Abstract
Taurine, glutamine, glutamate, aspartate, and alanine are the most abundant intracellular free amino acids in human heart. The myocardial concentration of these amino acids changes during ischemia and reperfusion due to alterations in metabolic and ionic homeostasis. We hypothesized that dilated left ventricle secondary to mitral valve disease has different levels of amino acids compared to the right ventricle and that such differences determine the extent of amino acids' changes during ischemia and reperfusion. Myocardial concentration of amino acids was measured in biopsies collected from left and right ventricles before cardioplegic arrest (Custodiol HTK) and 10 min after reperfusion in patients undergoing mitral valve surgery. The dilated left ventricle had markedly higher (P < 0.05) concentrations (nmol/mg wet weight) of taurine (17.0 ± 1.5 vs. 10.9 ± 1.5), glutamine (20.5 ± 2.4 vs. 12.1 ± 1.2), and glutamate (18.3 ± 2.2 vs. 11.4 ± 1.5) when compared to right ventricle. There were no differences in the basal levels of alanine or aspartate. Upon reperfusion, a significant (P < 0.05) fall in taurine and glutamine was seen only in the left ventricle. These changes are likely to be due to transport (taurine) and/or metabolism (glutamine). There was a marked increase in the alanine to glutamate ratio in both ventricles indicative of ischemic stress which was confirmed by global release of lactate during reperfusion. This study shows that in contrast to the right ventricle, the dilated left ventricle had remodeled to accumulate amino acids which are used during ischemia and reperfusion. Whether these changes reflect differences in degree of cardioplegic protection between the two ventricles remain to be investigated.
Keywords: Dilatation, Mitral valve surgery, Ischemia, Cardioplegia, Taurine, Glutamine, Glutamate, Alanine, Aspartate
Introduction
Cardioprotective strategies against ischemia and reperfusion are aimed at preserving intracellular metabolites and opposing disruption to ionic homeostasis. Evaluation of the efficacy of cardioplegic solutions has relied upon monitoring intracellular changes of key metabolites including amino acids [1-4]. During ischemia, anaerobic metabolism leads to the building up of lactic acid and a decrease in intracellular pH. The fall in pH triggers Na+/H+ exchanger to increase the intracellular Na+ concentration which is accentuated by the reduced Na-pump activity due to a fall in ATP. Reperfusion following prolonged ischemia can lead to irreversible damage caused by Ca2+ loading via the Na+/Ca2+ exchanger and generation of reactive oxygen species [4]. In addition to causing Ca2+ loading, it has been shown that ischemia-induced Na+ accumulation would also contribute to osmotic-induced cell swelling, which has been implicated as a cause of ischemia-induced sarcolemmal damage [5-7]. In response to cardiac insults/osmotic stress, heart cells release free amino acids [8] including the non-protein β-amino acid taurine [9-11]. Taurine is the main organic osmolyte in the heart [12], and its trans-sarcolemmal fluxes are dependent on the Na+ gradient [13]. This close link between Na+ (osmotic changes) and taurine has been supported by our study involving open heart surgery [1, 2, 11, 14-16]. These studies have demonstrated that the more the ischemically stressed the heart during cardioplegic arrest, the greater the fall of myocardial taurine following ischemia and reperfusion. We have shown that maneuvers that increase the intracellular concentration of Na+ in isolated guinea-pig hearts trigger a fall in myocardial taurine and that this fall was due to efflux, as the amino acid could be measured in the coronary effluent [13]. This is consistent with taurine being very slowly metabolized and the finding that its concentration is raised in the blood of patients following acute myocardial infarction, unstable angina, and cardiovascular surgery [17-19]. Subsequently it was proposed that preloading the heart with taurine before a cardiac insult would improve its ability to cope by opposing changes in intracellular Na+ and Ca2+ [11].
In addition to taurine, the principal free amino acids glutamine, glutamate, aspartate, and alanine also change in heart cells during cardiac insults in both experimental models and during open heart surgery [10, 11, 14]. However, unlike taurine, the changes in these amino acids involve metabolism and transport and influence the biosynthesis of several key compounds including ATP and glutathione [20].
Cardiac muscle adapts to changes in the body (e.g., substrate availability and physiologic demands) by modifying muscle function, altering the synthesis and degradation rates of specific proteins, and altering the use of substrates [21]. In hearts with mitral regurgitation, volume overload triggers structural, functional, and metabolic remodeling in the left ventricle [22]. In contrast and in the early stages of the disease, the right ventricle is not affected by these compensatory mechanisms, as it is not chronically overloaded. As a result, the cardiac pump is likely to end up having two ventricles with different metabolic states that are likely to respond differently to ischemic cardioplegic arrest. Therefore, this study was designed to investigate whether the level of amino acids in the dilated left ventricle is indeed different from the right ventricle, and whether this difference has any bearing on cellular changes in amino acids during cardioplegic arrest. In order to achieve our aim, we measured myocardial amino acids in both dilated left ventricle secondary to mitral valve regurgitation and normal right ventricle in patients with degenerative mitral valve disease at pre-ischemic level and following cardioplegic arrest and reperfusion.
