Abstract
The binding thermodynamics of the stereoisomers of fenoterol, (R,R')-, (S,S')- , (R,S')-, and (S,R')-fenoterol, to the β2-adrenergic receptor (β2-AR) have been determined. The experiments utilized membranes obtained from HEK cells stably transfected with cDNA encoding human β2-AR. Competitive displacement studies using [3H]CGP-12177 as the marker ligand were conducted at 4°, 15°, 25°, 30° and 37°C, the binding affinities calculated and the standard enthalpic (ΔH°) and standard entropic (ΔS°) contribution to the standard free energy change (ΔG°) associated with the binding process determined through the construction of van't Hoff plots. The results indicate that the binding of (S,S')- and (S,R')-fenoterol were predominately enthalpy-driven processes while the binding of (R,R')- and (R,S')-fenoterol were entropy-driven. All of the fenoterol stereoisomers are full agonists of the β2-AR, and, therefore, the results of this study are inconsistent with the previously described “thermodynamic agonist-antagonist discrimination”, in which the binding of an agonist to the β-AR is entropy-driven and the binding of an antagonist is enthalpy driven. In addition, the data demonstrate that the chirality of the carbon atom containing the β-hydroxyl group of the fenoterol molecule (the β-OH carbon) is a key factor in the determination of whether the binding process will be enthalpy-driven or entropy-driven. When the configuration at the β-OH carbon is S the binding process is enthalpy-driven while the R configuration produces an entropy-driven process.
Keywords: enantiomers, enantiospecific binding, thermodynamic agonist-antagonist discrimination, binding mechanisms, binding thermodynamics, chirality
1. Introduction
The thermodynamics of the binding of agonists and antagonists to β-adrenergic receptors (β-ARs) have been described as fundamentally different processes in which the binding of an agonist is enthalpy-driven while the binding of an antagonist is entropy-driven [1–3]. This observation has been generalized as the principle of “thermodynamic agonist-antagonist discrimination”, which has been defined as a relationship that when the binding of an agonist is entropy-driven, the binding of an antagonist is enthalpy driven, or vice versa. [4]. This principle has been recently reviewed and appears to hold for the β-AR, adenosine A1 and A2A, A2B and A3, cannabinoid CB1 and CB2, histamine H3, glycine, GABAA, serotonin 5-HT3, nicotinic acetylcholine and purinergic P2X3 receptors [4,5]. However, “thermodynamic agonist-antagonist discrimination” does not appear to apply to cholecystokinin CCK2, dopamine D2, histamine H1, δ- and μ-opiod, purinergic P2X1 and serotonin 5-HT1A receptors [4,5].
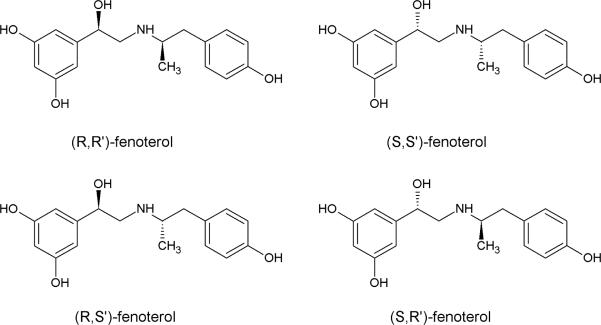
To our knowledge, the data used to establish “thermodynamic agonist-antagonist discrimination” at the β-AR were obtained using racemic mixtures or only one enantiomer of a chiral compound. One of these compounds was the agonist fenoterol. Fenoterol, is an asymmetric molecule that contains two chiral centers and, therefore, exists as 4 stereoisomers, (R,R')-, (S,S')- , (R,S')-, (S,R')-fenoterol, Fig. 1, where the configuration at the carbon containing the β-hydroxyl moiety, β-hydroxy carbon, is denoted as R or S and the configuration at the chiral center on the aminoalkyl portion of the molecule is denoted as R' or S'. The compound identified as “fenoterol” in the initial study by Wieland, et al. [3] was the racemic (50:50) mixture of (R,R')- and (S,S')-fenoterol, rac-fenoterol.
Figure 1.
Structures of the compounds used in this study.
