Abstract
To complete the metastatic journey, cancer cells have to disseminate through the circulation and extravasate to distal organs. However, the extravasation process, by which tumor cells leave a blood vessel and invade the surrounding tissue from the microcirculation, remains poorly understood at the molecular level. In this study, tumor cell adhesion to the endothelium (EC) and subsequent extravasation were investigated under various flow conditions. Results have shown polymorphonuclear neutrophils (PMNs) facilitate melanoma cell adhesion to the EC and subsequent extravasation by a shear-rate dependent mechanism. Melanoma cell-PMN interactions are mediated by the binding between intercellular adhesion molecule-1 (ICAM-1) on melanoma cells and β2 integrins on PMNs. In addition, the fluid convection affects the extent of activation of β2 integrins on PMNs by endogenously secreted interleukin 8 (IL-8) within the tumor microenvironment. Results also indicate that shear rate affects the binding kinetics between PMNs and melanoma cells, which may contribute to the shear-rate dependence of melanoma extravasation in a shear flow when mediated by PMNs.
Keywords: Cancer metastasis, tumor microenvironment, interleukin 8, inflammation, microcirculation
1 Introduction
Metastasis, the spread of cancer from a primary site and formation of new tumors in distant organs, is responsible for most cancer deaths. Previous studies have implicated heterotypic adhesion of tumor cells to the blood vessel endothelium (EC) and subsequent transendothelial migration are key components in the metastatic cascade (Glinsky, 1998). Human neutrophils (PMNs), which comprise 50–70% of circulating leukocytes, have been shown to promote melanoma adhesion and transendothelial migration in certain circumstances (Liang et al., 2005; Slattery et al., 2005; Liang et al., 2007). Both endothelial and melanoma cells express intercellular adhesion molecule-1 (ICAM-1), a potential ligand for β2 integrins (CD11a/CD18 or LFA-1; CD11b/CD18 or Mac-1) on PMNs. PMN-EC and PMN-melanoma cell adhesions may play roles in bringing tumor cells into a close proximity to the EC, thus facilitating subsequent tumor cell extravasation through the EC in a shear flow.
Melanoma cells have been found to constitutively secrete a number of chemokines, including interleukin 8 (IL-8) (Schadendorf et al., 1993). IL-8 has been shown to trigger the functional up-regulation of the ligand binding activity of the β2 integrins on PMNs (Seo et al., 2001). IL-8 has a wide range of proinflammatory effects and stimulates PMNs through CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2) on PMNs (Holmes et al., 1991; Murphy and Tiffany, 1991). This PMN stimulation appears to be essential for PMNs to firmly adhere to the EC and subsequently migrate to the surrounding tissue in response to inflammation (Springer, 1994).
It is now well known that a local fluid mechanical environment of the circulation critically affects the molecular pathways of cell-cell interactions. Studies have suggested that the blood-flow pattern will affect hematogenous metastasis (Weiss, 1992). Shear stress and shear rate are two important parameters that describe the behaviors of hemodynamic flow. Shear stress dictates the forces applied to cells either individually (e.g., increase the extent of cell deformation) or to the intermolecular bonds between cells. Shear rate governs the transport of cells to other cells, and thus modulates cell-cell collisions and cell-cell contact time. An increase in shear rate leads to a reduction in the interaction time during cell collisions (Taylor et al., 1996). Transport of signaling molecules, such as chemokines and other molecules in the medium, in a shear flow may affect the development of local concentrations of these molecules, which may lead to the activation of cells in the fluid environment. However, there is little understanding of how transport of chemokines under hydrodynamic shear conditions affects the activation of PMNs, which may in turn affect melanoma cell extravasation.
Cell adhesion is mediated by the formation of reversible bonds between receptors and ligands. The association rate, the rate at which a receptor and ligand will combine to form a bond, is dependent on physical factors such as the number of ligands available to a receptor and the distance between the receptor and ligand. It is not known how the properties of the shear flow affect the association rate of binding between PMN expressed β2 integrins and melanoma cell expressed ICAM-1.
In this study, interactions between PMNs and melanoma cells via binding of β2 integrins and ICAM-1 were examined to characterize the adhesion mechanism of melanoma extravasation in the presence of PMNs. In addition, the hydrodynamic effects of shear rate and shear stress on PMN-facilitated melanoma adhesion were investigated. The activation of PMNs in response to IL-8 secreted by melanoma cells under various flow conditions was characterized. Results indicate that PMN facilitated melanoma extravasation via the binding of β2 integrins on PMNs and ICAM-1 on melanoma cells. The adhesion to and subsequent extravasation of melanoma cell through the EC, mediated by PMNs, is modulated only by shear rate rather than by shear stress. Results also show the transport of tumor-liberated IL-8, which up-regulated the expression of β2 integrins on PMNs, and thereby the PMN-melanoma cell aggregation, is regulated by the shear rate.
2 Materials & Methods
2.1 Reagents
Mouse anti-human mAbs against LFA-1 (anti-CD11a) and Mac-1 (anti-CD11b) were purchased from Invitrogen (Carlsbad, CA). Human recombinant IL-8 was purchased from R&D systems (Minneapolis, MN). Other reagents were purchased from R&D systems unless otherwise indicated.
2.2 Cell preparations
WM9 (kindly provided by Dr. M. Herlyn, Wistar Institute, Philadelphia, PA) and C8161.c9 melanoma cells were cultured respectively in RPMI-1640 (Invitrogen) and DMEM/F12 (Invitrogen) supplemented with 10% Fetal Bovine Serum (FBS; Invitrogen) and 100 units/ml penicillin-streptomycin (Invitrogen) at 37°C and 5% CO2. In assays where anti-ICAM-1 blocking antibodies were used, cells were incubated with mAb (5μg/ml) for 30 minutes at 37°C prior to the start of an assay. IgG isotype control antibody was used to verify specificity of all blocking antibodies.
