Abstract
This article explores the notion that Freudian constructs may have neurobiological substrates. Specifically, we propose that Freud’s descriptions of the primary and secondary processes are consistent with self-organized activity in hierarchical cortical systems and that his descriptions of the ego are consistent with the functions of the default-mode and its reciprocal exchanges with subordinate brain systems. This neurobiological account rests on a view of the brain as a hierarchical inference or Helmholtz machine. In this view, large-scale intrinsic networks occupy supraordinate levels of hierarchical brain systems that try to optimize their representation of the sensorium. This optimization has been formulated as minimizing a free-energy; a process that is formally similar to the treatment of energy in Freudian formulations. We substantiate this synthesis by showing that Freud’s descriptions of the primary process are consistent with the phenomenology and neurophysiology of rapid eye movement sleep, the early and acute psychotic state, the aura of temporal lobe epilepsy and hallucinogenic drug states.
Keywords: freud, the ego, default-mode, prediction, free-energy
Introduction
In this synthesis we explore the notion that Freudian constructs may have real neurobiological substrates and could be usefully revisited in the context of modern neuroscience. It is worth noting that Freud had a formal training in neuroanatomy and was influenced by people like Helmholtz, who laid many of the foundations for theoretical neurobiology. Advances in empirical and theoretical neuroscience now allow us to recast some central Freudian ideas in a mechanistic and biologically informed fashion. Specifically, we note that the psychoanalytic distinction between the primary and secondary processes (as functions of the id and ego respectively) fit comfortably with modern notions of functional brain architecture, at both a computational and neurophysiological level. Although this may seem a rather abstract and ambitious synthesis, there is in fact an enormous amount of empirical evidence from neuropsychology, neuroimaging and psychopharmacology to support it.
In what follows we attempt to demonstrate consistencies between key Freudian ideas and recent perspectives on global brain function that have emerged in imaging and theoretical neuroscience. The intention is to demonstrate and develop the construct validity of the Freudian concepts. This should allow Freudian concepts to be operationalized and measured empirically and could enable a dialogue between psychoanalysts and neurobiologists. This may have implications for psychiatry to the extent that mechanistic theories of psychopathology appeal to either neurobiological or psychoanalytical constructs. We start by summarizing the key elements of the three areas that we want to relate to each other; namely the central Freudian constructs, the Helmholtzian or Bayesian brain framework and empirical findings from neuroimaging on the global organization of brain activity.
The primary and secondary process
Freud came to a realization that there are two fundamentally different modes of cognition (the primary and secondary process) through a study of ‘altered’ or ‘non-ordinary’ states of consciousness (e.g. Q115, Q333 and Q462 in Supplementary material). He recognized in certain non-ordinary states (e.g. dreaming and psychosis) a mode of cognition that is characterized by a primitive, animistic style of thinking. Freud conjectured that the exchange of neuronal energy is relatively ‘free’ in this mode and he named this the ‘primary process’. Simultaneously, Freud recognized in non-ordinary states the loss of certain functions, which are normally present in waking cognition. He ascribed these functions to a central organization (the ego) which works to minimize the mind’s free-energy. Freud named this function the ‘secondary process’ and defined its aim as one of converting ‘free energy’ into ‘bound energy’ (for a more thorough discussion of these central concepts of Freudian theory see the supporting quotes in the online Supplementary material, cited in Table 1):
We seem to recognize that nervous or psychical energy occurs in two forms, one freely mobile and another, by comparison, bound; we speak of [activations] and [hyperactivations] of psychical material, and even venture to suppose a [hyperactivation] brings about a kind of synthesis of different processes—a synthesis in the course of which free energy is transformed into bound energy … We hold firmly to the view that the distinction between the [primary] and the [secondary] state lies in dynamic relations of this kind, which would explain how it is that, whether spontaneously or with our assistance, the one can be changed into the other … We have found that processes in the unconscious or in the id obey different laws from those in the ego. We name these laws in their totality the primary process, in contrast to the secondary process which governs the course of events in the ego. (Q461, Freud, 1940)
Table 1.
List of quotations pertaining to the characteristics of the secondary process (and the ego) and primary process thinking (and the id)
| The ego and the secondary process | Relevant quotations from Freud |
|---|---|
| 1. Default energy store or reservoir, which possesses the property of being spontaneously or tonically active. | Q5, Q15, Q109, Q154, Q162, Q209, Q273, Q298, Q300, Q301, Q314, Q320, Q321, Q416, Q438, Q454 |
| 2. Receives and ‘contains’ or ‘represses’ endogenous excitation. | Q1, Q15, Q46, Q80, Q132, Q152, Q154, Q183, Q205, Q209, Q212, Q219, Q283, Q283, Q287, Q328, Q358, Q363, Q391, Q392, Q427, Q427, Q429, Q437, Q448, Q475 |
| 3. Minimizes free-energy. | Q2, Q8, Q18, Q70, Q199, Q200, Q283, Q285, Q307, Q314, Q321, Q366, Q373, Q410, Q461, Q483 |
| 4. Integrates or binds the primary process and its representational system (the id) into a broader, more cohesive, composite organization (the ego). | Q15, Q29, Q45, Q46, Q154, Q209, Q218, Q231, Q233, Q234, Q237, Q300, Q302, Q308, Q314, Q315, Q334, Q339, Q351, Q358, Q360, Q383, Q384, Q385, Q391, Q397, Q402, Q413, Q429, Q447, Q461, Q483 |
| 5. Specific ontogenetic development. | Q47, Q113, Q174, Q273, Q300, Q301, Q358, Q414, Q440, Q459, Q486 |
| 6. Supports reality-testing and perceptual processing. | Q15, Q19, Q23, Q39, Q51, Q153, Q234, Q258, Q259, Q310, Q350, Q356, Q363, Q373, Q375, Q380, Q392, Q427, Q428, Q429, Q448, Q482, Q485 |
| 7. Supports conscious awareness, cognition and directed attention. | Q10, Q21, Q27, Q39, Q40, Q153, Q154, Q204, Q234, Q238, Q249, Q254, Q334, Q372, Q380, Q427 |
| 8. Possesses internally and externally-focused components, which are inversely related (anti-correlated). | Q6, Q39, Q162, Q173, Q204, Q243, Q273, Q289, Q300, Q301, Q320, Q329, Q363, Q438, Q448, Q454, Q484 |
| 9. Excessive-engagement of internally-focused component and impoverished engagement of externally-focused network during pathological withdrawal; e.g. in depression and schizophrenia. | Q144, Q147, Q158, Q161, Q168, Q169, Q170, Q172, Q244, Q252, Q253, Q263, Q265, Q266, Q267, Q277, Q288, Q292, Q293, Q297, Q301, Q329, Q330, Q368, |
| 10. Failure of systems to minimize free-energy (suppress endogenous excitation) results in disturbed affect, cognition and perception; as seen in non-ordinary states such as dreaming and psychosis. | Q23, Q35, Q58, Q115, Q134, Q135, Q147, Q231, Q261, Q262, Q333, Q365, Q383, Q455, Q462, Q466, Q469, Q475, Q476, Q482, Q485 |
| The id and primary process thinking | Relevant quotations from Freud |
| 11. Characteristics of the system unconscious/the id and primary process thinking: i.e. a primitive, ‘magical’ or animisitic style of thinking, characterized neurophysiologically by ‘free’ movement of energy. One can think of primary process thinking in evolutionary terms as a ‘protoconsciousness’. | Q58, Q63, Q90, Q92, Q97, Q115, Q135, Q151, Q160, Q171, Q198, Q201, Q203, Q209, Q211, Q216, Q217, Q218, Q228, Q229, Q230, Q231, Q233, Q237, Q241, Q242, Q247, Q249, Q254, Q257, Q261, Q270, Q279, Q280, Q282, Q299, Q305, Q311, Q315, Q335, Q359, Q388, Q389, Q396, Q397, Q423, Q424, Q425, Q426, Q437, Q440, Q442, Q443, Q446, Q453, Q461, Q465, Q467, Q468, Q470, Q471, Q472, Q474, Q477, Q479, Q480, Q482, Q490, Q491 |
The quotations can be found in Supplementary material.
Free-energy and the Bayesian brain
In terms of theoretical and computational neuroscience, we will focus on Helmholtz’s suggestion that the brain is an inference machine (Helmholtz, 1866; Dayan et al., 1995); this idea is now a fundamental premise in neurobiology (Gregory, 1968). Key examples of this include the Bayesian brain (Knill and Pouget, 2004), predictive-coding (Rao and Ballard, 1998) and the free-energy principle (Friston, 2009). This framework assumes that the brain uses internal hierarchical models to predict its sensory input and suggests that neuronal activity (and synaptic connections) try to minimize the ensuing prediction-error or (Helmholtz) free-energy. This free-energy is a measure of surprise and is essentially the amount of prediction-error. It is an information theory quantity that, mathematically, plays the same role as free-energy in statistical thermodynamics. Free-energy is not an abstract concept; it can be quantified easily and is used routinely in modelling empirical data (Friston et al., 2007) and in neuronal simulations of perception and action (Friston et al., 2009).
The notion of a hierarchy is central here because it allows the brain to construct its own top-down prior expectations about sensory samples from the world. This resolves one of the key challenges facing the brain and also allows it to resolve ambiguities when inferring and representing the causes of exteroceptive and interoceptive sensations. Crucially, the hierarchical form of internal models (and associated neuroanatomy) (Felleman and Van Essen, 1991) entails a progression in the complexity of representations, as one proceeds up the hierarchy from thalamic nuclei and primary sensory cortex to association and paralimbic cortex (e.g. from sensations, through perceptions to concepts). This progression is reflected in the temporal extent of what is represented; with higher levels representing extended sequences of events that best account for the stream of sensory information represented in lower levels (see Kiebel et al., 2008 for a full discussion and simulations).
The hierarchical architecture may also accommodate the distinction between the Freudian primary and secondary processes, where the secondary process provides top-down predictions to reduce free-energy associated with the primary process (cf. converting free energy into bound energy). Under this mapping between Freudian and Helmholtzian models, one can link the energy associated with the primary process and the free-energy of Bayesian formulations. In both accounts, higher cortical areas are trying to organize activity in lower-levels through suppression of their free-energy.
Intrinsic brain networks and the default mode
Analyses of spontaneous fluctuations in the blood oxygen level dependent (BOLD) signal of functional magnetic resonance imaging (fMRI) during unconstrained ‘resting’ states (typically lying quietly with eyes closed or fixating on a cross) have identified a number of large-scale intrinsic networks (Beckmann et al., 2005; Damoiseaux et al., 2006). Of particular interest here is the so called ‘default-mode network’ (DMN), a network of regions that show high metabolic activity and blood flow at rest but which deactivate during goal-directed cognition (Raichle, 2001). Recent work has confirmed that the major nodes of the DMN are functionally and structurally connected (van den Heuvel et al., 2008; Greicius et al., 2009) and that this connectivity develops through ontogeny (Fair et al., 2008; Kelly et al., 2009). Another feature of the DMN is the inverse relationship of its neuronal activity with that of another large-scale intrinsic network; the ‘attention system’ (Fox et al., 2005; Fransson, 2005; Corbetta and Shulman, 2002). In this article, we pursue the idea that these intrinsic networks correspond to the high-levels of an inferential hierarchy, which function to suppress the free-energy of lower levels (i.e. suppress prediction errors with top-down predictions). We associate this optimization process with the secondary process. Furthermore, we associate failures of top-down control with non-ordinary states of consciousness, such as early and acute psychosis, the temporal-lobe aura, dreaming and hallucinogenic drug states. In what follows, we organize the evidence that speaks to the integration of neurobiological and psychoanalytic ideas and conclude with a defence of its value and potential utility.
