Introduction
The last few years have witnessed an explosive growth of interest in the use of B cell targeted therapies for the treatment of a wide assortment of autoimmune diseases. As recently reviewed elsewhere, the use of this approach –initially pioneered for the therapy of non-Hodgkin's B cell lymphomas and later for Rheumatoid Arthritis (RA) and Systemic Lupus SLE (SLE)- has spread both in clinical studies and clinical practice to multiple rheumatic diseases such as Sjogren's syndrome, vasculitis and dermatomyositis; neurological diseases (multiple sclerosis, neuromyelitis optica, antibody-mediated paraneoplastic syndromes and IgM-mediated polyneuropathy); endocrinopathies (including Type 1 Diabetes and Graves disease), dermatological conditions (autoimmune blistering diseases); and hematological syndromes (ITP, autoimmune hemolytic anemia and TTP) among many other clinical entities 1, 2. As it might be expected due to regulatory, ethical and financial considerations, and as formal, randomized, controlled prospective studies are completed, the main use of this therapeutic modality has centered on patients refractory to conventional therapy as dictated by current clinical practice for the disease in question. In this review, I shall summarize the status of knowledge regarding the rationale and evidence for the use of B cell depletion therapy (BCDT) in different autoimmune diseases. Diseases in which the use of BCDT has been most thoroughly tested or may otherwise provide useful information to guide current clinical utilization and/or future studies will represent the focus if this review.
This review will also focus on the use of B cell depletion induced by Rituximab as this drug is now commonly used in clinical practice and provides the vast majority of the information available. Rituximab is a chimeric mouse anti-human CD20 monoclonal antibody initially developed and FDA–approved for the treatment of follicular lymphoma. Due to the pattern of expression of CD20 Rituximab targets a wide swath of the B cell compartment spanning from pre-B cell to activated memory cells and a fraction of pre-plasma cells while largely sparing pro-B cells as well as fully differentiated plasma cells. Its pharmacokinetics and the multiple ways in which Rituximab mediates B cell depletion (including direct B cell apoptosis, complement-mediated cytotoxicity, antibody-dependent cell-mediated killing and immune complex decoy actions) have been extensively reviewed in multiple forums and will not be further discussed here 3. Similarly, other agents that directly or indirectly target different B cell subsets will not be reviewed here.
Rationale and working models to understand B cell depletion in autoimmune diseases
Autoantibodies are considered critical contributors to the pathogenesis of most if not all autoimmune diseases whether through the generation of pro-inflammatory circulating immune complexes (conventional Type3 hypersensitivity reaction), antibody-mediated cytotoxicity (type
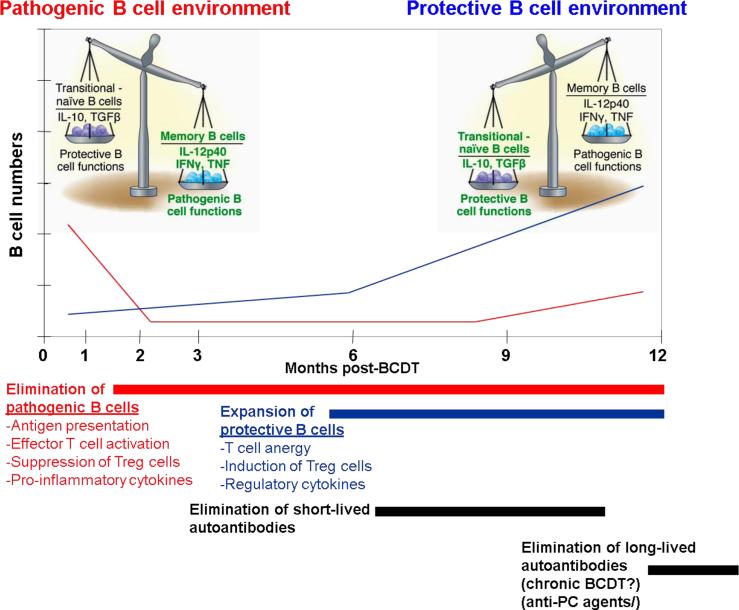
2 hypersensitivity) or as inducers of type IFN production 4, 5. Of note however, the actual contribution of autoantibodies to disease is far from clear in many conditions, may differ between diseases, within disease subsets and even for different types of autoantibodies within a single disease. It is also critical to consider that, as further discussed below in different sections, B cell depletion may also interrupt multiple and powerful antibody-independent pathogenic B cell functions which have been by now abundantly documented in animal models and to a lesser extent in humans and include autoantigen-presentation and activation of effector T cells, production of pro-inflammatory cytokines, inhibition of regulatory T cells and formation of ectopic lymphoid tissue within target organs 6, 7. AT the same time, antibody-independent B cell functions (REF), may also play protective roles in the regulation of autoimmunity and is therefore likely that the final outcome of BCDT therapy will rest upon the balance between the relative depletion and reconstitution of different B cell subsets mediating either protective or pathogenic functions 6. These considerations are essential to frame any discussion of the indications, outcome measurements and interpretation of results, whether positive or not, of BCDT. Moreover, the lessons learned from such considerations should help inform the design of more effective strategies (whether they are different, synergistic or complementary) (Figure 1).
