Abstract
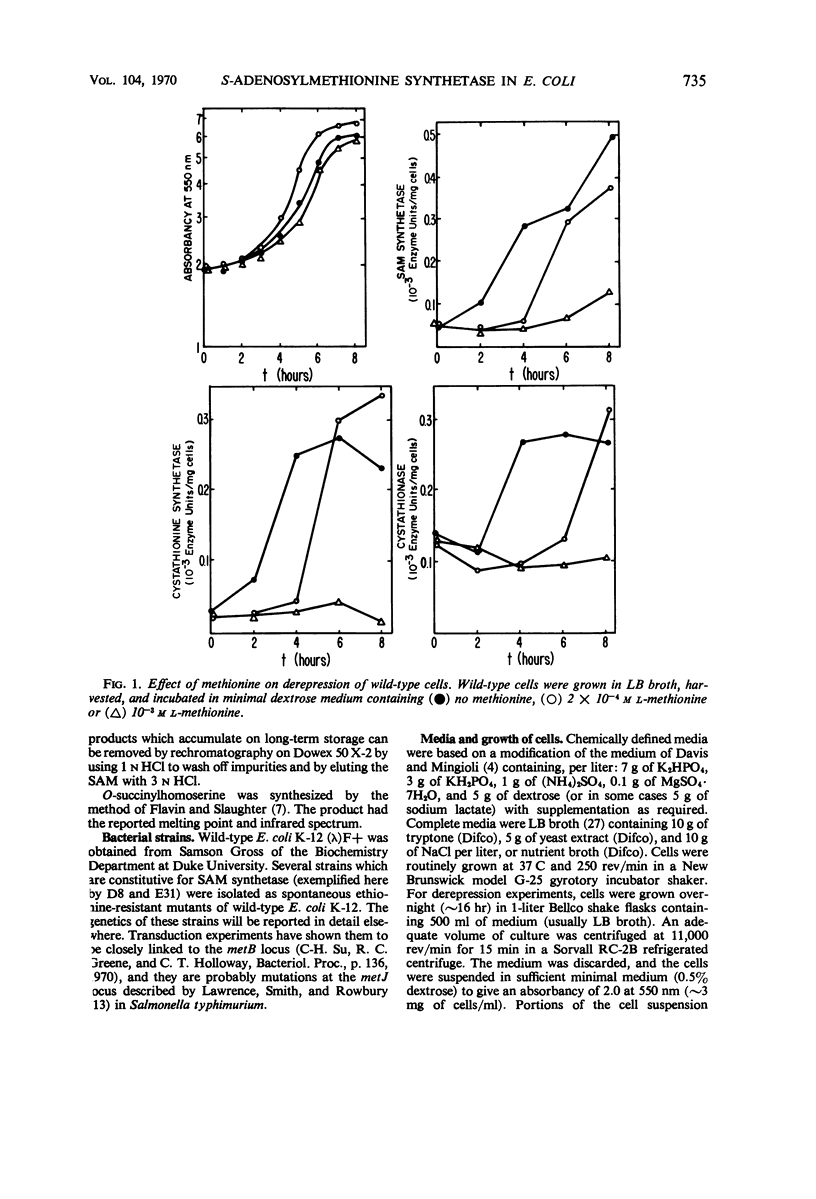
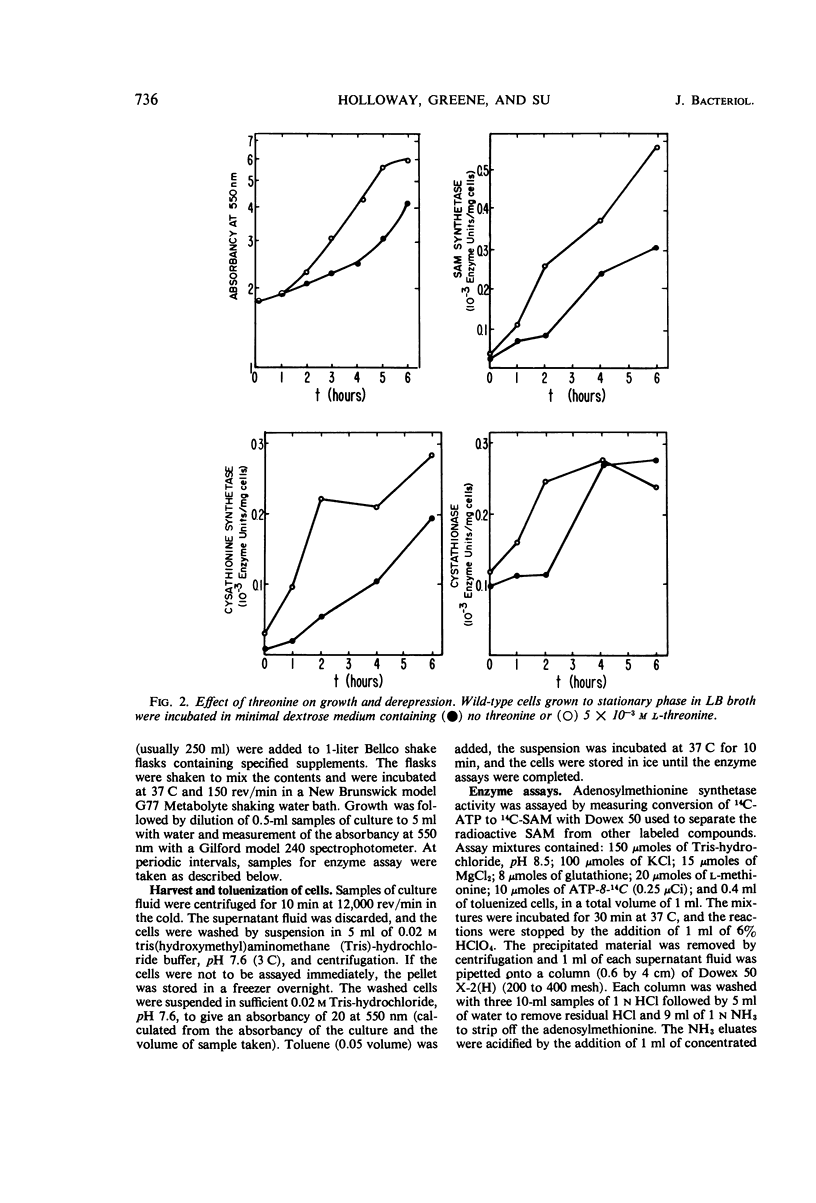
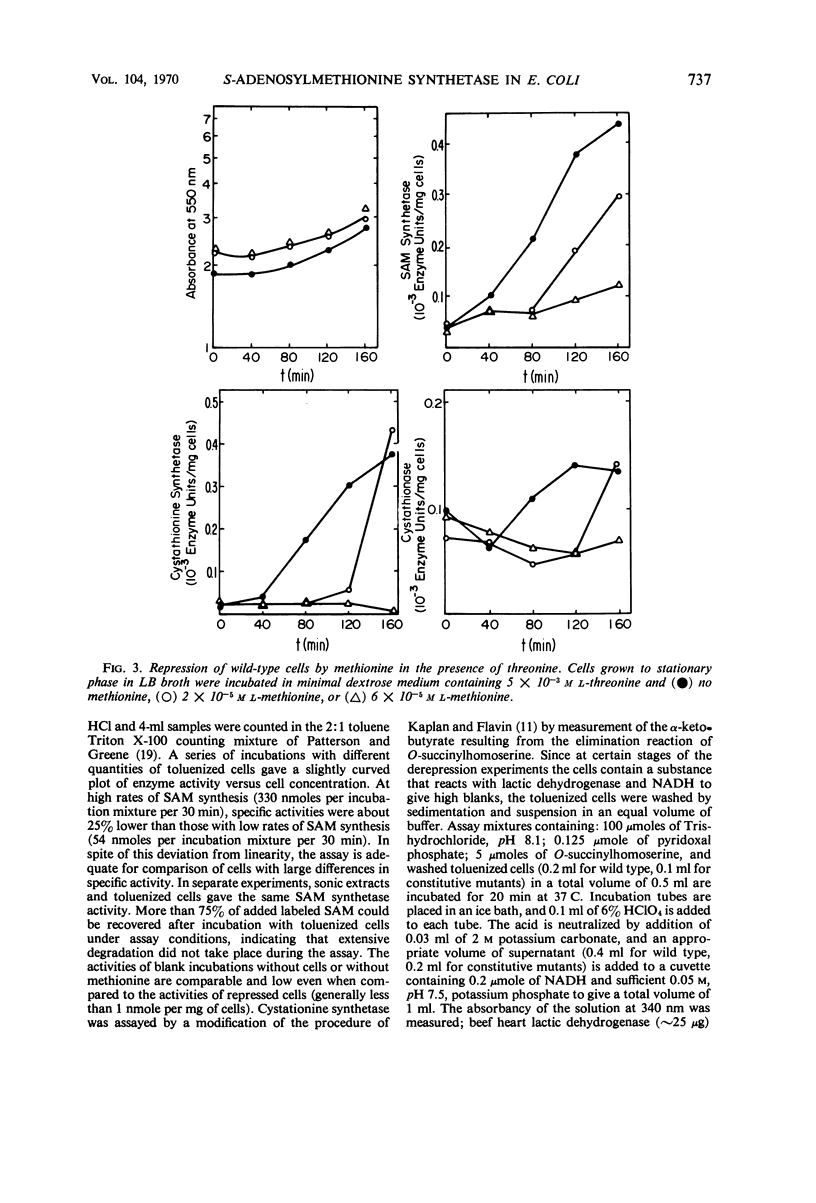
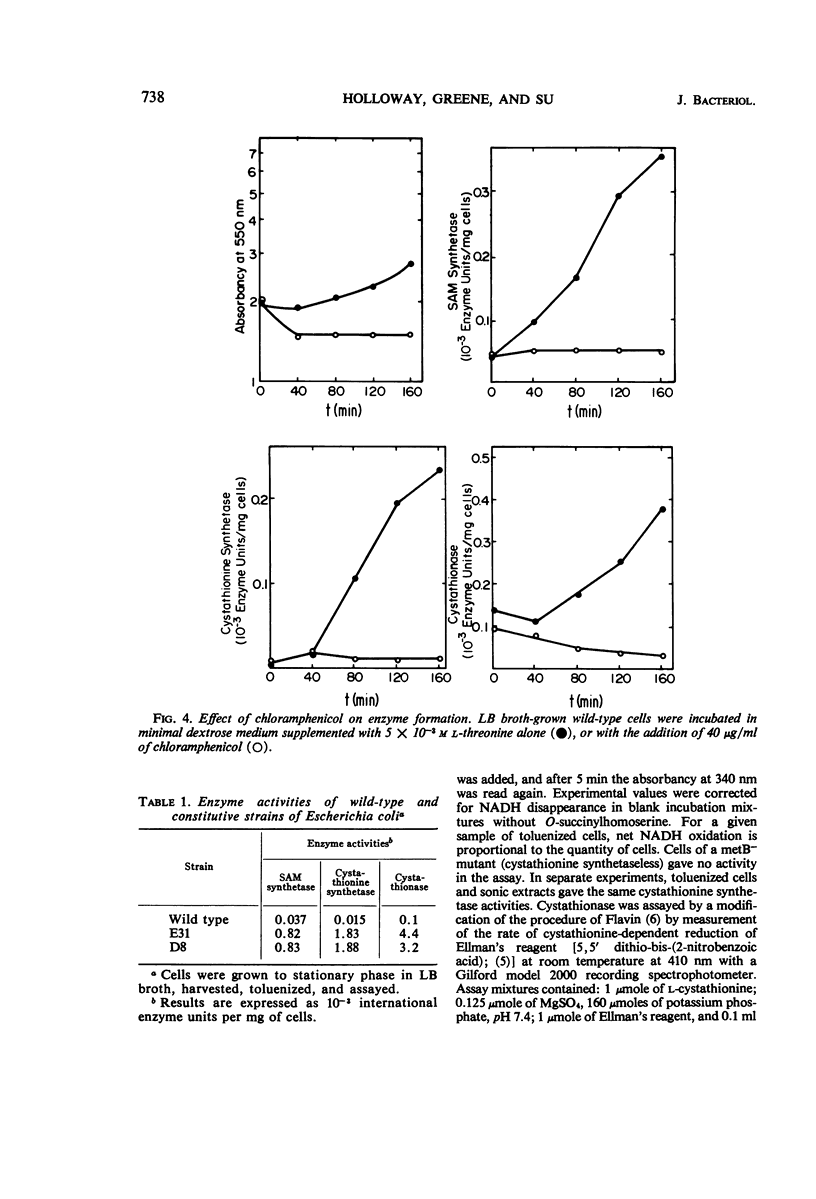
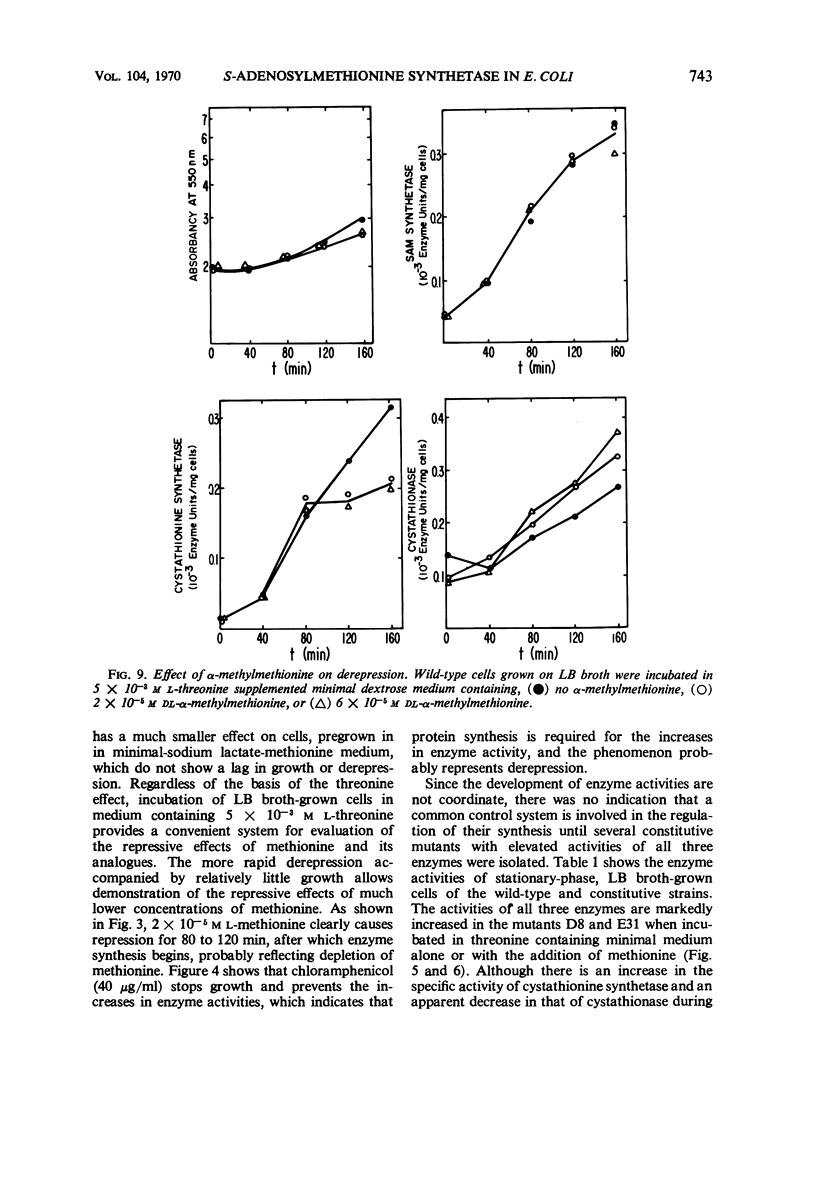
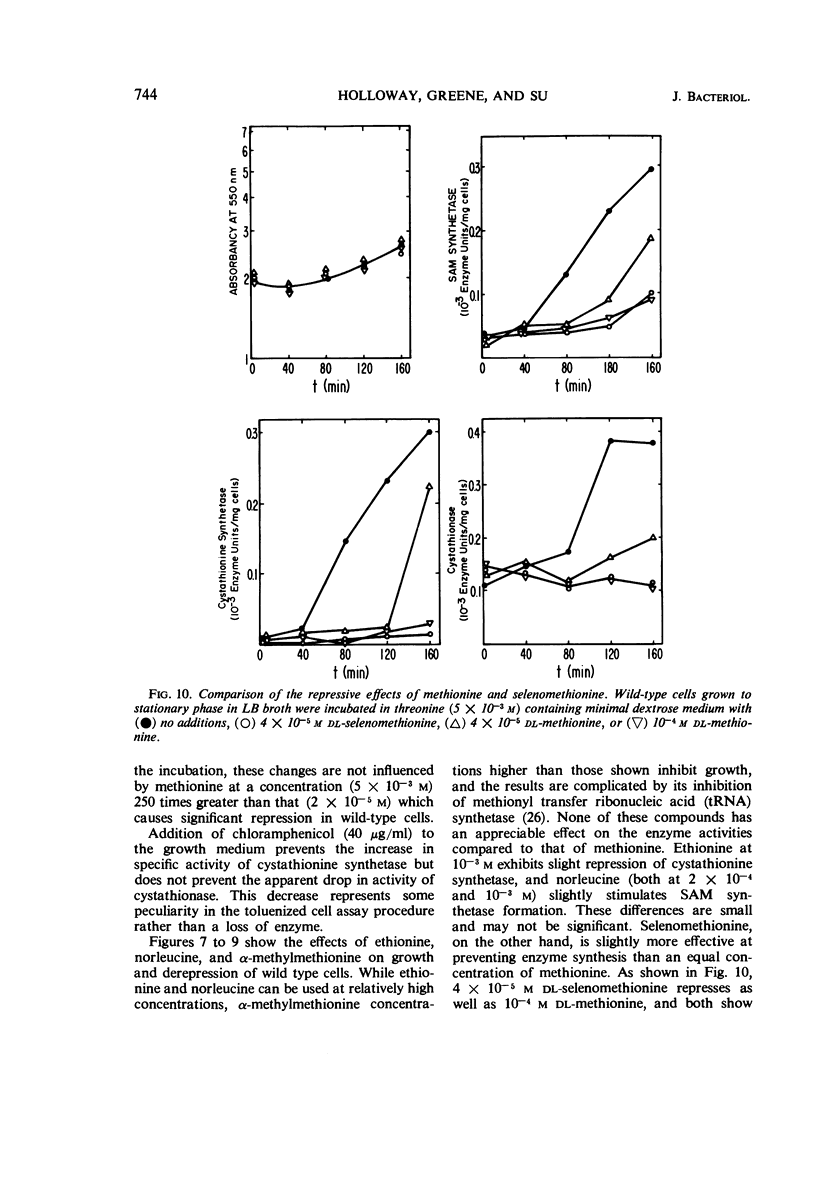
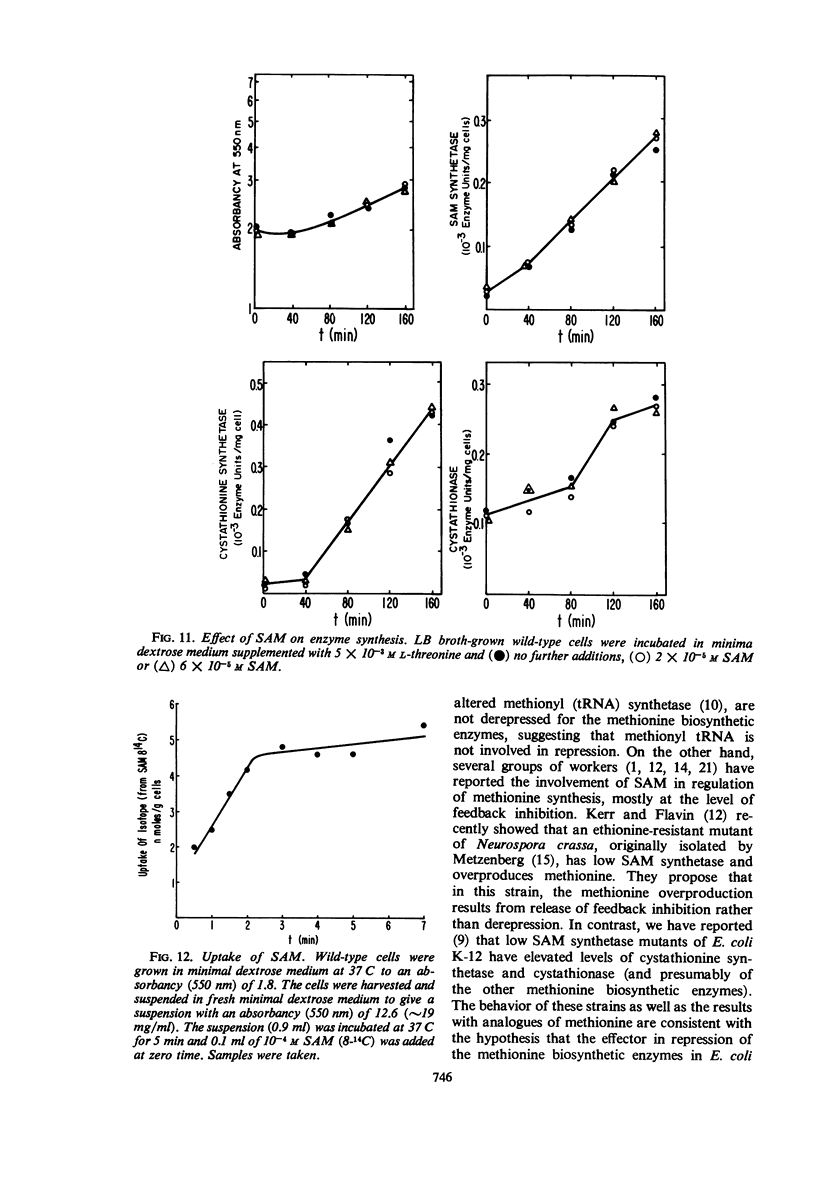
Addition of methionine to the growth medium of Escherichia coli K-12 leads to a reduction in the specific activity of S-adenosylmethionine (SAM) synthetase. Thus the enzyme appears to be repressible rather than inducible. Mutant strains (probably metJ−) are constitutive for SAM synthetase as well as for the methionine biosynthetic enzymes, suggesting that the regulatory systems for these enzymes have at least some elements in common. Cells grown to stationary phase in complete medium, which have low specific activities of the enzymes, were routinely