We have recently synthesized all of the stereoisomers of fenoterol and demonstrated that the chirality at the two chiral centers affected the magnitude of the interactions with the β2-AR. In these studies, the β2-AR binding affinities (Ki) and activities (EC50) were determined using a stably expressed cell line [6,7,]. The calculated Ki values differed for each stereoisomer and ranged from 7158 nM (S,S')-fenoterol to 164 nM (R,R')-fenoterol (25°C), see Table 1. The data also demonstrated that all of the fenoterol stereoisomers were full agonists, defined as ≥ 100% induced stimulation of cAMP accumulation relative to isoproterenol, with EC50cAMP values of 0.3 nM (R,R'), 4.70 nM (R,S'), 8.50 nM (S,R') and 580 nM (S,S') [7] and Supplemental Data, Figs. S1-4. The activity of the fenoterol stereoisomers also differed in a rat cardiomyocyte contractility model with EC50cardio values of 73 nM (R,R') [8], 575 nM (R,S') [7], 2,340 nM (S,R') [8], and 55,000 nM (S,S') [unpublished data]. In addition, we have also demonstrated that the stereochemistry of the fenoterol molecule determines the β2-AR coupling preference for G-proteins in cardiomyocyes, as (R,R')-fenoterol preferentially activates Gs signaling while (S,R')-fenoterol activates both Gs and Gi proteins [8].
Table 1.
The Ki values (nM ± SD) and Hill's coefficient (n ± SD) determined for the four stereoisomers of fenoterol, (R)-isoproterenol and rac-propranolol at five different temperatures; n = 3 for each determination unless otherwise indicated. ΔKi is a change in Ki values between the lowest and the highest temperature (i.e., Ki (4°C)-Ki (37°C)) and the significance, p, was calculated using a two-tailed t-test for independence of two means: Ki (4°C) and Ki (37°C); a p ≤ 0.05 is assumed a significant difference in ΔKi.
| Temp [°C] | (S,S')-(+)-fenoterol | (S,R')-(+)-fenoterol | (R,S')-(−)-fenoterol | (R,R')-(−)-fenoterol | (R)-isoproterenol | rac-propranolol* |
|---|---|---|---|---|---|---|
| 4°C | ||||||
| Ki | 2325 ± 124 | 1700 ± 372 | 2450 ± 246 | 198 ± 33 | 137 ± 19 | 0.43 ± 0.09 |
| n | 1.69 ± 0.20 | 1.62 ± 0.38 | 1.05 ± 0.18 | 0.87 ± 0.10 | 0.83 ± 0.03 | 1.12 ± 0.16 |
| 15°C | ||||||
| Ki | 3838 ± 295 | 2507 ± 232 | 2001 ± 200 | 164 ± 13 | 268 ± 38 | 0.44 ± 0.08 |
| n | 2.06 ± 0.14 | 1.95 ± 0.04 | 1.08 ± 0.19 | 0.92 ± 0.15 | 0.98 ± 0.13 | 1.14 ± 0.11 |
| 25°C | ||||||
| Ki | 7158 ± 663 | 4293 ± 374 | 2087 ± 114 | 164 ± 31 | 285 ± 23 | 0.46 ± 0.06 |
| n | 2.04 ± 0.04 | 1.63 ± 0.24 | 1.12 ± 0.11 | 0.88 ± 0.05 | 0.99 ± 0.08 | 1.10 ± 0.08 |
| 30°C | ||||||
| Ki | 6237 ± 252 | 4430 ± 268 | 1809 ± 132 | 156 ± 17 | 291 ± 10 | 0.50 ± 0.06 |
| n | 1.93 ± 0.13 | 1.57 ± 0.31 | 1.16 ± 0.04 | 1.00 ± 0.11 | 0.99 ± 0.03 | 1.13 ± 0.06 |
| 37°C | ||||||
| Ki | 14008 ± 5132 | 4440 ± 458 | 1709 ± 191 | 184 ± 22 | 347 ± 23 | 0.52 ± 0.08 |
| n | 2.06 ± 0.03 | 1.63 ± 0.25 | 1.21 ± 0.14 | 0.99 ± 0.06 | 1.00 ± 0.07 | 1.17 ± 0.11 |
|
| ||||||
| ΔKi | −11683 | −2740 | +741 | +14 | −210 | −0.09 |
|
| ||||||
| p | 0.017 | 0.013 | 0.015 | 0.574 | 0.003 | 0.014 |
Standard control in all experiments and n = 21
The objective of the current study was to further explore the role that stereochemistry plays in the interactions of fenoterol with the β2-AR through the determination of the binding thermodynamics of the four fenoterol stereoisomers. The results indicate that the binding of (S,S')-fenoterol and (S,R')-fenoterol were enthalpy-driven, while the binding of (R,R')-fenoterol and (R,S')-fenoterol was entropy-driven. Thus, the binding of these compounds to the β2-AR does not conform to the previously described “thermodynamic agonist-antagonist discrimination” and instead is similar to the processes described for the cholecystokinin CCK2, dopamine D2, histamine H1, δ- and μ-opiod, purinergic P2X1 and serotonin 5-HT1A receptors, in which agonists bind to the receptors with both enthalpy-driven and entropy-driven mechanisms. In addition, the data demonstrate that the chirality of the β-OH carbon is a key factor in the determination of whether the binding process will be enthalpy-driven or entropy-driven, with the S configuration resulting in an enthalpy-driven and the R configuration producing an entropy-driven process. To our knowledge this is the first report in which the binding thermodynamics of enantiomers differ not only quantitatively but also qualitatively, i.e. the binding of one enantiomer is enthalpy driven (ΔH° < 0) while the binding of the other is purely entropy-driven (ΔH° ≥ 0).
