Abstract
Cells from patients with genomic instability syndromes have high predisposition to cancer. However, little is known about whether these mutant cells have high susceptibility to oncogenic transformation. We have tested the hypothesis that a defect in maintaining genome integrity is necessary but not sufficient alone for oncogenic transformation and needs to collaborate with other signals in order to produce full oncogenic transformation. Using genetically matched primary cells deficient for the Fanconi complementation group C gene (Fancc) and the ataxia telangiectasia mutated gene (Atm), we found that certain forms of oncogenic activation and cooperation require a combination of genomic instability with increased expression of nucleophosmin (NPM) to prevent oncogenic stress-induced apoptosis or senescence. Intriguingly, co-expression of c-Myc and NPM leads to a synergistic increase in the proliferation rate in Fancc−/− or Atm−/− cells. Analysis of p53 stabilization and activation by c-Myc demonstrates that over-expression of NPM significantly reduces the accumulation of the activated p53 but not the stability of p53. Moreover, NPM is shown to enhance transforming activity of co-expressed Myc and Ras in wild-type and, to a greater degree, in Fancc−/− or Atm−/− cells, suggesting a role in oncogenic cooperation. Finally, a partial knockdown of NPM is sufficient to cause massive apoptosis in Fancc−/− or Atm−/− cells co-expressing c-Myc and Ras while sparing untransformed cells. Our study demonstrates a novel mechanism of NPM tumorigenesis by establishing NPM as a crucial inhibitor of oncogene-induced apoptosis and senescence.
Introduction
In respond to oncogenic activation, normal cells induce genetically encoded programs that prevent deregulated proliferation and thus protect multicellular organisms from cancer progression. Two such programs induced by oncogenic activation are apoptosis and senescence that are normally triggered by DNA damage or other stresses. Studies have demonstrated that over-expression of anti-apoptotic proteins such as Bcl-2 or deletion of apoptosis- and senescence-associated proteins such as p53 accelerates oncogene-induced tumorigenesis (1,2). The theory that has arisen from these findings is that oncogene-driven proliferation must be associated with inhibition of apoptosis and senescence to allow malignant outgrowth.
Oncogene-induced senescence is considered a tumor-suppressive mechanism employed by cells to maintain genomic stability and protect themselves from malignant transformation (3). For example, over-expression of activated Ha-ras can trigger senescence in normal fibroblasts and epithelial cells (4–6). The mechanism of oncogene-induced senescence is believed to involve the p16INK4a-retinoblastoma protein and the alternative reading frame (ARF)–p53 pathways that are also operative in other stress-induced senescence (7). To a large extent, cellular senescence triggered by oncogenic activation is dependent on the p19–ARF pathway-mediated stabilization of p53 (4,8,9). However, recent studies have shown that expression of oncogenic K-rasV12 or BRAFE600 in premalignant tumors induced senescence through activation of both p16INK4a-retinoblastoma and the ARF–p53 pathways (10,11).
It is believed that transformation is initiated by a combination of oncogenic mutational events and loss of the cellular checkpoints that prevent cell division in the presence of DNA damage (12). The initiation phase of cancer development can persist in otherwise normal tissue indefinitely until the occurrence of a second phase of events, known as promotion. Promotion can result from exposure of initiated cells to a variety of stimuli including the activation of certain oncogenes and reduced repair of DNA damage. In this context, cells with genomic instability are conceivably more susceptible to oncogenic transformation than normal cells. Indeed, cells from patients with genomic instability syndrome such as Fanconi anemia (FA), Bloom’s, Werner’s or ataxia telangiectasia have high predisposition to cancer due to inactivating mutations in the genes encoding proteins that play critical roles in the detection and repair of DNA damage and in maintaining genome integrity (13–17). We have tested the hypothesis that a defect in maintaining genome integrity is necessary but not sufficient alone for oncogenic transformation and needs to collaborate with other signals in order to produce full oncogenic transformation. Using genetically matched primary cells deficient for the FA complementation group C gene (Fancc) and the ataxia telangiectasia mutated gene (Atm), we found that certain forms of oncogenic activation and cooperation require a combination of genomic instability with increased expression of the nucleolar protein nucleophosmin (NPM) to prevent oncogenic stress-induced apoptosis or senescence.
NPM is a multifunctional protein that plays important roles in the regulation of cell proliferation and apoptosis (18). NPM is found to be more abundant in tumor and growing cells than in normal resting cells (19–23). Conversely, NPM expression is down-regulated in cells undergoing differentiation or apoptosis (24). In the context of tumorigenesis, NPM appears to be the target for certain transforming oncogenes such as c-Myc (25,26). In addition to over-expression in cancer cells, NPM is frequently found in the chromosomal translocation associated with several hematopoietic malignancies, such as acute promyelocytic leukemia, anaplastic large-cell lymphomas and myelodysplastic syndrome/acute myeloid leukemia (27–29). Strikingly, certain mutations in the NPM gene that cause aberrant cytoplasmic localization have been found in about one-third of patients with acute myelogenous leukemia, making it one of the most frequently mutated genes in acute myelogenous leukemia (30–32). Recently, studies with NPM-deficient mice have shown that loss of NPM compromises tumor suppressor ARF stability and genomic instability contributing to tumorigenesis (33,34).
