Abstract
During neuronal activity astrocytes function to remove extracellular increases in potassium, which are largely mediated by the inwardly-rectifying potassium channel Kir4.1, and to take up excess glutamate via glutamate transporter 1, a glial-specific glutamate transporter. Here we demonstrate that expression of both of these proteins is reduced by nearly 80% following a crush spinal cord injury in adult male rats, 7 days post-injury. This loss extended to spinal segments several millimetres rostral and caudal to the lesion epicentre, and persisted at 4 weeks post-injury. Importantly, we demonstrate that loss of these two proteins is not a direct result of astrocyte loss, as immunohistochemistry at 7 days and western blots at 4 weeks demonstrate a marked up-regulation in glial fibrillary acidic protein expression. Kir4.1 and glutamate transporter 1 expression were partially rescued by post-spinal cord injury administration of physiological levels of 17β-oestradiol (0.08 mg/kg/day) in vivo. Utilizing an in vitro culture system we demonstrate that 17β-oestradiol treatment (50 nM) is sufficient to increase glutamate transporter 1 protein expression in spinal cord astrocytes. This increase in glutamate transporter 1 protein expression was reversed and Kir4.1 expression reduced in the presence of an oestrogen receptor antagonist, Fulvestrant 182 780 suggesting a direct translational regulation of Kir4.1 and glutamate transporter 1 via genomic oestrogen receptors. Using whole-cell patch-clamp recordings in cultured spinal cord astrocytes, we show that changes in protein expression following oestrogen application led to functional changes in Kir4.1 mediated currents. These findings suggest that the neuroprotective benefits previously seen with 17β-oestradiol after spinal cord injury may be in part due to increased Kir4.1 and glutamate transporter 1 expression in astrocytes leading to improved potassium and glutamate homeostasis.
Keywords: inward rectifier potassium channel, astrocyte glutamate transporter, spinal cord injury, oestrogen, potassium buffering
Introduction
Astrocytes display a high resting K+ conductance that facilitates the uptake and buffering of neuronally released K+, and a very negative resting membrane potential which provides the energetic force for glutamate uptake. Both characteristics rely on the activity of inwardly rectifying Kir4.1 channels, the predominant potassium channel expressed by astrocytes throughout the CNS (Butt and Kalsi, 2006; Olsen and Sontheimer, 2008). Reduction of Kir4.1 using small hairpin RNA in cultured astrocytes (Olsen et al., 2006; Kucheryavykh et al., 2007) or transgenic elimination of the Kir4.1 gene (Kofuji et al., 2000; Neusch et al., 2001, 2006; Djukic et al., 2007) results in depolarized astrocytes that lack inwardly rectifying currents. These astrocytes are unable to buffer potassium and are deficient in glutamate uptake (Djukic et al., 2007; Kucheryavykh et al., 2007). Kir4.1 knockout animals present with seizures, ataxia and premature death (P10–P20) (Neusch et al., 2001; Djukic et al., 2007). Interestingly, the most severe pathology is observed in the spinal cord which becomes hypo-myelinated and shows severe spongiform vacuolization, axonal swelling and degeneration (Neusch et al., 2001).
Recent studies have demonstrated a loss of astrocytic Kir channel function in neurological diseases including temporal lobe epilepsy (Bordey and Sontheimer, 1998; Hinterkeuser et al., 2000), amyotrophic lateral sclerosis (Kaiser et al., 2006), retinal degeneration (Pannicke et al., 2005) and malignant gliomas (Olsen and Sontheimer, 2004). It was shown in spinal cord that astrocytic Kir4.1 channels prevent process swelling and hypertrophy (Dibaj et al., 2007). Surprisingly little is known concerning changes in Kir4.1 after acute spinal cord injury (SCI). With ∼250 000 people living with SCI, management presents a tremendous health care challenge in the United States, and despite extensive research, effective treatments for SCI are lacking (for review see Sribnick et al., 2005). Recent research suggests that 17β-oestradiol decreases lesion volume and attenuates apoptotic cell death following SCI (Sribnick et al., 2005), and pre-injury treatment with physiological levels of 17β-oestradiol to ovariectomized rats improved hind-limb locomotion (Chaovipoch et al., 2006) and significantly increased white matter sparing. Although the cellular and molecular targets of 17β-oestradiol are unclear, astrocytes may be important targets for oestrogen receptor signalling (for review, see Dhandapani and Brann, 2007). A recent study suggests improvements in hind-limb locomotion in adult male rats treated post-injury with 17β-oestradiol is due to early cytokine release and astrocyte activation preventing secondary damage and spreading during the first 7 days post-injury (Ritz and Hausmann, 2008). Additionally, 17β-oestradiol treatment has been shown to reduce reactive gliosis in vivo (Martinez and de Lacalle, 2007) and cause increases in glutamate aspartate transporter and glutamate transporter 1 (GLT-1) expression in vitro (Pawlak et al., 2005). Even at picomolar to low nanomolar concentrations 17β-oestradiol can modulate the activity and expression of K+ channels (Farkas et al., 2007).
These findings prompted us to examine whether astrocytic expression of Kir4.1 and GLT-1 may be altered after spinal cord crush/compression injury in vivo and whether oestrogen treatment could rescue their expression. Our findings suggest significant and sustained decreases in expression and function of both Kir4.1 and GLT-1 which spread several millimetres from the epicentre of the lesion. Moreover, we demonstrate a novel therapeutic benefit of 17β-oestradiol. At physiologically relevant doses, 17β-oestradiol partially rescued expression of Kir4.1 and GLT-1; effects that we show to be mediated by genomic oestrogen receptor signalling in astrocytes.
Materials and methods
All surgical protocols were in accordance with the National Institutes of Health guidelines and were carried out with approval from the Animal Care and Use Committee of the University of Alabama at Birmingham. Crush injuries to the mid-thoracic region were performed on adult male Sprague–Dawley rats (3-months-old, 225–275 g) as previously described (Chaovipoch et al., 2006) with slight modifications. Briefly, rats were anaesthetized with a ketamine–xylazine mixture (100/10 mg/kg, i.p.). A heating pad equipped with a temperature feedback control system (CMA Microdyalysis, Stockholm Sweden) and an anal temperature probe was used to maintain body temperature. The skin and muscle were then incised to expose the vertebral column. A laminectomy was performed in the mid-thoracic region (T8–T9). Micro-dissector forceps (0.6 mm wide smooth tip; RS-5096, Roboz Instruments) with a detent were carefully placed by hand between the dura and laminae. The spinal cord was extradurally compressed for 3 s. The forceps laterally compress the spinal cord to a blade separation of 0.5 mm, as measured with micro-callipers. Following compression, haemostasis was secured. The incised erector spinae musculature and skin were then closed in layers with chromic absorbable surgical suture (3/0). For those animals receiving oestrogen treatment, a pellet of 17β-oestradiol designed to provide sustained release over 21 days (0.5 mg pellet, released over 21 days in a 300 g rat = 0.08 mg/kg/day, Innovative Research of America) was implanted subcutaneously 30 min after the SCI with an 10 g trochar. After SCI, rats received Ringer’s solution for fluid replacement and hydration, carprofen (Rimadyl, 5 mg/kg, s.c.) for pain, and enrofloxacin (Baytril, 1 mg/kg, s.c) to curb infection for 14 days post-SCI. The bladders of paraplegic animals were emptied by gentle abdominal compression three times per day. Animals had access to food and water ad libitum.
