Abstract
The ongoing US Glatiramer Acetate (GA) Trial is the longest evaluation of continuous immunomodulatory therapy in relapsing-remitting multiple sclerosis (RRMS). The objective of this study was to evaluate up to 15 years of GA as a sole disease-modifying therapy. Two hundred and thirty-two patients received at least one GA dose since study initiation in 1991 (mITT cohort), and 100 (43%, Ongoing cohort) continued as of February 2008. Patients were evaluated every 6 months using the Expanded Disability Status Scale (EDSS). Mean GA exposures were 8.6 ±5.2, 4.81 ±3.69, and 13.6 ± 1.3 years and mean disease durations were 17, 13, and 22 years for mITT, Withdrawn and Ongoing cohorts, respectively. For Ongoing patients, annual relapse rates (ARRs) maintained a decline from 1.12±0.82 at baseline to 0.25 ± 0.34 per year; 57% had stable/improved EDSS scores (change ± 0.5 points); 65% had not transitioned to secondary progressive multiple sclerosis (SPMS); 38%, 18%, and 3% reached EDSS 4, 6, and 8. For all patients on GA therapy (the mITT cohort), ARRs declined from 1.18 ± 0.82 to 0.43 ± 0.58 per year; 54% had stable/improved EDSS scores; 75% had not transitioned to SPMS; 39%, 23%, and 5% reached EDSS 4, 6, and 8. In conclusion, multiple sclerosis patients with mean disease duration of 22 years administering GA for up to 15 years had reduced relapse rates, and decreased disability progression and transition to SPMS. There were no long-term safety issues.
Keywords: disability, Expanded Disability Status Scale, glatiramer acetate, long-term, relapsing-remitting multiple sclerosis, secondary progressive multiple sclerosis
Introduction
Multiple sclerosis (MS) is a lifelong, progressive disease and a majority of patients with relapsing–remitting (RRMS) disease develop secondary-progressive MS (SPMS) within two decades of diagnosis.1,2 Therefore, it is important to prevent accumulation of long-term disability and slow inflammatory and neurodegenerative MS pathology. Mechanisms related to relapse and remission during early MS may differ from those associated with an unremitting chronic progression of disease, and the findings of the multiple disease-modifying therapies (DMTs) trials in SPMS have demonstrated that they are for the most part unreliable in slowing the progression of disability over intervals up to 3 years (glatiramer acetate [GA], Copaxone; IFNβ-1a IM, Avonex; IFNβ-1a SC, Rebif; IFNβ-1b SC, Betaseron).3–8
As the availability of immunomodulatory therapy for MS is relatively recent (within the last two decades), data regarding their prospective long-term effects on disability is just beginning to be accumulated. Follow-up assessments of patients participating in the short-term controlled clinical trials of the interferon beta (IFNβ) drugs have found that patients continuing to take IFNβ drugs at the time of the later follow-up visit have better neurologic status than those who report more limited exposure to MS treatment.9–12 Conclusions based on the findings of these studies must be tempered by the studies’ methodological limitations: importantly, data were collected after considerable intervals during which patients were not monitored and patients had frequently discontinued, switched, or added other immunomodulators to the IFNβ drug under study.
Two recent prospective non-randomized open-label studies have attempted to assess the continuous use of DMTs for 7–8 years. Trojano et al.13 evaluated the effects of DMT use over 7 years of follow-up and Brown et al.14 over 25 years of follow-up (the DMT treatment period in the latter study was up to 8.4 years). Although caution must be used in evaluating the results of these studies due to the uncontrolled nature of open-label studies, the findings of these studies suggest that, as a group, DMTs delayed accumulation of disability in RRMS patients as measured by Expanded Disability Status Scale (EDSS) scores and conversion to SPMS compared with no treatment.
The ongoing US Glatiramer Acetate Trial is unique in that it is an open-label study that prospectively and regularly evaluates patients with RRMS on continuous therapy with a single immunomodulating agent for an extended period of time. Now at the 15th year of the study, patients ongoing in the study have an average of 13.6 years of GA treatment. Beginning in 1991, 251 RRMS patients enrolled in the multicenter placebo-controlled double-blind US pivotal trial. At the end of the double-blind placebo-controlled phase, placebo patients were crossed over to GA and the prospective open-label study was begun. The clinical effectiveness and safety of GA in this study have been reported at 2 and 3 years compared with placebo,15,16 and at 6, 8, and 10 years in the open-label study.17–19
This report further describes the long-term efficacy and safety of daily GA in carefully monitored patients who have received continuous GA as their sole DMT, beginning as early as 1991.