We used a transfected fibroblast L-cell line (Gopalan et al., 1997) that constitutively expresses human E-selectin and ICAM-1 as an adhesive substrate. This cell line (provided by Dr. S. Simon, University of California Davis), which expresses levels of E-selectin and ICAM-1 that are comparable to those found on human umbilical vein endothelial cells, provides a model to explore melanoma cell adhesion (Liang et al., 2008a). For experiments which required melanoma cells in suspension, cells were detached when confluent using 0.05% trypsin/versine (Invitrogen) and washed twice with fresh medium. Then the cells were re-suspended in fresh medium and allowed to recover for 1 hr while being rocked at 8 rpm at 37°C.
Following the Pennsylvania State University Institutional Review Board (IRB) approved protocols (#19311), fresh human blood was collected from healthy donors by venipuncture. PMNs were isolated using a Histopaque® (Sigma) density gradient by manufacturer’s instruction and kept at 4°C in Dulbecco’s PBS (D-PBS) containing 0.1% human serum albumin (HSA) for up to 4 hr before use. In assays where anti-CD11a or CD11b blocking antibodies were used, PMNs were incubated with mAb (5μg/ml) for 30 minutes at 37°C prior to the start of an assay. In the cases of PMN and melanoma cell co-culture, PMNs resuspended in RPMI 1640 medium supplemented with 5% FBS were added onto the confluent melanoma cell lines (1:1 ratio) directly and co-cultured for 4 hours. 95% melanoma cells stay alive after 4hr contact culture with PMNs (data not shown).
2.3 Flow-migration assay
Flow migration assay was measured in a modified 48-well chemotactic Boyden chamber consisting of a top and bottom plate separated by a gasket (Liang et al., 2005; Slattery et al., 2005). Prior to each experiment, a monolayer of EI cells was grown (typically 36 hours after cell seeding) on a sterile polyvinylpyrrolidone (PVP)-free polycarbonate filters (8 μm pore size; NeuroProbe, Gaithersburg, MD) pre-coated with fibronectin (30 μg/ml, 3 hours) (Sigma). The center 12 wells of bottom plate were filled with soluble chemoattractant type IV collagen (CIV; 100 μg/ml in RPMI 1640/0.1 % BSA) (BD Biosciences) and surrounding control wells were filled with medium (RPMI 1640/0.1 % BSA). We have shown that melanoma cells express α2β1 integrin receptors for soluble collagen IV protein and migrate toward collagen IV stimulation, which agrees with previous finding (Dong et al., 1994). Apparatus was assembled by placing filter on bottom plate followed by addition of a sealing gasket and top plate. The chamber was primed with 37°C medium to eliminate bubbles in the system. 5×105 melanoma cells alone or PMNs together with melanoma cells (5×105 of each) were put in the chamber under static or shear flow conditions for 4 hours in a 37°C, 5 % CO2 incubator. The migrated melanoma cells towards chemoattractant were stained with Protocol Brand Hema3 solution (Fisher Scientific, Pittsburgh, PA) and counted using an inverted microscope (Diaphot 330, Nikon, Japan) with NIH Image software (v. β4.0.2).
2.4 Detection of β2 integrins expression on PMNs
PMNs were incubated with saturating concentrations of primary mAbs directed against either LFA-1 (CD11a) or Mac-1 (CD11b) in PBS containing 1% BSA for 20 minutes at 4°C and then washed twice. After an additional 20 minutes incubation with TRITC-conjugated goat anti-mouse Fab2 fragment (1μg/106 cells; Jackson ImmunoResearch, West Grove, PA) at 4°C, the cells were washed twice and fixed with 2% formaldehyde and analyzed by a GUAVA personal flow cytometry (GUAVA technologies Inc., Burlingame, CA.).
2.5 Parallel-plate flow assay
Cell collision and adhesion experiments were performed in a parallel-plate flow chamber (Glycotech, Rockville, MD) mounted on the stage of a phase-contrast optical microscope (Diaphot 330, Nikon, Japan). A syringe pump (Harvard Apparatus, South Natick, MA) was used to generate a steady flow field in the flow chamber. A petri dish (35 mm) with a confluent EI cell monolayer was attached to the flow chamber. The field of view was 800 μm (direction of the flow) by 600 μm. The focal plane was set on the EI monolayer. The experiments were recorded to computer using a CCD camera (Cooke Corporation, Romulus, MI). The flow chamber was perfused with appropriate media over the EI monolayer for 2–3 minutes at a shear rate of 40 sec−1 for equilibration before the introduction of cells suspended in specific medium. In some cases, the medium viscosity was altered by adding 1% or 2% (wt/vol) dextran (2×106 Mr; Sigma). The viscosity of the medium was measured as 1.0 cP (no dextran), 2.0 cP (1% dextran), 3.2 cP (2% dextran) or 7.0 cp (4% dextran) at 37°C in a cone-plate viscometer (RotoVisco 1, Haake, Newington, NH).
To investigate the interactions of PMNs and melanoma cells, PMNs and WM9 (1×106 cells/ml each) were injected together into the flow chamber. After allowing PMNs and WM9 cells to contact the EI monolayer at a shear stress of 0.1–0.3 dyn/cm2 for 2 min, shear stresses were adjusted to the experimental range of 0.6–2 dyn/cm2 and kept constant for 5 minutes. Experiments were performed in triplicate and recorded for off-line analysis. The interactions between PMNs and melanoma cells were quantified using melanoma adhesion efficiency as follows (Liang et al., 2005):
The numerator is the number of melanoma cells arrested on the EI monolayer at the end of the entire flow assay as a result of collision between entering melanoma cells and tethered PMNs. The denominator is the total number of melanoma cells-PMN collisions near the EI monolayer surface and is counted as a transient accumulative parameter throughout the entire flow assay.
To measure the rolling velocity, PMNs (1×106 cells/ml) were perfused into the flow chamber at various flow rates. The interactions of PMNs with EI monolayer in the flow chamber were recorded for two minutes and the rolling velocity for each PMN was determined by tracking individual PMN with Image-ProPlus 5.0 (Media Cybernetics, Inc., Silver Spring, MD). The mean rolling velocity of a PMN under various shear stresses was calculated from 40 rolling PMNs.
2.6 Transport of IL-8 released by melanoma cells in a shear flow
A two-dimensional Couette flow model was used to simulate the transport of chemokines released by tumor cells in the circulation. The inputs used in the model are tabulated in Table 1.
Table 1.