Western blot analysis
Vertebrae from the lower thoracic to cervical region of the spinal cord were removed, the rootlets cut and the long section of cord removed while perfusing ice-cold phosphate buffered saline over the tissue. A 3 mm section including the lesioned area (1 mm) and 1 mm in either direction from the epicentre of the lesion was collected. We also collected tissue that extended an additional 3 mm both rostral and caudal from the initial section of tissue, for a combined region of 9 mm (Fig. 1). Tissue was placed in ice cold homogenization buffer (100 mM Tris, pH 7.5, 1% sodium dodecyl sulphate) supplemented with protease inhibitors and phosphatase inhibitors (Sigma). The tissue was mechanically homogenized and then sonicated for 10 s. For cultured spinal cord astrocytes, cells were rinsed twice with ice cold phosphate buffered saline and then collected in a small volume of homogenization buffer supplemented with protease inhibitors. Tissue and cell homogenates were centrifuged for 5 min at 12 000g at 4°C. Protein quantification was performed on the supernatant using a DC protein assay kit from Bio-Rad (Hercules, CA). Protein was heated to 60°C for 15 min in an equal volume of 2× sample buffer (100 mM Tris, pH 6.8, 10% sodium dodecyl sulphate in Laemmli- sodium dodecylsulphate, 600 mM β-mercaptoethanol). Equal amounts of protein were loaded into a 4–20% gradient pre-cast sodium dodecyl sulphate gel (Bio-Rad). Gels were transferred at 200 mA constant for 2 h at room temperature and membranes were blocked in blocking buffer (10% dried milk in Tris–buffered saline Tween-20). The Kir4.1 rabbit antibody was obtained from Alomone (1:750, Jerusalem, Israel). Blots were incubated for 90 min at room temperature. The membrane was then rinsed 3× for 15 min and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody for 60 min. After three 10 min washes membranes were developed with enhanced chemiluminesence (Amersham, Arlington Heights, IL) using an Image Station 4000 MM (Kodak). The blots were then stripped and re-probed with GLT-1 guinea pig (1:10 000), GFAP mouse (1:5000; Millipore) and glyceraldehyde 3-phosphate dehydrogenase mouse (Abcam 1:1000) or actin rabbit (Sigma) antibodies for a loading control. Protein expression was quantitated using the Kodak Image Station normalizing total Kir4.1, GLT-1 or glial fibrillary acidic protein (GFAP) protein to glyceraldehyde 3-phosphate dehydrogenase or actin expression in the same lane. Relative amounts of protein are reported in arbitrary units. The mouse oestrogen receptor antibody (DAKO) and rabbit oestrogen receptor beta (Calbiochem) were diluted according to manufacturers’ instructions.
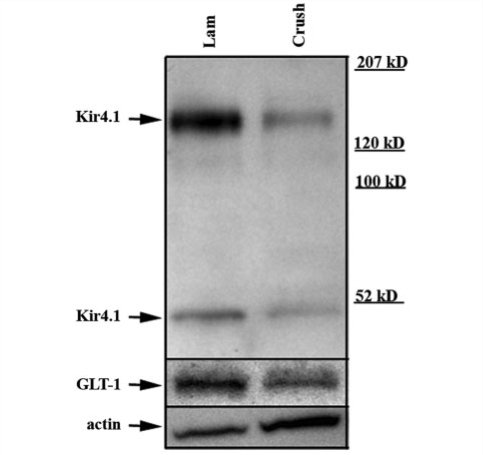
Figure 1.
A representative Western blot. Western blotting demonstrates significant decreases in Kir4.1 and GLT-1 seven days post-SCI. Representative western blot obtained from an injured and control animal probed with anti-Kir4.1 demonstrate significant decreases in immunoreactivity of a band at ∼200 kDa that corresponds to a tetramer and a band at ∼50 kDa corresponding to a monomer of Kir4.1. Significant decreases were detected for GLT-1. For a loading control this blot was probed with anti-actin.
Immunohistochemistry
Animals were anaesthetized with a peritoneal injection of ketamine (100 mg/kg) and perfused with a 4% paraformaldehyde solution for 30 min. The cord was removed and stored in 4% paraformaldehyde. After washing in phosphate buffered saline, 50 μM sections within 3 mm of the lesion area or the same region in control animals were cut using a Vibratome (Oxford Instruments). Sections were blocked for 1 h in 10% horse serum and 0.3% Triton-X100 in phosphate buffered saline (BB). Primary antibodies were diluted in BB 1:3 with phosphate buffered saline and incubated with slices overnight. The sections were then washed three times in diluted phosphate buffered saline incubating with fluorescein isothiocyanate or tetramethyl rhodamine iso-thiocyanate-conjugated secondary antibodies obtained from Molecular Probes for 60 min at room temperature. The slices were then washed two times with diluted BB, then incubated with 4′,6-diamidino-2-phenylindole (10–4 mg/ml; Sigma), and finally washed twice with phosphate buffered saline before being mounted onto glass coverslips. Fluorescent images were acquired with a Zeiss Axiovert 200M (München, Germany).
Slice preparation
Animals were decapitated and the spinal cord was removed and placed in ice cold calcium-free artificial cerebral spinal fluid (in mM, NaCl 116, KCl 4.5, MgCl2 0.8, NaHCO3 26.2, glucose 11.1, HEPES 5.0) for 30 s. The cord was then placed in liquid low melt agar at ∼30°C, which was quickly solidified by placing in a –30°C freezer on ice. Sections from ∼3 mm rostral to the lesion to 3 mm cadual to the lesion were cut at 250–300 µm using a Vibratome 3000 (Ted Pella, Inc., Redding CA) in calcium-free artificial cerebral spinal fluid. Before recording, slices were allowed to recover for at least one hour at room temperature in calcium-free artificial cerebral spinal fluid, which was continuously bubbled with 5% CO2/95%O2.
Slice electrophysiology
Whole-cell voltage-clamp recordings were made as described previously (Olsen et al., 2006). After slicing, slices were transferred after the recovery period to a Zeiss Axiskop fluorescence microscope (Zeiss, Thornwood, NY, USA) equipped with Nomarski optics. Signals were acquired using an Axopatch 200B amplifier, controlled by Clampex 9.0 software via a Digidata 1200B interface (Axon Instruments). Signals were filtered at 2 kHz and digitized at 5 kHz. Data acquisition and storage were conducted with the use of pClamp 9.0 (Axon Instruments). Resting membrane potentials were measured directly from the amplifier in I = 0 mode, ∼1 min after whole cell access was obtained. Whole-cell capacitance and series resistances were also measured directly from the amplifier, with the upper limit for series resistance being 12 MΩ and series resistance compensation adjusted to 80% to reduce voltage errors. Slices were continuously perfused with artificial cerebral spinal fluid with the addition of 2.0 mM CaCl2. The standard KCl pipette solution contained (in mM) 145 KCl, 1 MgCl2, 10 ethylene glycol tetraacetic acid, 10 HEPES sodium salt, pH adjusted to 7.3 with Tris–base. CaCl2 (0.2 mM) was added to the pipette solution just before recording, resulting in a free calcium concentration of 1.9 nM. Cells were continuously perfused at room temperature with oxygenated artificial cerebral spinal fluid containing 2 mM CaCl2. Drugs were added directly to these solutions. Unless stated otherwise, all drugs were purchased from Sigma (St. Louis, MO).