Methods
Patients
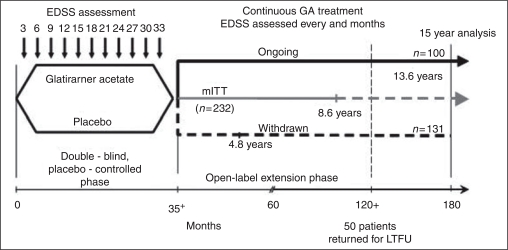
The patients with RRMS who have participated in this trial have been described previously.19 Briefly, patients who participated in the double-blind placebo controlled pivotal trial were asked to enroll in the open-label extension phase (Figure 1). Patients originally randomized to active treatment continued to take daily GA and those randomized to placebo switched to GA 20 mg/day SC. The modified intention-to-treat (mITT) population reported here differs from the original ITT cohort (n ¼ 251) of the pivotal trial in that the mITT cohort includes only patients who have received at least one dose of GA (19 patients initially randomized to placebo in the pivotal study did not participate in the open-label extension and are excluded from the mITT cohort). The mITT population is sub-divided into two patient cohorts, the Ongoing cohort, who are patients that continued in the study as of February 2008, and the Withdrawn cohort, who are patients that withdrew from the study at some point after starting GA therapy. At the time of data cut-off for this analysis, February 2008, 100 patients continued in the ongoing study (Ongoing cohort). Patients who had received GA from randomization had been treated for up to 15 years and patients originally randomized to placebo had been receiving GA for up to 13 years.
Figure 1.
Study design for the long-term glatiramer acetate (GA) study of patients who participated in the placebo-controlled study and were entered into the open-label study of GA. Patients on placebo in the placebo-controlled phase were switched to active treatment with GA in the open-label phase and those on GA in the placebo-controlled phase continued on it. The mean number of years of GA treatment for the Ongoing cohort was 13.6 years, for the mITT cohort was 8.6 years and for the Withdrawn cohort was 4.8 years.
Study design
The US Glatiramer Acetate Pivotal Trial, a double-blind, randomized, placebo-controlled study, was initiated in October 1991. All of the 11 original US academic centers continue to participate, and the Institutional Review Boards at all centers continue to approve participation in the study.
Neurological status is evaluated in the clinic every 6 months by EDSS. Patients are examined within 7 days if they experience symptoms suggestive of a relapse (appearance or reappearance of one or more neurologic abnormalities persisting for at least 48 hours, preceded by a stable or improving neurological state of at least 30 days duration). During the open-label phase, laboratory assessments (chemistry panel) and vital signs are documented at 6-month study visits. In most cases, patients are assessed by the same neurologists and study coordinators at each visit.
Any patient who discontinued daily GA for any reason, or who took another immunomodulator, was withdrawn from the study. Thus, while in the study, these RRMS patients received only continuous GA monotherapy for disease modification. Reasons for withdrawal were classified into three categories: (1) withdrawal due to adverse events, (2) lost-to-followup, and (3) withdrawal due to ‘patient decision’ or ‘other’. The reasons under the patient decision/other category were further divided into subcategories to gain a better understanding of why patients discontinued the study.
Fifteen-year analyses
Demographic characteristics were collected for the year prior to patients starting GA therapy. Time of ‘disease onset’ was defined as the time patients experienced their first MS symptom (reported by patients at study entry). Changes in disability were measured by mean yearly EDSS scores and mean changes in EDSS from GA start to the last observation while on GA in the mITT, Ongoing, and Withdrawn patient cohorts. EDSS at GA start was the EDSS score of the patient when they began GA therapy (i.e. at randomization for patients originally randomized to GA, and at entry into the open-label extension study for patients who were initially randomized to placebo). Categorical analyses of patients’ neurological status were performed; patients were classified as ‘stable/improved’ if EDSS scores were changed by ≤ 0.5 EDSS points, did not change, or decreased from onset of treatment; ‘worsened’ status was used to describe increases of more than 0.5 EDSS points.
Proportions of patients who reached confirmed scores of EDSS 4, 6, or 8 while on GA and time to these EDSS thresholds were obtained for the mITT, Ongoing, and Withdrawn cohorts (only patients who began GA therapy with EDSS scores lower than the EDSS threshold were included in these analyses).
Categorical analyses and proportions of patients who reached predefined EDSS thresholds (EDSS 4, 6, and 8) were also measured in patients of the mITT cohort stratified by EDSS score at GA start. The Low EDSS strata consisted of patients with EDSS 0 to 2.0 at GA start and the High EDSS strata included patients with EDSS ≤2.5 at GA start.
Definitions of confirmed progression to SPMS vary among neurologists and within the literature, since it is difficult to accurately determine the onset of chronic progression, even in patients who are evaluated frequently.9,10 11, In this study, SPMS was defined as an increase of >1.0 EDSS point sustained for 12 months, without relapses occurring during that period.
