. Huntington disease in children: genotype-phenotype correlation. Neuropediatrics. 2000;31:190–194. doi: 10.1055/s-2000-7461. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience. 2006;137:327–336. doi: 10.1016/j.neuroscience.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol. 2003;457:420–440. doi: 10.1002/cne.10541. [DOI] [PubMed] [Google Scholar]
- Reiner A, Del Mar N, Deng YP, Meade CA, Sun Z, Goldowitz D. R6/2 neurons with intranuclear inclusions survive for prolonged periods in the brains of chimeric mice. J Comp Neurol. 2007;505:603–629. doi: 10.1002/cne.21515. [DOI] [PubMed] [Google Scholar]
- Romero E, Cha GH, Verstreken P, Ly CV, Hughes RE, Bellen HJ, Botas J. Suppression of neurodegeneration and increased neurotransmission caused by expanded full-length huntingtin accumulating in the cytoplasm. Neuron. 2008;57:27–40. doi: 10.1016/j.neuron.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Coyle JT. Striatal lesions with kainic acid: neurochemical characteristics. Brain Res. 1977;127:235–249. doi: 10.1016/0006-8993(77)90538-8. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Seneca S, Fagnart D, Keymolen K, Lissens W, Hasaerts D, Debulpaep S, Desprechins B, Liebaers I, De Meirleir L. Early onset Huntington disease: a neuronal degeneration syndrome. Eur J Pediatr. 2004;163:717–721. doi: 10.1007/s00431-004-1537-3. [DOI] [PubMed] [Google Scholar]
- Shelbourne PF, Killeen N, Hevner RF, Johnston HM, Tecott L, Lewandoski M, Ennis M, Ramirez L, Li Z, Iannicola C, Littman DR, Myers RM. A Huntington’s disease CAG expansion at the murine Hdh locus is unstable and associated with behavioural abnormalities in mice. Hum Mol Genet. 1999;8:763–774. doi: 10.1093/hmg/8.5.763. [DOI] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Smeal RM, Keefe KA, Wilcox KS. Differences in excitatory transmission between thalamic and cortical afferents to single spiny efferent neurons of rat dorsal striatum. Eur J Neurosci. 2008;28:2041–2052. doi: 10.1111/j.1460-9568.2008.06505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington’s disease. Neuroscience. 2008;157:606–620. doi: 10.1016/j.neuroscience.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AJ, André VM, Cepeda C, de Lima M, Chandler SH, Levine MS. Alterations in N-methyl-d-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington’s disease. J Neurosci Res. 2005;82:377–386. doi: 10.1002/jnr.20651. [DOI] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Navia B, Douglas J. Differential expression of preproenkephalin and preprodynorphin mRNAs in striatal neurons: high levels of preproenkephalin expression depend on cerebral cortical afferents. J Neurosci. 1988;8:4755–4764. doi: 10.1523/JNEUROSCI.08-12-04755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg JM, Bjorkqvist M, Brundin P. Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol. 2009;8:765–774. doi: 10.1016/S1474-4422(09)70178-4. [DOI] [PubMed] [Google Scholar]
- van Dijk JG, van der Velde EA, Roos RA, Bruyn GW. Juvenile Huntington disease. Hum Genet. 1986;73:235–239. doi: 10.1007/BF00401235. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Murphy Z, Slow EJ, Leavitt BR, Hayden MR. Selective degeneration and nuclear localization of mutant huntingtin in the YAC128 mouse model of Huntington disease. Hum Mol Genet. 2005;14:3823–3835. doi: 10.1093/hmg/ddi407. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Gibson WT, Pearson J, Murphy Z, Lu G, Leavitt BR, Hayden MR. Body weight is modulated by levels of full-length huntingtin. Hum Mol Genet. 2006;15:1513–1523. doi: 10.1093/hmg/ddl072. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Walker AG, Miller BR, Fritsch JN, Barton SJ, Rebec GV. Altered information processing in the prefrontal cortex of Huntington’s disease mouse models. J Neurosci. 2008;28:8973–8982. doi: 10.1523/JNEUROSCI.2804-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147(Suppl 1):S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience. 2009;159:501–513. doi: 10.1016/j.neuroscience.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler VC, White JK, Gutekunst CA, Vrbanac V, Weaver M, Li XJ, Li SH, Yi H, Vonsattel JP, Gusella JF, Hersch S, Auerbach W, Joyner AL, MacDonald ME. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet. 2000;9:503–513. doi: 10.1093/hmg/9.4.503. [DOI] [PubMed] [Google Scholar]
- White JK, Auerbach W, Duyao MP, Vonsattel JP, Gusella JF, Joyner AL, MacDonald ME. Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat Genet. 1997;17:404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- Wood JD, MacMillan JC, Harper PS, Lowenstein PR, Jones AL. Partial characterisation of murine huntingtin and apparent variations in the subcellular localisation of huntingtin in human, mouse and rat brain. Hum Mol Genet. 1996;5:481–487. doi: 10.1093/hmg/5.4.481. [DOI] [PubMed] [Google Scholar]
- Woodman B, Butler R, Landles C, Lupton MK, Tse J, Hockly E, Moffitt H, Sathasivam K, Bates GP. The Hdh(Q150/Q150) knock-in mouse model of HD and the R6/2 exon 1 model develop comparable and widespread molecular phenotypes. Brain Res Bull. 2007;72:83–97. doi: 10.1016/j.brainresbull.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Wu N, Joshi PR, Cepeda C, Masliah E, Levine MS. Alpha-synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway. J Neurosci Res. 2009 doi: 10.1002/jnr.22327. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-d-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]