2. Materials and Methods
2.1. Materials
(R,R')-Fenoterol, (R,S')-fenoterol, (S,R')-fenoterol and (S,S')-fenoterol were synthesized as previously described [6]. [5,7-3H]-(-)-CGP-12177 was purchased from PerkinElmer (Shelton, CT), DMEM was purchased from Lonza Walkersville, Inc. (Walkersville, MD), Fetal Bovine Serum was purchased from Atlas Biologicals (Fort Collins, CO), Penicillin-Streptomycin andGeneticin (G418) were purchased from Invitrogen (Carlsbad, CA), Sodium Chloride and Calcium Chloride were purchased from Mallinckrodt (Phillipsburg NJ) and (±)-Propranolol, (-)-Isoproterenol, Tris-HCL, Trizma Base, Potassium Chloride, Magnesium Chloride, D-(+)-Glucose were purchased from Sigma-Aldrich (St. Louis, MO).
2.2 Membrane binding studies
The binding affinities, expressed as Ki values, were determined as previously described [6,7]. In brief, HEK cells stably transfected with cDNA encoding human β2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, CA) were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 0.05% penicillin-streptomycin with 400 μg/ml G418. The cells were scraped from the 150 × 25 mm plates and centrifuged at 500 × g for 5 min. The pellet was washed twice by homogenization in 50 mM Tris-HCl, pH 7.7 and centrifugation at 27,000 × g for 10 min. The pellet was resuspended in 25 mM Tris-HCl, containing 120 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, and 5 mM glucose, pH 7.4. The binding assays contained 0.3 nM [3H]CGP-12177 in a volume of 1.0 ml. Nonspecific binding was determined using 1 μM propranolol. Binding measurements were conducted in triplicate at 4°, 15°, 25°, 30° and 37°C. To reach equilibrium, the incubation lasted 1.5 h at 4°, and 1 h for the experiments conducted at 15°, 25°, 30°, and 37°C. The buffer was adjusted to maintain pH 7.4 at each temperature. As previously described (6,7), the data was used to construct binding curves, which were used to determine IC50 values and Hill coefficients using Prism software (GraphPad Software, Inc., San Diego, CA) running on a personal computer and Ki values were then determined using the Cheng-Prusoff transformation.
2.3 Calculations
The enthalpic (ΔH°) and entropic (ΔS°) contribution to the standard free energy change of binding ( ΔG° ) were determined using the following equation:
| (1) |
where Ki is equilibrium binding constant, R is the gas constant (8.314 J mol−1K−1) and T is the temperature of experiment in kelvin. The van't Hoff plots were constructed by plotting ln KA versus 1/T, and the slope and the intercept were used to estimate both ΔH° and ΔS° values. Since the binding affinity of (R,R')-fenoterol did not depend upon temperature, the correlation examined using eqn. (1) was not statistically significant. In this case, the slope was assumed to be zero and the intercept was calculated as the average of lnKA values determined over the temperature range.
3. Results
The effect of a decrease in temperature, from 37°C to 4°C, on the Ki values of the fenoterol stereoisomers is reported in Table 1. The agonist (R)-isoproterenol and the antagonist rac-propranolol were utilized as a prototypical agonist and antagonist as the thermondynamics of the β2-AR binding of these compounds have been previously examined [1–4]. The results obtained for these two compounds are also presented in Table 1. The binding affinity, Kd value, of the marker ligand, [3H]-CGP-12177, was also determined over the range of experimental temperatures and the results are presented in the Supplemental Data, Table S1. At each temperature, the Kd value of the marker at that temperature was used in the calculation of the Ki values of the displacers.
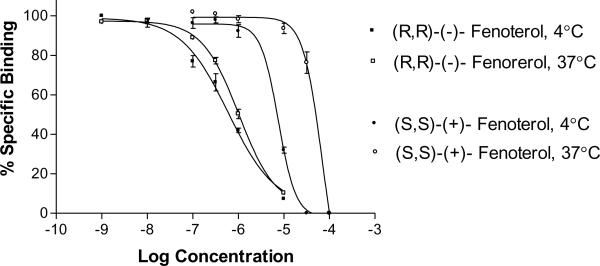
All of the binding curves constructed in this study were monophasic and indicative of an interaction with one homogenous class of binding sites, although steeper slopes were observed with (S,S')-fenoterol and (S,R')-fenoterol. This is illustrated by the binding curves for (R,R')-fenoterol and (S,S')-fenoterol at 4°C and 37°C, Fig. 2. Hill coefficients were determined for all of the binding experiments, Table 1. The calculated coefficients for (R,R')-fenoterol, (R,S')-fenoterol, (R)-isoproterenol and rac-propranolol were close to 1.0, while the Hill coefficients for (S,S')-fenoterol and (S,R')-fenoterol were significantly greater than 1.0. While the meaning of these differences has not been determined, they appear to be associated with the stereochemistry at the beta-hydroxy carbon and suggest that the chirality at this carbon affects the mode of binding.