Description and source of variables as inputs for Comsol simulation
| Variable | Description | Value | Source |
|---|---|---|---|
| Q | Volumetric Flow Rate | 0.025 ml/min, 0.04 ml/min and 0.08 ml/min | Experimental setting |
| DAB | IL-8 diffusion coefficient | 2.59×10−10 m2/s | (Butcher et al., 1999; Moghe et al., 1995) |
| C0 | IL-8 secreted by a melanoma cell | 1.52×10−4 mol/m3 | (Peng et al., 2007) |
| a | Distance from the center of channel to the wall | 63.5 μm | Parallel plate chamber geometry |
| ρ | Density of fluid | 1.0 ×103 kg/m3 | Known constant |
| u | Melanoma cell velocity | 9.046×10−5 m/s, 1.447×10−4 m/s and 2.894×10−4 m/s | Calculated |
| μ | Viscosity | 1.0 cP, 2.0 cP and 3.2 cP | Experimental setting |
IL-8 distribution under flow conditions was modeled computationally using the commercial software package Comsol Multiphysics 3.2 (Comsol, Stockholm, Sweden), which employs a finite element method to solve the governing partial differential equations. To simulate the IL-8 transport, the convection and diffusion application mode was selected and the Navier-Stokes application mode was used to calculate the velocity profile. The convection-diffusion equation solved is shown in Equation 1:
| (1) |
where ci is the IL-8 concentration, u is the velocity, and Di is the solute diffusion coefficient. The diffusion coefficient for IL-8 was obtained from literature (Moghe et al., 1995). It has been shown that for small proteins, the diffusion coefficient is proportional to molecular weight (Butcher et al., 1999). A constant flux was assigned at the cell surface and complete dilution was assumed very far from the cell; mathematically stated as:
where C0 is IL-8 concentration and R is the tumor cell radius. It was assumed that the secreted IL-8 would not be re-consumed by the tumor cell and C0 be uniform over the surface of the cell.
The velocity profile was calculated using the Navier-Stokes application mode. The fluid was assumed to be Newtonian, incompressible, steady and laminar. A no-slip boundary condition was implemented at the channel walls. Uniform velocity and constant pressure were prescribed at the inlet and outlet, respectively. In the fully developed region, the classical parabolic velocity profile (Poiseuille flow) was obtained.
The relation between diffusion coefficient and solvent viscosity can be calculated using the Stokes-Einstein equation as follows.
| (2) |
where DAB is the diffusion coefficient of solute A in solvent B, RA is the hydrodynamic radius of solute A, κ is Boltzmann’s constant, T is the Kelvin temperature, and μB is the solvent viscosity. Note that the Stokes-Einstein equation usually gives poor accuracy when solute A is a large molecule. Recently, a modification of the Stokes-Einstein equation for small molecules was proposed by Kooijman (Kooijman, 2002). However, both equations have shown that the diffusion co-efficient is inversely proportional to the solution viscosity.
2.7 PMN-melanoma cell binding association rate
A molecular model of receptor-ligand binding has been developed previously and has been used in simulations of cell-cell adhesion (Bell, 1978; Dembo et al., 1988). In this model, the association rate, kon, governs the likelihood of a receptor to form a bond with a ligand on another cell, whose equation is shown in Equation 3 (Jadhav et al., 2005):
| (3) |
where AL is the surface area on the ligand-bearing cell that is available to a receptor, nL is the number of ligands on a cell, nB is the number of bonds already formed. These values and the separation distance between two cells, ε are determined by the geometry and properties of the cells. The association rate for the receptor-ligand binding under zero-force conditions, , was determined for LFA-1 and Mac-1 binding with ICAM-1 in a companion effort (Hoskins and Dong, 2006). The bond spring constant, σts, and equilibrium length, λ as well as the Boltzmann’s constant, kb, were assigned values found in the literature (Dembo et al., 1988; Simon and Green, 2005).
2.8 Statistical analysis
All experimental results are reported as the mean ± standard error of the mean (SEM) unless otherwise stated. One-way ANOVA analysis was used for multiple comparisons and t-tests were used for comparisons between 2 groups. P < 0.05 was considered significant.
3 Results
3.1 PMNs facilitate melanoma cell extravasation under shear flow conditions
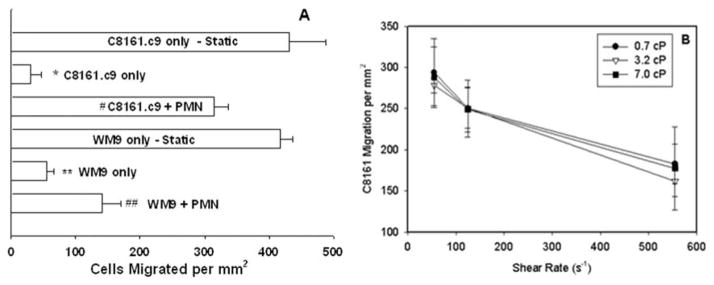
Melanoma cells extravasation under flow conditions was characterized using the flow-migration assay under both static and flow conditions. When exposed to a shear flow (0.4 dyn/cm2), extravasations of C8161.c9 and WM9 cells toward CIV were all significantly less than those under static conditions (Fig. 1A). Addition of PMN significantly enhanced tumor cell extravasation under flow conditions compared with melanoma cells only (Fig. 1A), which suggest that tumor cell extravasation in a shear flow is mediated by leukocytes.
Figure 1. PMNs facilitate melanoma cell extravasation under shear flow conditions.
Melanoma extravasation was investigated using flow migration chamber as described in “Materials and Methods”. (A) All cases were under a flow shear stress at 0.4 dyn/cm2 unless labeled “Static” (#P<0.01 with respect to C8161 static case; *P<0.05 with respect to C8161+PMN case; ##P< 0.01 with respect to WM9 static case; **P < 0.05 with respect to WM9+PMN case); (B) C8161c.9 extravasation in the presence of PMNs was investigated under various shear flow conditions. By changing the medium viscosity, the effects of shear rate and shear stress were able to be differentiated. Under the same shear rates, melanoma extravasation was at the similar level. At the same shear stress 4 dyn/cm2, the change of shear rates from 55.5 sec−1 (medium viscosity at 7.0 cP) to 555 sec−1 (medium viscosity at 0.7 cP) significantly reduced melanoma extravasation. All the values are mean ± S.E.M. for N ≥ 3.