Isolation of Kir4.1-mediated currents
We isolated Kir4.1 in slices and cultured astrocytes as previously described (Olsen and Sontheimer, 2007). Briefly, we repeated the same voltage step protocol before and after application of 100 µM Ba2+, which, at this concentration, is a specific inhibitor of Kir channels (Ransom and Sontheimer, 1995). A point-by-point subtraction of the corresponding current traces isolated the Ba2+-sensitive component of the whole cell current designated as the Kir current. This current typically showed a characteristic inactivation at very negative voltage steps as previously reported (Ransom and Sontheimer, 1995).
Cultured spinal cord astrocytes
Spinal cord astrocytes were cultured in phenol red-free media as previously described (Olsen et al., 2006). At 7 days in culture cells were treated with vehicle, 50 nM 17β-oestradiol or 50 nM 17β-oestradiol + 1 µM Fulvestrant or 13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a] phenanthrene-3,17-diol (ICI) 182 780 (a specific oestrogen receptor antagonist) for 24 or 48 h.
Statistical analysis
Current responses to varied voltage steps and ramps were analysed and measured in Clampfit (Molecular Devices), and the resulting raw data were graphed and plotted in Origin 6.0 (MicroCal, Northampton, MA). One-way ANOVAs were performed using Graphpad software (San Diego, CA) and P-values are reported in the text. Current amplitudes are reported as mean ± SE, with n indicating the number of cells sampled. For western blot quantification the relative amount of Kir4.1 protein in each lane was normalized to its loading control and then normalized to the total amount of protein in control lanes. A one-way ANOVA was used to analyse statistical differences in the relative amount of Kir4.1 protein between groups of animals. The data are reported as mean ± SE with n indicating the number of animals per condition.
Results
Kir4.1 and GLT-1 expression is decreased following spinal cord injury in vivo
Kir4.1 mediates the majority of the K+ resting conductance in spinal cord astrocytes and is largely responsible for their negative resting membrane potential (Olsen et al., 2007), which in turn provides the driving force necessary for glutamate uptake by astrocytes (Djukic et al., 2007; Kucheryavykh et al., 2007). Here we hypothesize that SCI in vivo will cause a loss of Kir4.1 similar to that we have previously observed in vitro (MacFarlane and Sontheimer, 1997) and that this loss will induce compromised K+ and glutamate homeostasis. As a first step towards testing this hypothesis, we examined total Kir4.1 protein expression following SCI. We harvested tissue for western blotting from adult male animals, 7 days post-injury. The tissue lysates included the lesion area (1 mm) and extended ∼1 mm in either direction from the lesioned area. A representative example of a control (laminectomy) and tissue obtained from an injured animal are shown in Fig. 1. We have previously demonstrated that the bands at ∼200 and 50 kDa represent Kir4.1, as these bands are absent in the knock-out animals (Olsen et al., 2006) and correspond to a tetramer and monomer of Kir4.1 subunits. When we analysed total Kir4.1 protein at these two molecular weights in five groups of animals (five control/five SCI) and normalized to a loading control, we demonstrate that there is an ∼85% loss of Kir4.1 protein at 7 days post-injury (Fig. 5B). It is important to note that loss of Kir4.1 alone is sufficient to decrease glutamate uptake (Djukic et al., 2007; Kucheryavykh et al., 2007) via depolarization of the resting membrane potential. However, a recent study examining GLT-1 levels following a SCI contusion demonstrated increased levels of GLT-1 that peak at 6 h (Vera-Portocarrero et al., 2002). Therefore, we also assessed levels of GLT-1 protein to determine if the up-regulation immediately following injury persists at 7 days following injury. We stripped and re-probed blots with a GLT-1 antibody. Our data demonstrate a band at ∼70 kDa that is markedly reduced to nearly 90% (n = 5 groups of animals) following SCI (Fig. 5B), suggesting that at 7 days post-SCI there is a significant loss of GLT-1, an integral astrocytic membrane protein.
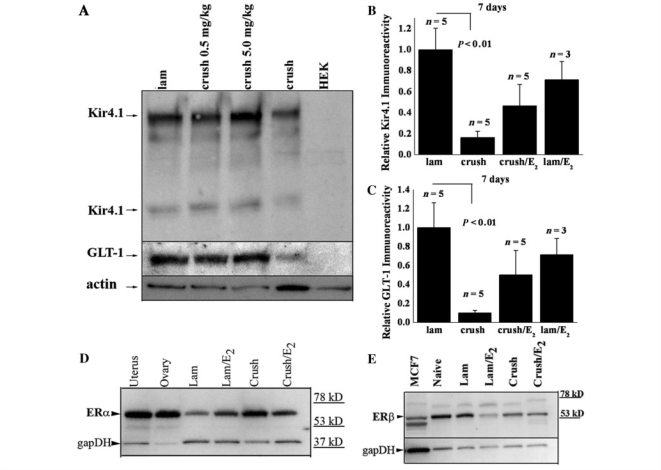
Figure 5.
Kir4.1 and GLT-1 loss is attenuated by 17β-oestradiol treatment. (A) Kir 4.1 and GLT-1 immunoreactivity levels are higher when animals are treated with 17β-oestradiol (E2). In this western blot from lysates obtained 7 days post-SCI, increasing the oestrogen dose led to increased levels of Kir4.1 and GLT-1 when compared to a loading control. (B) Averaged data from five independent groups of animals (3 laminectomy + E2) experiments show Kir 4.1 is reduced nearly 85% following a crush injury and physiological doses (0.5 mg/kg) of time-release 17β-oestradiol increase Kir4.1 in injured animals over 50%. There is no significant difference between laminectomy treated animals and injured animals treated with (0.5 mg/kg) of 17β-oestradiol or laminectomy treated animals treat with 17β-oestradiol. (C) Similar results were observed for GLT-1, approximately an 85% reduction in immunoreactivity following a crush injury and 17β-oestradiol increase GLT-1 in injured animals over 50%. (D, E) The alpha and beta oestrogen receptors are expressed in adult male spinal cord before and after injury at variable levels. Western blots from these animals were probed with antibodies to the oestrogen receptors α and β. Immunoreactivity demonstrates both receptors were present in all lysates. Uterus and ovary tissue from a post-partum rat were used as a positive control for oestrogen receptor-α and MCF7 cells, a breast cancer cell line, were used as a positive control for oestrogen receptor-β.