Figure 2.
The displacement of 0.3 nM [3H]CGP-12177 by (R,R')-fenoterol and (S,S')-fenoterol from membranes obtained from HEK293 cells stably transfected with cDNA encoding human β2-AR obtained at 4°C and 37°C. See text for experimental details.
In this study, the reduction in temperature produced a significant reduction in the Ki values for (S,S')-fenoterol and (S,R')-fenoterol with an affinity difference between the lowest and highest temperature, ΔKi of −11683 nM and −2740 nM, respectively. The significance of the difference was determined using a two-tailed t-test for independence of two means and the calculated p values were 0.017 ((S,S')-fenoterol) and 0.013 ((S,R')-fenoterol). There was a significant decrease in the Ki values for (R)-isoproterenol with decreasing temperature, ΔKi −210 nM (p = 0.003), which is consistent with the previously reported data [1–3]. The results are consistent with the description of the effect of temperature on the binding affinities of β2-AR agonists in which there are large increases in binding affinities with decreasing temperatures [1–3].
The opposite effect was observed with (R,S')-fenoterol, where the Ki values increased with decreasing temperature and the ΔKi value was +741 nM (p = 0.015). For (R,R')-fenoterol the ΔKi was +14 nM, but this difference was not statistically significant, p = 0.5740, as the ΔKi was lower than the average standard deviation of Ki determination which was 23 nM. In case of rac-propranolol the ΔKi was was also small (−0.09 nM) but this time the difference was statistically significant, p = 0.014. The data indicate that temperature had little effect on the affinity of (R,R')-fenoterol or rac-propranolol. The observed results for propranolol are consistent with the general observation that changes in temperature have little effect on the binding affinities of β2-AR antagonists [1,2], however inconsistent for full β2-AR agonists such as (R,R')-fenoterol and (R,S')-fenoterol.
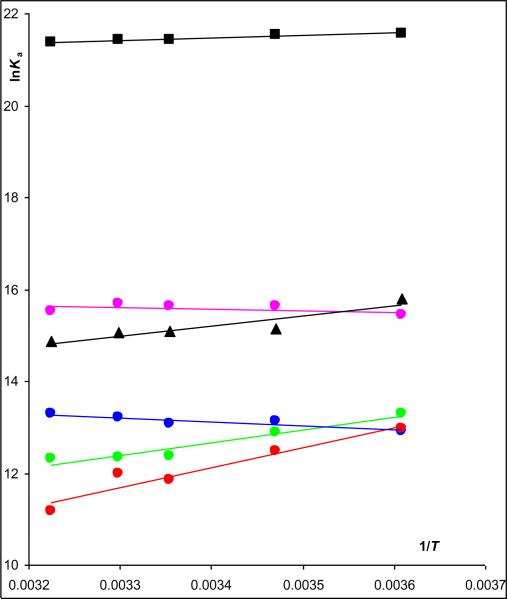
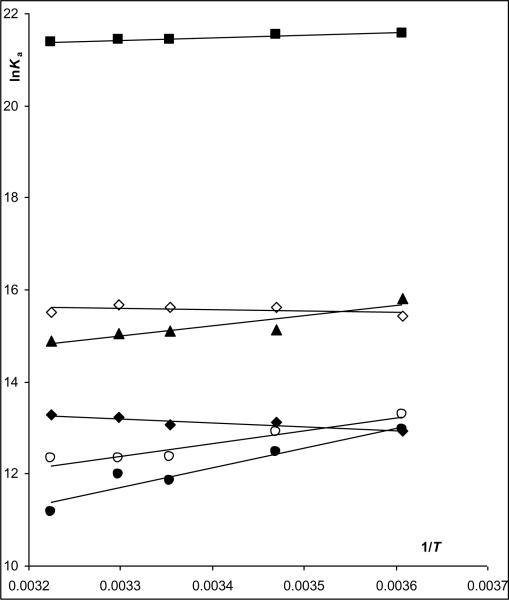
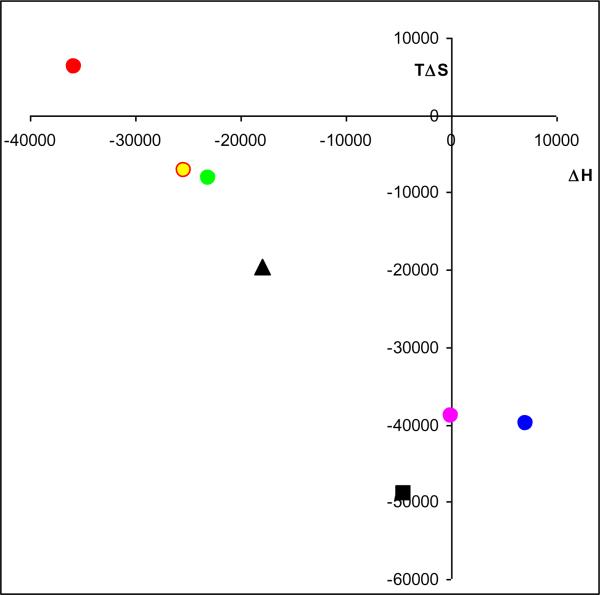
The data in Table 1 were used to construct van't Hoff plots, Fig.2, and to calculate the entropic (ΔS°) and enthalpic (Δ°) components of the binding process, Table 2. The van't Hoff plots for (S,S')-fenoterol, (S,R')-fenoterol and (R)-isoproterenol were linear with positive slopes, and the calculated ΔH° values were highly negative and the −TΔS° values ranged from −19.54 kJ mol-1 ((R)-isoproterenol) to close to zero for (S,S')-fenoterol and (S,R')-fenoterol. The results indicate that the binding of these compounds