Despite the link of NPM over-expression to proliferation in cancer cells, very little is known about the mechanism by which NPM influences the fundamental cellular processes during oncogene activation. We wished to investigate whether, under which conditions, NPM over-expression could bypass oncogene-induced apoptosis and senescence. Here we show that over-expression of NPM prevents apoptosis and senescence induced by oncogene activation in primary mouse embryo fibroblasts (MEFs) and appears to enhance oncogenic cooperation between ras and myc.
Materials and methods
Mice and cell culture
Fancc−/− mice were generated by interbreeding the heterozygous Fancc+/− mice [kindly provided by Dr Manuel Buchwald, University of Toronto (35)]. p53−/− and ARF−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and National Cancer Institute (Bethesda, MD), respectively. All experimental procedures conducted in this study were approved by the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital Medical Center. All mice have been maintained on a C57BL/6 background and genotyped routinely by polymerase chain reaction analysis. MEFs were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM non-essential amino acids, 55 μM β-mercaptoethanol and 10 μg/ml gentamycin.
Retroviral expression vectors and infection
The full-length human NPM (GeneBank sequence accession number BC009623) was amplified by polymerase chain reaction, using Pfu DNA polymerase (Stratagene, La Jolla, CA) and the following primers: forward, 5′-ATAAGAATGCGGCCGCCACCATGGAAGATTCGATGGACATG and reverse, 5′-GTCCTAGAAAGAGACTTCCTCCACTG. The resulting polymerase chain reaction fragments were sub-cloned into the Not1 and Xbal sites of p3 × FLAG-CMV-14 (Sigma, St. Louis, MO) to create p3 × FLAG-CMV-14-NPM. The flag-tagged NPM was then removed and sub-cloned into the retroviral vector pMSCVpuro (BD Biosciences Clontech, San Jose, CA) to create pMSCVpuro-NPM. The retroviral constructs MSCV-IRES-GFP-MycER (36) and MIEG3-H-ras-G12V are from Dr John L. Cleveland (St Jude Children’s Research Hospital) and Dr Yi Zheng (Cincinnati Children’s Hospital Medical Center), respectively. Retroviruses were prepared by the Viral Vector Core of Cincinnati Children’s Research Foundation (Cincinnati Children’s Hospital Medical Center), using 10 μg of each vector DNA. MEFs were infected at passage 2 with the pMSCVpuro or pMSCVpuro-NPM viruses in the presence of 4 μg/ml polybrane, and selected with puromycin (2 μg/ml). After 3 days of drug selection, the cells were then infected with the MycER or H-ras-G12V viruses or both without drug selection. To activate c-Myc, the MycER-transduced cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen (Sigma, St. Louis, MO).
Analysis of cell proliferation and apoptosis
For analysis of Myc-induced cell death, MEFs at passage 2 were infected with the empty pMSCVpuro vector or pMSCVpuro-NPM retroviruses. After 72 h puromycin selection, the cells were split and infected with the control vector or mycER viruses. Forty-eight hours after transduction, cells were cultured in the absence or presence of 125 nM 4-hydroxy tamoxifen. Cell viability was determined at the indicated time points by Trypan blue exclusion. Apoptosis was determined as percentage of cells with subdiploid DNA content by staining the cells with propidium iodide followed by flow cytometry. For proliferation analysis, cells were synchronized at G0 by culturing in medium supplemented with 0.1% fetal bovine serum for 96 h. MEFs were cultured for 24 h in normal growth medium supplemented with 10 μM bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO), harvested by trypsinization and fixed in 70% ethanol. BrdU-labeled cells fixed in 70% ethanol were treated with 2 N HCl (20 min at room temperature followed by addition of two volumes of 0.1 M sodium borate (pH 8.5). The cells were incubated with an anti-BrdU mouse monoclonal antibody, washed and incubated with fluorescein isothiocyanate-conjugated anti-mouse antibody. Cells were counterstained overnight with 5 μg of propidium iodide/ml containing 40 μg of RNase/ml. The stained cells were analyzed by flow cytometry.
Senescence-associated-β-galactosidase activity analysis
Senescence-associated-β-galactosidase (SA-β-gal) activity was determined using an SA-β-gal staining kit from Cell Signaling Technology (Beverly, MA) according to the manufacturer’s instruction. Briefly, cells were washed in phosphate-buffered saline and fixed in 2% formaldehyde–0.2% glutaraldehyde. Then the cells were washed and incubated at 37°C overnight with fresh SA-β-gal stain solution [1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside, 40 mM citric acid–sodium phosphate (pH 6.0), 150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide]. Senescent cells were identified as blue-stained cells by standard light microscopy, and a total of 500 cells were counted in random fields on a slide to determine the percentage of SA-β-gal-positive cells.
Immunoprecipitation and immunoblotting
Whole cell extracts (200 μg in 500 μl) were incubated with 20 μl of the anti-FLAG (M2, conjugated to agarose beads, Sigma, St. Louis, MO) or p53 (DO-1; Calbiochem, San Diego, CA) antibodies for 2 h at 4°C. Beads were washed three times and collected by centrifugation. For immunoblotting, samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Immunoblots were incubated with the antibodies specific for NPM, p53, p21 and ARF (all from Santa Cruz Biotechnologies, Santa Cruz, CA), Myc (Oncogene, Cambridge, MA), phospho-p53ser15 (Cell Signaling Technology, Beverly, MA) or β-actin (Sigma, St. Louis, MO) for 12–16 h at 4°C.