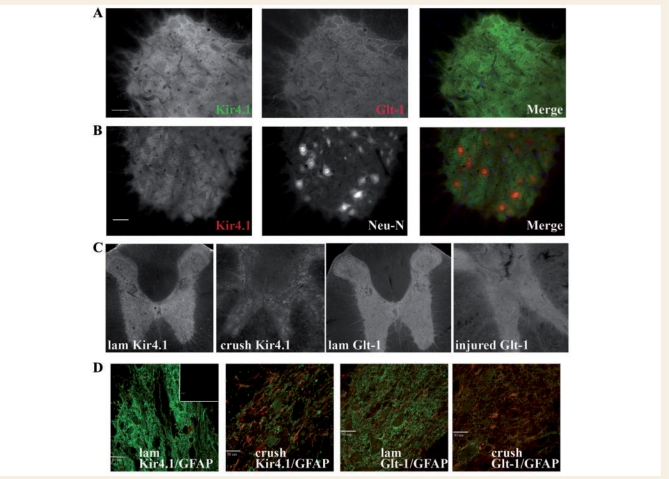
To confirm our western blot findings and to examine the pattern of loss of Kir4.1 and GLT-1 in spinal cord, we examined tissue in control and injured animals using immunohistochemistry and examining tissue under low power. Previous data have shown Kir4.1 to be glia specific with highest expression in grey matter astrocytes (Kaiser et al., 2006; Olsen and Sontheimer 2007), although oligodendrocytes also express Kir4.1 (Neusch et al., 2001). Slices were only compared to one another if they were immuno-stained concurrently and imaged identically. As we have shown previously, Kir4.1 and GLT-1 are most highly expressed in the grey matter, and their staining pattern is remarkably similar as demonstrated (Fig. 2A). Figure 2B demonstrates that Kir4.1 immunoreactivity in the adult male rat is quite distinct from the neuronal cell body marker Neu N as shown in the individual panels and the merged image. Low power images obtained from tissue within 1–2 mm rostral and caudal from the epicentre of the injury demonstrate a dramatic and uniform loss of Kir4.1 and GLT-1 throughout transverse sections of the spinal cord grey matter when compared to control slices from the same region (Fig. 2C). High resolution confocal images (Fig. 2D) from Kir4.1/GFAP and GLT-1/GFAP-labelled sections show diffuse staining covering the cell bodies and processes of astrocytes and the neuropil. Following injury, few neuronal cell bodies remain and the overall staining is attenuated, yet remains diffuse. These images also display an increase in GFAP in injured tissue.
Figure 2.
Immunohistochemistry confirms a decrease in Kir4.1 and GLT-1 in the injured spinal cord. (A) Kir4.1 and GLT-1 demonstrate a similar staining pattern in the adult male spinal cord as demonstrated by the merged image (Kir4.1 = green, GLT-1 = red in merged image, scale bar = 50 µm). (B) Kir4.1 immunoreactivity demarcates neuronal cell bodies which are shown in the middle panel by Neu-N labelling (a neuronal cell body marker) (Kir4.1 = green, Neu-N = red in merged image, scale bar = 50 µm). (C) Low power transverse sections demonstrate widespread loss of both Kir4.1 and GLT-1 in the injured spinal cord. (D) This loss is reiterated in high power confocal images of Kir4.1 (green) and GLT-11 (green) overlayed with GFAP (red) (inset is secondary alone, scale bar = 50 µm). GFAP immunoreactivity is increased in injured tissue.
Kir4.1 and GLT-1 loss following spinal cord injury is chronic and extends longitudinally from the epicentre of the lesion
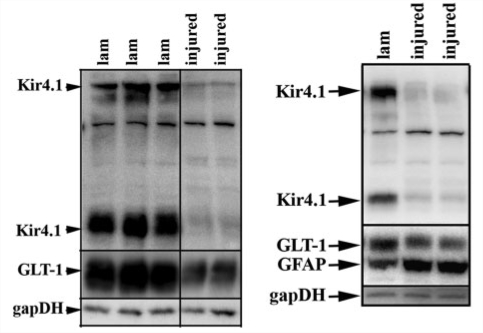
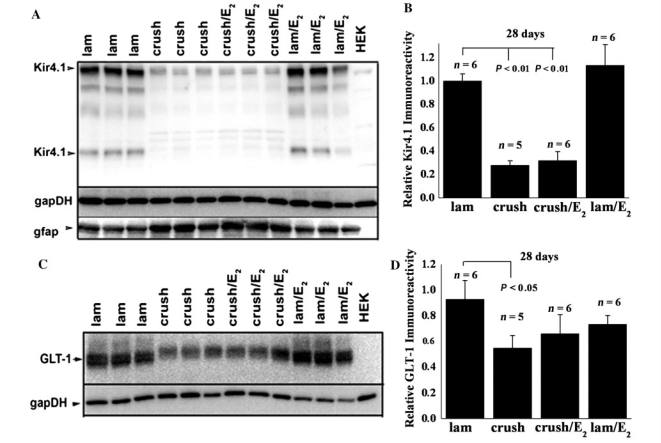
An important and clinically relevant aspect of SCI focuses on long-term changes that occur near the site of injury. To ascertain long term changes of Kir4.1 and GLT-1 we assessed protein levels by western blot 4 weeks post-SCI. We show two western blots at the 28-day time point. Each demonstrates similar losses of Kir4.1 and GLT-1 at 4 weeks post-injury compared to those seen at 7 days (Fig. 3A and B). Importantly Fig. 3B shows a marked up-regulation of GFAP in the two injured samples, demonstrating that the loss of Kir4.1 and GLT-1 is not due to an overall decrease in astrocytes. These results confirm our immunohistochemistry results from the 7-day time point which show higher GFAP immunoreactivity in spinal segments adjacent to the injury epicentre (Fig. 2D). This is not to say that there is not significant cell loss (astrocytes, neurons and oligodendrocytes) at the injury epicentre, however the GFAP up-regulation at spinal segments neighbouring the injury epicentre suggests that these cells have lost expression of Kir4.1 and GLT-1.
Figure 3.
Kir4.1 and GLT-1 remain low 4 weeks post-injury. (A) Western blot with tissue from three control and two injured animals demonstrates at 4 weeks post-SCI there is still a significant loss of GLT-1 and Kir4.1 when compared to glyceraldehyde 3-phosphate dehydrogenase (gapDH) as a loading control. (B) A second western blot demonstrating similar results to those seen in (A), but also demonstrating a marked increase in GFAP, suggesting the loss of Kir4.1 and GLT-1 is not due to a lack of astrocytes in the region we examined.
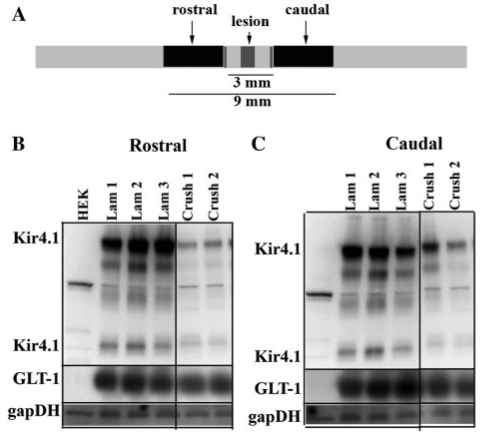
We next sought to determine if these chronic changes in protein expression were confined to the injury epicentre or if they extended longitudinally beyond the lesion. We hypothesized there would be a likely compensatory up-regulation of these two proteins rostral and caudal to the lesion as was demonstrated for astrocytic connexin-43, which increased nearly 3-fold, 10 mm in either direction from the epicentre of the lesion following a severe SCI (Lee et al., 2005). Figure 4A demonstrates the rostral and caudal regions we used for these studies and the distance from the epicentre of the lesion. Surprisingly, our western blot data from 4 weeks post-injury indicated that Kir4.1 and GLT-1 expression were substantially decreased in both the rostral and caudal segments and that these decreases were comparable to those observed at the epicentre at 7 days post-injury (Fig. 4B). Taken together, these data demonstrate a massive and wide-spread, concerted loss of the two proteins presumed to provide the major homeostatic functions of astrocytes, i.e. K+ buffering and glutamate homeostasis.
Figure 4.
Kir4.1 and GLT-1 loss extends longitudinally following SCI. (A) A cartoon depicting the distance rostral and caudal to the lesion used for western blotting. (B) This western blot from several animals 28 days post-SCI indicates Kir4.1 is significantly decreased as far as several millimetres in either direction from the epicentre of the lesion when compared to a loading control (glyceraldehyde 3-phosphate dehydrogenase; gapDH). This blot was subsequently stripped and blotted with anti-GLT-1 which also shows a significant loss that extends from the epicentre of the lesion. Human embryonic kidney (HEK) cells were used as a negative control.