This article comprises three sections: in the first, we review evidence that the development and functioning of the DMN is consistent with ego-functions and the secondary process. We focus specifically on the DMN’s containment of endogenous excitation and its suppression of systems engaged by exogenous stimuli. In the second, we review evidence that a loss of top-down control over limbic activity in hierarchically lower systems is equivalent to a loss of the ego’s control over the primary process. In the final section, we discuss the clinical relevance of these ideas.
Large-scale intrinsic networks, the secondary process and ego
In this section, we introduce the idea that Freud’s descriptions of the development and functioning of the ego resonate with the development and functioning of the DMN and its reciprocal exchanges with subordinate brain systems. Freud’s first useful account of the ego was given in his posthumously published Project for a Scientific Psychology (Freud, 1895). Inspired by the recent introduction of ‘neurone theory’ by Cajal and Waldeyer-Harz, Freud hypothesized three functionally-distinct classes of neurone: the ‘ψ neurones’, which receive endogenous input and make up the ego; the ‘neurones’, which are sensitive to exogenous input; and the ‘ω neurones’, which signal qualitative information. Although this neuronal classification system was abandoned by Freud soon after its conception, the ideas that inspired it remained a source of influence throughout his work. Several of Freud’s most important ideas were introduced and/or developed in the Project, including the notion that the ego is an organization that receives and contains/represses bottom-up endogenous excitation (Table 1, row 2; Q1, Q15, Q46 and Q429).
The secondary process, or ‘secondary process cognition’, is the mode of cognition of the ego; put simply, it is the mode of normal waking consciousness of adult humans (e.g. Q317). Freud described the secondary process as ‘inhibited’ and ‘bound’; in contradistinction to the primary process which is ‘free’ and ‘motile’ (Freud, 1895, 1900). The concept of ‘bound’ energy was attributed by Freud to ideas first expressed by Breuer in their Studies on Hysteria (Breuer and Freud, 1895). Breuer conjectured the existence of a system of tonically active neurons, forming a ‘reservoir of nervous tension’ (e.g. Q45, Q154 and Q233). It is significant that the primary and secondary processes owe their inception to observations of non-ordinary states of consciousness (e.g. row 10, Table 1; Q23, Q35, Q115, Q333 and Q462). We shall see later that compelling evidence for the existence of two distinct modes of cognition can be found in studies of non-ordinary states. In what follows, we review the functional anatomy of the default-mode and related networks and then consider these networks from a theoretical perspective.
Functional anatomy of the default-mode
The notion of the DMN originated in a paper by Marcus Raichle reviewing a pattern of blood flow, glucose metabolism and oxygen consumption in the resting-state, which consistently decreases during goal-directed cognition (Raichle, 2001); in other words, a high-level distributed system whose activity is reciprocally related to the activity in cortical areas subserving task or stimulus-bound processing. Raichle proposed that this pattern reflects a default mode of brain function and a functionally relevant physiological baseline (Raichle, 2001). Subsequent work has associated activity in the network identified by Raichle and others (Greicius et al., 2003; Beckmann et al., 2005; Damoiseaux et al., 2006; Fransson and Marrelec, 2008; Wu et al., 2009) with phenomena such as self-referential processing, autobiographical recollection, mind-wandering and theory-of-mind (Gusnard et al., 2001; Vincent et al., 2006; Mason et al., 2007; Buckner et al., 2008; see also Q332).
Regions specifically implicated in the DMN include the medial prefrontal cortex, the posterior cingulate cortex, the inferior parietal lobule, the lateral and inferior temporal cortex and the medial temporal lobes (Buckner et al., 2008; Fransson and Marrelec, 2008). Analyses of resting-state functional connectivity and diffusion tensor imaging have showed that the major nodes of the DMN are strongly interconnected (Greicius et al., 2003, 2009; Van den Heuvel et al., 2009) and that this connectivity matures through development (Fair et al., 2008; Kelly et al., 2009). Functional connectivity in the DMN is relatively weak in the elderly (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008) and in patients with attention deficit disorder (Castellanos et al., 2008) and impulse control disorders (Church et al., 2009). Interestingly, medial prefrontal cortex-posterior cingulate cortex connectivity is entirely absent in infants (Fransson et al., 2007). These findings imply that the DMN develops through ontogeny, in a manner that parallels the emergence of ego-functions (Table 1, row 5).
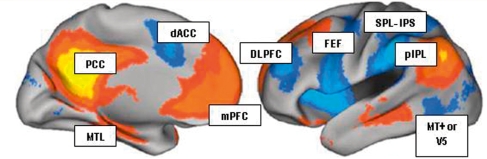
Figure 1.
The DMN (yellow/orange) and attention system (blue): resting state functional connectivity of three seed regions: the dorsal medial prefrontal cortex, ventral medial prefrontal cortex and hippocampal formation (medial temporal lobes). Positive correlations (yellow–orange) with all seeds were evident in the posterior cingulate (PCC), posterior inferior parietal lobule (pIPL) and medial prefrontal cortex (mPFC). Regions negatively correlated with these seeds constitute the attention system and include the superior parietal lobule (SPL), intraparietal sulcus (IPS), the motion-sensitive middle temporal area (MT+), the frontal eye fields (FEF) the dorsal anterior cingulate (dACC), the dorsolateral prefrontal cortex (DLPFC), the ventral premotor cortex and the frontal operculum. Image reproduced from Buckner et al. (2008), with permission.
Model and data-driven analyses of resting-state functional connectivity, diffusion tensor imaging analyses of structural connectivity and anatomical work in primates suggest that the medial temporal lobes are connected to the medial prefrontal cortex and posterior cingulate cortex nodes of the DMN (Catani et al., 2003; Vincent et al., 2006; Buckner et al., 2008; Fransson and Marrelec, 2008; Kahn et al., 2008; Saleem et al., 2008; van den Heuvel et al., 2008; Robinson et al., 2009). This is important because the medial temporal lobes contain key structures (e.g. the hippocampal formation, the amygdala, parahippocampal gyrus and entorhinal cortex) that play a role in mnemonic and hedonic or emotional processing. The evidence suggests that medial prefrontal cortex-medial temporal lobe functional and structural connectivity increases through ontogeny (Eluvathingal et al., 2007; Kelly et al., 2009) with a notable increase at puberty (Benes et al., 1989). A recent study found reduced medial prefrontal cortex-amygdala functional connectivity in patients with schizophrenia and an inverse correlation between connectivity and aggression in these patients (Hoptman et al., 2009). Preclinical work indicates that emotional extinction takes place via glutamatergic projections from the medial prefrontal cortex terminating on inhibitory interneurons in the medial temporal lobes (Rosenkranz and Grace, 2002; Rosenkranz et al., 2003) and a recent analysis of effective connectivity implied that activation of the rostral anterior cingulate drives inhibition of the amygdala in response to fearful faces (Stein et al., 2007).
There is a huge amount of clinical and preclinical evidence supporting the limbic-suppressive function of the medial prefrontal cortex (Hariri et al., 2000; Milad and Quirk, 2002; Rosenkranz and Grace 2002; Phillips et al., 2003; Phelps et al., 2004; Etkin et al., 2006; Milad et al., 2006). Functional neuroimaging studies have correlated primitive thought and emotion with decreased activity in the medial prefrontal cortex and increased activity in the medial temporal lobes (Pietrini et al., 2000; Dougherty et al., 2004), while suppression of these behaviours correlated with medial prefrontal cortex activations (Pietrini et al., 2000; Beauregard et al., 2001; Dougherty et al., 2004). The recollection of distressing memories and emotions in patients with post-traumatic stress disorder has also been found to correlate with medial prefrontal cortex deactivations and medial temporal lobe activations (Bremner et al., 1999; Shin et al., 2004, 2006; Hopper et al., 2007) and the blockade of these memories also correlated with medial prefrontal cortex activations (Lanius et al., 2002; Reinders et al., 2003, 2006). Damage to the ventromedial prefrontal cortex has long been associated with impaired impulse control (Grafmen et al., 1996; Anderson et al., 1999; Davidson et al., 2000; Kaplan-Solms and Solms, 2001; Solms and Turnbull, 2002). The medial prefrontal cortex sends dense projections to the ventral striatum (Ferry et al., 2000) and midbrain (Carr and Sesack, 2000). The ventral striatum displays functional connectivity with the midbrain, medial temporal lobes and higher-level nodes of the DMN (Postuma and Dagher, 2006; Di Martino et al., 2008; Gutman et al., 2009) and the midbrain and ventral striatum signal prediction-error and motivational-salience (Robbins and Everitt, 1996; Berridge and Robinson, 1998; Schultz, 2002; Kapur, 2005). In summary, the DMN comprises high-level cortical nodes such as the medial prefrontal cortex that exchange neuronal signals with subcortical systems and other association and polymodal cortex, especially the systems responsible for emotional learning and memory. Much of the evidence suggests that activation of the DMN suppresses activity in lower systems. We now consider these aspects of functional anatomy in the light of hierarchical inference and the secondary process.
Theoretical formulations of the default mode
Freud argued that the ego modulates both endogenous and exogenous excitation. Empirically, this can be seen in early and acute psychosis, the aura of temporal lobe epilepsy and hallucinogenic states, where affective (e.g. fear), mnemonic (e.g. moments of déjà vu or vivid recollection), perceptual (e.g. hallucinations) and cognitive (e.g. confused or muddled thinking) processing is perturbed (Bleuler, 1911; Epstein and Ervin, 1956; Cohen, 1964; Vollenweider et al., 1997) (Table 1, row 10; e.g. Q462). He further hypothesized that the ontogenetic/phylogenetic evolution of healthy, adult waking cognition depends on the formation of an equilibrium between the pressing forces of the primary process (entailed by the id) and the counter forces of the secondary process (entailed by the ego) (e.g. Q116). This description is remarkably consistent with contemporary models of cognition based on hierarchical Bayesian inference and Helmholtzian free-energy; where backward connections from higher cortical areas work to minimize the free-energy of lower areas (Mumford, 1992; Rao and Ballard, 1999; Friston, 2003; Kiebel et al., 2009).