Soft agar and foci formation assays
Cells (1 × 104) in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 0.45% low-gelling temperature agarose (Sigma) were plated onto six-well plates with 0.75% agarose underlay. Two weeks later, colonies (>40 cells) were counted. To determine the foci number, 5 × 104 cells were plated onto six-well plates in growth medium. Cell was cultivated for 15 days with changing medium every 3 days. Then the plates were stained with Wright–Giemsa (Fisher Scientific, Waltham, MA) and washed extensively.
RNA interference
The small interfering RNA (siRNA)-targeting NPM was designed using the siRNA target finder and design tool (http://www.ambion.com) and criteria suggested by Brummelkamp et al. (37). Two siRNA oligonucleotide duplexes, one targeting nucleotides 84–106 relative to the translation initiation codon of mouse NPM (GeneBank sequence accession number NM008722) and the other serving as the scramble control, were transfected into cells with Oligofectamine (Invitrogen) according to the manufacturer’s protocol. To achieve partial knockdown, NPM siRNA was used at 50, 100 and 150 nM whereas the scramble siRNA at 150 nM.
Results
NPM suppresses c-Myc-induced apoptosis and senescence and enhances proliferation in collaboration with c-Myc in Fancc−/− and Atm−/− cells
We recently demonstrated that NPM protects cells from apoptosis induced by DNA damage, hypoxia and oxidative stress (38,39). We wanted to know if NPM also functioned in cellular response to oncogenic stress. To this end, we assessed the ability of c-Myc to signal growth arrest in primary MEFs over-expressing NPM. Since we wished to test whether cells with genomic instability were more susceptible to oncogenic transformation than normal cells, we used two mouse models of genomic instability: one is deficient in the FA complementation group C gene [Fancc−/− (35)] and the other is deleted for the Atm gene [Atm−/− (40)]. We conducted two rounds of retro-viral infections: first with NPM and an empty vector and then with mycER that can be induced into the active conformation by addition of 4-hydroxy tamoxifen. We verified protein expression by western blot analysis in infected wild-type (WT) and Fancc−/− MEFs (Figure 1A). WT cells underwent senescence 48 h after c-Myc activation, which was reduced by over-expression of NPM (Figure 1B). Remarkably, c-Myc induced massive senescence in Fancc−/− and Atm−/− cells, and over-expression of NPM significantly decreased the oncogene-induced senescence in these cells (Figure 1B). These results indicate that NPM suppresses c-Myc-induced senescence in both WT and mutant cells. Similarly, analysis of apoptotic cell death showed that over-expression of NPM significantly reduced Myc-induced apoptosis both in WT cells and in Fancc−/− and Atm−/− mutant cells (Figure 1C).
Fig. 1.
Over-expression of NPM suppresses c-Myc-induced senescence and apoptosis in Fancc−/− and Atm−/− cells. (A) Expression of retrovirally encoding proteins in Fancc−/− and Atm−/− cells. WT, Fancc−/− or Atm−/− MEFs were infected at passage 2 with pMSCVpuro empty vector (V) or pMSCVpuro-NPM retrovirus. After 72 h puromycin selection, the cells were split and infected with the control vector (control) or MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen. Western blot analysis shows expression of the expected Flag-tagged NPM, endogenous NPM and c-Myc proteins. Fifty micrograms of total proteins were loaded in each lane, and equal loading was ensured by probing the blot with anti-β-actin. (B) NPM inhibits c-Myc-induced senescence. Cells described in (A) were further cultured for 48 h and stained for SA-β-gal. The graphs on the right are percentages of the cells stained positive for SA-β-gal quantified by counting a total of 100 cells in random fields on a slide. The data represent the mean ± SD of three independent experiments. (C) Cells described in (A) were analyzed for subdiploid DNA content by flow cytometry 24 h after transfer to medium containing 125 nM 4-hydroxy tamoxifen. Data shown represent mean ± SD of three independent experiments. *Statistical significance between Myc + V and Myc + NPM samples at P < 0.05. (D) Effect of NPM over-expression on proliferation of Myc-activated WT, Fancc−/− or Atm−/− cells. Cells synchronized at G0 were cultured for 24 h in normal growth medium supplemented with 10 μM BrdU, and level of BrdU incorporation was determined by flow cytometry. Data represent mean ± SD of three experiments. *Statistical significance between Myc + V and Myc + NPM samples at P < 0.05.