Figure 1. The different phases and mechanisms of action of B cell depletion therapy.
As indicated by multiple studies in different autoimmune diseases, clinical benefit may be evident shortly after the decline of peripheral B cells and before significant changes in autoantibody levels. These observations suggest that the initial benefit of this therapy is mediated at least in part by the elimination of antibody-independent pathogenic B cell functions. Chronologically, at least in some diseases, significant decreases in autoantibodies (such as dsDNA, ANCA and RF) may ensue after several weeks presumably reflecting the depletion of short-lived plasma cells secondary to the elimination of their B cell precursors and of a fraction of immature plasma cell expression some level of surface CD20. Whether chronic depletion of B cells induced by repeated courses of Rituximab will eventually result in significant declines of long-lived, CD20- plasma cells and of the autoantibodies they produce remains to be established. If so, this change (which could also potentially be achieved in combination with other agents that directly or indirectly impact plasma cell survival) could have profound consequences for the long-term course of the disease. In any case, animal experiments as well as observations in a subset of SLE patients with long-term remissions after BCDT strongly suggest that in at least some diseases or disease subsets, the delayed but critical benefit of rituximab might be the promotion of expansion of B cells with protective functions that might include the induction of T cell anergy, promotion of Treg cells and the production of regulatory cytokines. While for illustration purposes this figure assigns pathogenic and regulatory functions to memory and transitional/naïve cells respectively, the actual division of labor between B cell subsets remains to be elucidated and is likely to be significantly more complex than depicted here.
Currently, the relative contributions of the above mechanisms to different autoimmune manifestations and consequently, the actual mechanisms whereby B cell depletion may improve disease remain to be formally elucidated. B cell depletion was initially conceived for autoimmune diseases as an attempt to eliminate autoantibodies, an outcome which could be augmented by the concurrent use of other drugs (such as high-dose corticosteroids or cyclophosphamide 8). Indeed, B cells represent the cellular source of antibody-producing plasma cells (PC) and therefore, complete and sustained elimination of B cells would ideally result in interruption of the PC supply chain and the eventual elimination of autoantibody-producing PC. An obvious implication of the antibody-based model is that effective therapy could also induce elimination of the PC responsible for the maintenance of protective serological memory through the production of anti-microbial antibodies. This untoward effect however has not been generally observed with the possible exception of patients of pediatric age which may be more prone to developing hypogammaglobulinemia. The relative lack of impact of B cell depletion in global antibody and protective antibody levels can be explained if these antibodies derive from long-lived PC that are insensitive to Rituximab due to lack of expression of CD20 and which are relatively independent of precursor B cell for their replenishment or survival 9, 10. This model however, begs the question of whether PC of similar properties are also responsible for the generation of pathogenic autoantibodies and if so, whether the persistence of such PC could explain lack of response to Rituximab despite effective B cell depletion 10. By and large, these critical questions remain to be formally answered. However, it is well established that some autoantibodies such as Rheumatoid Factor (RF), anti-ds DNA, anti-C1q and anti-neutrophil-cytoplasm antibodies (ANCA) and possibly anti-phospholipid antibodies appear to decline weeks or months after Rituximab therapy probably reflecting their provenance from short-lived plasma cells that require B cell replenishment. In contrast, other autoantibodies such anti-cyclic citrullinated peptide antibodies decrease to a much lesser extent and yet others like anti-Smith/RNP and anti-Ro and La RNA-binding protein autoantibodies are impervious to BCDT most likely reflecting their production by long-lived plasma cells 11.