Anatomically speaking, forward connections originate in supragranular layers and terminate in layer four spiny stellate cells. They project from lower to higher-levels; e.g. from thalamic nuclei to primary sensory cortex or from secondary sensory cortex to tertiary sensory areas. Backward connections are more abundant and diffuse than forward connections and their effects are primarily modulatory. Backward connections originate in deep pyramidal cells (infragranular layers) of the cortex and target infra and supragranular layers of lower cortical areas. Based on Bayesian and Helmholtzian principles it has been proposed that forward connections convey prediction-errors that optimize representations in higher levels. These representations are then used to form predictions that are conveyed by backward connections to lower levels. These predictions suppress or cancel prediction-errors (free-energy) until they can be minimized no further (Friston, 2003, 2005). In this way, the brain optimizes its representation of the world by suppressing prediction-errors with reciprocal message passing between hierarchical levels to minimize free-energy. This suppression simply involves countering excitatory presynaptic inputs (from representational units to neurons encoding prediction-error) with top-down presynaptic inputs, mediated by inhibitory interneurons. When the representations at any level can be explained by top-down predictions from the level above, prediction-error is minimized and the representations are internally consistent over levels. The aim of this process is to optimize parsimonious explanations for what caused sensory input (Friston, 2003) and establish sensory predictions to guide action and behaviour (Friston et al., 2009). Crucially, this empirically-informed scheme (Sandell and Schiller, 1982; Girard and Bullier, 1989; Hupé et al., 1998; Kleinschmidt et al., 1998; Murray et al., 2002; Lachaux et al., 2005; Chen et al., 2008) recapitulates Freud’s 19th century conception and in particular his principle of constancy:
[We] have taken the view that the principle which governs all mental processes is a special case of Fechner’s “tendency towards stability”, and have accordingly attributed to the mental apparatus the purpose of reducing to nothing, or at least of keeping as low as possible, the sums of excitation which flow in upon it. (Freud, 1924; Q366)
It is significant that Freud cited as his inspiration for these ideas, Gustav Fechner, the founder of psychophysics and a contemporary of Helmholtz (e.g. Q307, Q353, Q366, Q379): the process of minimizing ‘the sums of excitation’ is exactly the same as minimizing the sum of squared prediction-error or free-energy in Helmholtzian schemes. This rests on the assumption that the brain explicitly encodes prediction-error with neuronal activity (excitation) that is suppressed or explained by backward (top-down) afferents.
As mentioned above, Freud argued that the ego modulates and suppresses both exogenous and endogenous signals (Table 1, rows 2 and 6). In neurobiological terms, exogenous signals correspond to interoceptive and exteroceptive signals from thalamic and unimodal sensory areas that convey sensory signals (prediction-errors) to polymodal and medial temporal lobe structures. Endogenous signals could be equated with subsequent bottom-up prediction errors (excitation) arising in limbic and paralimbic systems, which are passed up to high-level polymodal cortical areas that comprise the nodes of the default-mode.
Clearly, the principles that attend hierarchical inference under Helmholtzian schemes are generic and may apply to all hierarchically deployed brain systems. However, we will focus on the DMN; specifically, on medial prefrontal suppression of limbic and paralimbic activity, and associate this with the suppression of endogenous activity by the ego. We now consider how the ego modulates excitation evoked by stimuli from the external world.
Hierarchical brain systems
As discussed in the introduction, BOLD signal oscillations in the DMN are characterized by their inverse relation to those of another major intrinsic network, referred to as the attention system (Corbetta and Shulman, 2002; Fox et al., 2005; Fransson, 2005). As well as showing a spontaneous inverse relationship with the DMN, the attention system is activated during externally-directed cognition and deactivated during internally-directed cognition, whereas the opposite is true of the DMN (Buckner et al., 2008); implying a ‘give-and-take’ relationship (Raichle, 2009). Regions implicated in the attention system include the dorsolateral prefrontal cortex, the dorsal anterior cingulate cortex, the frontal eye fields, the extrastriate cortex (e.g. V5) the superior parietal lobule, the intraparietal sulcus and the ventral premotor cortex (Buckner et al., 2008). These regions are active during target detection, attention to spatial detail and hand-eye coordination (Corbetta and Shulman, 2002; Shulman, 2003). Moreover, many of these nodes have been associated with top-down control of primary sensory input (Friston and Büchel, 2000; Lachaux et al., 2005). High-level association cortices not only receive feedforward signals from sensory regions but also anticipate and reciprocate these inputs with backward connections conferring context-specificity and higher-level constraints (i.e. predictions) (Rao and Ballard, 1999; Friston, 2003, 2005; Angelucci and Bressloff, 2006).
Recent work has suggested that what we have referred to as the ‘attention system’ is in fact not a unified system. Based on independent component analyses of resting state BOLD activity, Seeley et al. (2007) have shown that the system described by Fox et al. (2005) and Fransson (2005) can be differentiated into a ‘salience system’ which includes the dorsal anterior cingulate cortex, frontoinsular cortices, amygdala and ventral midbrain; and a more dorsal and lateral cortical system (the ‘dorsal attention system’) which includes the dorsolateral prefrontal cortex, frontal eye fields, dorsal medial prefrontal cortex, intraparietal sulcus and superior parietal lobule. BOLD signal oscillations in both systems exhibit an inverse relationship with those in the posterior cingulate cortex of the DMN (Greicius et al., 2003) but the systems do not appear to be well integrated with each other. This differentiation has also been suggested by others (He et al., 2007; Dosenbach et al., 2008; Sridharan et al., 2008; Vincent et al., 2008).
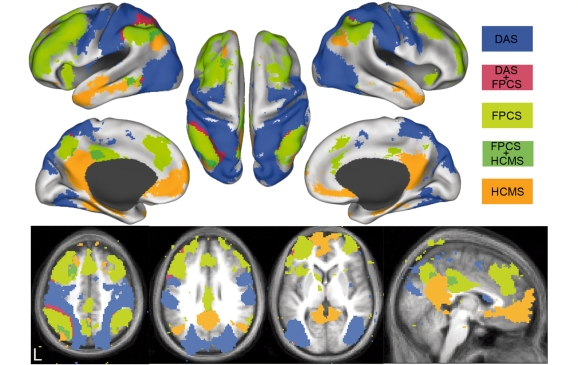
Figure 2.
Resting state functional connectivity in three cortical networks: (i) dorsal attention system (DAS, blue) using voxels in the middle temporal area and superior parietal lobule as regions of interest; (ii) the salience system (light green) using voxels in the anterior PFC and anterior inferior parietal lobule as regions of interest; and (iii) the default mode network (orange) using the hippocampal formation and posterior inferior parietal lobule as regions of interest. Overlap between the networks is shown in dark green (salience system and DMN) and red (dorsal attention systems and salience system). Image used with permission from Justin Vincent and Randy Buckner.
The picture that emerges is of a hierarchy of brain systems with the DMN at the top and the salience and dorsal attention systems at intermediate levels, above thalamic and unimodal sensory cortex. Under a Helmholtzian model, each system is trying to suppress the free-energy of its subordinates, through a process of optimizing predictions to reduce prediction-errors. This rests on recurrent message-passing between these systems that leads to self-organized activation patterns with a characteristic reciprocity or ‘give-and-take’ among levels. In this view, activation of the attention system may facilitate the suppression of exogenous excitation (Q258). Similarly, the DMN furnishes top-down control of the attentional and salience systems by explaining and thereby suppressing their activity. We next address the physiological basis of message-passing or interactions among brain regions that mediate this self-organized suppression.
Spontaneous BOLD oscillations and neuronal activity
Until recently, there had been some uncertainty about whether spontaneous BOLD-signal oscillations are generated by neuronal activity or non-neuronal physiological processes (Wise et al., 2004; Birn et al., 2006). Recent work has shown that spontaneous BOLD oscillations most probably have a neuronal origin (He et al., 2008; Nir et al., 2008; Shmuel and Leopold, 2008). Simultaneous fMRI and intracranial recordings in monkeys (Shmuel and Leopold, 2008) and humans (Nir et al., 2007) have identified spontaneous neuronal fluctuations that correlate with spontaneous BOLD fluctuations (Shmuel and Leopold, 2008). The neuronal fluctuations, which are coherent across the hemispheres, were evident in multiunit firing rates and local field potential gamma power. Stimulus evoked BOLD activations have also been shown to correlate positively with gamma power (Niessing et al., 2005). Gamma has been associated with attention, feature-binding and expectancy (Singer and Gray, 1995; Herrmann, 2000; Engel et al., 2001). This suggests that BOLD fluctuations reflect cortical coherence associated with gamma and secondary process cognition, particularly since the gamma frequency, recorded in higher-level cortical areas, has recently been shown to suppress lower frequencies in lower-level cortical areas (Chen et al., 2009). This leads us to predict that the fluctuations in gamma power evident in the large-scale intrinsic networks index the ongoing minimization of free-energy in subordinate levels of the hierarchy (Engel et al., 2001; Raichle, 2007) and could provide an empirical measure of the secondary process.
Generally speaking, oscillatory processes are ubiquitous in the brain and serve to couple remote neuronal populations. High frequency gamma has often been implicated in perceptual synthesis and binding (e.g. Singer, 2009); while theta rhythms have been most studied in the hippocampal system, where they are associated with (spatial) memory and exploration (e.g. Lisman and Redish, 2009). Crucially, theta and gamma are themselves coupled (e.g. Sirota et al., 2008), where slower theta oscillations may provide a temporal frame of reference for faster computations mediated at gamma frequencies. We will return to the oscillations and frequency-specific coupling in the brain in the next section.
So far we have discussed the importance of reciprocal or anti-correlated activity in the DMN and networks for goal-directed cognition. However, it should be noted that spontaneous fluctuations in the DMN continue during active cognition, just as spontaneous fluctuations in the dorsal attention system continue during rest (Hampson et al., 2002; Beckmann et al., 2005; Damoiseaux et al., 2006; Fox et al., 2007). This tonicity presumably primes structures to infer exogenous inputs (Fox et al., 2007; Raichle, 2007) and supports a background level of predictive coding (Hampson et al., 2002). Furthermore, spontaneous fluctuations in the BOLD signal, which can be as large in amplitude as evoked BOLD responses (Fox et al., 2007), have been shown to reflect variations in behaviour (Boly et al., 2007; Fox et al., 2007). Much of the brain’s vast energy budget is reserved for spontaneous neuronal activity (Fox and Raichle, 2007; Raichle, 2007). We speculate that spontaneous activity in the DMN reflects the constant containment of spontaneous endogenous activity—commensurate with Freud’s concept of repression (Q209), while spontaneous activity in the dorsal attention system indexes the continual monitoring and suppression of exogenous stimuli. This conjecture appeals to the Helmholtzian view of the brain as an inference engine that continually generates predictions and hypotheses that, when freed from the present (Kiebel et al., 2009), necessarily entails the past and future.
