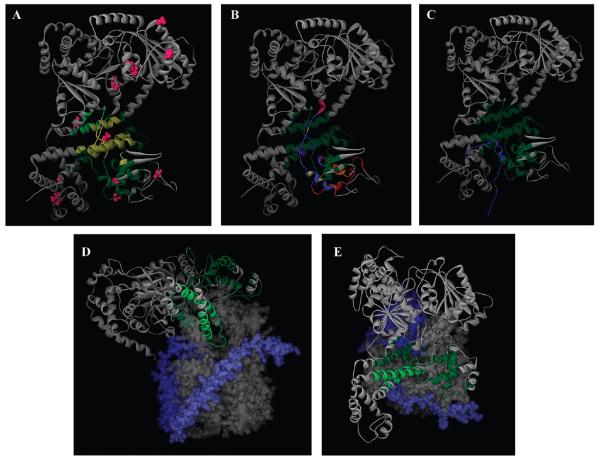
Figure 6. Location of the SecA signal peptide-binding domain.
(A) A model of the NMR structure of E. coli SecA (Protein Data Bank entry 2VDA structure 1) (38) is shown in a gray-colored ribbon representation. The SecA region in common for the SP22 and SP2 data sets is colored yellow, while those residues specific for SP22 and SP2 are colored dark and light green, respectively. The cysteine residues utilized for mapping are colored magenta. (B) Similar to panel A except the different SecA signal peptide-binding sites are color-coded for comparison to highlight overlapping and nonoverlapping regions: green, red, and magneta for nonoverlapping regions of our site compared to that determined by Musial-Siwek et al. (36) and Baud et al. (25), respectively; dark blue, orange, and light blue for single overlapping regions of our site with that determined by Gelis et al., Musial-Siwek et al., and Baud et al., respectively; purple and yellow for double overlapping regions of our site with that determined by both Gelis et al. and Musial-Siwek et al. or both Gelis et al. and Baud et al., respectively. (C) Comparison of the SecA-bound KRR-LamB signal peptide structure (Protein Data Bank entry 2VDA SP structure 1) with our FRET-mapped signal peptide-binding domain, which is colored green. The KRR-LamB signal peptide is colored blue, and its amino terminus is located at the bottom of the figure. (D) A model of the cocrystal structure of the Thermotoga maritima SecA–SecYEG complex (Protein Data Bank entry 3din) (10) viewed from the side. Most of SecA is shown in a gray-colored ribbon representation, SecY as a gray solid surface structure, SecE as a light blue solid surface structure, and SecG as a dark blue solid surface structure. The FRET-mapped SecA signal peptide-binding domain is colored dark green, except for those regions that fall within the two-helix finger, which are colored light green for the sake of clarity. SecA residues 668–741 are not shown for the sake of clarity of presentation of the two-helix finger. (E) Similar to panel D except viewed from the cytoplasm.