As recently reviewed elsewhere 12, the relative role of autoantibody decline in the clinical benefit of BCDT remains unclear with arguments for and against this conclusion. While it is likely that decreases in at least some autoantibodies may play an important role in the clinical benefit of Rituximab, it is important to point out that such correlation is limited as indicated by the significant clinical benefit often observed before significant changes in autoantibody levels and the frequent lack of correlation between autoantibody titers and disease response or relapse 11, 13, 14. Hence, careful studies of other parameters of B cell function will be critical to fully understand, monitor and augment the clinical benefits of Rituximab.
Rituximab in Rheumatoid Arthritis
RA remains the only non-malignant conditions for which Rituximab has received FDA approval. Based on strong clinical benefit in controlled studies, current practice centers on the use of Rituximab together with methotrexate for the treatment of cases refractory to anti-TNF agents 15. Such approach has also been shown to have a beneficial impact on radiological progression 16. While the duration of clinical benefit may vary and includes long-standing responses in a small fraction of patients, most patients who respond favorably to the initial treatment will experience relapses 6-12 after treatment with clinical reactivation roughly correlating with B cell repopulation. This observation has prompted the consensus recommendation of retreatment with repeated courses of Rituximab every 6-9 months for patients with disease reactivation 15. While more studies will be required to identify accurate biomarkers of response and retreatment, strong studies have already shown that the degree of B cell depletion initially obtained (even after the initial infusion in the first treatment cycle) appears to correlate with clinical benefit 17. Interesting studies, albeit limited in size, also suggest that lack of initial response may predict lack of response to subsequent cycles of Rituximab 18. Similarly, good responders to the initial treatment appear to respond equally well to repeated courses. Of significant importance, serious infusion reactions or other severe adverse effects including serious infections do not appear to increase in RA patients after several cycles of Rituximab treatment 19. Whether this safety profile can and will be maintained with indefinite treatment cycles and in essence chronic B cell depletion remains to be elucidated. Also of note, recent studies support the efficacy and safety of Rituximab treatment in RA patients who have failed other biological agents including abatacept 16, 17, 20-23
Rituximab in Multiple Sclerosis
Rituximab-induced BCDT has shown highly promising results in the relapsing remitting form of multiple sclerosis (RRMS) in a phase II double-blind study in which a very significant decrease in total and new gadolinium enhancing lesions was observed was observed as early as week 12 after treatment 14. This benefit was sustained over the 48 weeks of follow up after a single cycle of Rituximab was administered. In this study, the rapid clinical benefit observed in the absence of significant decreases in antibody levels suggested that at least in part the benefit of Rituximab could be mediated by the interruption of other pathogenic B cell functions such as antigen presentation and production of pro-inflammatory cytokines rather than mere autoantibody decrease 24. Similar improvements, starting as early as 4 weeks and lasting for at least 72 weeks after treatment have been observed in an open label, phase I trial using two courses of Rituximab 6 months apart 25. However, more definitive studies will be needed to fully establish the benefit of BCDT in RRMS and in particular its impact on cumulative disability and secondary progression, a degenerative phase of the disease that could be curtailed at least in part by early treatment of the initial inflammatory component 26. Disappointing results however have been recently published from a randomized double-blind placebo-controlled multicenter trial of rituximab in primary progressive MS 27. Of note, this study nonetheless identified significant improvement in younger patients when predetermined subset analyses were performed.