Methods
We studied 12 adult patients with dilated left ventricle secondary to mitral valve regurgitation undergoing mitral valve repair/replacement surgery during 2007/2008. Dilation was confirmed when Indexed Left Ventricular End Diastolic Volume (ILVEDV) was ≥76 ml/m2. Mean ILVEDV in this study was 95 ± 4 ml/m2. The main inclusion criterion was that patients had a normal right ventricle as per the following echocardiographic criteria: volume at 2/3 of the left ventricle, right to left ventricle diameters ratio less than 0.6; triangular shape; free wall thickness less than 5–6 mm and TAPSE of more than 20 mm (TAPSE: tricuspid annular plane systolic excursion). Patients' characteristics are shown in Table 1. Exclusions included severe tricuspid regurgitation, systolic pulmonary pressure >35 mmHg, coronary disease, aortic valve disease, and type I & II diabetes mellitus. Patients had no history of arrhythmias and were in sinus rhythm. The investigation conforms to the principles outlined in the Declaration of Helsinki. Local hospital ethical approval as well as patient consent was obtained. Most of the patients (n = 10) were using the following medications: ACE inhibitors (e.g., enalapril) and diuretics (e.g., Frusemide).
Table 1.
Patients' characteristics
| Age (years) | 53 ± 3 |
| Sex (male/female) | 8/4 |
| NYHA (I/II) | 5/7 |
| Systemic hypertension (Yes/No) | 5/7 |
| Indexed left ventricular end diastolic volume (ml/m2) | 95 ± 4 |
| Ejection fraction (%) | 64 ± 2 |
| BSA (m2) | 1.86 ± 0.05 |
| Degree of MR (3+/4+) | 8/4 |
| Cardioplegia infusion time (min) | 10.6 ± 0.4 |
| Cardiopulmonary bypass (min) | 123 ± 8 |
| Cross-clamp time (min) | 107 ± 7 |
Mean ± SEM
NYHA New York Heart Association, BSA body surface area, MR mitral regurgitation
Surgical and anesthetic techniques
A standard circuit of cardiopulmonary bypass (CPB) was used with a 40-lm filter, a Stockert roller pump, and a fiber membrane oxygenator (Dideco, Sorin, Cobe). Priming solution of the extracorporeal circuit included 1,000 ml of pH 7.4 balanced electrolyte solution, 500 ml of plasma expanding reagent (6% hydroxyethyl starch), 100 ml of 18% mannitol, and 5,000 IU of Heparin. Non-pulsatile flow was used at a rate of 2.4 l/m2/min. Systemic temperature was kept at 34°C. A left ventricular vent was inserted in all patients via the right superior pulmonary vein. Heparin IV was used at a dose of 300 UI/Kg to achieve a target ACT of 480 s.
For induction and maintenance of anesthesia, propofol infusion was used at 2–2.8 μg/ml (5.4–7.5 mg/Kg/min) combined with remifentanyl infusion at 3.5–7.60 μg/ml (0.13–0.28 μg/Kg/min.) using a Diprifusor pump. Neuro-muscular blockade was achieved with 0.6 mg/Kg of Rocuronium infused at 0.12 mg/Kg/min. Normocapnia was maintained and the lungs were ventilated with a mixture of air/O2 = 0.5, with low flow ventilation technique. Mean arterial blood pressure of 60–70 mmHg was maintained with volume or increments of Metaraminol 0.5–1.0 mg. Following transfer to intensive care unit, sedation was maintained with propofol infusion at 1–2 μg/ml combined with remifentanyl infusion at 2.0–2.5 μg/ml.
Myocardial protection was achieved using ice-cold Custodiol (Bretschneider's HTK-solution) cardioplegia. Infusion of cardioplegia following institution of cardiopulmonary bypass and aortic cross clamping was 10.5 ± 0.5 min at a rate of 200 ml/min giving a mean total infusion volume of 2083 ± 93 ml. This resulted in a maximum drop in myocardial temperature to approximately 10°C which then gradually increases to reach around 25°C toward the end of cardioplegic arrest.
Collection of ventricular biopsies and extraction of metabolites
Transmural biopsies of the left ventricular apical or anterior-free wall and the right ventricular-free wall (3–7 mg wet weight) were taken using a Trucut needle (Baxter Healthcare Corporation, Northbrook, IL). Two biopsies were collected from each ventricle; the first biopsy was taken 5 min after institution of cardiopulmonary bypass before aortic cross-clamping (basal or pre-ischemic) and the second after 10 min of reperfusion following removal of the aortic cross-clamp. Each specimen was frozen immediately in liquid nitrogen until processing the analysis of metabolites. A research technician blind to the operative technique performed the extraction and the analyses.
Determination of amino acids in biopsy specimens
Free amino acids were measured in all biopsies collected. Amino acids were determined using HPLC according to the Pico-Tag method of Water as reported earlier [2]. Concentration of metabolites was expressed as nmol/mg wet weight.
Reperfusion injury
Markers of injury and stress were measured in blood samples collected from the radial artery. Post-operative release of creatine kinase (CK-MB) was used as a measure of myocardial injury. Plasma lactate was also measured early after reperfusion to assess the degree of metabolic ischemic stress. Elevated blood lactate concentration during cardiac surgery is an indicator of hypoxia and anaerobic glycolysis. Accumulated cardiac lactate during ischemia is normally released from heart cells immediately upon reperfusion [23].