In order to differentiate the effects of shear rate and shear stress on melanoma extravasation in the presence of PMNs, dextran was used to vary the medium viscosity. In order to keep shear stress (τ = μγ̇) constant while varying shear rate (γ̇), cell suspension medium viscosity (μ) was varied by adjustments in medium dextran (2 ×106Mr) concentration. Under the same shear rate (55.5, 125, or 555 sec−1), the extravasation of melanoma cells was not significantly different (Fig. 1B). In contrast, when shear stress is the same at 4 dyn/cm2, the increase of shear rate from 55.5 (medium viscosity at 7.0 cP) to 555 sec−1 (medium visocisty at 0.7 cP) dramatically reduced melanoma extravasation (Fig. 1B).
3.2 Co-culture of melanoma cells and PMNs increases Mac-1 expression on PMNs
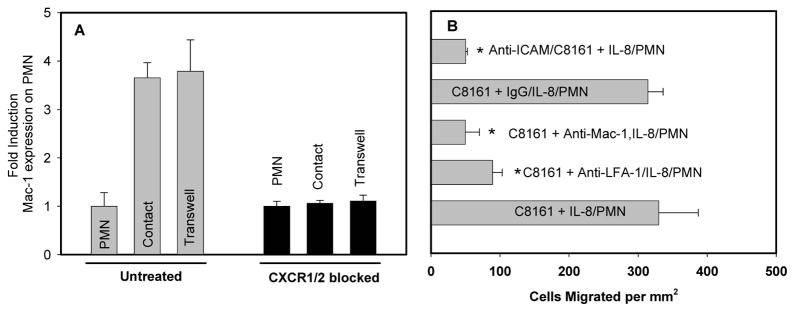
Untreated PMN or PMN treated with blocking antibodies for CXCR1 and CXCR2 were co-cultured with C8161c.9 melanoma cells, either in contact (Contact) or separated by a Transwell insert (Transwell). Both contact and Transwell co-cultures between untreated PMN and melanoma cells experienced a nearly 4-fold increase in Mac-1 expression levels over those cultured alone for 4 hours. PMNs treated with mAb blocking CXCR1 and CXCR2 showed no change in Mac-1 levels after co-cultured with C8161c.9 cells compared with PMNs cultured alone (Fig. 2A).
Figure 2. Melanoma extravasation in the presence of PMNs is mediated by the binding of adhesion molecules.
(A) Melanoma cells were cultured in 6-well plate until confluence. After replacing the old medium with fresh medium, PMNs were added on top of the confluent melanoma cells directly (1:1 ratio). After 4 hours co-culture in a 37°C, 5 % CO2 incubator, PMNs were collected. Mac-1 expression on PMN after co-culture with C8161c.9 melanoma cells was measured using flow cytometry. Values were normalized to background Mac-1 expression on PMN alone and shown as means ± SEM; (B) ICAM-1 on C8161.c9, LFA-1 or Mac-1 on PMNs was blocked using mAb (5μg/ml) and the extravasation of melanoma cells was then investigated using the flow migration assay under 0.4 dyn/cm2. Disrupting C8161-PMN aggregation by blocking ICAM-1 on melanoma significantly reduces melanoma cell extravasation. Both Mac-1 and LFA-1 contributed to PMN-mediated melanoma extravasation. *P <0.05 with respect to C8161+PMN case. All the values are mean ± S.E.M. for N ≥ 3.
To elucidate the possibility that adhesion molecules could be responsible for melanoma-PMN interactions, ICAM-1 on C8161c.9 or β2 integrin on PMNs was functionally blocked respectively and the extravasation of melanoma cells was then examined. Blocking ICAM-1 on melanoma cells significantly reduced melanoma extravasation even in the presence of PMNs (Fig. 2B). In addition, the blocking of either LFA-1 or Mac-1 interrupted melanoma extravasation. These results indicate that PMNs facilitate melanoma cell extravasation through the binding of ICAM-1 on melanoma cells and β2 integrin on PMNs (Fig. 2B).
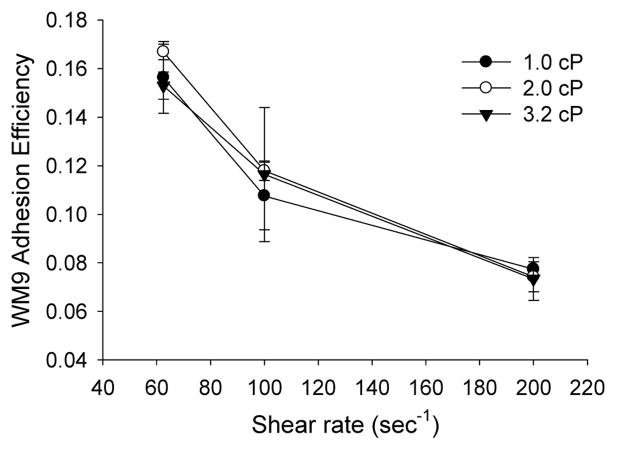
3.3 Melanoma cells adhesion to the EC via PMNs is shear rate dependent
To further understand how interactions of PMNs and melanoma cells result in enhanced tumor cell extravasation, the adhesion of melanoma cells on the endothelial surface in the presence of PMNs was characterized over a range of flow conditions using the parallel-plate flow assay. Previous results indicated that there was few WM9 cells adhesion to the EI monolayer without PMNs; however, the presence of PMNs enhanced WM9 cells adhesion to the EI monolayer via the tethered PMNs (Liang et al., 2005). The different effects of shear stress and shear rate were analyzed by using dextran to vary the medium viscosity. For a fixed shear rate 62.5 sec−1, the elevation of shear stress from 0.6 to 2 dyne/cm2 did not alter WM9 cell adhesion to the EI monolayer via PMNs significantly (Fig. 3). When shear stress was held constant at 2 dyn/cm2 and shear rate varied from 62.5 to 200 sec−1, WM9 adhesion via PMNs decreased dramatically. These results illustrate that melanoma cell adhesion to the endothelium in the presence of PMNs is regulated by the local hydrodynamic shear rate, or the contact time (∝ 1/γ̇), not by the shear stress (Fig. 3).