Rituximab in ANCA-mediated vasculitis
Early open label studies of Rituximab suggested a striking benefit of this intervention in refractory ANCA-mediated vasculitis (AMV). One striking feature of at least some of the initial studies was a steep decline in ANCA titers 28, 29. The interpretation of these changes is complicated by the concomitant use of high dose corticosteroids in one study 28 and the observation in another study that clinical response often preceded significant decline in autoantibody titers 30. Nevertheless, these studies dovetailed nicely with strong experimental evidence indicating a pathogenic role of ANCA autoantibodies and opened the door to large, randomized controlled studies currently underway (RITUXVAS and RAVE). Meanwhile, several subsequent reports appear to substantiate the potential benefit of rituximab at least for the systemic, inflammatory component of the disease process 31. This potential has been perhaps best summarized by a recent retrospective analysis of a multicenter cohort of 65 patients treated with Rituximab for refractory AMV. Only 2% experienced no favorable response while 75% of patients achieved complete remission. Interestingly, close to 60% of patients experienced a relapse and in approximately 50% of them B cell reaccumulation preceded relapse. While ANCA levels fell significantly in most patients, no correlation was observed between ANCA positivity or titer elevation and relapse 32.
The response to BCDT of the more chronic, granulomatous manifestations of the disease including head and neck manifestations remains controversial with initial reports pointing to lack of response and more recent studies suggesting that Rituximab may also provide substantial benefit for granulomatous complications of ANCA-mediated vasculitis 33-36.
Rituximab in Type 1 Diabetes
While most immunological and therapeutic studies of type 1 diabetes (T1D), have concentrated on T cells, autoantibodies are almost universal markers of this disease and when appropriately used represent a major risk factor for disease development. This fact, together with numerous animal studies indicating a major role for B cells in the pathogenesis of T1D led to the design and implementation of a clinical trial of Rituximab in patients with new onset T1D whose initial results were recently presented at the annual meeting of the American Diabetes Association held in New Orleans in June 2009 37. The very limited results available in the public domain indicate a protective effect of Rituximab in disease progression in newly diagnosed patients as indicated by statistically significant differences in meal stimulation test-induced C-peptide values between patients treated with rituximab versus placebo treated patients after 1 year of follow-up. These promising results are in keeping with several papers published over the last couple of years indicating significant benefit for other B cell targeting therapies (anti-CD20, anti-CD22, anti-BLyS), in animal models of autoimmune diabetes 37,38-42. Of great interest, these studies uncovered different mechanisms of action of BCDT including elimination of pathogenic B cells, induction of T regulatory cells and expansion of B cell regulatory cells with protective functions that include the production of IL-10. Combined, these observations generate great hope and provide a major impetus to pursue future studies of B cell therapies to ameliorate established disease, prevent or delay full development in early cases and even prevent disease onset by early treatment of high risk patients.
Rituximab in SLE
The actual benefit of Rituximab in SLE continues to baffle physicians and scientists alike. From a pathophysiological standpoint, this disease characterized by high levels of multiple autoantibodies and florid abnormalities of B cell homeostasis and activation would appear to be an ideal target for BCDT. Indeed, and in keeping with this notion, early open label studies indicted substantial benefit in SLE including induction of long-term remissions with a single course of Rituximab in the absence of concomitant therapy. Yet, two relatively large, well controlled, randomized studies of non-nephritis SLE and of lupus nephritis (Explorer and Lunar) failed to meet their corresponding primary and secondary endpoints leading to the general conclusion that Rituximab is not superior to conventional therapy in SLE 43. These negative results stand in stark contrast to the accumulating experience of expert clinicians which continues to indicate substantial clinical efficacy of Rituximab in refractory SLE mostly when administered in combination with cyclophosphamide 44, 45.