Statistical analysis
Data were expressed as mean ± standard error (SE). Comparison between continuous variables was made using a non-parametric test (Wilcoxon's signed rank test). All statistical analyses mentioned were performed with the aid of a computerized software package, Statview for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Basal myocardial taurine and α-amino acids in left and right ventricles
The basal (pre-ischemic) intracellular concentration of amino acids in left and right ventricles is shown in Table 2. The slowly metabolizing non-protein amino acid taurine was present at a significantly higher level in the left compared to right ventricle. The same trend was also seen for the total principal protein-free α-amino acids. The difference in total protein amino acids was due to higher myocardial concentrations in left ventricle in glutamine and glutamate but not in aspartate or alanine. The total amino acids in the right ventricle were significantly less than those in the left ventricle (approximately 62% of left ventricular amino acid pool). Despite the large difference in the total free amino acid pool between the two ventricles, the percentage of individual amino acids in each ventricle remained the same. These were 28% versus 27% for taurine, 33% versus 33% for glutamine, and 30% versus 29% for glutamate in left versus right ventricle, respectively.
Table 2.
Basal myocardial amino acid concentration in left and right ventricles
| Amino acid | Left ventricle (nmol/mg wet weight) |
Right ventricle (nmol/mg wet weight) |
|---|---|---|
| Taurine | 17.05 ± 1.54 | 10.90 ± 1.56* |
| Glutamine | 20.49 ± 2.36 | 12.12 ± 1.23* |
| Glutamate | 18.34 ± 2.17 | 11.35 ± 1.49* |
| Aspartate | 2.53 ± 0.18 | 1.95 ± 0.32 |
| Asparagine | 0.59 ± 0.09 | 0.53 ± 0.08 |
| Alanine | 2.65 ± 0.35 | 2.18 ± 0.36 |
| Total free amino acids | 61.7 ± 4.2 | 39.0 ± 4.6* |
| Total α-amino acids | 44.6 ± 3.2 | 28.1 ± 3.2* |
Mean ± SEM
P < 0.05 versus left ventricle
Changes in myocardial amino acids in left and right ventricles following cardioplegic arrest and reperfusion
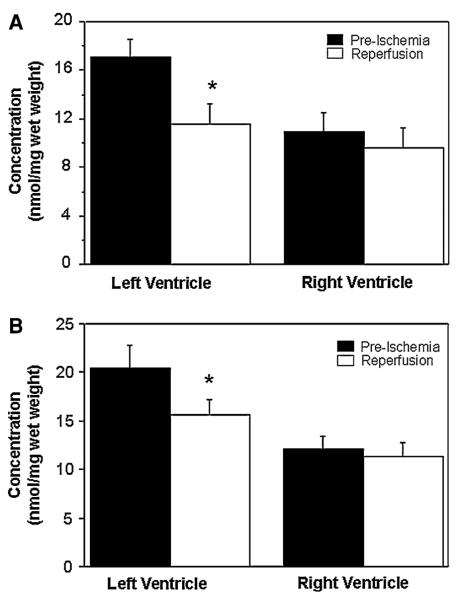
There were changes in the myocardial concentration of amino acids, measured in the second biopsy (collected 10 min after reperfusion) when compared to the preischemic basal levels. These changes were different for both ventricles (Figs. 1, 2). There were no changes in the concentrations of taurine and glutamine in the right ventricle as a result of cardioplegic arrest and reperfusion (Fig. 1). However, there was a marked and significant fall in the myocardial concentration of taurine (from 17.0 ± 1.5 to 11.6 ± 1.6 nmol/mg weight, P < 0.05) and glutamine (from 20.5 ± 2.3 to 15.6 ± 1.6 nmol/mg weight, P < 0.05) in the left ventricle.
Fig. 1.
Changes in myocardial taurine (a) and glutamine (b) during cardioplegic arrest and reperfusion. The concentrations of taurine and glutamine in biopsies collected from both left and right ventricles before cross-clamping the aorta (pre-ischemia) and after 10 min reperfusion. Data are shown as mean ± SEM (n = 12). *P versus pre-ischemia in corresponding ventricle
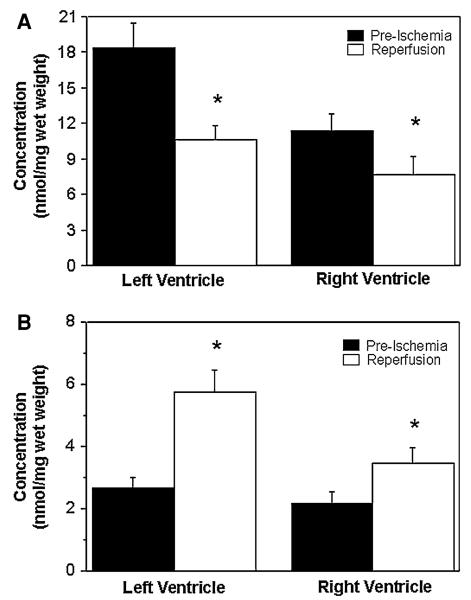
Fig. 2.