The annualized relapse rate (ARR) was calculated for each patient by dividing the total number of relapses by the number of years of exposure to GA.
Statistical methods
Propensity score analyses. As the study has been running continuously for more than 15 years, there was concern that there might be a treatment bias between the Withdrawn and Ongoing cohorts due to either their demographic characteristics at GA start or their initial randomization into a GA or placebo treatment group in the original double-blind study. A propensity score is a statistical method 21,22 that can be used to partly adjust for bias of baseline characteristics between the ongoing and the withdrawal cohorts, by equating these subgroups according to these covariates. The propensity scores were constructed for each patient using a logistic regression fitted to the data with the cohort (either Ongoing or Withdrawn) as the dependent variable and the following as independent variables: age at disease onset, age at GA start, gender, disease duration, relapses 2 years prior, EDSS, and randomization grouping. A stepwise algorithm was used to find the most explanatory variables from the above-stated characteristics, second-order interactions and third-order interactions.
Analyses of clinical endpoints. Descriptive statistics are reported for changes from GA start in ARR, changes from GA start in EDSS scores, and yearly EDSS scores. Kaplan-Meier survival methods were used to estimate the median time to EDSS 4, 6, and 8 while on GA. Chi-squared analyses were used for binary variables (the proportion of patients reaching EDSS scores 4, 6, and 8) and /-tests were used for continuous variables (last EDSS score while on GA and change from GA start to last EDSS); and to compare the outcomes between stratified patients with EDSS scores 0-2 and those with EDSS scores ≤2.5 at GA start. The percent of mITT, Ongoing, and Withdrawn patients who transitioned to SPMS over the course of the study are reported.
Results
Patients
A total of 232 patients who had received at least one dose of GA since study inception comprised the mITT cohort. One patient discontinued after receiving GA but before undergoing neurological evaluation; therefore, the efficacy evaluable mITT cohort included 231 patients. As of February 2008, 100 out of the 232 patients (43%) remained in the study and comprised the Ongoing cohort. The Withdrawn cohort consisted of 131 patients.
Propensity scores. The descriptive statistics for the patient characteristics used as independent variables in the propensity score analyses are presented in Table 1. There were no significant differences between the Ongoing and Withdrawn cohorts for patient characteristics except for EDSS at GA start (t-test, p = 0.0053). There were no significant differences in the percentage of patients in the Ongoing or Withdrawn cohorts due to their original randomization group (chi-squared test, p = 0.3022). For the Ongoing cohort, 50 patients were randomized to GA (50%) and 50 patients were randomized to placebo (50%). For the Withdrawn cohort, 75 patients were randomized to GA (57%) and 57 were randomized to placebo (43%, p = 0.3522). The final model following the implementation of the logistic regression model included the following variables: EDSS at GA start, age at GA start, gender and the interaction between age at GA start and gender. There were no differences in the cohorts’ propensity scores (mITT mean score = 0.43 ±0.12, Ongoing = 0.47 ±0.12 and Withdrawn = 0.40 ±0.12). This indicates that there was a similar distribution of their patient characteristics at GA start and therefore it was not appropriate to adjust the subsequent analyses of the clinical endpoints using the propensity scores.
Table 1.
Patient characteristics at glatiramer acetate start
| Characteristic | mITT cohort n = 232 | Ongoing cohort n = 100 | Withdrawn cohort n = 132 |
| Age at multiple sclerosis disease onset (mean ±SD) | 27.40 ± 6.18 | 28.06 ± 6.31 | 26.90 ± 6.05 |
| Age (mean ± SD) | 35.5 ± 6.4 | 35.1 ± 5.8 | 33.8 ± 6.5 |
| Gender (% female) | 73% | 70% | 76% |
| Mean relapse rate 12 months before GA start | 1.18 ± 0.82 | 1.12 ± 0.82 | 1.23 ± 0.83 |
| Expanded Disability Status Scale score (mean ± SD) | 2.8 ± 1.50 | 2.5 ± 1.3 | 3.0 ± 1.6 |
| Disease duration (years, mean±SD) | 8.3 ± 5.12 | 8.4 ± 5.14 | 8.13 ± 5.12 |
GA, glatiramer acetate.
Stratification by EDSS scores at GA start. Patients of the mITT cohort stratified by EDSS score at GA start included 103 patients in the Low EDSS (0-2.0) stratum and 129 patients in the High (≤2.5) EDSS stratum. At GA start, mean ± SD and range of EDSS scores in the mITT were 1.5 ±0.6 (range: 0-2.0) in the Low strata, and 3.9 ±1.1 (range: 2.5-7.0) in the High strata. Patients in the High EDSS strata at GA start tended to be slightly older than those in the Low EDSS strata (36.4 ±6.4 years versus 34.7 ±6.1 years, respectively) and to have longer mean disease duration at study start (9.0 ± 5.4 years versus 7.3 ± 4.7 years, respectively).