17β-oestradiol treatment partially rescues Kir4.1 and GLT-1 expression
Previous studies have indicated that 17β-oestradiol treatment confers neuroprotection following SCI. This includes several different doses in male and female rats, using different injury and treatment paradigms. Indeed, in the same injury paradigm used in these studies, 17β-oestradiol treatment following injury in male rats reduced apoptosis, increased white matter sparing and increased neuronal survival (Kachadroka et al., 2010). Furthermore, these animals displayed improved hind-limb motor function. Due to the non-specific effects of steroidal hormones, several molecular targets of 17β-oestradiol treatment have been identified in these studies. Because previous work has shown that 17β-oestradiol affects several potassium channels and increases GLT-1 and glutamate aspartate transporter expression, we sought to investigate if these two proteins, fundamental for normal astrocyte function, were affected by 17β-oestradiol treatment. Thus, we hypothesized that post-SCI administration of 17β-oestradiol may reduce secondary damage in part by improving K+ and glutamate homeostasis. To test this hypothesis, we examined Kir4.1 and GLT-1 expression 7 days post-injury near the epicentre of the injury in animals treated with 17β-oestradiol. For those experiments, animals received a pellet of 17β-oestradiol designed to provide constant, sustained release of 17β-oestradiol for up to 21 days. Our data indicate that after 7 days of treatment, Kir4.1 and GLT-1 expression is partially rescued by 17β-oestradiol treatment at two different doses (Fig. 5A). These studies were repeated on four additional groups of animals using the physiological dose of 17β-oestradiol (0.5 mg pellet or 0.08 mg/kg/day), and results are summarized in Figs 5B and C. These data demonstrate a significant difference (P < 0.01) between control and injured animals, which is lost when the animals are treated with oestrogen, suggesting that at physiological 17β-oestradiol doses, Kir4.1 and GLT-1 expression is markedly enhanced over untreated injured animals. Importantly, we probed these same tissues for both oestrogen receptor alpha (Fig. 5D) and beta (Fig. 5E) subunits and demonstrate that both receptors are present in adult male animals. We also examined injured tissue 4 weeks after injury treated with 17β-oestradiol. Representative western blots demonstrate that the increase in Kir4.1 (Fig. 6A) and GLT-1 (Fig. 6C) in injured tissue treated with 17β-oestradiol is lost by the 4-week time point. Mean data for both proteins summarized in Fig. 6B and D from at least five animals per group suggest the increased levels of these two proteins at 7 days after injury is transient and lost by the time of lesion stabilization. Furthermore, GLT-1 appears to show some recovery by the 4-week time point (Fig. 6D) relative to the levels seen at 7 days (Fig. 5C).
Figure 6.
Increased Kir4.1 and GLT-1 expression in 17β-oestradiol-treated injured animals is transient. (A) A representative blot from three groups of animals demonstrates that there is a significant loss of Kir4.1 at 28 days in injured animals that is unchanged following oestrogen treatment. (B) Densitometry normalizing Kir4.1 levels to glyceraldehyde 3-phosphate dehydrogenase from at least five animals per condition demonstrate that Kir4.1 levels are similar in injured animals treated with a placebo to those treated with 0.5 mg/kg of 17β-oestradiol. (C) A representative western blot from the same group of three animals as in A shows GLT-1 levels are similar between injured animals and injured animals treated with 17β-oestradiol. (D) Mean data from five animals per group demonstrate an increase in overall protein by 4 weeks post-injury relative to the 7-day time point and with little difference between injured animals and those injured animals treated with 17β-oestradiol.
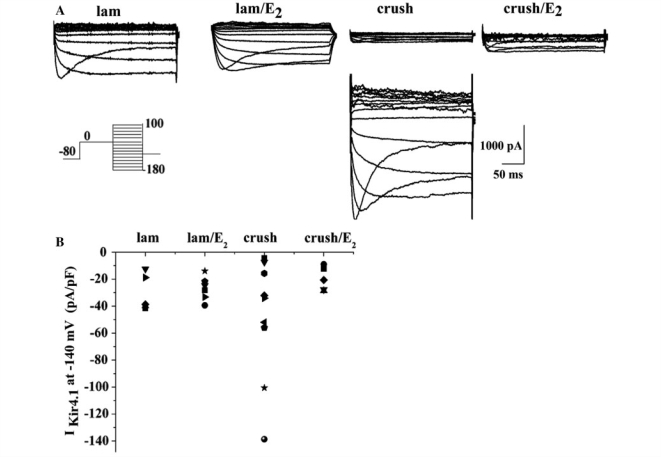
To examine whether these changes in Kir4.1 expression are also reflected in the biophysical properties of astrocytes, we performed whole-cell voltage-clamp recording from control and injured animals treated with 17β-oestradiol. For these experiments we made acute (200–300 µm) sections 1–3 mm in either direction from the injury epicentre from adult male rats, 7 days post-injury. It is important to note that these experiments could not be performed at the epicentre of the lesion because the lesion itself was too fragile to section. The overall success rate of obtaining recordings from astrocytes was quite low throughout this study. Visibility into the tissue was very limited because of the age of the animals and high degree of myelination; therefore, we selected cells in the grey matter based on responses to linear voltage ramps and voltage steps. Three cell types were commonly observed: (i) neurons that displayed spontaneous electrical activity and spiked in response to a linear voltage ramp; (ii) astrocytes that displayed time- and voltage-sensitive currents, and relatively low input resistances (25–300 MΩ); and (iii) a cell type with very high input resistances (1–4 GΩ), depolarized resting membrane potentials (–20 to –40 mV) and small outwardly rectifying currents (150–200 pA). In injured animals, the third cell type was observed with the highest frequency, leading us to the conclusion that this cell type probably represents an immune cell responding to injury. The data in Fig. 7A represent recording from the second cell type often referred to as complex or intermediate rectifying astrocytes. To activate Kir4.1-mediated currents in these cells, we used a specific voltage-step protocol as described previously (Olsen et al., 2007) and isolated these currents using 100 µM Ba2+ (a concentration demonstrated to completely inhibit Kir4.1 mediated currents, see ‘Materials and methods’ section). Representative traces in Fig. 7A show Ba2+-sensitive Kir4.1-mediated currents in control animals; control animals treated with 17β-oestradiol, injured animals, and injured animals treated with oestrogen. From these recordings we determined the Kir current density (expressed in pA/pF) as the Ba2+-sensitive conductance at −140 mV normalized to the whole cell capacitance which correlates with cell size. We successfully recorded a total of 25 cells in which we also obtained the barium subtraction, five in control, nine in injured untreated, five in injured oestrogen treated and six laminectomy treated with oestrogen, from 20 animals. In control animals, Kir current density values (Fig. 7B) fell within a narrow range of –10 to –45 pA/pF. Surprisingly, however, injured animals showed a large degree of scatter in current density values with some cells showing barely recordable currents yet others expressing currents at densities exceeding that of control cells by over 4-fold as shown in Fig. 7A. Importantly, this variability was reduced to below control values in all injured animals treated with 17β-oestradiol treatment (Fig. 7B). These data suggest that cells do not show a homogenous reduction in Kir4.1 after injury as we initially expected. However, given the small number of cells from which we were able to obtain reliable recordings it is difficult to draw strong conclusions from them. In light of the overall reduction in Kir4.1 protein observed by western blot which samples the entire affected tissue, it is plausible that many cells in which Kir4.1 expression had been lost may have escaped our recordings. Importantly, however, the data also suggest that treatment with 17β-oestradiol following injury normalizes Kir current expression both in amplitude and degree of variability i.e. scatter close to that observed in uninjured animals. To examine this question and more specifically to ascertain whether 17β-oestradiol can regulate Kir4.1 channel expression directly, we next turned to a simplified preparation, namely short term primary cultures of spinal cord astrocytes and examined their response to 17β-oestradiol treatment.