Next, we determined whether inhibition of Myc-induced apoptotic cell death by NPM over-expression resulted in an increase in cell proliferation. BrdU incorporation experiments show that Fancc−/− or Atm−/− cells proliferated slower than their WT counterparts (% BrdU-positive cells were 17.7 ± 4.2 and 16.3 ± 2.5 in Fancc+/+ or Atm+/+ cells, respectively, in comparison with 6.9 ± 2.2 and 5.3 ± 2.8, respectively, in Fancc−/− or Atm−/− cells; Figure 1D). Over-expression of NPM led to a modest reduction of proliferative cells in the WT but not in Fancc−/− and Atm−/− cells (Figure 1D). This is consistent with others’ observation that over-expressed NPM suppresses WT MEF cell growth (42). Significantly, co-expression of c-Myc and NPM led to a synergistic increase in the proliferation rate in Fancc−/− and Atm−/− cells, whereas this effect was not observed in WT cells (Figure 1D). These results suggest that NPM might cooperate with Myc oncogene to promote proliferation in Fancc−/− and Atm−/− cells.
NPM inhibits Myc-induced p53 activation
NPM protects cells from stress-induced apoptosis through a mechanism involving p53 (12,36,39,41). Given that part of c-Myc oncogenic stress is generated through the ARF–p53 pathway (1–3) and that NPM plays important roles in regulating both the activity and stability of the ARF and p53 proteins (42), we reasoned that NPM is a component of the oncogene–ARF–p53 network. To test this notion, we analyzed p53 stabilization and activation by c-Myc. We found that c-Myc induced accumulation of p53 in WT cells (Figure 2A and B), which is in line with previous observations (4). c-Myc induced p53 phosphorylation at Ser15 (43), an activated form that is also induced by DNA damage and other stresses (44), and this correlated with the expression of the p53 target p21WAF1 (Figure 2A and B). Over-expression of NPM signifi-cantly reduced the accumulation of the activated p53 (p53Ser15) and p21WAF1. Notably, NPM over-expression appeared to increase p53 stability in both control and Myc-expressing cells, which is consistent with previous report (42). More profound Myc induction of p53 was observed in Fancc−/− as well as in Atm−/− cells (Figure 2A and B). Again, NPM over-expression significantly reduced the oncogene-induced activation of p53 and expression of p21WAF1 in these mutant cells. Surprisingly, c-Myc also induced expression of p53Ser15 and p21WAF1 in Atm−/− cells, which was suppressed by NPM over-expression (Figure 2B).
Fig. 2.

Over-expression of NPM inhibits Myc-induced p53 activation. Genetically matched WT and Fancc−/− (A) or WT and Atm−/− (B) MEFs were infected at passage 2 with pMSCVpuro empty vector (V) or pMSCVpuro-NPM retrovirus. After 72 h puromycin selection, the cells were split and infected with the control vector (control) or MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen (4-OHT). Whole cell extracts were then prepared and 50 μg of total proteins from each sample was analyzed by western blot for expression of p53Ser15, p53, p21WAF1, NPM and c-Myc. β-Actin was used as a loading control. (C) WT and Fancc−/− cells were infected at passage 2 with pMSCVpuro empty vector or pMSCVpuro-NPM retrovirus. After 72 h puromycin selection, the cells were infected with MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence or absence of 125 nM 4-hydroxy tamoxifen for 48 h. Whole cell extracts were then prepared and 100 μg of total proteins from each sample was analyzed by western blot for expression of γH2AX and p53Ser15. β-Actin was used as a loading control. (D) Cells described in (C) were treated with mitomycin C (0.5 μM) for 24 h in the presence of 125 nM 4-hydroxy tamoxifen for 48 h. Whole cell extracts were then prepared and 100 μg of total proteins from each sample was analyzed by western blot for expression of γH2AX and p53Ser15. β-Actin was used as a loading control.
It is possible that the effect of NPM over-expression on p53 activation was not due to the inhibition of p53 activity but to the suppression of oncogene-induced DNA damage in the cells. It has been shown that NPM−/− cells accumulate high levels of damaged DNA with consequent checkpoint activation (34), suggesting a role of NPM in maintaining DNA integrity. To test this possibility, we determined the effect of NPM over-expression on oncogene-induced DNA damage/response in WTand Fancc−/− cells by examining two well-established DNA damage response markers, phosphorylated histone H2AX (γH2AX) that forms foci in response to DNA double-strand breaks and phosphorylated p53 at Ser15 (p53Ser15) (43,44). We found that high levels of p53Ser15 and γH2AX could be detected after 48 h of c-Myc activation in WT and Fancc−/− cells (Figure 2C). Over-expression of NPM reduced substantially both p53Ser215 and γH2AX in these cells. Moreover, NPM over-expression eliminated most of p53Ser15 and γH2AX induced by DNA cross-linking agent mitomycin C (Figure 2D). These results suggest that NPM may function to limit oncogene-induced DNA damage to a threshold of DNA damage that can support cell survival.