were primarily enthalpy-driven. In contrast, the van't Hoff plot for (R,S')-fenoterol was linear with a negative slope, the calculated ΔH° value was close to zero (7.1 kJ mol−1) and the −TΔS° value was −40.0 kJ mol−1. In case of (R,R')-fenoterol, where van't Hoff correlation was not statistically significant, the slope (and ΔH°) was assumed to be zero and the intercept was calculated by averaging the lnKi values over the temperatures. The intercept was then used to calculate ΔS° and the −TΔ° values for (R,R')-fenoterol, − 38.8 kJ mol−1. The results for (R,S')-fenoterol, (R,R')-fenoterol and rac-propranolol indicate that the binding processes for these compounds were primarily entropy-driven. The thermodynamic parameters, ΔH° and TΔS°, for each compound were plotted following the approach reviewed by Borea, et al [4], Figure 3. The resulting figure presents a visual representation of the thermodynamic data and a simplified classification of ligand-receptor interactions according to their presence in quadrants labeled as enthalpy driven, enthalpy-entropy driven or entropy-driven processes. The TΔS° and ΔH° plot using the data from this study indicated that the binding of (S,S')-fenoterol to the β2-AR was within the quadrant associated with a purely enthalpy controlled process, the binding of (S,R')-fenoterol, (R)-isoproterenol and rac-propranolol were located within the quadrant associated with an enthalpy-entropy driven process and the binding of (R,S')-fenoterol and (R,R')-fenoterol and were within the quadrant associated with a purely entropy controlled process, Fig. 3. The results obtained with (R)-isoproterenol and rac-propranolol were consistent with previously reported plots [4]. When the previously reported ΔH° and TΔS° parameters for rac-fenoterol [1] were plotted, the compound was placed within the quadrant associated with an enthalpy-entropy driven process and lay between (S,S')- and (R,R')-fenoterol, Fig. 3. This is consistent with the data from this study as results for the rac-fenoterol would reflect the contribution of both enantiomers. In addition, it is interesting to note that the Kd values associated with the binding of [3H]-(−)-CGP-12177 were temperature dependent and decreased with increasing temperature, Supplemental Data, Table S1. The data was used to construct a van't Hoff plot which was linear (R2 = 0.972) and which allowed the calculation of ΔH= +20.9 (± 2.0) kJ/mol and −TΔS= −76.4 (± 2.0) kJ/mol. The results locate the compound within the quadrant associated with an enthalpy controlled process as would be expected for an antagonist.
Table 2.