In addition to the functional importance of spontaneous neuronal activity in intrinsic networks, the give-and-take between the default system and task-positive systems appears to be vital for active cognition and conscious awareness (Pomarol-Clotet et al., 2008; He and Raichle, 2009; Whitfield-Gabrieli et al., 2009). Functional connectivity within the DMN has been shown to increase through ontogeny (Fair et al., 2008; Kelly et al., 2009), decrease in ageing (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008) and to be underdeveloped in patients with impaired impulse control (Church et al., 2009). These findings imply that functional connectivity in the DMN (Hampson et al., 2006) and the dorsal attention system (Seeley et al., 2007) provides an index of cognitive aptitude but not necessarily active cognition (Larson-Prior et al., 2009). Ineffective deactivation of the DMN has been associated with cognitive error in healthy subjects (Li et al., 2007; Eichele et al. 2008) and negative symptoms in schizophrenia (Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009) and depression (Grimm et al., 2009; Sheline et al., 2009). Functional connectivity in the DMN is not significantly altered in sleep, sedation or coma (Boly et al., 2008; Larson-Prior et al., 2009) but the give-and-take between the DMN and its anti-correlated networks is (He and Raichle, 2009) (see Q238).
Summary and synthesis
In this section, the secondary process was considered in relation to large-scale intrinsic networks working to predict and suppress excitation (Helmholtz free-energy) in subordinate systems. The concept of the secondary process entailed by ego-functions was associated with the suppressive effect of the DMN on its subcortical nodes and anti-correlated networks. Functional connectivity between limbic (e.g. the hippocampus and amygdala) structures and major nodes of the DMN during rest (Buckner et al., 2008; Di Martino et al., 2008) supports the notion that the systems enacting ego-functions have evolved to receive and control endogenous excitation that underlies mnemonic and hedonic processing. In the next section, we focus on the primary process and specifically how it is manifest in non-ordinary states of consciousness.
The phenomenology of primary process thinking
In this section we describe the phenomenology of non-ordinary states of consciousness that have been associated with primary process thinking. The primary process is not generally regarded as a serious topic of science but the phenomenology of certain non-ordinary states compel us to consider its importance. Psychoanalysis owes its origins to observations of non-ordinary states (Table 1, row 10; e.g. Q23, Q35, Q115, Q333; Q462). An early observation that has remained at the core of the Freudian model is that there exists in the mind an archaic mode of cognition, which under normal waking conditions is effectively suppressed (Q315). Freud saw this ‘primary’ mode as belonging to an ontogenetically and phylogenetically primitive system, which he referred to initially as ‘the unconscious’ (Breuer and Freud, 1895), later as ‘the system unconscious’, ‘system Ucs’ or just ‘Ucs’ (Freud, 1900, 1915 b) and eventually as ‘the it’ (Freud, 1923) (note: Freud’s original term for ‘the id’ was ‘das es’ and should really have been translated ‘the it’, just as his original term for ‘the ego’, ‘das Ich’, should have been translated ‘the I’; for simplicity however, we will use the familiar terms ‘id’ and ‘ego’).
Thus, the term ‘the id’ was introduced relatively late by Freud (Freud, 1923) as a new name for ‘the unconscious’ in its systematic sense (i.e. ‘the system unconscious’ or ‘system Ucs’) (Freud, 1900, 1915 b). Freud wrote relatively less about the id than the system unconscious but the two are essentially synonymous (see Q422, Q423, Q458 and Q461). Freud’s decision to rename the system unconscious ‘the id’ was motivated by his acknowledgement that aspects of the ego are also unconscious (in the descriptive sense) and processes in the id can become conscious. The introduction of the id was useful in this respect as it resolved ambiguities relating to the descriptive meaning of ‘unconscious’. Referred to as ‘the id’, the unconscious could be understood more explicitly as a system subserving a specific mode of cognition (e.g. Q461).
The characteristics of primary process thinking are clearest when contrasted against those of the secondary process: just as the characteristics of the primary process only become manifest in certain non-ordinary states, the characteristics of the secondary process only really become evident when they are lost. For example, Freud considered timelessness to be a major characteristic of the id and time perception to be a function of the ego (e.g. Q424). The notion of timelessness is difficult to comprehend from the vantage of normal waking consciousness but becomes clearer if we consider the phenomenology of states such as the temporal lobe aura: ‘Time seems endless’ (Epstein and Ervin, 1956); acute psychosis: ‘Time slowed down, much more experience could be crowded into a brief time span’ (Bowers, 1965) and the hallucinogenic drug state: ‘[Under the influence of drugs such as LSD, one has] the feeling that so much was “seen” that “hours” or “days” or “aeons” must have passed’ (Masters and Houston, 2000). Recent work involving the serotoninergic hallucinogen, psilocybin, has shown that hallucinogen-induced impairments in temporal perception are dose-dependent (Wackermann et al., 2008). Furthermore, recent formulations of the free-energy principle suggest that there is a systematic increase in temporal coherence in higher-level structures (Kiebel et al., 2009). Thus, impaired temporal perception is a property of primary process thinking that has the potential to be measured psychophysically; thus bringing previously intangible phenomena into the scientific realm. Four other qualities of primary process thinking that can be assessed empirically include the following.
Sensations of fear or dread, e.g. in the aura of temporal lobe epilepsy: ‘I feel afraid, as if something awful might happen’ (Williams, 1956); early psychosis: ‘Suddenly Fear, agonizing, boundless, Fear, overcame me, not the usual uneasiness of unreality, but real fear, such as one knows at the approach of danger, of calamity’ (Sechehaye, 1951); the hallucinogenic drug state: ‘I found myself all at once on the brink of panic’ (Huxley, 1954); and dreaming: ‘Fear is the most frequently occurring dream emotion’ (Bulkeley, 2009).
Perceptual distortions/visual hallucinations, e.g. in the aura of temporal lobe epilepsy: ‘The surroundings feel strange and unfamiliar’ (Hansen and Brodtkorb, 2003); early psychosis: ‘It wasn’t really unreal; it was just strange, funny, different’ (Cutting and Dunne, 1989); and the hallucinogenic drug state: ‘The room and furniture were distorted, strange and terrifying’ (LSD, Cohen, 1964).
Déjà vu, recollective or reliving phenomena, e.g. in the aura of the temporal lobe epilepsy: ‘I went back to all that occurred in my childhood’ (Hughlings-Jackson, 1879); early psychosis: ‘My whole world seemed to cave in—I kept thinking about my birthplace and my past’ (Cutting and Dunne, 1989); and the hallucinogenic drug state: ‘I started to cry uncontrollably and nothing could have stopped it—it was like a dam giving way. At first I didn’t know what I was weeping about, but soon became aware that I was reliving childhood experiences of which I had scarcely any conscious knowledge. Until today I had remembered only fragments, but now the entire sequence reeled off as from a microfilm that was securely stored within my head’ (LSD, Cohen, 1964).
Disturbance to the sense-of-self, e.g. in the aura of temporal lobe epilepsy: ‘I felt that I disappeared’ (Johanson et al., 2008); early psychosis: ‘When I look at somebody my own personality is in danger. I am undergoing a transformation and myself is beginning to disappear’ (Chapman, 1966); and the hallucinogenic drug state: ‘I felt the relaxing of the self boundaries’ (LSD, Cohen, 1964).
Other characteristics of primary process thinking include a fear of losing control of ones mind, a general sense of the peculiarity of things, euphoria, grandiosity, paranoia and suspiciousness, thought-disturbances, bizarre thought-content and an increased interest in mystical, magical or animistic notions. All these phenomena could easily be assessed using subjective rating scales as a global measure of ego-disturbance or primary process thinking. However, an association between the primary process and dreaming, acute psychosis, temporal lobe aura and hallucinogenic states can be motivated at a purely phenomenological level. Crucially, all these states have been compared with each other previously; e.g. psychosis and dreaming (Freud, 1900; Jung, 1907; Bleuler, 1911); psychosis and the temporal lobe aura (Slater and Beard, 1963; Bear, 1979; Ferguson and Rayport, 2006); psychosis and the hallucinogenic drug state (Behringer, 1927; Bowers and Freedman, 1966; Gouzoulis et al., 1994); dreaming and the temporal lobe aura (Rodin et al., 1955; Penfield and Perot, 1963; Mahl et al., 1964); dreaming and the hallucinogenic drug state (Grof, 1975; Fischman, 1983; Callaway, 1988); and the temporal lobe aura and the hallucinogenic drug state (Bercel et al., 1956; Schwarz et al., 1965; Balestrieri, 1967). It is also worth noting that dreaming (Freud, 1900), psychosis (Freud, 1900; Bleuler, 1911), the temporal lobe aura (Kubie, 1952; Robin et al., 1955; Delgado et al., 1956; Epstein and Ervin, 1956; Ostow, 1957; Mahl et al., 1964; Horowtiz et al., 1968) and the hallucinogenic drug state (Busch and Johnson, 1950; Sandison et al., 1954; Cattell, 1957; Martin, 1957; Eisner, 1959; Cohen, 1964; Abramson, 1967; Horowitz et al., 1968; Grof, 1975) have all been described as states conducive to the emergence of primary process thinking. In the remainder of this section we will review evidence that these states, which clearly display a related phenomenology, also possess a related neurophysiology.
Neurophysiology of the primary process
In this section, we show that brain states associated with primary process thinking have common neurophysiological substrates. Intracranial electroencephalography (EEG) recordings in medial temporal structures, the superior temporal gyrus and the visual association cortex, after high-frequency stimulation of the perirhinal cortex, reveals bursts of synchronous high-amplitude theta activity spreading from the medial temporal lobes to the visual association cortices during the hallucinatory revival of past experiences (Barbeau et al., 2005). This activity is consistent with Freud’s speculations about the processes underlying dreaming and related states (e.g. Q97 and Q98). Similar activity has been recorded in the medial temporal lobes of other epileptic patients during states of hallucinosis and recollection (Rodin et al., 1955; Heath, 1961; Stevens et al., 1969) and increased theta power has been recorded over the medial temporal lobes during recollection using magnetoencephalography (Guderian and Düzel, 2005).
In the 1950s and early 1960s, activity was recorded in cortical and subcortical structures in a large number of patients experiencing acute psychotic episodes (Heath, 1954; Lesse et al., 1955; Sem-Jacobsen et al., 1956; Heath and Mickle, 1960; Sherwood, 1962; Heath and Walker, 1985). Subcortical contacts revealed conspicuous activities, which were generally not seen in the cortex or at the scalp (Sem-Jacobsen et al., 1956; Heath and Mickle, 1960). In actively psychotic patients, spiking and bursts of high-amplitude synchronous activity (of variable frequency but often theta) were recorded in the septum (which until the mid-1970s included the nucleus accumbens) (Heath, 1954; Stevens, 1999) amygdala and hippocampus (Sem-Jacobsen et al., 1956; Heath and Mickle, 1960; Sherwood, 1962). This activity was specific to these regions, was most pronounced when the psychosis was most florid and was absent when the symptoms remitted (Heath and Mickle, 1960).