Figure 7.
Acute slice recording from adult controls and injured spinal cord astrocytes demonstrates a high degree of variability of Kir4.1 currents in injured animals. (A) Representative Ba2+-sensitive Kir4.1 currents recordings in response to a voltage step protocol (inset) from each group of animals is depicted. In the injured animals, there was a high degree of variability in the amplitude of the Ba2+ subtracted traces; therefore we show several examples for this group of cells. (B) Data from all cells in which we obtained a barium subtraction are depicted in this scatter plot.
17β-oestradiol modulation of Kir4.1 and GLT-1 is dependent on genomic oestrogen receptor signalling
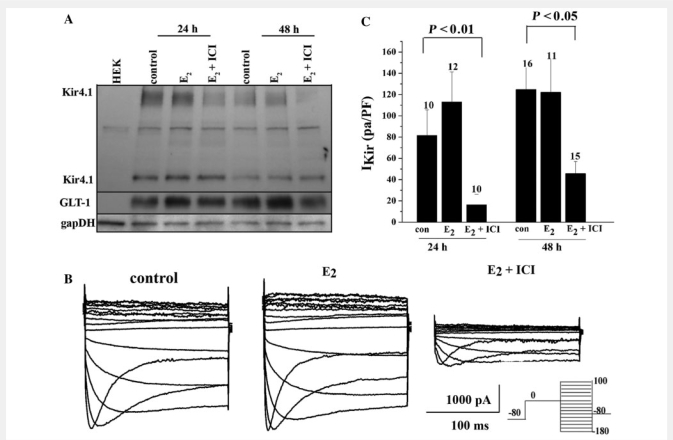
To assess the role of the oestrogen receptor in the modulation of Kir4.1 and GLT-1 by 17β-oestradiol, we utilized spinal cord astrocytes cultures (>95% astrocytes purity) as previously described (Macfarlane and Sontheimer, 1997; Olsen et al., 2006). Turning to an in vitro model permitted a rigorous and manageable means to examine biochemical and functional changes in protein that occur following 17β-oestradiol treatment. Seven days after plating, a time when cultured astrocytes express robust Kir4.1 mediated currents, we exposed them to 50 nM 17β-oestradiol for 24–48 h, or 17β-oestradiol + 1 µM ICI 182 780, an oestrogen receptor antagonist. We examined Kir4.1 and GLT-1 protein expression by western blotting. We demonstrate there is no significant change in Kir4.1 following 17β-oestradiol treatment. However as demonstrated by others, 17β-oestradiol treatment modulates GLT-1 expression. Reductions in both proteins were observed when these astrocytes were treated with 17β-oestradiol + 1 µM ICI 182 780 (Fig. 8A). Similar studies in cultured cortical astrocytes showed GLT-1 expression was increased with 17β-oestradiol treatment and this increase was reversed upon co-application of the antagonist ICI 182 780. These changes correlated with a corresponding change in these cells ability to clear glutamate as described by others (Liang et al., 2002; Pawlak et al., 2005). In order to address functional changes in Kir4.1 expression, we performed whole cell voltage-clamp electrophysiology where the experimenter was blinded to the condition. Representative recordings (Fig. 8B) revealed Kir4.1, Ba2+-sensitive currents in cultured spinal cord astrocytes demonstrated an identical current profile to those seen in acute slices, and were significantly reduced (P < 0.01) by 48 h treatment with 17β-oestradiol (50 nM) + 1 µM ICI 182 780 (1 µM) when compared to vehicle alone. Mean data from >10 cells per group demonstrate similar significant decreases at 24 and 48 h. Interestingly, we did not observe an increase in normalized whole cell Kir4.1 with 17β-oestradiol treatment, suggesting ‘normal’ Kir4.1 channel expression is dependent on genomic oestrogen receptor signalling.
Figure 8.
Kir4.1 and GLT-1 expression in cultured spinal cord astrocytes are modulated via oestrogen receptor signalling. (A) A western blot with spinal cord astrocytes treated for 24 or 48 h with 17β-oestradiol (50 nM) shows a decrease in Kir4.1 expression when treated with 17β-oestradiol (50 nM) + the oestrogen receptor antagonist ICI 182 780 (1 µM) when compared to a loading control. GLT-1 demonstrated an up-regulation in expression levels at 24 and 48 h when treated with 17β-oestradiol and values returned back to control levels when treated with 17β-oestradiol (50 nM) + the oestrogen receptor antagonist ICI 182 780 (1 µM). (B) Representative Ba2+-sensitive Kir4.1 currents recordings in response to a voltage step protocol (shown in inset) demonstrate a significant decrease in current amplitude in cells treated with 17β-oestradiol + the oestrogen receptor antagonist ICI 182 780 for 48 h. (C) Mean data from cells treated for 24 or 48 h with 17β-oestradiol (50 nM) or 17β-oestradiol (50 nM) + the oestrogen receptor antagonist ICI 182 780 (1 µM) demonstrate a significant (P < 0.01 at 24 and P < 0.05 at 48 h) decrease in Kir4.1 barium-sensitive currents at each time point. E2 = 17β-oestradiol.
Discussion
Using a widely accepted in vivo SCI model, this study demonstrates the rapid and sustained loss of two astrocytic proteins believed to be critical in providing neuroprotection in the CNS, the glutamate transporter, GLT-1 and the K+ channel, Kir4.1. These proteins work hand-in-hand to clear the extracellular space of neuronally released K+ and glutamate. While Kir4.1 can function independently, the activity of GLT-1 depends on the negative membrane potential provided by Kir4.1 establishing a sufficient electrochemical gradient for glutamate import. These results would predict a loss of ion and transmitter homeostasis in the injured cord, albeit the compromised extracellular space at the lesion after injury precluded us to examine changes in K+ and glutamate concentrations directly. Instead, we used changes in protein expression as a surrogate marker. In doing so, we were surprised by the spatial extent to which these proteins were lost, extended nearly 10 mm of the spinal cord, and the time course of loss showed essentially no recovery after 4 weeks. These important and unexpected findings are consistent with the irreversible loss of spinal cord function and sustained paralysis typical of patients with SCI. However, this does not imply that loss of astrocytic support is mechanistically involved in the primary injury phase. Instead, we suggest that loss of these astrocytic homeostatic functions may be detrimental to the prolonged secondary injury phase where ion and neurotransmitter homeostasis dysregulation are unopposed by glial support mechanisms that would normally be beneficial.
This is the first study examining Kir4.1 changes in SCI in vivo. However, other studies have demonstrated changes in these proteins in acute and chronic neurological conditions (for review see Olsen and Sontheimer, 2008). Acute CNS injuries, epilepsy and most neurodegenerative diseases demonstrate reactive gliosis, a conspicuous morphological change, characterized by cell body hypertrophy, thickened cell processes and increased GFAP expression (Pekny and Pekna, 2004). Following mechanical SCI, GFAP expression levels have been shown to increase within 1 h and remain elevated chronically (Dusart and Schwab, 1994). In the spinal cord, gliotic regions may provide structural stability to lesioned tissue, however gliosis also forms persistent barriers obstructing axonal re-growth (Profyris et al., 2004; Rolls et al., 2009).