Linear regression of the Van't Hoff relations and the estimation of ΔS° values [kJ/mol/K] and ΔH° values [kJ/mol]; TΔS° [kJ/mol] and ΔG° values for T = 300 °K [kJ/mol]; data presented as (± SE) for fenoterol stereoisomers, (R)-isoproterenol and rac-propranolol
| (S,S')-fenoterol | (S,R')-fenoterol | (R,S')-fenoterol | (R,R')-fenoterol | (R)-isoproterenol | Rac-propranolol | |
|---|---|---|---|---|---|---|
| R2 | 0.926 | 0.921 | 0.858 | 0.204 (1) | 0.844 | 0.919 |
| Slope | 4309 ± 703 | 2768 ± 469 | −856 ± 201 | --- | 2169 ± 537 | 542 ± 93 |
| intercept | −2.52 ± 2.39 | 3.25 ± 1.59 | 16.03 ± 0.68 | 15.57 ± 0.1(2) | 7.84 ± 1.8 | 19.63 ± 0.32 |
| Δ H ° | −35.8 ± 5.8 | −23.0 ± 3.9 | 7.1 ± 1.7 | 0 | −18.03 ± 4.5 | −4.5 ± 0.78 |
| Δ S ° | −0.021 ± 0.020 | 0.027 ± 0.013 | 0.133 ± 0.006 | 0.129 ± 0.001(2) | 0.065 ± 0.015 | 0.164 ± 0.002 |
| − T Δ S ° | −6.3 ± 6.0 | 8.1 ± 4.0 | 40.0 ± 1.7 | 38.8 ± 0.2 | 19.5 ± 4.5 | 48.95 ± 0.79 |
| ΔG° | −29.5 ± 8.3 | −31.1 ± 5.6 | −32.9 ± 2.4 | −38.8 ± 0.2 | −37.6 ± 6.4 | −53.5 ± 1.1 |
– linear correlation not statistically valid so no slope reported (and assumed to be 0)
– assuming no temperature dependence, the intercept value was calculated as the average of lnKi values determined in different temperatures (± SD).
Figure 3.
(Online version) Van't Hoff plots for (S,S')-fenoterol ( ), (S,R')-fenoterol (
), (S,R')-fenoterol ( ), (R,S')-fenoterol (
), (R,S')-fenoterol ( ), (R,R')-fenoterol (
), (R,R')-fenoterol ( ), rac-propranolol (∎) , (R)-isoproterenol (▴).
), rac-propranolol (∎) , (R)-isoproterenol (▴).
(Black and White print version) Van't Hoff plots for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴).
4. Discussion
All of the fenoterol stereoisomers used in this study are full agonists of the β2-AR, and, therefore, the results of this study are inconsistent with the hypothesis that agonist binding to the β-AR [1] and β2-AR [2] is an enthalpy-driven process. Instead, the data indicate that agonist binding to the β2-AR is similar to the processes described for the cholecystokinin CCK2, dopamine D2, histamine H1, δ- and μ-opiod, purinergic P2X1 and serotonin 5-HT1A receptors, in which agonists bind to the receptors with both enthalpy-driven and entropy-driven mechanisms [4,9].
Since the physicochemical properties of enantiomers are essentially equivalent, the thermodynamic differences between the two enantiomeric pairs, i.e. (R,R')- and (S,S')-fenoterol and (R,S')- and (S,R')-fenoterol, reflect the specific binding interactions with the β2-AR. The designation of the binding process as enthalpy-driven or entropy-driven is the sum of the enthalpic (ΔH°) and entropic (ΔS°) contributions of these interactions; in which ΔH° is associated with hydrogen bond formation, polar interactions and van der Waals interactions and ΔS° reflects hydrophobic interactions produced by an increase in solvent entropy arising from the conformational changes in the receptor involving hydrophobic groups and the release of water upon binding [3,5,10].
Based on the data from this study, the chirality of the carbon atom containing the β-hydroxyl group of the fenoterol molecule (the β-OH carbon) is a key factor in the determination of whether the binding process will be enthalpy-driven or entropy-driven. When the configuration at the β-OH carbon is S, the binding process is enthalpy-driven while the R configuration produces an entropy-driven process. While the effect of the stereochemistry at the β-OH carbon on the thermodynamics of agonist binding to the β2-AR has not been previously examined, the chirality at the β-OH carbon has been shown to affect the binding kinetics, affinities and agonist activities, c.f. [8, 11–14]. These differences have been associated with enantioselective binding interactions between the β-OH moiety and the Asn-293 residue in TM6 of the protein, in which the R-configuration produced the more favorable complex [11]. Another description of the effect of the chirality of the β-OH carbon on agonist activities and binding kinetics was based upon the assumption that the β2-AR exists in an inactive (R) state and one or more ligand-specific active conformations (R*n) and that stereochemistry plays a role in the conformational response to agonist binding [12,13]. A recent computational study of the docking of enantiomeric β2-AR agonists and antagonists with a human β2-AR model has raised the possibility that (R)- and (S)-enantiomers interact with different residues on the receptor [14].