Changes in myocardial glutamate (a) and alanine (b) during cardioplegic arrest and reperfusion. The concentrations of glutamate and alanine in biopsies collected from both left and right ventricles before cross-clamping the aorta (pre-ischemia) and after 10 min reperfusion. Data are shown as mean ± SEM (n = 12). *P versus pre-ischemia in corresponding ventricle
The effect of ischemic cardioplegic arrest and reperfusion on the concentration of glutamate and alanine was qualitatively similar for both ventricles, but the extent of the changes was different (Fig. 2). In both ventricles, there was a fall in glutamate and a rise in tissue alanine. However, the changes were markedly higher in the left ventricle. There were no significant changes in myocardial aspartate in both left and right ventricles.
Myocardial injury and metabolic stress during mitral valve surgery
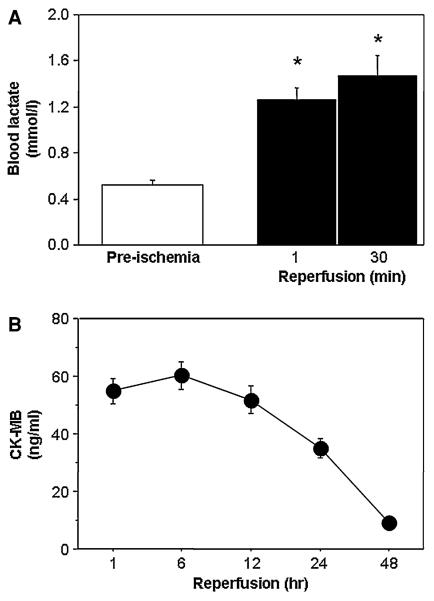
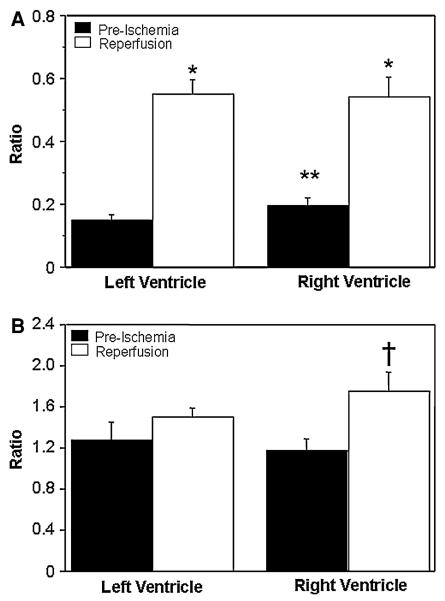
Figure 3a shows that there is a significant rise in the blood lactate at the onset of reperfusion (release of aortic cross-clamp), which remained elevated for 30 min afterward. In order to assess the stress in individual ventricles, two markers (ratios) of metabolic stress [24, 25] were calculated. They are the alanine/glutamate and glutamine/glutamate ratios. The alanine/glutamate ratio was markedly elevated in both ventricles (Fig. 4a). However, the glutamine/glutamate ratio was significantly higher upon reperfusion in the right ventricle (Fig. 4b). Surgery was associated with significant cardiac injury as seen by significant release of CK-MB which peaks 1 h after reperfusion (Fig. 3b).
Fig. 3.
Ischemic cardioplegic stress (a) and reperfusion injury (b). Lactate release following the release of cross-clamp was used to assess the metabolic ischemic stress early after reperfusion. Reperfusion injury as measured by post-operative release of creatine kinase (CK-MB). Data are shown as mean ± SEM (n = 12). CK-MB values at 1, 6, 12, and 24 h were significantly higher than 48 h. *P versus pre-ischemia in the corresponding ventricle
Fig. 4.
Markers of myocardial metabolic stress. The alanine/glutamate (a) and glutamine/glutamate (b) ratios in left and right ventricles calculated before cross-clamping the aorta (pre-ischemia) and after 10 min reperfusion. Data are shown as mean ± SEM (n = 12). *P versus pre-ischemia in the corresponding ventricle. **P versus pre-ischemia in the left ventricle. †P versus all other values
Discussion
In this study, we show a number of novel observations. First, the dilated left ventricle secondary to mitral regurgitation has markedly higher levels of taurine, glutamine, and glutamate than that of the right ventricle. Second, upon reperfusion following cardioplegic arrest, amino acids and metabolic stress in both ventricles reach similar levels. Finally, cardioplegic arrest triggers changes in the left ventricle that reflects metabolic remodeling back toward the status of the right ventricle. It is likely that these differences between the two ventricles would be associated with differences in vulnerability to ischemic cardioplegic arrest and reperfusion.
The “dilated” left ventricle has higher levels of amino acids compared to the “normal” right ventricle
Mitral regurgitation triggers a volume overload in the left ventricle that leads to cardiac remodeling, myocardial dysfunction, and eventually heart failure [26]. During the early stages of the disease (which largely applies to our study), patients remain relatively asymptomatic in New York Heart Association I or II due to physiologic compensation by left ventricle dilation and hypertrophy. Patients selected in this study had relatively “normal” right ventricle and with one exception, none had heart failure.
This study demonstrates that chronic volume overload triggers significant increase in the concentration of taurine in the left ventricle. This effect is quite marked, as taurine concentration in the left ventricle is nearly double that of the concentration in the right ventricle. Although it is extremely difficult to obtain fresh biopsy from a “normal” left ventricle, our earlier study on biopsies collected from viable myocardium of the left ventricle of patients with coronary disease (no hypertrophy or dilatation) had taurine levels at 9–10 nmol/mg wet weight [11, 14]. Such values are very similar to the levels found in the right ventricle of patients recruited for this study (Table 2). This suggests that the differences in the levels of taurine between the dilated left and normal right ventricles are due to disease progression. This conclusion is supported by study on dogs where a failing right ventricle had significantly higher taurine levels compared to the left ventricle [27].