Inhibition of Myc-induced apoptosis by NPM requires a functional p53–ARF pathway
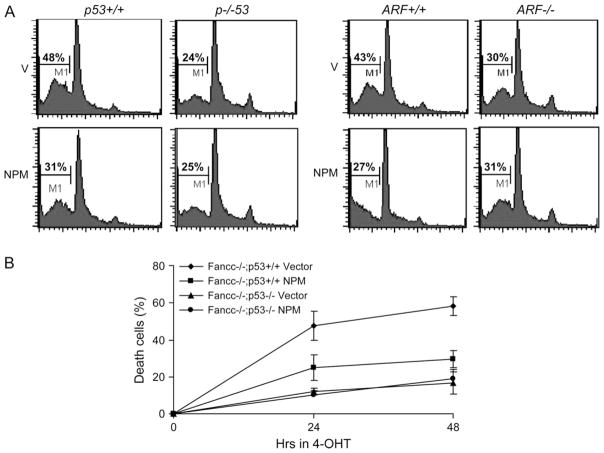
To investigate the role of NPM in oncogene–ARF–p53 signaling under more defined conditions, we analyzed c-Myc-induced apoptosis in p53−/− and ARF−/− primary MEFs over-expressing NPM. Myc-induced apoptosis was mitigated in both p53−/− and ARF−/− cells compared with their c-Myc-activated WT counterparts (Figure 3A). Notably, over-expression of NPM in either p53−/− or ARF−/− cells did not lead to significant reduction of apoptotic cells, as compared with the cells of same genotype but harboring the control vector. To further substantiate these observations, we co-expressed c-Myc and NPM in primary MEF cells from Fancc−/− mice deficient for p53 (Fancc−/− p53−/−). We reasoned that the double-knockout model would provide strong evidence that NPM suppresses c-Myc-induced apoptosis through a mechanism involving p53. Indeed, over-expression of NPM did not result in a significant further decrease in Myc-induced cell death in Fancc−/− p53−/− cells compared with the same parent cells infected with empty vector viruses (Figure 3B). Taken together, these results demonstrate that inhibition of c-Myc-induced apoptosis by NPM over-expression requires a functional ARF–p53 pathway.
Fig. 3.
NPM inhibits Myc-induced apoptosis requires a functional p53–ARF pathway. (A) Effect of NPM over-expression on Myc-induced apoptosis in p53- and ARF-null cells. Genetically matched p53 or ARF WT and null MEFs were infected with pMSCVpuro empty vector (V) or pMSCVpuro-NPM retrovirus. After 72 h puromycin selection, the cells were infected with the MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen (4-OHT) for 24 h and analyzed for subdiploid DNA content by flow cytometry. (B) p53-dependent cell death in Fancc−/− cells expressing activated c-Myc. p53+/+, Fancc−/− or p53−/− and Fancc−/− MEFs were infected with pMSCVpuro empty vector (V) or pMSCVpuro-NPM retrovirus. After 72 h puromycin selection, the cells were infected with the MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen and cell viability was measured at 0, 24 and 48 h after transfer to 4-hydroxy tamoxifen-containing medium.
The observation that NPM engages in the oncogene–ARF–p53 network prompted us to investigate NPM–ARF and NPM–p53 interactions in cells over-expressing NPM. Intriguingly, we found that c-Myc induced NPM–p53 association that was greatly enhanced in Fancc−/− cells (Figure 4A). However, there was no significant difference in c-Myc-induced NPM–ARF interaction between WT and these mutant cells. Interestingly, c-Myc-induced NPM–ARF association was independent of p53 in WT or Fancc−/− cells, since it was also observed in these cells that were deficient for p53 under the same conditions (Figure 4A). Similar results were obtained with Atm-deficient cells (Figure 4B). In addition, reciprocal immunoprecipitation of the cell extracts with antibody specific for p53 showed enhanced c-Myc-induced NPM–p53 interaction in Fancc−/− cells (Figure 4C). These results suggest that NPM exerts its inhibitory role on p53 activation induced by c-Myc, which may be mediated through NPM–p53 interaction.
Fig. 4.

c-Myc-induced NPM–ARF and NPM–p53 interactions in Fancc−/− and Atm−/− cells. WT or Fancc−/− in p53 WT or null background (A) or Atm WT or null (B) MEFs were infected with pMSCVpuro-NPM retroviruses. After 72 h puromycin selection, the cells were infected with the control vector (control) or MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen for 24 h. Whole cell extracts (500 μg in 500 μl) were incubated with 20 μl of the anti-FLAG (M2) antibodies conjugated to agarose beads. The input (left panel; 50 μg per lane) and anti-Flag immunocomplexes were analyzed by immunoblotting with antibodies specific for p53, p19ARF or Flag peptides. (C) WT or Fancc−/− cells were infected with pMSCVpuro-NPM retroviruses. After 72 h puromycin selection, the cells were infected with the control vector (control) or MycER viruses. Forty-eight hours after transduction, cells were cultured in the presence of 125 nM 4-hydroxy tamoxifen for 24 h. Whole cell extracts (500 μg in 500 μl) were incubated with 20 μl of the anti-p53 (DO-1) antibodies conjugated to agarose beads. The input (left panel; 50 μg per lane) and anti-p53 immunocomplexes were analyzed by immunoblotting with antibodies specific for NPM or p53. Note that one-fifth of total immunoprecipitates from c-Myc-expressing cells were loaded. IgG, IgG-heavy chain.