Intracranial recordings in subjects administered the hallucinogenic drugs LSD and mescaline revealed spiking and bursts of high-amplitude activity in the medial temporal lobes similar to that recorded in the acutely psychotic patients (Schwarz et al., 1956; Sem-Jacobsen et al., 1956; Monroe et al., 1957; Heath and Mickle, 1960; Chapman et al., 1962). LSD and related drugs were used extensively in the 1950s and 60s as adjuncts to psychoanalytic psychotherapy (Abramson, 1967; Grinspoon and Bakalar, 1979). Spontaneous recollections of a similar nature to those associated with the temporal lobe aura (e.g. Penfield and Perot, 1963; Barbeau et al., 2005) have been reported after ingestion of LSD and psilocybin (e.g. Sandison et al., 1954; Grof, 1975; Vollenweider et al., 1997). High-amplitude bursts of low-frequency/theta activity have also been recorded in the human hippocampus in rapid eye movement (REM) sleep (Brazier, 1968; Freemon and Walter, 1970; Giaquinto, 1973; Moiseeva and Aleksanyan, 1976; Mann et al., 1997; Yu et al., 1997; Bódizs et al., 2001; Cantero et al., 2003) and LSD given to humans immediately prior to (Toyoda, 1964; Muzio et al., 1966) or during sleep (Torda, 1968) has been shown to promote REM sleep and dreaming. These studies provide converging evidence that a specific mode of cognition (primary process thinking), rests on brain states, which possess a characteristic neurophysiology.
There are also some interesting examples of medial temporal activities being influenced by psychiatric interview (Heath, 1954, 1964; Lesse et al., 1955). Sporadic bursts of high-amplitude synchronous activity recorded intracranially in the medial temporal lobes (Heath, 1954, 1964; Lesse et al., 1955) were detected as personal memories, with strong emotional content, were touched on. The activity desynchronized if the patient attended to his environment (Lesse et al., 1955) or carried out a mathematical problem (Heath, 1954, 1964).
The abnormal limbic activity recorded in the temporal lobe aura, acute psychosis, the hallucinogenic drug state and REM sleep is often seen in the theta range (Sem-Jacobsen et al., 1955; Heath et al., 1955–56; Schwarz et al., 1956; Sem-Jacobsen et al., 1956; Monroe et al., 1957; Heath and Mickle, 1960; Chapman et al., 1962; Sherwood, 1962; Cantero et al., 2003; Barbeau et al., 2005), although bursts of high-amplitude fast activity were also seen (e.g. Lesse et al., 1955; Heath et al., 1955-56). Hippocampal theta in animals is reliably associated with locomotion, orienting and REM sleep (Kahana et al., 2001) and also long-term potentiation (Hölscher et al., 1997). Hippocampal theta depends on inputs from the septal nuclei, a major theta generator (Petsche et al., 1962; Winson 1978) and another site from which the abnormal activity was recorded in non-ordinary states in humans (e.g. Heath, 1954; Sherwood, 1962). As well as providing conditions for encoding new experiences, hippocampal theta facilitates the retrieval of past experiences (Hasselmo et al., 2002 Barbeau et al., 2005). Scalp recordings of increased theta power associated with goal-directed cognition (Burgess and Gruzelier, 2000; Krause et al., 2000; Onton et al., 2005) are unlikely to relate to the high-amplitude bursts seen in the septum and medial temporal lobes during the non-ordinary states of consciousness described above (Gevins et al., 1997; Kahana et al., 2001; Buzsaki 2002; Raghavachari et al., 2006). The cortex is capable of generating its own theta (Silva et al., 1991; Raghavachari et al., 2006) and intracranial work in humans has provided more evidence for low-amplitude, high-frequency oscillations in the hippocampus during attentiveness than for theta (Heath, 1954, 1964; Lesse et al., 1955; Halgren et al., 1978; Arnolds et al., 1980; Huh et al., 1990; Meador et al., 1991; Caplan et al., 2001; Axmacher et al., 2007). Moreover, in the non-ordinary states, activity recorded from the scalp and in the cortex is generally low-amplitude, high-frequency and desynchronous (Heath and Mickle, 1960; Chapman et al., 1962; Rodin et al., 1966; Cantero et al., 2003); such activity is highly characteristic of REM sleep (Jouvet, 1965; Maquet et al., 1996; Braun et al., 1998; Cantero et al., 2003; Wehrle et al., 2007) and other cortical ‘up’ states (Steriade et al., 2001) such as those induced by serotoninergic hallucinogens (Lambe and Aghajanian, 2006).
Based on empirical findings (e.g. Cañive et al., 1996; Jeanmonod et al., 1996, 2003; Llinás et al., 1998, 1999) it has been proposed that bursts of limbic theta, recorded in the cortex as increased gamma, can index the positive symptoms of various neurological and psychiatric disorders (Llinás et al., 1999; Jeanmonod et al., 2003; Llinás and Steriade, 2006). Under normal conditions, cortical gamma readily suppresses low-frequency oscillations (Chen et al., 2009). This function is analogous to the secondary process, but in pathological states and dreaming, limbic activity is more anarchic (e.g. Oertel et al., 2007; Wehrle et al., 2007) and the cortex must work harder to contain it (Llinás et al., 1999; Jeanmonod et al., 2003; Llinás and Steriade, 2006).
Recent intracranial EEG work in humans, using subdural electrodes recorded theta phase-modulation of high-frequency (80–150 Hz) gamma power (Canolty et al., 2006). Theta modulation of gamma power was evident at rest but also during behavioural tasks. Theta-gamma coupling was highest at the trough of the theta phase. Moreover, electrodes showing the highest mean theta power also showed the strongest theta-gamma coupling. These findings imply that theta modulates coupling between theta and gamma and a number of researchers have suggested that theta may promote long-range coupling in cortical networks (e.g. von Stein and Sarnthein, 2000; Buzsaki, 2006).
Summary and synthesis
Integrating these findings, we propose that high-amplitude low-frequency (e.g. theta) discharges in limbic and paralimbic regions index the free-energy of the Helmholtzian scheme and mediate the primary process of the Freudian scheme. In waking cognition, low-frequency limbic oscillations couple to (i.e. entrain) gamma in the cortex (Canolty et al., 2006; Llinás and Steriade, 2006) enabling the activity of the cortex to explain and thereby contain the activity of the limbic regions (Engel et al., 2001; Friston, 2003; Chen et al., 2009). In non-ordinary states, this function may be perturbed (e.g. in the case of hallucinogenic drugs, through actions at modulatory post-synaptic receptors) (Aghajanian and Marek, 1997), compromising the hierarchical organization and suppressive capacity of the intrinsic networks.
To investigate these phenomena further, neuroimaging measures of functional and effective connectivity could be employed to assess whether e.g. phasic events in REM sleep or the hallucinogenic drug state correlate with an increased limbic input to higher-level association cortices. It might transpire that in these states, limbic discharges become capable of traversing systems, which they are unable to do under normal conditions. For example, it might be possible to observe limbic discharges influencing activity in visual association areas (see Barbeau et al., 2005). One might expect limbic activity to be suppressed by higher-level regions of the DMN in normal waking cognition but not in non-ordinary states. This might explain the difference between the experience of day-dreaming in the resting-state (Mason et al., 2007, Q284 and Q332) and hallucinosis in non-ordinary states (Q97), where limbic activity is released from top-down control. The mechanisms of this release have been discussed previously in terms of perceptual inference and synaptic gain (Friston, 2005b; Stephan et al., 2009), where the major determinant of synaptic gain is neuronal synchronization.
Discussion
In this article we have explored the notion that Freud’s descriptions of the secondary process are consistent with the functional anatomy of large-scale intrinsic networks. We have proposed that intrinsic networks self-organize into hierarchical frameworks, in order to suppress the free-energy of their subordinate levels. This was associated with the function of the secondary process. We hypothesized that spontaneous fluctuations in neuronal activity in cortical nodes of the DMN function to suppress or contain otherwise anarchic and unconstrained endogenous activity in limbic and paralimbic systems, while fluctuations in subordinate networks anti-correlated with the DMN predict and counter prediction errors induced by exogenous sensory input in sensory and visceral systems.
Given the nature of this synthesis, different readers will find merit in different aspects of it. For example, some readers may see value in relating inferential coding to intrinsic networks and regard this as a potentially useful perspective on functional anatomy. Others may take the formal similarity between Freudian formulations and functionalist interpretations of neuronal processes as evidence for their construct validity. For example, the remarkable overlap between Freud’s theories and modern neurobiology may engage clinicians and academics who are more familiar with (and receptive to) Freud’s work (Table 2). Developing these points of contact may help anchor Freudian concepts to measurable biological phenomena and inform psychoanalytic thinking. As has been argued previously (Kandel, 1999; Solms, 2009), this process may be important for psychoanalysis. Furthermore, given the enduring, albeit marginal, influence of psychoanalysis in psychiatry, it may benefit psychiatry if psychoanalysis is properly grounded in neuroscience. This is the agenda of the Neuro-Psychoanalysis movement (www.neuro-psa.org.uk) and should assist the process of separating premises that have construct validity from those which do not.
Table 2.
Some points of contact between Freud’s account of the mind and empirical findings in neurobiology
|
Freud’s writings contain many useful heuristics for exploring global brain function, especially in non-ordinary states of consciousness. Indeed, the Freudian model owes its origins to inferences based on unconstrained states, whereas the cognitive-behavioural approach is uncertain in this domain (Morcom and Fletcher, 2007). Science usually analyses phenomena extrospectively but in the mind-sciences especially, certain phenomena demand that we look both inwards and outwards - even if introspection entails some compromise and a confrontation with our ‘it’. Freud’s theories were conceived through a study of non-ordinary states, his schooling in neurology and a readiness to introspect. If they were built on false inference and loose philosophy, it is unlikely they would have endured in the way that they have. For those opposed to Freud, who would rather see his constructs dissolved into pure phenomenology and neurobiology, we put up little resistance (e.g. Q176). Phenomenology and neurobiology can stand alone. The Freudian model adds a framework for an integrated understanding of psychopathological phenomena. Once the full-character of non-ordinary states and cognition are understood, this framework may dissolve naturally.
The synthesis attempted in this article is intended to facilitate a more comprehensive understanding of psychological and neurobiological phenomena; addressing topics which have hitherto been considered incompatible with the cognitive paradigm (e.g. Morcom and Fletcher, 2007). The Freudian model should not impede hypothesis testing but rather facilitate it by emphasizing the importance of studying the phenomenology, neurophysiology and neurodynamics of different modes or states of cognition; and by indicating where we might look for anomalies. For example, altered functional connectivity between limbic and cortical nodes of the DMN may predict symptoms of ego-disturbance or primary process thinking. Identifying the neurobiological signature of ego-disturbance or primary process thinking may provide new insights into the pathogenesis of schizophrenia, given that related symptoms are prevalent in the prodromal phase (Møller and Husby, 2000; Parnas and Handset, 2003; Häfner and Maurer, 2006). Another symptom cluster, which might benefit from a Freudian treatment, is the withdrawal seen in depression and schizophrenia. The association between ego–libido and object–libido and the give-and-take between the DMN and its anti-correlated networks may be especially relevant here:
All that we know about [libido] relates to the ego, in which at first the whole available quota of libido is stored up. We call this state absolute, primary narcissism. It lasts till the ego begins to invest the ideas of objects with libido, to transform narcissistic libido [ego–libido] into object–libido. Throughout the whole of life the ego remains the great reservoir from which libidinal investments are sent out to objects and into which they are also once more withdrawn. (Freud, 1940; Q454)
The notion of displacing energy from a default store to networks concerned with scrutinizing the external world is consistent with the functional relationship of the DMN to its anti-correlated networks, where e.g. activity is displaced from the DMN to the dorsal attention system during goal-directed cognition (Raichle et al., 2001):
We see also, broadly speaking, an antithesis between ego–libido and object–libido. The more of one is employed, the more the other becomes depleted. (Freud, 1914; Q173)
It is interesting that Freud’s notion of a finite ‘reservoir’ of energy and the reciprocal patterns of activation between the DMN and subordinate networks both fit comfortably with hierarchical minimization of free-energy. This minimization entails recurrent message-passing between hierarchical brain systems that try to suppress the free-energy at all levels (this scheme is also called predictive coding; e.g. Jehee and Ballard, 2009). The ensuing dynamics mean that increased neuronal activity at one level suppresses neural activity encoding prediction-error in another, leading to reciprocal patterns of activation and deactivation; see Murray et al. (2002) for a nice empirical example of this in the visual system and Friston and Stephan (2005) for a simulation in the auditory system. In brief, the ‘reservoir’ of free-energy is constantly primed by surprising or unaccountable exchanges with the sensorium and is distributed throughout the hierarchy in an attempt to minimize its expression at any one level.