We have previously described the binding of fenoterol and fenoterol derivatives as involving interactions with two binding areas of the β2-AR [6,7]. In this model, the “catechol” portion of the molecule interacts with the area created by the transmembrane (TM) helices defined by TM3, TM5 and TM6 (“catecholamine site”). The binding of agonists and antagonists within this area has been extensively studied, c.f. [11–13] and attributed to a combination of ionic, hydrogen bonding and π–π or π-hydrogen bond interactions. It is reasonable to assume that this process would be predominately described as an enthalpy-driven process. Fenoterol also contains a 4-hydroxyphenyl moiety on the aminoalkyl portion of the molecule, Fig. 1, and additional binding interactions have been suggested between the aromatic substituent and a second binding area defined by TM3, TM6 and TM7 (“hydrophobic site”). These interactions include hydrophobic and π–π or π-hydrogen bond interactions and hydrogen bond formation involving the 4-hydroxy moiety [6,7]. It is reasonable to assume that binding within this site would be predominantly described as an entropy-driven process.
The results of this study are consistent with a binding process that includes interactions with both of the identified binding areas and in which the relative importance of these interactions is indicated by the thermodynamic profile. Thus, for (R,R') and (R,S')-fenoterol, the interactions of the aminoalkyl portion of the molecule within the “aromatic site” are of greater importance in the formation and stabilization of the fenoterol-β2-AR complex than the interactions with the binding area defined as the “catecholamine site”. The inverse situation is suggested by the data obtained with (S,S')- and (S,R')-fenoterol.
The data does not permit a definitive determination of whether the change from an enthalpy-driven process to an entropy-driven process is a result of enantioselective interactions with different residues in the receptor or the induction of enantiomer-specific conformations. In the current study, [3H]-CGP-12117 was used as the marker ligand. CGP-12117 is a high-affinity neutral antagonist which acts at the “catecholamine site,” although at high concentrations the compound binds to at least one additional site on the β1-AR and acts as an agonist [15]. Thus, the enantioselective differences in the Ki values reflect the relative affinity of the fenoterol stereoisomers for an inactive receptor state. The determination of the effect of the stereochemistry at the β-hydroxy carbon on the binding to an active state of the receptor would provide additional insight into this issue. We have recently reported the synthesis of [3H]-labeled (R,R')-4-methoxyfenoterol [16], an analogue of fenoterol with full β2-AR agonist activity [7,8]. Displacement studies using this compound as the marker ligand are currently in progress and the results will be reported elsewhere.
Supplementary Material
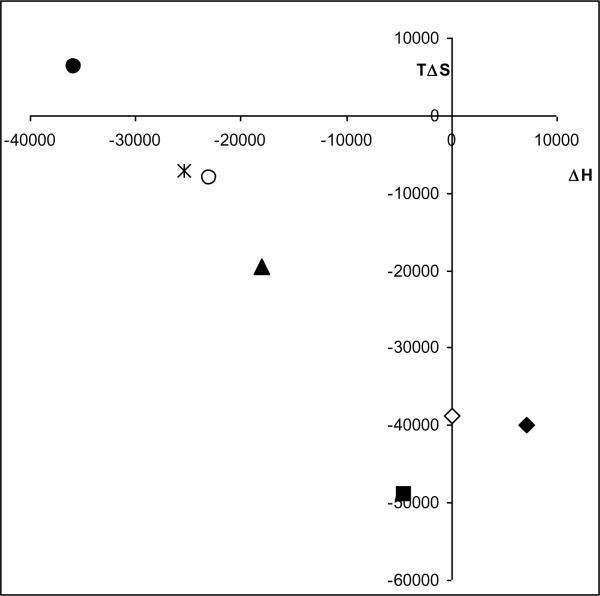
Figure 4.
(Online version) Enthalpy/entropy relation plot for (S,S')-fenoterol ( ), (S,R')-fenoterol (
), (S,R')-fenoterol ( ), (R,S')-fenoterol (
), (R,S')-fenoterol ( ), (R,R')-fenoterol (
), (R,R')-fenoterol ( ), rac-propranolol (∎), (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol (
), rac-propranolol (∎), (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol ( ) by Weiland et.al [1].
) by Weiland et.al [1].
(Black and White print version) Enthalpy/entropy relation plot for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol ( ) by Weiland et.al [1].
) by Weiland et.al [1].