NPM over-expression enhances oncogenic cooperation between myc and ras
Whereas over-expression of either c-Myc or Ras can activate p53 that leads to growth arrest, co-expression of both oncogenes in primary MEFs results in a low level of transformation. Based on our observations that NPM over-expression inhibits c-Myc-induced p53 activation (Figure 2) and that co-expression of c-Myc and NPM leads to a synergistic increase in the proliferation rate in Fancc−/− and Atm−/− cells (Figure 1D), we speculated that NPM might function to enhance oncogenic cooperation between myc and ras. To test this hypothesis, NPM-over-expressing WT and Fancc−/− or Atm−/− MEFs were infected with MycER, activated H-ras-G12V or both viruses, and transforming activity of the cells were then analyzed in soft agar (Figure 5A and B) and foci formation (Figure 5C) assays. Although insufficient for MycER or H-ras-G12V alone to transform the primary WT MEFs, NPM over-expression was able to enhance transforming activity of co-expressed Myc and activated Ras in WT and, to a greater degree, in Fancc−/− and Atm−/− cells (Figure 5A and B). Intriguingly, we found that Fancc−/− or Atm−/− MEFs co-expressing NPM and c-Myc or activated Ras were able to form colonies and foci, suggesting that NPM may function to enhance oncogene cooperation in these mutant cells.
Fig. 5.
NPM over-expression enhances oncogenic cooperation in Fancc−/− and Atm−/− primary cells. (A) WT or Fancc−/− MEFs were infected at passage 2 with pMSCVpuro-NPM retroviruses. After 72 h puromycin selection, the cells were split and infected with the control vector (control) or MycER, H-ras-G12Vor both MycER and H-ras-G12V viruses. Forty-eight hours after transduction, 1 × 104 cells were plated in each well of six-well plate in triplicate in semi-solid medium containing 125 nM 4-hydroxy tamoxifen. Two weeks after plating, colonies were counted and photographed. The numbers at upper right corners are colonies per plate. (B) WT or Atm−/− MEFs were infected at passage 2 with pMSCVpuro-NPM retroviruses. After 72 h puromycin selection, the cells were split and infected with the control vector (control), MycER, H-ras-G12V or both MycER and H-ras-G12V viruses. Forty-eight hours after transduction, 1 × 104 cells were plated in each well of six-well plate in triplicate in semi-solid medium containing 125 nM 4-hydroxy tamoxifen. Two weeks after plating, colonies were counted and data represent mean ± SD of three experiments. *Statistical significance between Myc + Ras and NPM + Myc + Ras samples at P < 0.05. (C) Foci formation. Cells (1 × 104) described in (A) and (B) were plated and cultured in growth medium containing 125 nM 4-hydroxy tamoxifen for two weeks. Then the cells were fixed, stained and colonies were counted. Data represents mean ± SD of three experiments performed in triplicate. *Statistical significance between Myc + Ras and NPM + Myc + Ras samples at P < 0.05.
NPM down-regulation renders oncogene-transformed Fancc−/− and Atm−/− cells hypersensitive to apoptosis
On the basis of the observation that NPM over-expression enhanced oncogenic transformation, we wanted to know whether inhibition of NPM expression would augment oncogenes-induced apoptosis. To this end, the Fancc−/− and Atm−/− cells provided a unique system, as these cells are more susceptible not only to oncogenes-induced apoptosis (Figure 1) but also to oncogenic transformation (Figure 5) probably because of their genomic instability (13,40). We thus sought to inhibit NPM expression by RNA interference (siRNA) in Fancc−/− and Atm−/− MEFs co-expressing MycER and H-Ras-G12V, which efficiently transform the Fancc−/− and Atm−/− cells (Figure 5). To avoid high levels of apoptotic cell death in untransformed cells, we transfected the cells with a dose of siRNA that reduced ~50% of NPM expression (Figure 6A). Strikingly, a partial knockdown of NPM was sufficient to cause massive apoptosis in Fancc−/− or Atm−/− cells co-expressing c-Myc and activated Ras (Figure 6B). In contrast, this partial NPM inactivation did not result in significant apoptosis in WT cells (Figure 6B). These results suggest that oncogene-transformed Fancc−/− or Atm−/− cells may require NPM to prevent apoptosis and probably maintain the transformed state.
Fig. 6.

Partial NPM inactivation by RNA interference sensitizes Fancc−/− and Atm−/− cells to oncogene-induced apoptosis. (A) Levels of NPM protein in genetically matched Fancc or Atm MEFs were infected at passage 2 with pMSCVpuro-NPM retroviruses. After 72 h puromycin selection, the cells were co-infected with the MycER and H-ras-G12V viruses. Forty-eight hours after transduction, cells were transfected with NPM siRNA (50, 100 and 150 nM) or the scramble siRNA (150 nM). Another 36 h later, the cells were analyzed by western blotting for NPM expression level, which was also normalized for loading by probing the western blot with antibody for β-actin. (B) Partial NPM silencing causes transformed Fancc−/− and Atm−/− cell death as analyzed by measurement of fractional subdiploid DNA content after staining with propidium iodide and analysis by flow cytometry. Cells described in (A) were cultured in the presence of 125 nM 4-hydroxy tamoxifen for additional 24 h. Data reflect means ± SD of two independent experiments performed. *Statistical significance between Scramble and siNPM samples at P < 0.05.