Figure 3. Effects of shear stress and shear rate on melanoma adhesion to the EC in the presence of PMNs.
The parallel plate flow chamber was perfused with appropriate medium over the EI monolayer for 2–3 minutes at a shear rate of 40 sec−1 for equilibration and then PMNs and WM9 cells were injected into the flow chamber through the syringe pump at a predetermined concentration (1×106 cells/ml) of each. The cell-cell interactions in shear flow were then recorded and analyzed off-line. WM9 adhesion efficiency was defined in “Materials and Methods”. Values are mean ± S.E.M. for N ≥ 3.
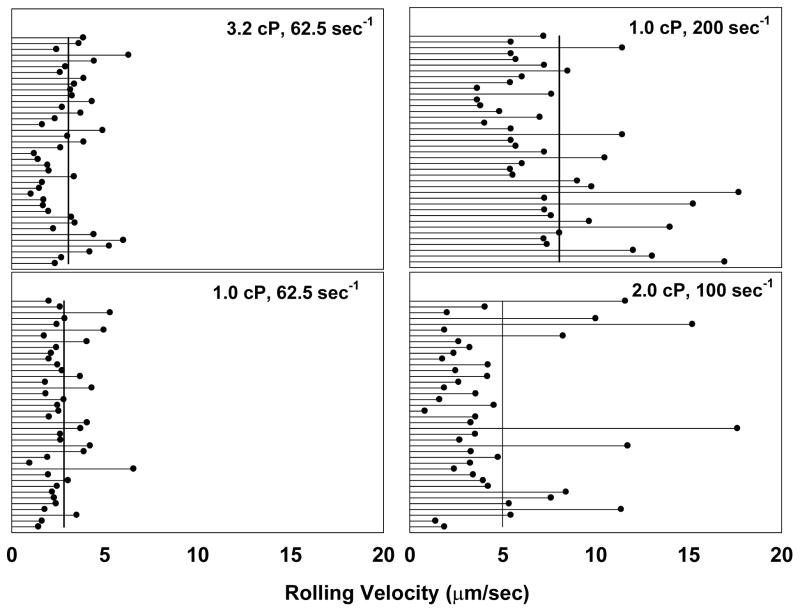
3.4 Rolling velocity of PMN under flow conditions
Previous study has shown that PMN-facilitated melanoma adhesion is a multiple step process which begins with PMNs tethering on the EC, followed by melanoma cells adhering to tethered PMNs, thus bringing tumor cells into close contact with the EC (Liang et al., 2005). Since the PMN tethering step is an important step in this process, PMN rolling velocity was measured under various shear conditions. Results indicate that PMN rolling velocity was dependent on the shear rate (Fig. 4). Under the same shear rate, an increase in shear stress does not change the PMN velocity significantly. However, under the same shear stress, an increase in shear rate results in higher PMN rolling velocity.
Figure 4. PMN rolling velocity.
PMNs (1×106 cells/ml in DPBS containing 0.1% HSA) were perfusion into a parallel plate flow chamber at various flow rates. The interactions of PMNs with EI monolayer in the parallel plate flow chamber were recorded for two minutes using a CCD camera and the rolling velocity for each PMN was determined by tracking individual PMN with Image-ProPlus 5.0. The line in each figure indicated the mean rolling velocity of a PMN under various shear stresses calculated from total 40 rolling PMNs.
3.5 Expression of LFA-1 and Mac-1 on a rolling PMN upon stimulation of the convective diffusion of IL-8 from a moving tumor cell under shear flow
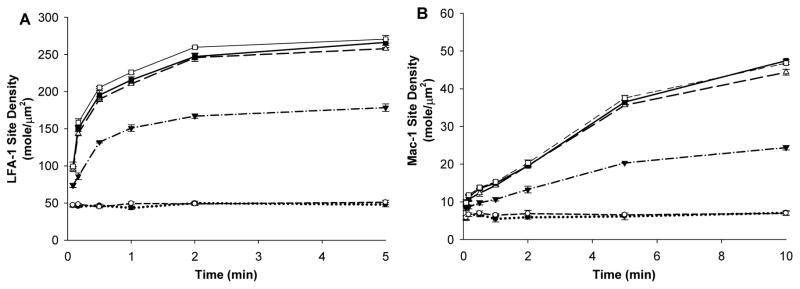
The numbers of LFA-1 and Mac-1 expressed by PMNs at rest were previously determined to be 45 LFA-1/μm2 and 5 Mac-1/μm2 (Simon and Green, 2005). To further determine the expression of LFA-1 and Mac-1 on PMNs upon stimulation, PMNs were treated with various concentrations (0.01, 0.1, 1, 5 and 10nM) of IL-8 for various time periods (5, 10, 30, 60, 120, 300, and 600 sec). By converting the fluorescence intensity changes to a ratio of activated to non-activated intensity and using the known baseline expressions, the site density of LFA-1 and Mac-1 on the activated PMNs was estimated. The expressions of Mac-1 and LFA-1 were upregulated upon IL-8 stimulation in a time- and dose-dependent manner (Fig. 5). After the stimulation with a low IL-8 concentration of 0.01 nM, Mac-1 and LFA-1 were not upregulated. Mac-1 and LFA-1 expressions started to increase upon stimulation when IL-8 concentration reached 0.1 nM, indicating a possible threshold concentration for IL-8 to activate LFA-1 and Mac-1. In addition, there were no significant differences in LFA-1 or Mac-1 expressions after increasing IL-8 concentration from 1nM to 5nM or 10nM, which implies that there might be a saturating concentration for IL-8 to up-regulate LFA-1 and Mac-1 expressions. Based on these experimental data, curve fitting was used for the equations to derive LFA-1/Mac-1 expressions upon stimulation, either with a specific IL-8 concentration but in various time durations, or with various IL-8 concentrations but at a specific time point.
Figure 5. Mac-1 and LFA-1 expression profiles on PMNs after IL-8 stimulation.