used for derepression experiments. The lag in growth and derepression when these cells are incubated in minimal medium is shortened by threonine. Ethionine, norleucine, and α-methylmethionine are poor substrates or nonsubstrates for SAM synthetase and are ineffective repressors. Selenomethionine, a better substrate for SAM synthetase than methionine, is also slightly more effective at repression than methionine. Although SAM is considered to be a likely candidate for the corepressor in the control of the methionine biosynthetic enzymes, addition of SAM to the growth medium does not cause repression. Measurement of SAM uptake shows that too little is taken into the cells to have a significant effect, even if it were active in the control system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P. Regulation of branched biosynthetic pathways in bacteria. Science. 1969 Aug 8;165(3893):556–562. doi: 10.1126/science.165.3893.556. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FLAVIN M. Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J Biol Chem. 1962 Mar;237:768–777. [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Synthesis of the succinic ester of homoserine, a new intermediate in the bacterial biosynthesis of methionine. Biochemistry. 1965 Jul;4(7):1370–1375. doi: 10.1021/bi00883a022. [DOI] [PubMed] [Google Scholar]
- Greene R. C. Kinetic studies of the mechanism of S-adenosylmethionine synthetase from yeast. Biochemistry. 1969 Jun;8(6):2255–2265. doi: 10.1021/bi00834a004. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Methionyl transfer RNA synthetase mutants of Salmonella typhimurium which have normal control of the methionine biosynthetic