Discussion
The NPM protein is over-expressed in a variety of human cancers, and has been proposed to have both oncogenic and tumor-suppressing activities depending on its level of expression and cellular localization (12,45). The study presented here demonstrates a novel mechanism of NPM tumorigenesis by establishing NPM as a crucial inhibitor of oncogene-induced apoptosis and senescence. Since the activation of the ARF–p53 pathway in response to oncogene activation is considered a primary event toward apoptosis, our finding that NPM possesses oncogenic activity, at least in part, through the inhibition of oncogene-induced p53 activation is particularly relevant. In addition, our results give a mechanistic explanation for the high levels of NPM expression in cancer cells.
NPM has been shown to interact with p53 and stabilize the tumor suppressor (42). However, MEFs deleted for the Npm gene actually show increased stability of p53, resulting in elevated apoptosis and senescence (33,34). NPM also binds to and stabilizes ARF (46), the other tumor suppressor of the ARF–p53 pathway that signals cell-growth arrest and apoptosis in response to oncogenic activation (47). The acute myelogenous leukemia-associated NPM mutant proteins (NPMc+), which are cytoplasmic (30–32), retain the ability to bind ARF but impair its stability (45). Interestingly, while the suppression of oncogene-induced apoptosis by NPM requires a functional ARF–p53 pathway (Figure 3), it appears that NPM–ARF association is not critical for the NPM response to c-Myc activation (Figure 4). In addition, over-expression of NPM in c-Myc-activated cells does not affect the stability of either ARF or p53 (Figure 2). These observations support the hypothesis that NPM exerts its inhibitory role on oncogenes-induced p53 activation induced by c-Myc, which may be mediated through NPM–p53 interaction.
Perhaps the most interesting illustration of the NPM effects is the observation that its over-expression enhances oncogenic cooperation in primary cells. It is well known that over-expression of NPM leads to increased cell proliferation and inhibition of apoptotic cell death (12). It is thus conceivable that the anti-apoptotic or proliferation-promoting (or both) function of the NPM protein may have contributed to the observed oncogenic activity of NPM. It is particularly striking to observe the profound enhancement of oncogenic cooperation by NPM over-expression in cells deficient in the FA or ATM DNA damage repair/response pathway. Both FA and ataxia telangiectasia are genomic instability syndromes with a high cancer predisposition (13,40). The combined effect of genomic instability with NPM over-expression may have contributed to enhanced oncogenic cooperation and increased transformation in Fancc−/− and Atm−/− cells. This may also suggest that a defective FA or ATM tumor-suppressing mechanism is necessary but not sufficient alone for oncogenic transformation and needs to collaborate with other signals (such as NPM over-expression) in order to produce full oncogenic transformation. Thus, some forms of oncogenic activation and cooperation may require a combination of elevated level of NPM with genomic instability to direct the cell toward the proliferative state instead of the apoptotic or senescent state. If this hypothesis is correct, then inhibition of NPM expression by a controlled gene knockdown should be sufficient to signal for apoptosis and/or senescence in oncogenically transformed mutant cells although having little effect on untransformed cells. Indeed, our study shows that reduced NPM expression renders oncogene-transforming Fancc−/− or Atm−/− cells hypersensitive to apoptosis while sparing untransformed cells.
Acknowledgments
We thank Dr Manuel Buchwald (Hospital for Sick Children, University of Toronto) for the Fancc+/− mice, Dr. John L. Cleveland (St Jude Children’s Research Hospital) for retroviral vector MSCV-IRES-GFP-MycER, Dr. Yi Zheng (Cincinnati Children’s Hospital Medical Center) for MIEG3-H-ras-G12V, Dr Xiaoling Zhang for technical assistance and discussion and The Vector Core of Cincinnati Children’s Research Foundation (Cincinnati Children’s Hospital Medical Center) for the preparation of retroviruses. Q.P. thanks Dr Grover Bagby (Oregon Health Science University) for continued support. This work was supported in part by a Leukemia Research Foundation grant, a Trustee grant and National Institutes of Health grants R01 CA50519 (DJC), R01 CA109641 and R01 HL076712 (QP).
Abbreviations
- ATM
ataxia telangiectasia mutated gene
- BrdU
bromodeox-yuridine
- FA
Fanconi anemia
- Fancc
Fanconi complementation group C gene
- MEF
mouse embryo fibroblast
- NPM
nucleophosmin
- SA-β-gal
senescence-associated-β-galactosidase
- siRNA
small interfering RNA
- WT
wild type
Footnotes
Conflict of Interest Statement: None declared.