PMNs were treated with various concentration of IL-8 for various durations. Following treatment, the LFA-1 and Mac-1 expressions on PMNs were detected using flow cytometry. Site density data were calculated by multiplying the ratio of activated to non-activated fluorescence intensity and the known expression numbers of Mac-1 (5 mole/μm2) and LFA-1 (45 mole/μm2) on non-activated PMNs (Simon and Green, 2005). (A) LFA-1 expression on PMNs after IL-8 stimulation; (B) Mac-1 expression on PMNs after IL-8 stimulation. Values were mean ± SEM for N≥3.○, No stimulation; ●, 0.01 nM; ▼;, 0.1 nM; △, 1 nM; ■, 5 nM; □, 10 nM.
To investigate how the convective diffusion of IL-8 secreted by melanoma cells affects the expression of LFA-1 and Mac-1 on PMNs, which in turn may modify tumor cell adhesion to the EC within the tumor microenvironment, Comsol Multiphysics was used to simulate the convective diffusion of IL-8 from a single moving tumor cell under various flow conditions. To simulate the system, a tumor cell was assumed to be in the free stream and its velocity was derived from the shear flow conditions using the Poiseuille flow solution. A PMN was assumed to roll on the EC and its velocity was measured experimentally. The concentration of IL-8 secreted by the melanoma cell was reported previously by (Peng et al., 2007). Simulation could predict the local IL-8 concentration near a rolling PMN and the time that the PMN is stimulated by the IL-8 under flow conditions. Combining experimental data (Fig. 5), we can therefore determine the LFA-1/Mac-1 expression profile upon convective IL-8 stimulation.
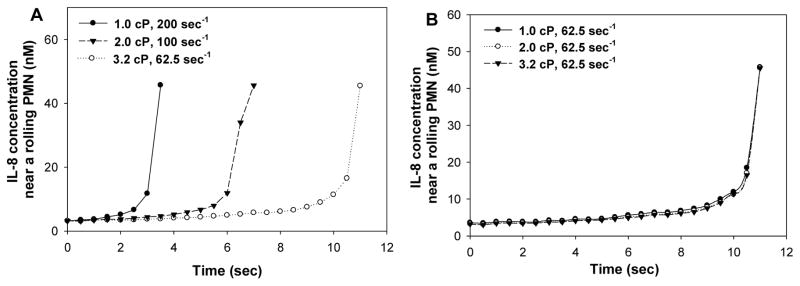
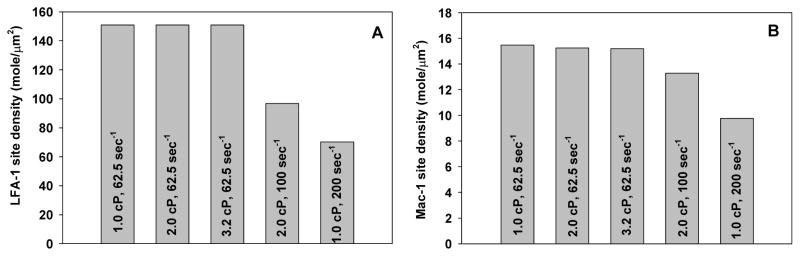
Results indicate that changing in shear rate alters the IL-8 concentration near a rolling PMN at different time points (Fig. 6). When the shear stress was kept constant at 2 dyn/cm2, increase in shear rates from 62.5 to 200 sec−1 would shorten the stimulation time duration of IL-8 on PMNs (Fig. 6A). In contrast, if the shear rate was kept constant at 62.5 sec−1, the resulting IL-8 concentration near a rolling PMN and the IL-8 stimulation time duration would remain constant under various shear stresses (Fig. 6B). These data strongly suggest that the IL-8 transport in a convective flow field affects the local concentration near a rolling PMN as well as the stimulation time, which could subsequently affect the expressions of LFA-1 and Mac-1 on PMNs, as shown next in Fig. 7. At a constant shear rate 62.5 sec−1, varying the shear stress by changing the viscosity did not modify the expression of LFA-1 or Mac-1 on PMNs (left three bars of Fig. 7A or Fig. 7B); however, at a constant shear stress 2 dyn/cm2, LFA-1 and Mac-1 expression was reduced when shear rate was increased (right three bars of Fig. 7A or Fig. 7B). These results indicate that under shear flow conditions, the LFA-1 and Mac-1 expressions on PMNs upon IL-8 stimulation from tumor cell is dependent on shear rate.
Figure 6. The concentration of IL-8 near a rolling PMN and the time it activates the PMN under various flow conditions.
Comsol Multiphysics was used to simulate the convective diffusion of IL-8 as described in “Materials and Methods”, which gave the concentration profile of IL-8 near a rolling PMN. The activation time of IL-8 on the PMN was determined by calculating the duration a melanoma cell used to reach the PMN. (A) The higher shear rate let tumor cell travel faster towards PMN, resulting in shorter period of stimulation on PMN under same shear stress but different shear rates; (B) IL-8 concentration near a rolling PMN and the time it activated the PMN was about the same under same shear rate but different shear rates.
Figure 7. LFA-1 and Mac-1 expressions on PMN after activation of IL-8 from tumor cell under various shear flow conditions.
LFA-1 and Mac-1 site densities on the PMN under various shear conditions were derived using the site density expression data as exemplified in Fig. 5 and the simulation data shown in Fig. 6. (A) Mac-1 expression; (B) LFA-1 expression. At the same shear rate, LFA-1 and Mac-1 expressions on the PMN did not have much difference although the shear stresses were different. In contrast, when the shear stress is the same, the increase of shear rates reduced LFA-1 and Mac-1 expressions on the PMN.