While it is worth noting that subset analysis of Explorer appears to indicate a significant benefit of Rituximab on serological responses and also a clinical benefit in African-americans and Hispanic patients, the results have been nonetheless disappointing and have prompted a reexamination of study design, outcome measurements and length of follow-up, all areas of significant concern for the studies in question 46. Moreover, these trials have brought to the fore, once again, severe limitations in our knowledge and ability to define disease heterogeneity thereby restricting the investigators ability to identify disease subsets that might be responsive to BCDT. Finally, the negative results obtained with Rituximab also raise concerns regarding the potential of this drug to eliminate not only pathogenic B cells but also regulatory B cells. As illustrated in Figure 1, the latter activity might help reconcile diverging results in different diseases or between disease subsets within a single autoimmune condition if in fact expansion of regulatory B cells are be critical to disease recovery in some cases but not in others. Thus, diseases in which B cell regulatory cells play no major role would benefit from sustained BCDT resulting in elimination of autoantibodies and pathogenic B cells. These cases would be most suitable for recurrent treatment cycles as it has become the practice in RA. In contrast, diseases where post-depletion expansion of a B cell regulatory population may be critical would not benefit from repeated cycles of Rituximab which might delay the emergence of the protective cells. Of note, this caveat would apply to both SLE studies which included a second course of treatment six months after the initial one irrespective of clinical response or type of B cell repopulation. The possibility that indiscriminate B cell depletion (whether in scope or timing of depletion) may not be the best avenue in all cases is indirectly supported by encouraging results recently reported with drugs that have weaker and more selective effects on different B cell subsets such as belimumab and epratuzumab. Undesirable consequences of the elimination of B cells have been powerfully illustrated by a recent report of increased allograft rejection in renal transplant patients treated with Rituximab 47. Clearly, understanding the relative merits of depleting or facilitating the expansion of specific B cell subsets remains a major challenge in the field whose resolution would undoubtedly lead to more intelligent design of drugs and combination therapies aimed at achieving such goals.
Clinical use and monitorization
In most clinical situations Rituximab is now used as two 1 g IV infusions two weeks apart. There is no evidence that the original “lymphoma” regimen of 4 infusions of 375 mg/m2 provides significant advantages and has the drawback of inconvenience to the patient. As discussed in multiple reviews, concurrent medication with corticosteroids and antihistamines is typically used to minimize infusion reactions which may be more frequent in SLE. When measured by conventional flow cytometry and defined as a level below 5 CD19+ cells/μl of blood, good B cell depletion is almost universally observed with the exception of approximately 10% of SLE patients, a fraction that is further decreased by concomitant use of corticosteroids or cyclophosphamide. While routine monitorization of B cell counts or quality of B cell reconstitution does not currently guide clinical use of the drug, these parameters may be informative and should be encouraged at least in research settings. As previously discussed, it is now common practice to retreat patients with RA every 6-9 months and when repeated up to 6-8 times this approach has not yet resulted in any significant increase in major adverse effects although serum IgM levels appear to significantly decline with repeated infusions. For any other diseases, as illustrated by SLE, Rituximab is used by and large as an off-label indication for conditions refractory to conventional therapy.
While serious infections do not appear to be substantially increased even with repeated administration of Rituximab, the report of several cases of Progressive Multifocal Leukoencephalopathy in non-HIV, autoimmune patients treated with Rituximab has created significant concern 48-50. Thus far, only 5 cases of PML have been reported in Rituximab-treated autoimmune diseases: 2 cases of SLE, one case of RA and also single cases of Wegener's granulomatosis and other vasculitis. The interpretation of these reports is complicated by the occurrence of PML in similar situations in the absence of Rituximab and therefore whether this drug actually increases the risk of PML remains to be formally determined. However, given the relatively small number of SLE patients treated with Rituximab, this possibility should be entertained specially in patients previously treated with multiple other drugs (as it was the case for both SLE patients and the RA case who also suffered from oropharyngeal cancer). Accordingly, current recommendations include obtaining informed consent before starting treatment and careful monitorization of symptoms of PML. Should the suspicion arise on the basis of clinical surveillance, proper diagnosis should be pursued and Rituximab discontinued if established. Whether routine surveillance of JC viral replication in asymptomatic patients will prove of value to predict development of clinical infection remains to be determined (51).