In addition to volume overload-induced accumulation of taurine (demonstrated in this study), we have previously shown the same trend occurring in hypertrophic left ventricle due to pressure overload secondary to aortic stenosis [16]. Pressure stress has also been shown to elevate levels of vitreous taurine in a rabbit model of cataract surgery [28]. These studies indicate that mechanical stress is likely to be a key trigger of taurine accumulation. This mechanical stress could induce osmotic changes that would eventually lead to taurine accumulation. An alternative explanation could be stress (disease)-induced changes in taurine transporter activity and expression. It has been suggested that taurine accumulation due to congestive heart failure is the result of adrenergic stimulation of the taurine transporter [29].
Taurine is a β-amino acid that is very slowly metabolized and is the major osmolyte in heart cells [12]. Therefore, it can be argued that the accumulated taurine can be used to remove the excess Na+ and help the heart to cope better with subsequent cardiac insults [13, 30] as well as having anti-apoptotic and anti-necrotic effects [31-33]. Although taurine has been implicated in the regulation of intracellular Na+ (via the taurine transporter or by altering Na+ channel activity [34]), this amino acid has also been linked to Ca2+ cycling in myocytes [34-36]. Whether taurine accumulation does alter Na+ and Ca2+ concentration in dilated myocardium is not presently known.
The higher concentration of glutamine in the left ventricle will have effects on transport and metabolism [37]. Like taurine, it will influence Na+ levels as a result of the increased Na-glutamine symport activity. Glutamine is also metabolically active and acts as a nitrogen donor for the biosynthesis of a number of compounds such as nucleotides and amino acids including glutamate [20]. In fact, the concentration of glutamate in the left compared to the right ventricle was proportionately the highest (220% higher) when compared to other amino acids. Although glutamate can be used as substrate for energy production especially during metabolic stress, it also has strong antioxidant activity in cardiomyocytes [38, 39]. Due to its cardioprotective effects, glutamate has been used to supplement cardioplegia [40-42]. The observation that alanine/glutamate ratio is lower in the left ventricle (Fig. 4a) could indicate that this ventricle has relatively less metabolic stress. However, it should be emphasized that this is a chronic effect which could also result from disease-induced changes in amino acid transporters and metabolism.
Only the left ventricle shows a marked fall in taurine and glutamine following ischemia and reperfusion
Both left and right ventricles are exposed to long duration (107 ± 7 min) of cardioplegic arrest. During this period, the heart will be exposed to ischemic/osmotic stress which will involve disruption in metabolic and ionic homeostasis. In particular, there will be accumulation of lactate which appears in the effluent following the release of the cross-clamp (Fig. 3). In addition, Na+ will be accumulated by heart cells which will be accentuated by hypothermia [10, 11, 43]. The main routes for removing intracellular Na+ upon reperfusion can cause injury (e.g., Na+/Ca2+ exchanger loads cells with Ca2+, whereas Na-pump uses the much needed ATP). In addition, there are two important and fast symports that transport Na+ with glutamine and taurine. Evidence for taurine-Na symport is seen in the left ventricle as changes in tissue taurine are due to transport [30]. An efflux of taurine (and glutamine) would suggest that a significant build up in Na+ has occurred during cardioplegic arrest and early after reperfusion. Alternatively, and a more likely explanation is that, the left ventricle has a much greater gradient for efflux than the right ventricle. The fall of taurine in the left ventricle was approximately 6 nmol/mg wet weight (Fig. 2). This would be equivalent to at least at 6 mmol/l assuming 80% of the cardiac tissue is water. It has been suggested that for every time the transporter carries one molecule of taurine, it also carries between 1 and 3 Na+ [29]. Therefore, the fall in taurine would be accompanied by at least 6 mmol/l Na+. This is a significant concentration; had it not been removed, it would have caused further Ca2+ loading via Na+/Ca2+ exchanger. The reason for the right ventricle not showing a significant fall in taurine is likely to be due to the unavailability of sufficient energy in the taurine gradient or that Na accumulation was not high enough to co-transport with taurine. In an earlier study [14], we provided an estimate of the Na+ electrochemical energy across myocardial sarcolemma at approximately 14.5 kJ mol−1. We proposed that if we assume a stoichiometry of 1:1, then this amount of energy would be enough to maintain a taurine gradient of (~10 mmol/l in tissue) and 0.04 mmol/l in plasma [14]. Clearly the elevated levels of taurine (17 nmol/mg wet weight, Table 2) in the left ventricle would have higher energy available which would be used to efflux Na+ against its concentration gradient. Like taurine transport, glutamine transport is Na+ dependent, faster than other amino acids transporters, and is largely responsible for the changes in tissue glutamine during cardiac insults [37, 44]. It is evident that the dilated left ventricle is remodeled to accumulate taurine and glutamine which can be used to efflux Na+ during ischemia and reperfusion. At the end of reperfusion, the left ventricle has similar levels of amino acids compared to the right ventricle. Whether these acute effects also alter the activity of taurine and glutamine transporters is not known, but as the two ventricles are clearly different in their response, such a possibility is unlikely within the period of cardioplegic arrest.