For Permissions, please email: journals.permissions@oxfordjournals.org
References
- 1.Strasser A, et al. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 2.Elson A, et al. The MMTV/c-myc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene. 1995;11:181–190. [PubMed] [Google Scholar]
- 3.Bringold F, et al. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35:317–329. doi: 10.1016/s0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JJ, et al. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13:2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin AW, et al. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc Natl Acad Sci USA. 2001;98:5025–5030. doi: 10.1073/pnas.091100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano M, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 7.Sharpless NE, et al. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–168. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates S, et al. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 9.Palmero I, et al. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- 10.Collado M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 11.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 12.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy RD, et al. The Fanconi anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 14.Bischof O, et al. Regulation and localization of the Bloom syndrome protein in response to DNA damage. J Cell Biol. 2001;153:367–380. doi: 10.1083/jcb.153.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, et al. WRN, the protein deficient in Werner syndrome, plays a critical structural role in optimizing DNA repair. Aging Cell. 2003;2:191–199. doi: 10.1046/j.1474-9728.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 17.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 18.Grisendi S, et al. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- 19.Chan WY, et al. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry. 1989;28:1033–1039. doi: 10.1021/bi00429a017. [DOI] [PubMed] [Google Scholar]
- 20.Feuerstein N, et al. Identification of numatrin, the nuclear matrix protein associated with induction of mitogenesis, as the nucleolar protein B23. Implication for the role of the nucleolus in early transduction of mitogenic signals. J Biol Chem. 1988;263:10608–10612. [PubMed] [Google Scholar]
- 21.Nozawa Y, et al. Expression of nucleophosmin/B23 in normal and neoplastic colorectal mucosa. J Pathol. 1996;178:48–52. doi: 10.1002/(SICI)1096-9896(199601)178:1<48::AID-PATH432>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Subong EN, et al. Monoclonal antibody to prostate cancer nuclear matrix protein (PRO:4-216) recognizes nucleophosmin/B23. Prostate. 1999;39:298–304. doi: 10.1002/(sici)1097-0045(19990601)39:4<298::aid-pros11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, et al. Genes preferentially expressed in embryo stomach are predominantly expressed in gastric cancer. Cancer Res. 1992;52:3372–3377. [PubMed] [Google Scholar]
- 24.Patterson SD, et al. Reduced numatrin/B23/nucleophosmin labeling in apoptotic Jurkat T-lymphoblasts. J Biol Chem. 1995;270:9429–9436. doi: 10.1074/jbc.270.16.9429. [DOI] [PubMed] [Google Scholar]
- 25.Zeller KI, et al. Characterization of nucleophosmin (B23) as a Myc target by scanning chromatin immunoprecipitation. J Biol Chem. 2001;276:48285–48291. doi: 10.1074/jbc.M108506200. [DOI] [PubMed] [Google Scholar]
- 26.Neiman PE, et al. Analysis of gene expression during myc oncogene-induced lymphomagenesis in the bursa of Fabricius. Proc Natl Acad Sci USA. 2001;98:6378–6383. doi: 10.1073/pnas.111144898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redner RL. Variations on a theme: the alternate translocations in APL. Leukemia. 2002;16:1927–1932. doi: 10.1038/sj.leu.2402720. [DOI] [PubMed] [Google Scholar]
- 28.Chiarle R, et al. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood. 2003;101:1919–1927. doi: 10.1182/blood-2002-05-1343. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda-Kato N, et al. The t(3;5)(q25.1;q34) of myelodysplastic syndrome and acute myeloid leukemia produces a novel fusion gene, NPM-MLF1. Oncogene. 1996;12:265–275. [PubMed] [Google Scholar]
- 30.Falini B, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 31.Alcalay M, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem cell maintenance. Blood. 2005;106:899–902. doi: 10.1182/blood-2005-02-0560. [DOI] [PubMed] [Google Scholar]
- 32.Boissel N, et al. Prevalence, clinical profile, and prognosis of NPM mutations in AML with normal karyotype. Blood. 2005;106:3618–3620. doi: 10.1182/blood-2005-05-2174. [DOI] [PubMed] [Google Scholar]
- 33.Grisendi S, et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature. 2005;437:147–153. doi: 10.1038/nature03915. [DOI] [PubMed] [Google Scholar]
- 34.Colombo E, et al. Nucleophosmin is required for DNA integrity and p19Arf protein stability. Mol Cell Biol. 2005;25:8874–8886. doi: 10.1128/MCB.25.20.8874-8886.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, et al. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat Genet. 1996;12:448–451. doi: 10.1038/ng0496-448. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson JA, et al. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol Cell Biol. 2004;24:1560–1569. doi: 10.1128/MCB.24.4.1560-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brummelkamp TR, et al. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 38.Li J, et al. Hypoxia-induced nucleophosmin protects cell death through inhibition of p53. J Biol Chem. 2004;279:41275–41279. doi: 10.1074/jbc.C400297200. [DOI] [PubMed] [Google Scholar]
- 39.Li J, et al. Nucleophosmin regulates cell cycle progression and stress response in hematopoietic stem/progenitor cells. J Biol Chem. 2006;281:16536–16545. doi: 10.1074/jbc.M601386200. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 41.Maiguel DA, et al. Nucleophosmin sets a threshold for p53 response to UV radiation. Mol Cell Biol. 2004;24:3703–3711. doi: 10.1128/MCB.24.9.3703-3711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo E, et al. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat Cell Biol. 2002;4:529–533. doi: 10.1038/ncb814. [DOI] [PubMed] [Google Scholar]
- 43.Hemann MT, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shieh SY, et al. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 45.Colombo E, et al. Delocalization and destabilization of the Arf tumor suppressor by the leukemia-associated NPM mutant. Cancer Res. 2006;66:3044–3050. doi: 10.1158/0008-5472.CAN-05-2378. [DOI] [PubMed] [Google Scholar]
- 46.Bertwistle D, et al. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol Cell Biol. 2004;24:985–996. doi: 10.1128/MCB.24.3.985-996.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]