enzymes. Biochim Biophys Acta. 1969 Jun 17;184(1):233–236. doi: 10.1016/0304-4165(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Flavin M. Cystathionine gamma-synthetase of Salmonella. Catalytic properties of a new enzyme in bacterial methionine biosynthesis. J Biol Chem. 1966 Oct 10;241(19):4463–4471. [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. The regulation of methionine synthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970 Apr 10;245(7):1842–1855. [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. W., Ravel J. M., Shive W. Multimetabolite control of a biosynthetic pathway by sequential metabolites. J Biol Chem. 1966 Nov 25;241(22):5479–5480. [PubMed] [Google Scholar]
- Metzenberg R. L. Repair of multiple defects of a regulatory mutant of Neurospora by high osmotic pressure and by reversion. Arch Biochem Biophys. 1968 May;125(2):532–541. doi: 10.1016/0003-9861(68)90611-5. [DOI] [PubMed] [Google Scholar]
- PATTE J. C., LE BRAS G., LOVINY T., COHEN G. N. [Retro-inhibition and repression of the homoserine dehydrogenase of Escherichia coli]. Biochim Biophys Acta. 1963 Jan 8;67:16–30. doi: 10.1016/0006-3002(63)91793-1. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- PIGG C. J., SORSOLI W. A., PARKS L. W. INDUCTION OF THE METHIONINE-ACTIVATING ENZYME IN SACCHAROMYCES CEREVISIAE. J Bacteriol. 1964 Apr;87:920–923. doi: 10.1128/jb.87.4.920-923.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. Further studies on the repression of methionine synthesis in Escherichia coli. J Gen Microbiol. 1961 Jan;24:129–144. doi: 10.1099/00221287-24-1-129. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Rowbury R. J. Resistance to norleucine and control of methionine synthesis in Escherichia coli. Nature. 