Ischemia and reperfusion trigger a rise in alanine and a fall in glutamate in both left and right ventricles
In contrast to the changes in taurine and glutamine, which occur only in the left ventricle, there are similar changes in glutamate and alanine during cardioplegic arrest and reperfusion in both ventricles. Glutamate is used during anaerobic energy production, as it is known to enhance anaerobic energy formation [45]. Evidence for its metabolic use is the accumulation of the byproduct alanine. In both left and right ventricles, there is a fall in glutamate and a rise in tissue alanine that is consistent with anaerobic metabolic stress. However, the fact that the fall in glutamate was higher in the left ventricle suggests more substrate-related energy production in the left ventricle. More evidence to support an apparently better left ventricle response to the cardioplegic insult is seen in the relatively lower glutamine/glutamate ratio in the left ventricle compared to the right ventricle (Fig. 4). It has been suggested that a lower ratio would reflect the generation of more energy in the cardiomyocytes in response to an increased energy demand [24]. Whether this left ventricular beneficial metabolic adaptation is also translated into reduced reperfusion injury compared to right ventricle is not presently known and requires further investigation.
Implication for myocardial protection during mitral valve surgery
In this study we have shown that the dilated left ventricle due to mitral regurgitation remodels (adapts) to improve its ability to accumulate taurine. Following cardioplegic arrest and reperfusion, the dilated ventricle (not the normal right ventricle) sustains a loss of taurine (and glutamine). Whether the loss of these amino acids confers better protection possibly by removing ischemia-induced Na+ loading requires further study involving separate assessment of injury in both ventricles. An important factor that could have influenced the loss of amino acids is the type of cardioplegia used in this study. Custodiol HTK is an intracellular type solution with low Na+ concentration. This is different from the more popular St. Thomas hyperkalemic extracellular type solution which has high Na+ concentration. Clearly the Na+ concentration gradient during cardioplegic arrest is crucial in determining the extent of amino acid transport across the sarcolemma. Therefore, a further study involving the use of a hyperkalemic extracellular type solution is needed to address this issue.
One interesting possibility arising from this study is that the disease of the left ventricle has induced an up-regulation of the taurine transporter activity and expression. If this is the case, then the left and not the right ventricle will benefit from increased availability of extracellular taurine. Therefore, it would be interesting to see whether dietary taurine supplementation to patients with mitral valve disease prior to surgery would increase taurine levels in dilated ventricle. The inclusion of taurine in the cardioplegia is not an option, as this could load heart cells with Na+.
Acknowledgments
We would like to acknowledge the help and support of the staff in the cardiac surgery unit at Ospedale Civile “Umberto I,” Venezia-Mestre, Italy. This study was supported by the British Heart Foundation and the Garfield Weston Trust.
References
- 1.Caputo M, Bryan AJ, Calafiore AM, et al. Intermittent antegrade hyperkalaemic warm blood cardioplegia supplemented with magnesium prevents myocardial substrate derangement in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 1998;14:596–601. doi: 10.1016/s1010-7940(98)00247-4. doi:10.1016/S1010-7940(98)00247-4. [DOI] [PubMed] [Google Scholar]
- 2.Caputo M, Dihmis WC, Bryan AJ, et al. Warm blood hyperkalaemic reperfusion (‘hot shot’) prevents myocardial substrate derangement in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 1998;13:559–564. doi: 10.1016/s1010-7940(98)00056-6. doi:10.1016/S1010-7940(98)00056-6. [DOI] [PubMed] [Google Scholar]
- 3.Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther. 2001;89:29–46. doi: 10.1016/s0163-7258(00)00102-9. doi: 10.1016/S0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 4.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. doi:10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tritto FP, Inserte J, Garcia-Dorado D, et al. Sodium/hydrogen exchanger inhibition reduces myocardial reperfusion edema after normothermic cardioplegia. J Thorac Cardiovasc Surg. 1998;115:709–715. doi: 10.1016/S0022-5223(98)70337-X. doi:10.1016/S0022-5223(98)70337-X. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Dorado D, Oliveras J. Myocardial oedema: a preventable cause of reperfusion injury? Cardiovasc Res. 1993;27:1555–1563. doi: 10.1093/cvr/27.9.1555. doi:10.1093/cvr/27.9.1555. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Meana M, Garcia-Dorado D, Gonzalez MA, et al. Effect of osmotic stress on sarcolemmal integrity of isolated cardiomyocytes following transient metabolic inhibition. Cardiovasc Res. 1995;30:64–69. [PubMed] [Google Scholar]