3.6 The binding kinetics of melanoma cell adhesion to PMN in a shear flow is affected by the shear rate
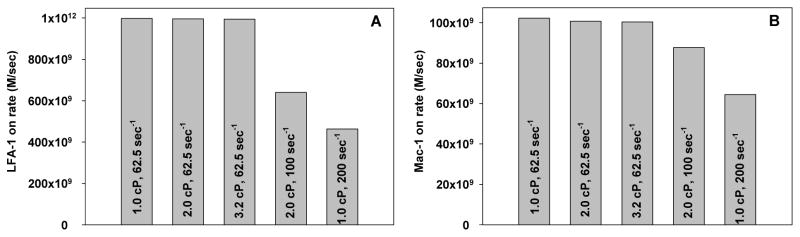
To quantify the increase in binding potential when PMNs are stimulated by the IL-8 from melanoma cells, the association rate governing the binding of the cells was calculated. The likelihood of binding between two cells is determined by the number and availability of adhesion molecules on both cells, as well as the intrinsic binding properties of the molecules. In the present study, Equation 3 was used to calculate the association rate for an LFA-1 or a Mac-1 molecule binding with an ICAM-1 molecule when a single melanoma cell was assumed to be 1 cm from a single rolling PMN under various shear conditions. The surface densities of LFA-1 and Mac-1 molecules on a PMN stimulated by melanoma cell-secreted IL-8 (Fig. 7) were used for nl. A constant separation distance and contact area were assumed for all six shear flow conditions simulated.
The association rates indicate that the likelihood of an LFA-1/ICAM-1 bond forming between a melanoma cell and PMN is almost doubled when the shear rate is decreased from 200 sec−1 to 62.5 sec−1 under a constant shear stress (Fig. 8A). For Mac-1/ICAM-1 binding, the likelihood increases by more than twice when the shear rate is decreased under fixed shear stress (Fig. 8B). When the shear rate remains constant, however, the binding potential for both molecules remains constant even when shear stress is increased. These results imply that the on-rate binding kinetics of ICAM-1/β2-integrin mediated melanoma cell adhesion to PMN in a shear flow is affected by the shear rate, not the shear stress, due to the convective IL-8 activation.
Figure 8. Association rate for LFA-1 and Mac-1 binding to ICAM-1 under various shear flow conditions.
On-rates for (A) LFA-1 and (B) Mac-1 binding to ICAM-1 when they are up-regulated due to the convective diffusion of IL-8 secreted by a melanoma cell. The on-rates are dependent on the shear rate and not the shear stress.
4 Discussion
Melanoma cell interaction with the EC is distinct from PMN-EC adhesion under shear conditions (Liang et al., 2005). Expression of ICAM-1 in human malignant melanoma cells has been seen to increase with the metastatic potentials (Natali et al., 1990). Inhibiting ICAM-1 expression on melanoma cells reduces the metastatic capacity of the melanoma cells (Miele et al., 1994), supporting a role of ICAM-1 in metastasis. Since interactions between ICAM-1 and β2 integrins are necessary for PMNs transmigration through the EC in a shear flow, it might be expected that melanoma-expressed ICAM-1 can promote the aggregation with PMNs. Both endothelial and melanoma cells express ICAM-1, the ligand for β2 integrins on PMNs; therefore, PMN-melanoma cell adhesion in the circulation may be a possible mechanism for bringing tumor cells into close proximity to the EC, thus facilitating their subsequent extravasation through the EC. Our results have shown that PMNs influence melanoma cell extravasations under dynamic flow conditions. Blocking either ICAM-1 molecules on melanoma cells or β2 integrins on PMNs inhibits melanoma extravasation, which further indicates that a β2 integrins/ICAM-1 binding mechanism is involved in melanoma extravasation mediated by PMNs. Such mediation is shown to be regulated by endogenously produced IL-8 within the tumor microenvironment (Peng et al., 2007).
Hydrodynamic forces play an important role in regulating melanoma cell adhesion and extravasation in the microcirculation. Shear rate modulates the cell-cell collision frequency and contact time, which is determined by the relative velocities of cells in the fluid. Previous studies have shown that tethering of PMNs on the EC is both shear rate and shear stress dependent (Liang et al., 2005). Our studies have indicated that melanoma cell adhesion to the EC mediated by PMNs is dependent on shear rate, rather than shear stress, suggesting a different mechanism of PMN-facilitated melanoma cell adhesion to the EC from the PMN-EC tethering. Whereas PMNs are able to adhere anywhere on the inflamed EC that is relatively a flat surface, melanoma cells could only adhere to PMNs that are spherical and only sporadically tethered to the EC monolayer. A lower shear rate increases the time that cells are in contact, thus allowing more melanoma cells-PMN binding to the endothelial surface with a resulting increase in melanoma cell adhesion to the EC. A recent study investigating the binding kinetics of PMNs and melanoma cells showed that the interactions between these two types of cells in a shear flow are dependent on shear rate only (Liang et al., 2008b). Their finding suggests that the shear-rate-dependent binding of PMNs and melanoma cells may be the dominant step of PMN-facilitated melanoma adhesion to the EC.
Chemokines, which comprise the largest family of cytokines, are small secreted proteins that regulate leukocyte transport by mediating the adhesion of leukocytes to the ECs, which is the initial step in transendothelial migration and tissue invasion (Zlotnik and Yoshie, 2000). Specifically, the up-regulation of LFA-1 and Mac-1 on PMNs upon stimulation by IL-8 has been reported (Lum et al., 2002; Seo et al., 2001). Our results have found that this up-regulation of LFA-1 and Mac-1 on PMNs is both time and dose dependent. In addition, our results have confirmed that there exist both a threshold and a saturating concentration of IL-8 in stimulating LFA-1 and Mac-1 on PMNs as shown previously by other groups (Lum et al., 2002; Seo et al., 2001). The response of LFA-1 upon IL-8 stimulation is rapid, while Mac-1 responds slowly in a later time period. Furthermore, our results here have shown that Mac-1 expression on PMNs increased ~4-fold after co-culture with melanoma cells. The blocking of CXCR1 and CXCR2 on PMNs inhibits dramatically the ability of PMNs to increase Mac-1 expression after co-culture with melanoma cells, suggesting that melanoma-secreted IL-8 could be responsible for the Mac-1 up-regulation on PMNs. A numerical model was employed in our study here to investigate how the convective diffusion of IL-8 affects PMNs in a shear flow. Results have shown that the local concentration of IL-8 near a rolling PMN and the stimulation time are shear rate dependent. Thus, the up-regulation of LFA-1 and Mac-1 on the stimulated PMN is also shear rate dependent. The binding between melanoma cells and the IL-8 stimulated PMNs follows the same shear rate dependent trend, which implies IL-8 convective diffusion contributes to the shear rate dependence of melanoma cell adhesion to the EC mediated by PMNs in a hydrodynamic shear flow.