Acknowledgements
This work was supported in part by funds from the National Institute on Aging Intramural Research Program, by the National Institutes on Aging under contract number N01AG-3-1009 and the Foundation for Polish Science (FOCUS 4/2006 Programme).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Weiland GA, Minneman KP, Molinoff PB. Fundamental difference between the molecular interactions of agonists and antagonists with the β-adrenergic receptor. Nature. 1979;281:114–117. doi: 10.1038/281114a0. [DOI] [PubMed] [Google Scholar]
- [2].Contreras ML, Wolfe BB, Molinoff PB. Thermodynamic properties of agonist interactions with the beta adrenergic receptor-coupled adenylate cyclase system. I. high- and low-affinity states of agonist binding to membrane-bound beta adrenergic receptors. J Pharm Exp Ther. 1986;237:154–64. [PubMed] [Google Scholar]
- [3].Miklavc A, Kocjan D, Mavri J, Koller J, Hadzi D. On the fundamental difference in the thermodynamics of agonist and antagonist interactions with β-adrenergic receptors and the mechanism of entropy-driven binding. Biochem Pharm. 1990;40:663–9. doi: 10.1016/0006-2952(90)90299-z. [DOI] [PubMed] [Google Scholar]
- [4].Borea PA, Dalpiaz A, Varani K, Gilli P, Gilli G. Can Thermodynamic Measurements of Receptor Binding Yield Information on Drug Affinity and Efficacy? Biochem Pharm. 2000;60:1549–1556. doi: 10.1016/s0006-2952(00)00368-3. [DOI] [PubMed] [Google Scholar]
- [5].Merighi S, Simioni C, Gessi S, Varani K, Borea PA. Binding thermodynamics at the human cannabinoid CB1 and CB2 receptors. Biochem Pharmacol. 2010;79:471–77. doi: 10.1016/j.bcp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- [6].Jozwiak K, Khalid C, Tanga MJ, Berzetei-Gurske I, Jimenez L, Kozocas JA, et al. Comparative molecular field analysis of the binding of the stereoisomers of fenoterol and fenoterol derivatives to the β2 adrenergic receptor. J. Med. Chem. 2007;50:2903–15. doi: 10.1021/jm070030d. [DOI] [PubMed] [Google Scholar]
- [7].Jozwiak K, Woo AHY, Tanga MJ, Toll L, Jimenez L, Kozocas JA, et al. Comparative Molecular Field Analysis of Fenoterol Derivatives: A Platform Towards Highly Selective and Effective β2 Adrenergic Receptor Agonists. Bioorg. Med. Chem. 2010;18:728–736. doi: 10.1016/j.bmc.2009.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Woo AYH, Wang TB, Zeng X, Zhu W, Abernethy DR, Wainer IW, et al. Stereochemistry of an Agonist Determines Coupling Preference of β2-Adrenoceptor to Different G Proteins in Cardiomyocytes. Mol Pharm. 2009;75:158–165. doi: 10.1124/mol.108.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harper EA, Mitchell EA, Griffin EP, Kalindjian SB. Thermodynamic analysis does not allow discrimination of agonists and antagonists at human CCK2S-receptors. Eu J Pharmacol. 2008;581:1–12. doi: 10.1016/j.ejphar.2007.11.055. [DOI] [PubMed] [Google Scholar]
- [10].Borea PA, Dalpiaz A, Varani K, Gessi S, Gilli G. Binding thermodynamics at A1 and A2A adenosine receptors. Life Sci. 1996;59:1373–88. doi: 10.1016/0024-3205(96)00311-6. [DOI] [PubMed] [Google Scholar]
- [11].Wieland K, Zuurmond HM, Krasel C, IJzerman AP, Lohse MJ. Involvement of ASN-293 in stereospecific agonist recognition and in activation of the β2-adrenergic receptor. Proc Natl Acad Sci USA. 1996;93:9276–81. doi: 10.1073/pnas.93.17.9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Seifert R, Wenzel-Seifert K, Gether U, Kobilka BK. Functional differences between full and partial agonists: evidence for ligand-specific receptor conformations. J Pharmacol Exp Ther. 2001;297:1218–26. [PubMed] [Google Scholar]
- [13].Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. Sequential Binding of Agonists to the β2 Adrenoceptor. J Bio Chem. 2004;279:686–91. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- [14].Soriano-Ursúa MA, Trujillo-Ferrara JG, Alvarez-Cedillo J, Correa-Basurto Docking studies on a refined human β2 adrenoceptor model yield theoretical affinity values in function with experimental values for R-ligands, but not for S-antagonists. J Mol Model. doi: 10.1007/s00894-009-0563-5. DOI 10.1007/s00894-009-0563-5, published online 11 July 2009. [DOI] [PubMed] [Google Scholar]
- [15].Baker JG, Proudman RGW, Hawley NC, Fischer PM, Hill SJ. Role of Key Transmembrane Residues in Agonist and Antagonist Actions at the Two Conformations of the Human β1-Adrenoceptor. Mol Pharm. 2008;74:1246–60. doi: 10.1124/mol.108.048371. [DOI] [PubMed] [Google Scholar]
- [16].Kozocas JA, Bupp JE, Tanga MJ, Pluhar JT, Wainer IW. Synthesis of Tritium Labeled (R,R)-4-Methoxyfenoterol. J Labl Comp Radiopharmaceut. doi: 10.1002/jlcr.1703. DOI 10.1002/jlcr.1703, published online 3 December 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.