Recent work has shown reduced task-evoked suppressions of DMN activity in schizophrenia (Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009) the severity of which correlated positively with connectivity in the DMN (Whitfield-Gabrieli et al., 2009). These findings support the observation that there is a reduced engagement with the external world in schizophrenia (see Table 1, row 9 and especially Q168 and Q170). In this article we have proposed that the brain's functional anatomy is organized hierarchically to ensure that free-energy is minimized in the most efficient way. Organized in this manner, the brain explains internal and external events and effectively discriminates between them. However, assuming that the development and maintenance of this organization is use-dependent, it will be jeopardized if the individual withdrawals from the external world. If the brain's hierarchical organization begins to breakdown, there may be an ensuing confusion over, among other things, what are internal and external sensations. This may be especially relevant during puberty, when the ego is forced to negotiate new demands from internal and external sources and through this, develop an adult ego. According to our model, the development of an adult ego (a properly functional DMN) is necessary to contain internal excitations and coordinate engagements with the external world. If this is not achieved, systems normally inhibited by the DMN (e.g. the salience system) may slip from its control. In the ensuing chaos, the patient may develop delusions as a compromise strategy for containing the increase in free-energy. Thus, from the free-energy perspective, withdrawal, psychomotor poverty and delusional thinking may be last resorts for someone who finds everything surprizing and unpredictable. See Fletcher and Frith (2009) and Corlett et al. (2009) for a free-energy (predictive coding) treatment of false inference in schizophrenia.
As in schizophrenia, Freud recognized that a retreat from the external world is also characteristic of depression. In depression however, emphasis was laid on a loss of an intense object-love. Freud argued that the patient reacts to this loss by targeting the aggression felt towards the lost object back upon his/her own ego:
There is no difficulty in reconstructing [the] process of [melancholia]. An object-choice, an attachment of the libido to a particular person, had at one time existed; then, owing to a real slight or disappointment coming from this loved person, the object-relationship was shattered … But the free libido was not displaced on to another object; it was withdrawn into the ego … Thus the shadow of the object fell upon the ego and the latter could henceforth be judged by a special agency, as though it were the forsaken object … One or two things may be directly inferred with regards to the preconditions and effects of a process such as this. On the one hand, a strong fixation to the loved object must have been present; on the other hand, in contradiction to this, the object-[investment] must have had little power of resistance … This contradiction seems to imply that the object-choice had been effected on a narcissistic basis, so that the object-[investment], when obstacles [came] in its way, [could] regress to narcissism. (Freud, 1917b, Q267)
As in schizophrenia, recent work has shown a reduced task-induced suppression of DMN activity in depression (Grimm et al., 2009; Sheline et al., 2009) and these reductions correlated positively with depression severity and ratings of hopelessness (Grimm et al., 2009). Reduced blood flow and activation in the dorsolateral prefrontal cortex and hyper-perfusion, metabolism and activity in limbic and medial prefrontal regions are also reliably associated with depression (e.g. Mayberg et al., 2005, 2007; Drevets et al., 2008). These findings support the notion of a withdrawal from the external world and a pathological self-focus in depression, consistent with the Freudian account (Table 1, row 9).
Conclusion
The first section of this article reviewed evidence that the development and functioning of the DMN and its functional relationship with its anti-correlated networks is consistent with that of the ego. In the second we described the phenomenology of primary process thinking, reviewed evidence that it can be observed in certain non-ordinary states and cited studies indicating that these states share a common neurophysiology. In the final section we sought to justify the synthesis and show how reference to the Freudian model might be used to understand clinically relevant phenomena in neurobiological terms.
This article does not address the efficacy of psychoanalysis as a treatment (see Fonagy, 2003 for a relevant review and Q478). Our focus is on the validity of Freudian constructs in relation to global phenomena and related theories that have recently emerged in systems neuroscience.
Finally, this synthesis was compelled by the links between psychopathology and the neurophysiology of certain non-ordinary states of consciousness, and between the functional organization of intrinsic brain networks and the secondary process as described by Freud. The synthesis is empirically-led, as are the methods we recommend for testing and applying it. The neurobiological phenomena addressed in this synthesis are central topics in contemporary neuroscience and the Freudian concepts are principal components of his model, where these components can be traced to his schooling in neurology and the influence of people like Meynert, Helmholtz, Fechner, Hering, Herbart, Charcot and Hughlings-Jackson.
Funding
KJF was funded by the Wellcome Trust. RCH is supported by the Beckley Foundation and has recently received a research grant from the Neuropsychoanalysis Association.
Supplementary material
Supplementary material containing relevant quotations of Freud is available at Brain online.
Supplementary Material
Acknowledgements
We would like to thank our reviewers for very helpful guidance in presenting and extending these ideas.
Glossary
Abbreviations
- BOLD
blood oxygen level dependent
- DMN
default-mode network
- fMRI
functional magnetic resonance imaging
- REM
rapid eye movement
References
- Abramson HA. The Second International Conference on the Use of LSD in Psychotherapy. New York: The Bobbs-Merrill Company; 1967. [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacol. 1997;36:589–99. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. [Review] Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- Arnolds DE, Lopes da Silva FH, Aitink JW, Kamp A, Boeijinga P. The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalogr Clin Neurophysiol. 1980;50:324–8. doi: 10.1016/0013-4694(80)90160-1. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 2007;27:7807–16. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrieri A. On the Action Mechanisms of LSD. In: Abramson HA, editor. The Use of LSD in Psychotherapy. New York: Bob-Merrill Company; 1967. [Google Scholar]
- Barbeau E, Wendling F, Régis J, Duncan R, Poncet M, Chauvel P, et al. Recollection of vivid memories after perirhinal region stimulations: synchronization in the theta range of spatially distributed brain areas. Neuropsychologia. 2005;43:1329–37. doi: 10.1016/j.neuropsychologia.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Bear DM. Temporal lobe epilepsy – a syndrome of sensory-limbic hyperconnection. Cortex. 1979;15:357–84. doi: 10.1016/s0010-9452(79)80064-7. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer K. Der Meskalinrausch. Berlin: Springer; 1927. [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–93. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Bercel NA, Travis LE, Olinger LB, Dreikurs E. Model psychoses induced by LSD-25 in normals. I. Psychophysiological investigations, with special reference to the mechanism of the paranoid reaction. AMA Arch Neurol Psychiatry. 1956;75:588–611. doi: 10.1001/archneurpsyc.1956.02330240026003. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bettelheim B. Freud and Man’s Soul. New York: Vintage Books; 1982. [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–48. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press; 1911. [Google Scholar]
- Bódizs R, Kántor S, Szabó G, Szûcs A, Erõss L, Halász P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001;11:747–53. doi: 10.1002/hipo.1090. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–92. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann NY Acad Sci. 2008;1129:119–29. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M. The onset of psychosis–a diary account. Psychiatry. 1965;28:346–58. doi: 10.1080/00332747.1965.11023442. [DOI] [PubMed] [Google Scholar]
- Bowers MB, Jr, Freedman DX. “Psychedelic” experiences in acute psychoses. Arch Gen Psychiatry. 1966;15:240–8. doi: 10.1001/archpsyc.1966.01730150016003. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesensten NJ, Gwadry F, Carson RE, Varga M, et al. Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science. 1998;279:91–5. doi: 10.1126/science.279.5347.91. [DOI] [PubMed] [Google Scholar]
- Brazier MA. Studies of the EEG activity of limbic structures in man. Electroencephalogr Clin Neurophysiol. 1968;25:309–18. doi: 10.1016/0013-4694(68)90171-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer J, Freud S. London: Vintage; 1895. Studies of hysteria. Vol. 2, Standard edn. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bulkeley K. Seeking patterns in dream content: A systematic approach to word searches. Conscious Cogn. 2009;18:905–16. doi: 10.1016/j.concog.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Burgess AP, Gruzelier JH. Short duration power changes in the EEG during recognition memory for words and faces. Psychophysiology. 2000;37:596–606. [PubMed] [Google Scholar]
- Busch AK, Johnson WC. L.S.D. 25 as an aid in psychotherapy; preliminary report of a new drug. Dis Nerv Syst. 1950;11:241–3. [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. [Review] Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Callaway JC. A proposed mechanism for the visions of dream sleep. Med Hypotheses. 1988;26:119–24. doi: 10.1016/0306-9877(88)90064-3. [DOI] [PubMed] [Google Scholar]
- Cañive JM, Lewine JD, Edgar JC, Davis JT, Torres F, Roberts B, et al. Magnetoencephalographic assessment of spontaneous brain activity in schizophrenia. Psychopharmacol Bull. 1996;32:741–50. [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–8. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86:368–80. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Cattell JP. Use of drugs in psychodynamic investigations. In: Hoch PH, Zubin J, editors. Experimental psychopathology. New York: Grune and Stratton; 1957. pp. 218–35. [Google Scholar]
- Chapman LF, Walter RD, Adey WR, Crandall PH, Rand RW, Brazier MAB, et al. Altered electrical activity of human hippocampus and amygdala induced by LSD. Physiologist. 1962;5:118. [Google Scholar]
- Chapman J. The early symptoms of schizophrenia. Br J Psychiatry. 1966;112:225–51. doi: 10.1192/bjp.112.484.225. [DOI] [PubMed] [Google Scholar]
- Chen CC, Henson RN, Stephan KE, Kilner JM, Friston KJ. Forward and backward connections in the brain: a DCM study of functional asymmetries. Neuroimage. 2009;45:453–62. doi: 10.1016/j.neuroimage.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132(Pt 1):225–38. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. The beyond within – the LSD story. New York: Atheneum; 1964. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Frith CD, Fletcher PC. From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology (Berl) 2009;206:515–30. doi: 10.1007/s00213-009-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J, Dunne F. Subjective experience of schizophrenia. Schizophr Bull. 1989;15:217–31. doi: 10.1093/schbul/15.2.217. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dayan P, Hinton GE, Neal RM. The Helmholtz machine. Neural Computation. 1995;7:889–904. doi: 10.1162/neco.1995.7.5.889. [DOI] [PubMed] [Google Scholar]