While the impact of Rituximab on pre-existing immunological memory remains to be established (mostly in patients with chronic depletion), current guidelines for immunization 22, 10, include trying to administer the seasonal influenza vaccine and other clinically indicated vaccines as required to boost pre-existing immunity (tetanus boost) before treatment (ideally, a 4 week intervening period would maximize the memory B cell boost expected from the vaccine albeit as little as 1-2 weeks is likely to substantially enhance the generation of at least short-lived plasma cell responses). The necessary window of time required to optimize vaccine responses to neoantigens (hepatitis vaccines when indicated for high risk patients and travel related vaccines) before administration of Rituximab in unknown. However, it would make sense to administer such vaccines as early as possible (several weeks to promote the generation and expansion of memory) and to make every effort to do so before treatment. It remains to be determined whether pre-existing serological and cellular memory will be eventually erased in patients treated with chronic B cell depletion. Should that occur, these patients might need to be vaccinated anew against the same agents as in early life.
Acknowledgements
Supported in part by NIH U19 AI56390, Rochester Autoimmunity Center of Excellence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Looney RJ. B cells as a therapeutic target in autoimmune diseases other than rheumatoid arthritis. Rheumatology. 2005;44:ii13–7. doi: 10.1093/rheumatology/keh618. [DOI] [PubMed] [Google Scholar]
- 2.Dörner T, Isenberg D, Jayne D, Wiendl H, Zillikens D, Burmester G. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmunity Reviews. doi: 10.1016/j.autrev.2009.08.007. In Press. Accepted Manuscript - doi:10.1016/j.autrev.2009.08.007 < http://dx.doi.org/10.1016/j.autrev.2009.08.007>. [DOI] [PubMed]
- 3.Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease--the immune complex decoy hypothesis. Nat Clin Pract Rheumatol. 2007;3:86–95. doi: 10.1038/ncprheum0424. [DOI] [PubMed] [Google Scholar]
- 4.Martin F, Chan AC. B Cell Immunobiology in Disease: Evolving Concepts from the Clinic. Annual Review of Immunology. 2006;24 doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 5.Lövgren T, Maija-Leena E, Gunnar E, Alm V, Rönnblom L. Induction of interferon-a production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis & Rheumatism. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 6.Manjarrez-Orduno N, Quach TD, Sanz I. B Cells and Immunological Tolerance. J Invest Dermatol. 2009;129:278–88. doi: 10.1038/jid.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund FE. Cytokine-producing B lymphocytes -- key regulators of immunity. Current Opinion in Immunology. 2008;20:332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JCW, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology. 2001;40:205–11. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proceedings of the National Academy of Sciences. 2008:0800555105. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Looney RJ, Srinivasan R, Calabrese LH. The effects of rituximab on immunocompetency in patients with autoimmune disease. Arthritis & Rheumatism. 2008;58:5–14. doi: 10.1002/art.23171. [DOI] [PubMed] [Google Scholar]
- 11.Cornec D, Avouac J, Youinou P, Saraux A. Critical analysis of rituximab-induced serological changes in connective tissue diseases. Autoimmunity Reviews. doi: 10.1016/j.autrev.2009.01.007. In Press. Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 12.Leandro MJ, Torre Idl. Translational Mini-Review Series on B Cell-Directed Therapies: The pathogenic role of B cells in autoantibody-associated autoimmune diseases – lessons from B cell-depletion therapy. Clinical & Experimental Immunology. 2009;157:191–7. doi: 10.1111/j.1365-2249.2009.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism. 2004;50:2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 14.Hauser SL, Waubant E, Arnold DL, et al. B-Cell Depletion with Rituximab in Relapsing-Remitting Multiple Sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 15.Smolen JS, Emery P, Keystone EC, et al. Consensus Statement on the Use of Rituximab in Patients With Rheumatoid Arthritis. Ann Rheum Dis. 2006:ard.2006.061002. doi: 10.1136/ard.2006.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keystone EC, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in rheumatoid arthritis patients with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2008:ard.2007.085787. doi: 10.1136/ard.2007.085787. [DOI] [PubMed] [Google Scholar]