- 8.Schaffer SW, Solodushko V, Kakhniashvili D. Beneficial effect of taurine depletion on osmotic sodium and calcium loading during chemical hypoxia. Am J Physiol Cell Physiol. 2002;282:C1113–C1120. doi: 10.1152/ajpcell.00485.2001. [DOI] [PubMed] [Google Scholar]
- 9.Suleiman MS, Chapman RA. Calcium paradox in newborn and adult guinea-pig hearts: changes in intracellular taurine and the effects of extracellular magnesium. Exp Physiol. 1993;78:503–516. doi: 10.1113/expphysiol.1993.sp003702. [DOI] [PubMed] [Google Scholar]
- 10.Suleiman MS, Chapman RA. Changes in the principal free intracellular amino acids in the Langendorff perfused guinea pig heart during arrest with calcium-free or high potassium media. Cardiovasc Res. 1993;27:1810–1814. doi: 10.1093/cvr/27.10.1810. doi:10.1093/cvr/27.10.1810. [DOI] [PubMed] [Google Scholar]
- 11.Suleiman MS, Fernando HC, Dihmis WC, et al. A loss of taurine and other amino acids from ventricles of patients undergoing bypass surgery. Br Heart J. 1993;69:241–245. doi: 10.1136/hrt.69.3.241. doi:10.1136/hrt. 69.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huxtable RJ. Taurine and the heart. Cardiovasc Res. 1993;27:1136–1137. doi: 10.1093/cvr/27.6.1136a. doi:10.1093/cvr/27.6.1136a. [DOI] [PubMed] [Google Scholar]
- 13.Suleiman MS, Rodrigo GC, Chapman RA. Interdependence of intracellular taurine and sodium in guinea pig heart. Cardiovasc Res. 1992;26:897–905. doi: 10.1093/cvr/26.9.897. doi:10.1093/cvr/26.9.897. [DOI] [PubMed] [Google Scholar]
- 14.Suleiman MS, Moffatt AC, Dihmis WC, et al. Effect of ischaemia and reperfusion on the intracellular concentration of taurine and glutamine in the hearts of patients undergoing coronary artery surgery. Biochim Biophys Acta. 1997;1324:223–231. doi: 10.1016/s0005-2736(96)00225-8. doi:10.1016/S0005-2736(96)00225-8. [DOI] [PubMed] [Google Scholar]
- 15.Ascione R, Gomes WJ, Angelini GD, et al. Warm blood cardioplegia reduces the fall in the intracellular concentration of taurine in the ischaemic/reperfused heart of patients undergoing aortic valve surgery. Amino Acids. 1998;15:339–350. doi: 10.1007/BF01320898. doi:10.1007/BF01320898. [DOI] [PubMed] [Google Scholar]
- 16.Lotto AA, Ascione R, Caputo M, et al. Myocardial protection with intermittent cold blood during aortic valve operation: antegrade versus retrograde delivery. Ann Thorac Surg. 2003;76:1227–1233. doi: 10.1016/s0003-4975(03)00840-3. doi:10.1016/S0003-4975(03)00840-3. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MW, Lombardini JB. Elevated blood taurine levels after myocardial infarction of cardiovascular surgery: is there any significance? Adv Exp Med Biol. 1981;139:191–205. doi: 10.1007/978-1-4757-0402-0_13. [DOI] [PubMed] [Google Scholar]
- 18.Lombardini JB, Bricker DL. Effects of cardiovascular surgery on blood concentrations of taurine and amino acids. Proc Soc Exp Biol Med. 1981;167:498–505. doi: 10.3181/00379727-167-41204. [DOI] [PubMed] [Google Scholar]
- 19.Lombardini JB, Cooper MW. Elevated blood taurine levels in acute and evolving myocardial infarction. J Lab Clin Med. 1981;98:849–859. [PubMed] [Google Scholar]
- 20.Rennie MJ, Bowtell JL, Bruce M, et al. Interaction between glutamine availability and metabolism of glycogen, tricarboxylic acid cycle intermediates and glutathione. J Nutr. 2001;131:2488S–2490S. doi: 10.1093/jn/131.9.2488S. discussion 2496S–7S. [DOI] [PubMed] [Google Scholar]
- 21.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. doi:10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 22.Conway MA, Bottomley PA, Ouwerkerk R. Mitral regurgitation: impaired systolic function, eccentric hypertrophy, and increased severity are linked to lower phosphocreatine/ATP ratios in humans. Circulation. 1998;97:1716–1723. doi: 10.1161/01.cir.97.17.1716. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Suleiman MS. Cariporide enhances lactate clearance upon reperfusion but does not alter lactate accumulation during global ischaemia. Pflugers Arch. 2003;447:8–13. doi: 10.1007/s00424-003-1134-8. doi:10.1007/s00424-003-1134-8. [DOI] [PubMed] [Google Scholar]
- 24.Roncalli J, Smih F, Desmoulin F, et al. NMR and cDNA array analysis prior to heart failure reveals an increase of unsaturated lipids, a glutamine/glutamate ratio decrease and a specific transcriptome adaptation in obese rat heart. J Mol Cell Cardiol. 2007;42:526–539. doi: 10.1016/j.yjmcc.2006.11.007. doi:10.1016/j.yjmcc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Suleiman MS, Dihmis WC, Caputo M, et al. Changes in myocardial concentration of glutamate and aspartate during coronary artery surgery. Am J Physiol. 1997;272:H1063–H1069. doi: 10.1152/ajpheart.1997.272.3.H1063. [DOI] [PubMed] [Google Scholar]