Numerous studies have reported that gliosis alters astrocytic biophysical properties, notably reverting to those seen in immature astrocytes, characterized by depolarized resting membrane potentials, high input resistances and little Kir mediated currents (for review see Olsen and Sontheimer, 2008). These changes can be readily induced in vitro by inflicting a mechanical scar (MacFarlane and Sontheimer, 1997). These biophysical alterations may involve blood-borne signals. Focal blood–brain barrier disruption in vivo or exposure of brain slices to albumin, resulted in down-regulation of Kir4.1 (Ivens et al., 2007), that increased K+ accumulation, neuronal hyperexcitability and epileptiform activity. Because SCI includes grey matter haemorrhage at the lesion centre, blood-born agents may also be responsible for the observed loss of Kir4.1. In spinal cord we would expect see a comparable dysregulation of K+ following the loss of Kir4.1. Additionally, the loss of Kir4.1 in spinal cord astrocytes after injury may markedly alter inflammatory responses as this channel has been shown to prevent glial process swelling under osmotic stress in acute spinal cord slices (Dibaj et al., 2007). Clearly, these studies indicate Kir4.1 plays an important role in spinal cord astrocytes however, Kir4.1 regulation following injury requires further study.
While our experiments showed a massive and sustained loss of Kir4.1 channel protein expression, our electrophysiological recordings paint a more nuanced picture, suggesting that not all astrocytes near the injury lose Kir4.1. We found that in normal and injured slices obtaining recordings was difficult particularly since myelin density obscured cell visibility and morphologic identity. Thus it was unrealistic to record from a reasonable number of animals so we limit our data interpretation and simply suggest that some astrocytic subpopulations lose Kir4.1 while others retain the channel. Furthermore, because we identified the cells we recorded visually and microglia and oligodendrocytes precursor cells exist in the control and injured spinal cord, we can not rule out the possibility that some of the cells included in our study were not astrocytes. However, since the biochemical data indicate a massive loss of Kir4.1, we would argue that most of the injury-affected astrocytes escaped our electrophysiological recordings. Our immunohistochemistry data suggest that the majority of the Kir4.1 protein is located in grey matter astrocytes, however spinal cord oligodendrocytes also express Kir4.1, and therefore some Kir4.1 in western blots may also be attributable to a loss in oligodendrocytes. It is also possible that some of the astrocytes that displayed significant Kir currents after injury did so by a compensatory up-regulation of other Kir genes, a question that deserves further examination in the future. The weak rectification, high Ba2+ sensitivity, and inactivation at very negative potentials of the recorded currents however, are most consistent with the properties of Kir4.1, suggesting that a subpopulation of astrocytes retained functional Kir4.1 channels.
17β-oestradiol effects on Kir4.1 following injury
Another interesting finding pertains to the partial recovery of Kir4.1 expression in oestrogen treated animals and that astrocytic Kir4.1 expression is directly regulated by oestrogen receptors. Numerous studies have reported on neuroprotective benefits conferred by 17β-oestradiol in brain and spinal cord. In spinal cord specifically, female rats tend to have improved hind-limb motor function compared to age-matched male counterparts following injury (Farooque et al., 2006). Similarly, exogenous 17β-oestradiol treatment in pre- and post-menopausal ovariectomized female rats significantly increased hind-limb locomotion, white matter sparing and decreased apoptosis (Chaovipoch et al., 2006). Sribinick and colleagues found that post-injury administration of 17β-oestradiol following SCI to male rats reduced lesion volume, decreased oedema and decreased markers of inflammation and apoptosis (Sribnick et al., 2005, 2006). And a recent study using the same injury and treatment paradigm used in this investigation demonstrated increased neuronal survival and improved hind-limb function in 17β-oestradiol-treated animals (Kachadroka et al., 2010). Interestingly, another recent study demonstrated a progressive loss of Kir4.1 that proceeded clinical symptoms in the ventral horn in a mouse model of amyotrophic lateral sclerosis (Kaiser et al., 2006). These authors used cultured spinal motor neurons to demonstrate that increasing [K+]o was toxic in a concentration and time dependent manner. The above studies suggest that the increased neuronal survival and resulting improvements in locomotor skills may be in part due to improved K+ homeostasis following 17β-oestradiol treatment. Our results also indicate that the increase in Kir4.1 we observed at 7 days was transient and by 4 weeks post-injury as Kir4.1 levels in oestrogen treated injured animals were similar to animals treated with a placebo. It is possible the increased Kir4.1 levels during lesion stabilization and thus increased K+ homeostasis lead to increased neuronal survival and improved functional recovery.
Several recent studies have indicated that astrocytes are targets of oestrogen receptor signalling. Acute treatment with 300 nM 17β-oestradiol decreased calcium rises and wave propagation in mechanically stimulated astrocytes (Rao and Sikdar, 2007). Importantly, reactive gliosis, as assessed by GFAP immunoreactivity, is attenuated to control levels in ovariectomized females treated with the 17β-oestradiol, in contrast to ovariectomized females treated with a placebo which demonstrate significantly higher levels of GFAP immunoreactivity (Martinez and de Lacalle, 2007). These studies suggest that a potential effect of 17β-oestradiol may be the attenuation of the astrogliosis, which may include maintaining astrocytes in a non-reactive state. This could account for a preservation of Kir4.1 and GLT-1 expression. Although we can not say which cell type is expressing 17β-oestradiol receptors or what receptor is the target of 17β-oestradiol in these studies, our data demonstrate that oestrogen receptor-α and oestrogen receptor-β are present in the spinal cord of these adult male animals. Also, others have demonstrated that spinal cord astrocytes express both receptors in vivo (Platania et al., 2003) and in vitro (Platania et al., 2005).
The regulation of Kir4.1 expression by oestrogen receptor signalling had not been previously studied. Since our in vivo data show that Kir4.1 is preserved in the presence of 17β-oestradiol, we used primary cultures of rat spinal cord astrocytes to examine direct effects of 17β-oestradiol on Kir4.1. Both biochemical and biophysical data suggest that Kir4.1 is transcriptionally regulated by oestrogen receptor inhibition, which causes a marked reduction in Kir4.1 protein and current. It is important to note that these experiments were performed in the presence of 10% serum, which contains 17β-oestradiol, and may explain why we did not observe increases in channel activity or a significant increase in Kir4.1 protein. It is therefore possible that we are underestimating the 17β-oestradiol effects on our spinal cord astrocyte cultures. However, in a follow-up series of experiments (data not shown) we injured cultured spinal cord astrocytes in the presence or absence of 17β-oestradiol and or a 17β-oestradiol receptor antagonist. For these studies the medium was charcoal-filtered to remove serum oestrogen. As in the data in Fig. 8, 17β-oestradiol had little influence on Kir currents, yet the 17β-oestradiol antagonist significantly reduced Kir expression. Hence, taken together, these data suggest that Kir4.1 expression is under the control of 17β-oestradiol receptor signalling.