1965 May 29;206(987):962–963. doi: 10.1038/206962a0. [DOI] [PubMed] [Google Scholar]
- Rowbury R. J., Woods D. D. The regulation of cystathionine formation in Escherichia coli. J Gen Microbiol. 1966 Jan;42(1):155–163. doi: 10.1099/00221287-42-1-155. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., DAINKO J. L., STANFORD S. M. Improved procedure for the isolation of S-adenosylmethionine and S-adenosylethionine. Arch Biochem Biophys. 1959 Jul;83(1):28–34. doi: 10.1016/0003-9861(59)90006-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger S. Inhibition of growth of Escherichia coli and of homoserine O-transsuccinylase by alpha-methylmethionine. J Bacteriol. 1967 Aug;94(2):327–332. doi: 10.1128/jb.94.2.327-332.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Genetic studies on bacteriophage P1. Virology. 1968 Dec;36(4):564–574. doi: 10.1016/0042-6822(68)90188-8. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Taylor R. T., Dickerman H., Weissbach H. Control of one-carbon metabolism in a methionine-B12 auxotroph of Escherichia coli. Arch Biochem Biophys. 1966 Nov;117(2):405–412. doi: 10.1016/0003-9861(66)90429-2. [DOI] [PubMed] [Google Scholar]
- Truffa-Bachi P., Cohen G. N. Some aspects of amino acid biosynthesis in microorganisms. Annu Rev Biochem. 1968;37:79–108. doi: 10.1146/annurev.bi.37.070168.000455. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- WIJESUNDERA S., WOODS D. D. Suppression of methionine synthesis in Escherichia coli by growth in the presence of this amino acid. J Gen Microbiol. 1960 Feb;22:229–241. doi: 10.1099/00221287-22-1-229. [DOI] [PubMed] [Google Scholar]