- 17.Dass S, Rawstron AC, Vital EM, Henshaw K, McGonagle D, Emery P. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis & Rheumatism. 2008;58:2993–9. doi: 10.1002/art.23902. [DOI] [PubMed] [Google Scholar]
- 18.Thurlings RM, Vos K, Gerlag DM, Tak PP. Disease activity-guided rituximab therapy in rheumatoid arthritis: The effects of re-treatment in initial nonresponders versus initial responders. Arthritis & Rheumatism. 2008;58:3657–64. doi: 10.1002/art.24035. [DOI] [PubMed] [Google Scholar]
- 19.Keystone E, Fleischmann R, Emery P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: An open-label extension analysis. Arthritis & Rheumatism. 2007;56:3896–908. doi: 10.1002/art.23059. [DOI] [PubMed] [Google Scholar]
- 20.Edward Keystone RFPEDEFRvVJBMDABGFACJUMWCSAS Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: An open-label extension analysis. Arthritis & Rheumatism. 2007;56:3896–908. doi: 10.1002/art.23059. [DOI] [PubMed] [Google Scholar]
- 21.Thurlings KV Rogier M., Gerlag Daniëlle M., Tak Paul P. Disease activity-guided rituximab therapy in rheumatoid arthritis: The effects of re-treatment in initial nonresponders versus initial responders. Arthritis & Rheumatism. 2008;58:3657–64. doi: 10.1002/art.24035. [DOI] [PubMed] [Google Scholar]
- 22.Smolen JS, Keystone EC, Emery P, et al. Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:143–50. doi: 10.1136/ard.2006.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breedveld F, Agarwal S, Yin M, et al. Rituximab Pharmacokinetics in Patients With Rheumatoid Arthritis: B-Cell Levels Do Not Correlate With Clinical Response. J Clin Pharmacol. 2007;47:1119–28. doi: 10.1177/0091270007305297. [DOI] [PubMed] [Google Scholar]
- 24.McFarland HF. The B Cell -- Old Player, New Position on the Team. N Engl J Med. 2008;358:664–5. doi: 10.1056/NEJMp0708143. [DOI] [PubMed] [Google Scholar]
- 25.Amit Bar-Or PAJCDACMSSLHKEWSGRJFMPNSSA Rituximab in relapsing-remitting multiple sclerosis: A 72-week, open-label, phase I trial. Annals of Neurology. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 26.Hauser SL. Multiple Lessons for Multiple Sclerosis. N Engl J Med. 2008;359:1838–41. doi: 10.1056/NEJMe0806738. [DOI] [PubMed] [Google Scholar]
- 27.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: Results of a randomized double-blind placebo-controlled multicenter trial. Annals of Neurology. 2009;9999:NA. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 28.Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis & Rheumatism. 2005;52:262–8. doi: 10.1002/art.20718. [DOI] [PubMed] [Google Scholar]
- 29.Stasi R, Stipa E, Del Poeta G, Amadori S, Newland AC, Provan D. Long-term observation of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis treated with rituximab. Rheumatology. 2006;45:1432–6. doi: 10.1093/rheumatology/kel098. [DOI] [PubMed] [Google Scholar]
- 30.Smith KGC, Jones RB, Burns SM, Jayne DRW. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: Remission, relapse, and re-treatment. Arthritis & Rheumatism. 2006;54:2970–82. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- 31.Aouba A, Pagnoux C, Bienvenu B, Mahr A, Guillevin L. Analysis of Wegener's Granulomatosis Responses to Rituximab: Current Evidence and Therapeutic Prospects. Clinical Reviews in Allergy and Immunology. 2008;34:65–73. doi: 10.1007/s12016-007-8026-1. [DOI] [PubMed] [Google Scholar]
- 32.Jones RB, Ferraro AJ, Chaudhry AN, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis & Rheumatism. 2009;60:2156–68. doi: 10.1002/art.24637. [DOI] [PubMed] [Google Scholar]