- Delgado JR, Hamlin H, Higgins JW, Mahl GF. Behavioral changes during intracerebral electrical stimulation. AMA Arch Neurol Psychiatry. 1956;76:399–419. doi: 10.1001/archneurpsyc.1956.02330280057006. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL, Deckersbach T, Marci C, Loh R, Shin LM, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105:6173–8. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner B. Observations on possible order within the unconscious. In: Bradley PB, Deniker P, Radouco-Thomas C, editors. Neuro-Psychopharmacology. Proc. 1st International Congress for Neuro-Psychopharmacology. London: Elsevier; 1959. pp. 439–41. [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–8. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Epstein AW, Ervin F. Psychodynamic significance of seizure content in psychomotor epilepsy. Psychosom Med. 1956;18:43–55. doi: 10.1097/00006842-195601000-00003. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Rayport M. Id, ego, and temporal lobe revisited. Int Rev Neurobiol. 2006;76:21–31. doi: 10.1016/S0074-7742(06)76002-0. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Ongür D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–70. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Fischman LG. Dreams, hallucinogenic drug states, and schizophrenia: a psychological and biological comparison. Schizophr Bull. 1983;9:73–94. doi: 10.1093/schbul/9.1.73. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Fonagy P. Psychoanalysis today. World Psychiatry. 2003;2:73–80. [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anti-correlated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104:15531–6. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Freemon FR, Walter RD. Electrical activity of human limbic system during sleep. Compr Psychiatry. 1970;11:544–51. doi: 10.1016/0010-440x(70)90017-9. [DOI] [PubMed] [Google Scholar]
- Freud S. Papers on hypnotism and suggestion. Standard edn. Vol. 1. London: Vintage; pp. 1888–92. [Google Scholar]
- Freud S. Project for a scientific psychology. Standard edn. Vol. 1. London: Vintage; 1895. [Google Scholar]
- Freud S. The interpretation of dreams. London: Penguin; 1900. [Google Scholar]
- Freud S. Three essays on the theory of sexuality. Standard edn. Vol. 7. London: Vintage; 1905. [Google Scholar]
- Freud S. A case of obsessional neurosis. Standard edn. Vol. 10. London: Vintage; 1909. [Google Scholar]
- Freud S. On narcissism. Standard edn. Vol. 14. London: Vintage; 1914. [Google Scholar]
- Freud S. Instincts and their vicissitudes. Standard edn. Vol. 14. London: Vintage; 1915a. [Google Scholar]
- Freud S. The unconscious. Standard edn. Vol. 14. London: Vintage; 1915b. [Google Scholar]
- Freud S. The metapsychology of dreams. Standard edn. Vol. 14. London: Vintage; 1917a. [Google Scholar]
- Freud S. Mourning and melancholia. Standard edn. Vol. 14. London: Vintage; 1917b. [Google Scholar]
- Freud S. A child is being beaten: a contribution to the study of the origin of sexual perversions. Standard edn. Vol. 17. London: Vintage; 1919. [Google Scholar]
- Freud S. Beyond the pleasure principle. Standard edn. Vol. 18. London: Vintage; 1920. [Google Scholar]
- Freud S. Group psychology. Standard edn. Vol. 18. London: Vintage; 1921. [Google Scholar]
- Freud S. The ego and the id. Standard edn. Vol. 19. London: Vintage; 1923. [Google Scholar]
- Freud S. The economic problem of masochism. Standard edn. Vol. 19. London: Vintage; 1924. [Google Scholar]
- Freud S. Negation. Standard edn. Vol. 19. London: Vintage; 1925a. [Google Scholar]
- Freud S. Inhibitions symptoms and anxiety. Standard edn. Vol. 20. London: Vintage; 1926. [Google Scholar]
- Freud S. New introductory lectures on psychoanalysis. Standard edn. Vol. 22. London: Vintage; 1933. [Google Scholar]
- Freud S. Moses and monotheism. Standard edn. Vol. 23. London: Vintage; 1939. [Google Scholar]
- Freud S. An outline of psychoanalysis. Standard edn. Vol. 23. London: Vintage; 1940. [Google Scholar]
- Friston KJ, Büchel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci USA. 2000;97:7591–6. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Learning and inference in the brain. Neural Netw. 2003;16:1325–52. doi: 10.1016/j.neunet.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005a;360:815–36. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Hallucinations and perceptual inference. Behavioral and Brain Sciences [0140-525X] 2005b;28:764–6. [Google Scholar]
- Friston K, Mattout J, Trujillo-Barreto N, Ashburner J, Penny W. Variational free energy and the Laplace approximation. Neuroimage. 2007;34:220–34. doi: 10.1016/j.neuroimage.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Friston K, Stephan KE. Free energy and the brain. Synthese. 2007;159:417–458. doi: 10.1007/s11229-007-9237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a rough guide to the brain? Trends Cogn Sci. 2009;13:293–301. doi: 10.1016/j.tics.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Daunizeau J, Kiebel SJ. Reinforcement learning or active inference? PLoS ONE. 2009;4:e6421. doi: 10.1371/journal.pone.0006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–85. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Giaquinto S. Sleep recordings from limbic structures in man. Confin Neurol. 1973;35:285–303. doi: 10.1159/000102849. [DOI] [PubMed] [Google Scholar]
- Girard P, Bullier J. Visual activity in area V2 during reversible inactivation of area 17 in the macaque monkey. J Neurophysiol. 1989;62:1287–302. doi: 10.1152/jn.1989.62.6.1287. [DOI] [PubMed] [Google Scholar]
- Gouzoulis E, Hermle L, Sass H. Psychedelic experiences at the onset of productive episodes of endogenous psychoses. Nervenarzt. 1994;65:198–201. [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–8. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Gregory RL. Perceptual illusions and brain models. Proc R Soc Lond B. 1968;171:179–96. doi: 10.1098/rspb.1968.0071. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–43. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Psychedelic drugs reconsidered. New York: Basic Books; 1979. [Google Scholar]
- Grof S. Realms of the human unconscious. London: Souvenir Press; 1975. [Google Scholar]
- Guderian S, Düzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15:901–12. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–82. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H, Maurer K. Early detection of schizophrenia: current evidence and future perspectives. World Psychiatry. 2006;5:130–8. [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Babb TL, Crandall PH. Human hippocampal formation EEG desynchronizes during attentiveness and movement. Electroencephalogr Clin Neurophysiol. 1978;44:778–81. doi: 10.1016/0013-4694(78)90212-2. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–62. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BA, Brodtkorb E. Partial epilepsy with “ecstatic” seizures. Epilepsy Behav. 2003;4:667–3. doi: 10.1016/j.yebeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hassin RR, Uleman JS, Bargh JA. The new unconscious. New York: Oxford University Press; 2005. [Google Scholar]
- He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci. 2009;13:302–9. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105:16039–44. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG. Studies in schizophrenia. Cambridge, Massachusetts: Harvard University Press; 1954. [Google Scholar]
- Heath RG, Mickle WA, Monroe RR. Characteristics of recordings from various specific subcortical nuclear masses in the brain of psychotic and non-psychotic patients. Trans Am Neurol Assoc. 1955 –56; 80th Meeting: 17–2. [PubMed] [Google Scholar]
- Heath RG, Mickle WA. Evaluation of seven years experience with depth electrode studies in human patients. In: Ramey ER, O’Doherty DS, editors. Electrical studies of the unanethetized brain. New York: Harper and Brothers; 1960. pp. 214–47. [Google Scholar]
- Heath RG. Common characteristics of epilepsy and schizophrenia: clinical observation and depth electrode studies. Epilepsy Behav. 1961;6:633–45. doi: 10.1016/j.yebeh.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Heath RG. Role of pleasure in behaviour. New York: Hoeber; 1964. [Google Scholar]
- Heath RG, Walker CF. Correlation of deep and surface electroencephalograms with psychosis and hallucinations in schizophrenics: a report of two cases. Biol Psychiatry. 1985;20:669–74. doi: 10.1016/0006-3223(85)90102-7. [DOI] [PubMed] [Google Scholar]
- Helmholtz Hvon. Concerning the perceptions in general. In: Treatise on physiological optics. (3rd edn) 1866;III (translated by J. P. C. Southall 1925 Opt. Soc. Am. Section 26, reprinted New York: Dover, 1962) [Google Scholar]
- Herrmann CS. Gamma activity as a functional correlate of cognition. special issue. Int J Psychophysiol. 2000;38:vii–viii. [PubMed] [Google Scholar]
- Hobson JA. The dream drugstore: chemically altered states of consciousness. Cambridge, Massachusetts: MIT Press; 2001. [Google Scholar]
- Hobson JA. 13 Dreams freud never had. New York: Pi Press; 2005. [Google Scholar]
- Hölscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17:6470–7. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–25. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AM, et al. Amygdalofrontal Functional Disconnectivity and Aggression in Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp012. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MJ, Adams JE, Rutkin BB. Visual imagery on brain stimulation. Arch Gen Psychiatry. 1968;19:469–86. doi: 10.1001/archpsyc.1968.01740100085013. [DOI] [PubMed] [Google Scholar]
- Hughlings-Jackson J. Lectures on Epilespy. Br Med J. 1879;1:141–3. doi: 10.1136/bmj.1.944.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh K, Meador KJ, Lee GP, Loring DW, Murro AM, King DW, et al. Human hippocampal EEG: effects of behavioral activation. Neurology. 1990;40:1177–81. doi: 10.1212/wnl.40.8.1177. [DOI] [PubMed] [Google Scholar]
- Hupé JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature. 1998;394:784–7. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- Huxley A. The doors of perception. New York: Harper and Row; 1954. [Google Scholar]
- Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119(Pt 2):363–75. doi: 10.1093/brain/119.2.363. [DOI] [PubMed] [Google Scholar]
- Jeanmonod D, Schulman J, Ramirez R, Cancro R, Lanz M, Morel A, et al. Neuropsychiatric thalamocortical dysrhythmia: surgical implications. Neurosurg Clin N Am. 2003;14:251–65. doi: 10.1016/s1042-3680(02)00116-x. [DOI] [PubMed] [Google Scholar]
- Jehee JF, Ballard DH. Predictive feedback can account for biphasic responses in the lateral geniculate nucleus. PLoS Comput Biol. 2009;5:e1000373. doi: 10.1371/journal.pcbi.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson M, Valli K, Revonsuo A, Wedlund JE. Content analysis of subjective experiences in partial epileptic seizures. Epilepsy Behav. 2008;12:170–82. doi: 10.1016/j.yebeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–20. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Paradoxical sleep – a study of its nature and mechanisms. Prog Brain Res. 1965;18:20–6. doi: 10.1016/s0079-6123(08)63582-7. [DOI] [PubMed] [Google Scholar]
- Jung CG. Über die Psychologie der Dementia Praecox. Halle: Marhold; 1907. [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–44. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–39. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. Biology and the future of psychoanalysis: a new intellectual framework for psychiatry revisited. Am J Psychiatry. 1999;156:505–24. doi: 10.1176/ajp.156.4.505. [DOI] [PubMed] [Google Scholar]
- Kaplan-Solms K, Solms M. Clinical studies in neuro-psychoanalysis. New York: Other Press; 2001. [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–57. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, Daunizeau J, Friston KJ. A hierarchy of time-scales and the brain. PLoS Comput Biol. 2008;4:e1000209. doi: 10.1371/journal.pcbi.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Büchel C, Zeki S, Frackowiak RS. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc Biol Sci. 1998;265:2427–33. doi: 10.1098/rspb.1998.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill DC, Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27:712–9. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmäki L, Koivisto M, Saarela C, Häggqvist A, Laine M, et al. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000;111:2071–8. doi: 10.1016/s1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Kubie LS. Some implications for psychoanalysis of modern concepts of the organization of the brain. Psychoanal Q. 1952;22:21–68. [PubMed] [Google Scholar]
- Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, et al. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology. 2006;31:1682–9. doi: 10.1038/sj.npp.1300944. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–11. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489–9. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse H, Heath RG, Mickle WA, Monroe RR, Miller WH. Rhinencephalic activity during thought. J Nerv Ment Dis. 1955;122:433–40. doi: 10.1097/00005053-195511000-00003. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–8. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Redish AD. Prediction, sequences and the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2009;364:1193–201. doi: 10.1098/rstb.2008.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. [Review] Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–9. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. [Review] J Neurophysiol. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Macdonald N. Living with schizophrenia. Can Med Assoc J. 1960;82:218–21. [PMC free article] [PubMed] [Google Scholar]
- Mahl GF, Rothenberg A, Delgado JM, Hamlin H. Psychological responses in the human to Intracerebral electrical stimulation. Psychosom Med. 1964;26:337–68. doi: 10.1097/00006842-196407000-00005. [DOI] [PubMed] [Google Scholar]
- Mann C, Simmons J, Wilson C, Engel J, Bragin A. EEG in human hippocampus, amygdala and entorhinal cortex during REM and NREM sleep. Sleep Res. 1997;26:27. [Google Scholar]
- Maquet P, Péters J, Aerts J, Delfiore G, Degueldre C, Luxen A, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–6. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Martin JA. L.S.D. (lysergic acid diethylamine) treatment of chronic psychoneurotic patients under day-hospital conditions. International Journal of Social Psychiatry. 1957;3:188–95. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters R, Houston J. The varieties of psychedelic experience. New York: Park Street Press; 2000. [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–30. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Thompson JL, Loring DW, Murro AM, King DW, Gallagher BB, et al. Behavioral state-specific changes in human hippocampal theta activity. Neurology. 1991;41:869–72. doi: 10.1212/wnl.41.6.869. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Moiseeva NI, Aleksanyan ZA. Activity of neuronal populations of human subcortical structures during sleep. Electroencephalogr Clin Neurophysiol. 1976;41:467–75. doi: 10.1016/0013-4694(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Møller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26:217–32. doi: 10.1093/oxfordjournals.schbul.a033442. [DOI] [PubMed] [Google Scholar]
- Monroe RR, Heath RG, Mickle WA, Llewellyn RC. Correlation of rhinencephalic electrograms with behavior; a study on humans under the influence of LSD and mescaline. Electroencephalogr Clin Neurophysiol. 1957;9:623–42. doi: 10.1016/0013-4694(57)90084-6. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–82. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Mumford D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol Cybern. 1992;66:241–51. doi: 10.1007/BF00198477. [DOI] [PubMed] [Google Scholar]
- Murray SO, Kersten D, Olshausen BA, Schrater P, Woods DL. Shape perception reduces activity in human primary visual cortex. Proc Natl Acad Sci USA. 2002;99:15164–9. doi: 10.1073/pnas.192579399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio JN, Roffwarg HP, Kaufman E. Alterations in the nocturnal sleep cycle resulting from LSD. Electroencephalogr Clin Neurophysiol. 1966;21:313–24. doi: 10.1016/0013-4694(66)90037-x. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronised gamma oscillations. Science. 2005;309:948–51. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96:5268–73. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–85. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11:1100–8. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel V, Rotarska-Jagiela A, van de Ven VG, Haenschel C, Maurer K, Linden DE. Visual hallucinations in schizophrenia investigated with functional magnetic resonance imaging. Psychiatry Res. 2007;156:269–73. doi: 10.1016/j.pscychresns.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–56. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Ostow M. Psychic function of temporal lobe as inferred from seizure phenomena. AMA Arch Neurol Psychiatry. 1957;77:79–85. doi: 10.1001/archneurpsyc.1957.02330310089015. [DOI] [PubMed] [Google Scholar]
- Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44:121–34. doi: 10.1053/comp.2003.50017. [DOI] [PubMed] [Google Scholar]
- Penfield W, Jasper S. Epilepsy and the functional anatomy of the human brain. New York: Little, Brown and Co; 1954. [Google Scholar]
- Penfield W, Perot P. The brain’s record of auditory and visual experience. A final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolak G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol. 1962;42:202–1. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Graffman J. Neural correlates of imaginal aggressive behaviour assessed by positron emission tomography in healthy subjects. Am J Psychiatry. 2000;157:1772–1781. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–93. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–34. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–8. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Reinders AA, Nijenhuis ER, Paans AM, Korf J, Willemsen AT, den Boer JA. One brain, two selves. Neuroimage. 2003;20:2119–25. doi: 10.1016/j.neuroimage.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Reinders AA, Nijenhuis ER, Quak J, Korf J, Haaksma J, Paans AM, et al. Psychobiological characteristics of dissociative identity disorder: a symptom provocation study. Biol Psychiatry. 2006;60:730–40. doi: 10.1016/j.biopsych.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Revonsuo A. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Cambridge: Cambridge University Press; 2003. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–36. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2009;31:173–84. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin EA, Mukder DW, Faucett RL, Bickford RG. Psychologic factors in convulsive disorders of focal origin. AMA Arch Neurol Psychiatry. 1955;74:365–74. doi: 10.1001/archneurpsyc.1955.02330160015004. [DOI] [PubMed] [Google Scholar]
- Rodin E, Luby E. Effects of LSD-25 on the EEG and photic evoked responses. Arch Gen Psychiatry. 1966;14:435–41. doi: 10.1001/archpsyc.1966.01730100099013. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–37. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–64. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–9. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Schiller PH. Effect of cooling area 18 on striate cortex cells in the squirrel monkey. J Neurophysiol. 1982;48:38–48. doi: 10.1152/jn.1982.48.1.38. [DOI] [PubMed] [Google Scholar]
- Sandison RA, Spencer AM, Whitelaw JD. The therapeutic value of lysergic acid diethylamide in mental illness. J Ment Sci. 1954;100:491–507. doi: 10.1192/bjp.100.419.491. [DOI] [PubMed] [Google Scholar]
- Scarone S, Manzone ML, Gambini O, Kantzas I, Limosani I, D'Agostino A, et al. The dream as a model for psychosis: an experimental approach using bizarreness as a cognitive marker. Schizophr Bull. 2008;34:515–22. doi: 10.1093/schbul/sbm116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schwarz BE, Sem-Jacobsen CW, Petersen MC. Effects of mescaline, LSD-25, and adrenochrome on depth electrograms in man. AMA Arch Neurol Psychiatry. 1956;75:579–87. doi: 10.1001/archneurpsyc.1956.02330240017002. [DOI] [PubMed] [Google Scholar]
- Sechehaye M. Autobiography of a schizophrenic girl. New York: Grune and Stratton; 1951. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sem-Jacobsen CW, Petersen MC, Lazarte JA, Dodge HW, Jr, Holman CB. Intracerebral electrographic recordings from psychotic patients during hallucinations and agitation. Am J Psychiatry. 1955;112:278–88. doi: 10.1176/ajp.112.4.278. [DOI] [PubMed] [Google Scholar]
- Sem-Jacobsen CW, Petersen MC, Dodhe HW, Jr, Lynge HN, Lazarte JA, Holman CB. Intracerebral electrographic study of 93 psychotic patients. Acta Psychiatr Neurol Scand Suppl. 1956;106:222–6. doi: 10.1111/j.1600-0447.1956.tb04748.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood SL. Electrographic depth recordings from the brains of psychotics. Ann NY Acad Sci. 1962;96:375–85. doi: 10.1111/j.1749-6632.1962.tb50130.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–6. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d'Avossa G, et al. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–97. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251:432–5. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–86. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Singer W. Distributed processing and temporal codes in neuronal networks. Cogn Neurodyn. 2009;3:189–96. doi: 10.1007/s11571-009-9087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–97. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E, Beard AW. The schizophrenia-like psychoses of epilepsy, V: discussion and conclusions. J Neuropsychiatry Clin Neurosci. 1963;7:372–8. doi: 10.1176/jnp.7.3.372. [DOI] [PubMed] [Google Scholar]
- Solms M. Oral presentation at the 10th Annual International Congress for Neuropsychoanalysis. Paris, 2009. [Google Scholar]
- Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. In Pace-Schott EF, Solms M, Blagrove M, Harnad S, editors. Sleep and dreaming. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Solms M, Turnbull O. The brain and the inner world. London: Karnac; 2002. [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Mark VH, Erwin F, Pacheco P, Suematsu K. Deep temporal stimulation in man. Long latency, long lasting psychological changes. Arch Neurol. 1969;21:157–69. doi: 10.1001/archneur.1969.00480140057006. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Lochery M. Temporal lobe epilepsy: origin and significance of simple and complex auras. J Neurol Neurosurg Psychiatry. 1987;50:673–81. doi: 10.1136/jnnp.50.6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torda C. Contribution to serotonin theory of dreaming (LSD infusion) N Y State J Med. 1968;68:1135–8. [PubMed] [Google Scholar]
- Toyoda J. The effects of chlorpromazine and imipramine on the human nocturnal sleep cycle. Folia Psychiatrica et Neurologica Japonica. 1964;18:198–221. [Google Scholar]
- van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28:10844–51. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–41. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–72. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. [Review] Int J Psychophysiol. 2000;38:301–13. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb Cortex. 1999;9:137–50. doi: 10.1093/cercor/9.2.137. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, Vollenweider FX. Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci Lett. 2008;435:51–5. doi: 10.1016/j.neulet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Webster R. Why freud was wrong. London: Harper Collins; 1995. [Google Scholar]
- Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Auer DP, Pollmächer T, et al. Functional microstates within human REM sleep: first evidence from fMRI of a thalamocortical network specific for phasic REM periods. Eur J Neurosci. 2007;25:863–71. doi: 10.1111/j.1460-9568.2007.05314.x. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–3. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–64. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Wu CW, Gu H, Lu H, Stein EA, Chen JH, Yang Y. Mapping functional connectivity based on synchronized CMRO fluctuations during the resting state. Neuroimage. 2009;45:694–701. doi: 10.1016/j.neuroimage.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Rayport M, Farison JB, Dennis MJ, Choi YS. Computer analysis of human depth EEG in different sleep stages. Biomed Sci Instrum. 1997;33:184–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.