- 26.Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52:319–326. doi: 10.1016/j.jacc.2008.02.084. doi:10.1016/j.jacc.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MB, Mead RJ, Welty JD. Free amino acids in congestive heart failure. J Mol Cell Cardiol. 1973;5:139–147. doi: 10.1016/0022-2828(73)90047-3. doi: 10.1016/0022-2828(73)90047-3. [DOI] [PubMed] [Google Scholar]
- 28.Yagihashi T, Wakabayashi Y, Kezuka J, et al. Changes in vitreous amino acid concentrations in a rabbit model of cataract surgery. Acta Ophthalmol Scand. 2007;85:303–308. doi: 10.1111/j.1600-0420.2006.00829.x. doi:10.1111/j.1600-0420.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 29.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 30.Chapman RA, Suleiman MS, Earm YE. Taurine and the heart. Cardiovasc Res. 1993;27:358–363. doi: 10.1093/cvr/27.3.358. doi:10.1093/cvr/27.3.358. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Ohyabu Y, Takahashi K, et al. Taurine renders the cell resistant to ischemia-induced injury in cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol. 2003;41:726–733. doi: 10.1097/00005344-200305000-00009. doi:10.1097/00005344-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Takatani T, Takahashi K, Uozumi Y, et al. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol. 2004;287:C949–C953. doi: 10.1152/ajpcell.00042.2004. doi: 10.1152/ajpcell.00042.2004. [DOI] [PubMed] [Google Scholar]
- 33.Takatani T, Takahashi K, Uozumi Y, et al. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem Biophys Res Commun. 2004;316:484–489. doi: 10.1016/j.bbrc.2004.02.066. doi:10.1016/j.bbrc.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 34.Bkaily G, Jaalouk D, Sader S, et al. Taurine indirectly increases [Ca]i by inducing Ca2+ influx through the Na(+)Ca2+ exchanger. Mol Cell Biochem. 1998;188:187–197. doi:10.1023/A:1006806925739. [PubMed] [Google Scholar]
- 35.Schaffer SW, Nguyen K, Ballard C, et al. Regulation of Ca2+ transport by insulin and taurine. Interaction at the level of the Na(+)-Ca2+ exchanger. Adv Exp Med Biol. 1996;403:551–560. doi: 10.1007/978-1-4899-0182-8_59. [DOI] [PubMed] [Google Scholar]
- 36.Bkaily G, Haddad G, Jaalouk D, et al. Modulation of Ca2+ and Na+ transport by taurine in heart and vascular smooth muscle. Adv Exp Med Biol. 1996;403:263–273. doi: 10.1007/978-1-4899-0182-8_28. [DOI] [PubMed] [Google Scholar]
- 37.Rennie MJ, Tadros L, Khogali S, et al. Glutamine transport and its metabolic effects. J Nutr. 1994;124:1503S–1508S. doi: 10.1093/jn/124.suppl_8.1503S. [DOI] [PubMed] [Google Scholar]
- 38.King N, McGivan JD, Griffiths EJ, et al. Glutamate loading protects freshly isolated and perfused adult cardiomyocytes against intracellular ROS generation. J Mol Cell Cardiol. 2003;35:975–984. doi: 10.1016/s0022-2828(03)00182-2. doi:10.1016/S0022-2828(03)00182-2. [DOI] [PubMed] [Google Scholar]
- 39.Williams H, King N, Griffiths EJ, et al. Glutamate-loading stimulates metabolic flux and improves cell recovery following chemical hypoxia in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:2109–2119. doi: 10.1006/jmcc.2000.1474. doi:10.1006/jmcc.2000.1474. [DOI] [PubMed] [Google Scholar]
- 40.Robertson JM, Vinten-Johansen J, Buckberg GD, et al. Safety of prolonged aortic clamping with blood cardioplegia. I. Glutamate enrichment in normal hearts. J Thorac Cardiovasc Surg. 1984;88:395–401. [PubMed] [Google Scholar]
- 41.Rosenkranz ER, Okamoto F, Buckberg GD, et al. Safety of prolonged aortic clamping with blood cardioplegia. III. Aspartate enrichment of glutamate-blood cardioplegia in energy-depleted hearts after ischemic and reperfusion injury. J Thorac Cardiovasc Surg. 1986;91:428–435. [PubMed] [Google Scholar]
- 42.Rosenkranz ER, Okamoto F, Buckberg GD, et al. Safety of prolonged aortic clamping with blood cardioplegia. II. Glutamate enrichment in energy-depleted hearts. J Thorac Cardiovasc Surg. 1984;88:402–410. [PubMed] [Google Scholar]
- 43.Suleiman MS, Chapman RA. Effect of temperature on the rise in intracellular sodium caused by calcium depletion in ferret ventricular muscle and the mechanism of the alleviation of the calcium paradox by hypothermia. Circ Res. 1990;67:1238–1246. doi: 10.1161/01.res.67.5.1238. [DOI] [PubMed] [Google Scholar]
- 44.Carter JM, Bell NJ, Suleiman MS. The use of Langendorff perfused guinea-pig heart to study the efflux of amino acids from heart cells. Biochem Soc Trans. 1996;24:482S. doi: 10.1042/bst024482s. [DOI] [PubMed] [Google Scholar]
- 45.Pisarenko OI. Mechanisms of myocardial protection by amino acids: facts and hypotheses. Clin Exp Pharmacol Physiol. 1996;23:627–633. doi: 10.1111/j.1440-1681.1996.tb01748.x. doi:10.1111/j.1440-1681.1996.tb01748.x. [DOI] [PubMed] [Google Scholar]