- 33.Aries PM, Hellmich B, Both M, et al. Lack of efficacy of Rituximab in Wegener's Granulomatosis with refractory granulomatous manifestations. Ann Rheum Dis. 2005:ard.2005.044420. doi: 10.1136/ard.2005.044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor SRJ, Salama AD, Joshi L, Pusey CD, Lightman SL. Rituximab is effective in the treatment of refractory ophthalmic Wegener's granulomatosis. Arthritis & Rheumatism. 2009;60:1540–7. doi: 10.1002/art.24454. [DOI] [PubMed] [Google Scholar]
- 35.Pero MMD, Chaudhry A, Jones RB, Sivasothy P, Jani P, Jayne D. B-cell depletion with rituximab for refractory head and neck Wegener's granulomatosis: a cohort study. Clinical Otolaryngology. 2009;34:328–35. doi: 10.1111/j.1749-4486.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 36.Seo P, Specks U, Keogh KA. Efficacy of Rituximab in Limited Wegener’s Granulomatosis with Refractory Granulomatous Manifestations. The Journal of Rheumatology. 2008;35:2017–23. [PubMed] [Google Scholar]
- 37.O'Neill SK, Liu E, Cambier JC. Change you can B(cell)eive in: recent progress confirms a critical role for B cells in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2009 doi: 10.1097/MED.0b013e32832e06a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SH, Tedder TF. Targeting B-cells Mitigates Autoimmune Diabetes in NOD Mice: What Is Plan B? Diabetes. 2009;58:1479–81. doi: 10.2337/db09-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiu Y, Wong CP, Bouaziz J-D, et al. B Lymphocyte Depletion by CD20 Monoclonal Antibody Prevents Diabetes in Nonobese Diabetic Mice despite Isotype-Specific Differences in Fc{gamma}R Effector Functions. J Immunol. 2008;180:2863–75. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 40.Bouaziz J-D, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proceedings of the National Academy of Sciences. 2007:0709205105. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C-y, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–67. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorina P, Vergani A, Dada S, et al. Targeting CD22 reprograms B cells and reverses autoimmune diabetes. Diabetes. 2008:db08–0420. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanz I. Ther conundrum of B cell depletion in SLE. Nature Reviews Rheumatology. 2009;5:304–5. doi: 10.1038/nrrheum.2009.100. [DOI] [PubMed] [Google Scholar]
- 44.Lu T, Ng KP, Cambridge G, et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at university college london hospital: The first fifty patients. Arthritis Care & Research. 2009;61:482–7. doi: 10.1002/art.24341. [DOI] [PubMed] [Google Scholar]
- 45.Ramos-Casals M, Soto M, Cuadrado M, Khamashta M. Rituximab in systemic lupus erythematosusA systematic review of off-label use in 188 cases. Lupus. 2009;18:767–76. doi: 10.1177/0961203309106174. [DOI] [PubMed] [Google Scholar]
- 46.Merrill JT, xx a, aa x, et al. Efficacy and Safety of Rituximab in Patients with Moderately to Severely Active Systemic Lupus Erythematosus (SLE): Results from the Randomized, Double-blind Phase II/III Study EXPLORER. ACR. 2008 Abstract L12 2008. [Google Scholar]
- 47.Clatworthy MR, Watson CJE, Plotnek G, et al. B-Cell-Depleting Induction Therapy and Acute Cellular Rejection. N Engl J Med. 2009;360:2683–5. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genovese MC, Breedveld FC, Emery P, et al. Safety of biologic therapies following rituximab treatment in rheumatoid arthritis patients. Ann Rheum Dis. 2009:ard.2008.101675. doi: 10.1136/ard.2008.101675. [DOI] [PubMed] [Google Scholar]
- 49.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra therapies for rheumatoid arthritis: meta-analyses of randomized placebo-controlled trials. Ann Rheum Dis. 2008:ard.2007.083188. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safety of Biologic Therapy in Rheumatoid Arthritis and Other Autoimmune Diseases: Focus on Rituximab.
- 51.Kamar N, Mengelle C, Rostaing L. Incidence of JC-Virus Replication After Rituximab Therapy in Solid-Organ Transplant Patients. American Journal of Transplantation. 2009;9:244–5. doi: 10.1111/j.1600-6143.2008.02499.x. [DOI] [PubMed] [Google Scholar]