Expression of GLT-1 after injury and the effects of 17β-oestradiol
While the major objective of our study was to examine changes in Kir4.1 following SCI, we also paid attention to changes in the astrocytic glutamate transporter GLT-1. This is in part due to the generally held notion that these two proteins cooperate functionally, whereby the activity of Kir4.1 assures a sufficient electrochemical potential to drive glutamate uptake via GLT-1. Hence it would not be unexpected to see a functional loss of GLT-1 if Kir4.1 is compromised. Indeed, our study suggests that both proteins are similarly affected by injury and are indeed similarly rescued by 17β-oestradiol. While the here reported oestrogen effects on Kir4.1 are entirely novel, several groups have looked at oestrogen regulation of astrocyte specific glutamate transporters. For example, cultured astrocytes treated with 17β-oestradiol demonstrate significant up-regulation of GLT-1 and glutamate aspartate transporter mitochondrial RNA and protein. And glutamate uptake was reversed with ICI 182 780 treatment (Pawlak et al., 2005). In a second study, human astrocytes derived from cortex of patients with Alzheimer’s disease were treated with 100 nM 17β-oestradiol for 48 h, which resulted in increased glutamate uptake and protein expression of glutamate aspartate transporter and GLT-1, although no changes were observed in astrocytes cultured from non-demented brain (Liang et al., 2002). A third study demonstrated that after 24 h of treatment, higher doses of 17β-oestradiol (1 and 100 μM) led to decreased glutamate clearance in primary cultured cortical astrocytes (Sato et al., 2003). Spinal cord astrocytes were never investigated, yet our own data indicate prominent expression of both receptors in spinal cord in vivo when compared to a positive control. GLT-1 expression both in vivo and in vitro, were markedly increased following 17β-oestradiol treatment. Importantly, in our in vitro cultured astrocytes we were able to demonstrate that expression could be modulated with the oestrogen receptor antagonist ICI 182 780. Hence at least in the spinal cord, astrocytic expression of Kir4.1 and GLT-1 are both regulated via oestrogen receptor signalling.
Conclusion
Our studies demonstrate that astrocytic Kir4.1 and GLT-1 expression is lost over wide regions in the spinal cord after injury and that this loss persists for at least 4 weeks. Both proteins are regulated by 17β-oestradiol receptors and treatment of injured animals with 17β-oestradiol causes a partial restoration or preservation of expression of both proteins. In light of their critical role in ensuring K+ and glutamate homeostasis in the spinal cord, the effects of oestrogen on these two proteins may contribute to the previously reported pronounced behavioural benefits seen in animals treated with oestrogen. These findings also indicate that 17β-oestradiol may be used to maintain or increase expression of Kir4.1 and/or GLT-1 in other injury or disease conditions that present with a loss of these astrocytic proteins. The ready availability of oestrogen and ease of administration warrants more extensive studies on its regulation of Kir4.1 and GLT-1 and potential broader neuroprotective effect.
Funding
National Institutes of Health grants RO1-NS36692, RO1-NS31234 and R21NS052559.
Glossary
Abbreviations
- GFAP
glial fibrillary acidic protein
- GLT-1
glutamate transporter 1
- SCI
spinal cord injury
References
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med. 2006;10:33–44. doi: 10.1111/j.1582-4934.2006.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17beta-estradiol is protective in SC injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–52. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–5. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Dibaj P, Kaiser M, Hirrlinger J, Kirchhoff F, Neusch C. Kir4.1 channels regulate swelling of astroglial processes in experimental SC edema. J Neurochem. 2007;103:2620–8. doi: 10.1111/j.1471-4159.2007.04979.x. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–65. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat SC. Eur J Neurosci. 1994;6:712–24. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Farkas I, Varju P, Liposits Z. Estrogen modulates potassium currents and expression of the Kv4.2 subunit in GT1-7 cells. Neurochem Int. 2007;50:619–27. doi: 10.1016/j.neuint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, et al. Gender-related differences in recovery of locomotor function after SC injury in mice. Spinal Cord. 2006;44:182–7. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schröder W, Hager G, Seifert G, Blümcke I, Elger CE, et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Europ J Neurosci. 2000;12:2087–96. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–47. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Kachadroka S, Hall AM, Niedzielko TL, Chongthammakun S, Floyd CL. Effect of endogenous androgens on 17β-estradiol-mediated protection after spinal cord injury in male rats. J Neurotrauma. 2010;27:1–16. doi: 10.1089/neu.2009.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Maletzki I, Hulsmann S, Holtmann B, Schulz-Schaeffer W, Kirchhoff F, et al. Progressive loss of a glial potassium channel (KCNJ10) in the SC of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;99:900–12. doi: 10.1111/j.1471-4159.2006.04131.x. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: Phenotypic impact in retina. J Neurosci. 2000;20:5733–40. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, et al. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–81. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L. Glial and neuronal connexin expression patterns in the rat SC during development and following injury. J Comp Neurol. 2005;489:1–10. doi: 10.1002/cne.20567. [DOI] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J Neurochem. 2002;80:807–14. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci. 1997;17:7316–29. doi: 10.1523/JNEUROSCI.17-19-07316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L, de Lacalle S. Astrocytic reaction to a lesion, under hormonal deprivation. Neurosci Lett. 2007;415:190–3. doi: 10.1016/j.neulet.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Papadopoulos N, Muller M, Maletzki I, Winter SM, Hirrlinger J, et al. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol. 2006;95:1843–52. doi: 10.1152/jn.00996.2005. [DOI] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21:5429–38. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Campbell SL, Sontheimer H. Differential distribution of kir4.1 in SC astrocytes suggests regional differences in k+ homeostasis. J Neurophysiol. 2007;98:786–93. doi: 10.1152/jn.00340.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Higashimori H, Campbell SL, Hablitz JJ, Sontheimer H. Functional expression of K(ir)4.1 channels in SC astrocytes. Glia. 2006;53:516–28. doi: 10.1002/glia.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia. 2004;46:63–73. doi: 10.1002/glia.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannicke T, Uckermann O, Iandiev I, Biedermann B, Wiedemann P, Perlman I, et al. Altered membrane physiology in Muller glial cells after transient ischemia of the rat retina. Glia. 2005;50:1–11. doi: 10.1002/glia.20151. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204:428–37. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- Platania P, Laureanti F, Bellomo M, Giuffrida R, Giuffrida-Stella AM, Catania MV, et al. Differential expression of estrogen receptors alpha and beta in the SC during postnatal development: localization in glial cells. Neuroendocrinology. 2003;77:334–40. doi: 10.1159/000070899. [DOI] [PubMed] [Google Scholar]
- Platania P, Seminara G, Aronica E, Troost D, Vincenza CM, Angela SM. 17beta-estradiol rescues spinal motoneurons from AMPA-induced toxicity: a role for glial cells. Neurobiol Dis. 2005;20:461–70. doi: 10.1016/j.nbd.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing SC injury. Neurobiol Dis. 2004;15:415–36. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J Neurophysiol. 1995;72:333–46. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- Ritz M, Hausmann O. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–88. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–41. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Sato K, Matsuki N, Ohno Y, Nakazawa K. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor alpha. J Neurochem. 2003;86:1498–505. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of SC injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84:1064–75. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute SC injury in rats. J Neurosci Res. 2005;82:283–93. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Mills CD, Ye Z, Fullwood SD, McAdoo DJ, Hulsebosch CE, et al. Rapid changes in expression of glutamate transporters after SC injury. Brain Res. 2002;927:104–10. doi: 10.1016/s0006-8993(01)03329-7. [DOI] [PubMed] [Google Scholar]