Our studies demonstrate a novel finding that PMNs can facilitate melanoma cell adhesion to and subsequent extravasation through the EC within the circulation. In addition, PMN-facilitated melanoma cell adhesion to and subsequent extravasation through the EC is regulated by shear rate alone through a mechanism of shear-rate-dependent IL-8 signaling. An understanding of the mechanisms that regulate cancer metastasis will lead to the ability to design more effective therapy for cancer. The present study elucidates how cancer induces immunoediting via intercellular communications between tumor cells and leukocytes within the circulation and thus fosters new approaches to cancer treatment through anti-inflammatory therapeutics.
Acknowledgments
The authors thank Dr. Scott Simon (UC Davis, CA) for providing EI cells and Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) for providingWM9 cells. This work was supported by the National Institutes of Health grant CA-97306, CA-125707 and National Science Foundation grant CBET-0729091.
The authors appreciate the support of the Penn State General Clinical Research Center (GCRC), the staff that provided nursing care. The GCRC is supported by NIH Grant M01-RR-10732.
Footnotes
An invited manuscript by the Editor-in-Chief, MCB, for a special issue in a celebration of Dr. Y.C. Fung’s 90th birthday
References
- 1.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 3.Dembo M, Torney DC, Saxaman K, Hammer D. The reaction-limited kinetics of membrane-tosurface adhesion and detachment. Proc R Soc London. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Aznavoorian S, Liotta L. Two phases of pseudopod protrusion in tumor cells revealed by a micropipette. Microvasc Res. 1994;47:55–67. doi: 10.1006/mvre.1994.1005. [DOI] [PubMed] [Google Scholar]
- 5.Glinsky G. Anti-adhesion cancer therapy. Cancer Metastasis Rev. 1998;17:177–185. doi: 10.1023/a:1006050302406. [DOI] [PubMed] [Google Scholar]
- 6.Gopalan P, Smith C, Lu H, Berg E, Mcintire L, Simon S. PMN CD18-dependent arrest on ICAM-1 in shear flow can be activated through L-selectin. J Immunol. 1997;158:367–375. [PubMed] [Google Scholar]
- 7.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of human interleukin-8 receptor. Science. 1991;253:1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 8.Hoskins MH, Dong C. Kinetics analysis of binding between melanoma cells and neutrophils. Mol Cell Biomech. 2006;3:79–87. [PMC free article] [PubMed] [Google Scholar]
- 9.Jadhav S, Eggleton CD, Konstantopoulos K. A 3-D computational model predicts that cell deformation affects selectin-mediated leukocyte rolling. Biophys J. 2005;88:96–104. doi: 10.1529/biophysj.104.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooijman HA. A modification of the Stokes-Einstein equation for diffusivities in dilute binary mixtures. Ind Eng Chem Res. 2002;41:3326–3328. [Google Scholar]
- 11.Liang S, Slattery M, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Experimental Cell Research. 2005;310:282–292. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang S, Sharma A, Peng HH, Robertson RP, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Research. 2007;67:5814–5820. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang S, Slattery M, Wagner D, Simon SI, Dong C. Hydrodynamic Shear Rate Regulates Melanoma-Leukocyte Aggregations, Melanoma Adhesion to the Endothelium and Subsequent Extravasation. Ann of Biomed Eng. 2008a;36:661–671. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang S, Fu C, Wagner D, Guo H, Zhan D, Dong C, Long M. Two-dimensional kinetics of β2-integrin and ICAM-1 bindings between neutrophils and melanoma cells in a shear flow. Am J Physiol Cell Physiol. 2008b;294:C743–C753. doi: 10.1152/ajpcell.00250.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lum AFH, Green CE, Lee GR, Staunton DE, Simon SI. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J Biol Chem. 2002;277:20660–20670. doi: 10.1074/jbc.M202223200. [DOI] [PubMed] [Google Scholar]
- 16.Miele M, Bennett C, Miller B, Welch D. Enhanced metastatic ability of TNF-a treated maliganant melanoma cells is reduced by intercellular adhesion molecule-1 (ICAM-1) antisense oligonucleotides. Exp Cell Res. 1994;214:231–241. doi: 10.1006/excr.1994.1253. [DOI] [PubMed] [Google Scholar]
- 17.Moghe PV, Nelson RD, Tranquillo RT. Cytokine-stimulated chemotaxis of human neutrophils in a 3-D conjoined fibrin gel assay. J Immunol Meth. 1995;180:193–211. doi: 10.1016/0022-1759(94)00314-m. [DOI] [PubMed] [Google Scholar]
- 18.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 19.Natali PG, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, Ferrone S. Differential expression of intercellular adhesion molecle 1 in primary and metastatic melanoma lesions. Cancer Res. 1990;50:1271–1278. [PubMed] [Google Scholar]
- 20.Peng H, Liang S, Henderson AJ, Dong C. Regulation of Interleukin-8 Expression in Melanoma-Stimulated Neutrophil Inflammatory Response. Exp Cell Res. 2007;313:551–559. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. Arch Dermatol Res. 1993;151:2667–2675. [PubMed] [Google Scholar]
- 22.Seo SM, Mcintire LV, Smith CW. Effects of IL-8, Groa, and LTB4 on the adhesive kinetics of LFA-1 and Mac-1 on human neutrophils. Am J Physiol Cell Physiol. 2001;281:C1568–C1578. doi: 10.1152/ajpcell.2001.281.5.C1568. [DOI] [PubMed] [Google Scholar]
- 23.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 24.Slattery M, Liang S, Dong C. Distinct Role of Hydrodynamic Shear in Leukocyte-facilitated Tumor Cell Extravasation. Am J Physiol Cell Physiol. 2005;288:C831–C839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:311–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AD, Neelamegham S, Hellums JD, Smith CW, Simon SI. Molecular dynamics of the transition from L-selectin to b2 integrin dependent neutrophil adhesion under defined hydrodynamic shear. Biophys J. 1996;71:3488–3500. doi: 10.1016/S0006-3495(96)79544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis. 1992;10:191–199. doi: 10.1007/BF00132751. [DOI] [PubMed] [Google Scholar]
- 28.Zlotnik A, Yoshie O. Chemokines: a new classification system and their immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]