Patient discontinuation. Eight patients left the study since the previously reported patient follow-up in November 2003.19 The reasons for withdrawal at the termination visit were categorized by the study sites as patient decision. Reasons included: increase in relapses (n = 1), progression of disability (n = 1), patient believes they have benign MS (n = 1), tired of traveling to investigative site (n = 1), and want to try another immuno-modulator (n = 4; three natalizumab and one interferon-ß-1a SC). Discontinuation reasons for all patients (n=132) who have left the trial since 1991 are listed in Table 2.
Table 2.
Reasons for patient withdrawal
| Reason for withdrawal | n | Patient decision/other subcategories∗ | n |
| Total | 132 | Total | 95 |
| Lost to follow-up | 14 | Patient perception of disease worsening | 29 |
| Adverse event | 23 | Desire to switch or combine therapies | 26 |
| Patient decision/other | 95 | Difficulty, inability, or unwillingness to adhere to study protocol | 32 |
| Pregnancy | 8 |
∗Patient decision/other subcategories were derived from written comments provided by Withdrawn patients at their final visit.
Fifteen-year analysis
At the time of the 15-year analysis, the mean disease duration from disease onset was 22.0 years for the Ongoing cohort, and for the total mITT cohort and the Withdrawn cohort while in the study was 17 and 13 years, respectively. All Ongoing patients had completed the 15-year clinical visit of the study; however, the patients in the Ongoing cohort who were switched from placebo to GA therapy at the end of the placebo-controlled trial (n = 50) had a GA exposure of 12.3 ±0.10 years (range: 11.7-12.5) and those who started GA at randomization (n = 50) have a mean GA exposure of 14.9 ±0.10 years (range: 14.6-15.3). Thus, the mean (±SD) number of GA treatment years for the Ongoing cohort was 13.6 ± 1.3 (range: 11.7-15.3) years. The mean number of GA treatment years was 8.6 ± 5.2 (range: 0.2-15.3) for the mITT cohort, and 4.8 ±3.7 (range: 0.2-14.2) years in the Withdrawn cohort. Mean (±SD) and median ages of the Ongoing cohort were 50 ±5.9 years and 49.9 years. Mean (±SD) and median ages for the mITT cohort were 44.2±8.4 years and 45.1 years, for the Withdrawn cohort, 33.8 ±6.5 years and 34.5 years. The ARR in the Ongoing Cohort declined from 1.12 ±0.82 before starting GA therapy to 0.25 ±0.34 at the 15-year analysis. The change in ARR from GA start to the last observed value on GA therapy in the study for the mITT cohort declined from 1.18 ±0.82 to 0.43 ±0.58 and for the Withdrawn cohort from 1.23 ±0.83 to 0.56 ±0.68.
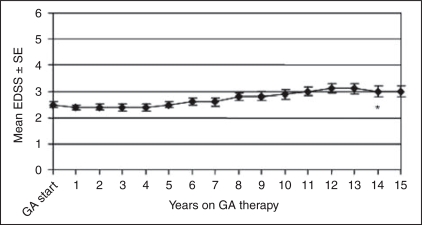
Changes in mean EDSS scores from GA start to last observation while on GA were 0.96 ± 1.8, 0.6 ±2.0, and 1.0 ±1.7 for the mITT, Ongoing, and Withdrawn cohorts (Table 3). Yearly mean (±SE) EDSS scores for the Ongoing patients are shown in Figure 2. Overall, 54% of the mITT, 57% of the Ongoing cohort and 52% of the Withdrawn cohort while on GA had stable/improved EDSS scores. Statistics of mITT, Ongoing, and Withdrawn patients who progressed to SPMS while in the study are shown in Table 4. Twenty-five percent of the mITT cohort and 17% of the Withdrawn patients reached SPMS while on GA and 35% of the Ongoing cohort have converted to SPMS at the time of the 15-year analyses.
Table 3.
Changes in Expanded Disability Status Scale from glatiramer acetate start to the last observation while on glatiramer acetate for the 15-year analysis
| While on GA | mITT cohort n ¼ 231 | Ongoing cohort n ¼ 100 | Withdrawn cohort n ¼ 131 |
| Time from GA start to last observation (years) | |||
| Mean ± SD | 5.59 ± 5.23 | 13.57 ± 1.32 | 4.81 ± 3.69 |
| Median | 9.94 | 13.56 | 3.79 |
| Range | 0.18-15.30 | 11.68-15.30 | 0.18-14.19 |
| EDSS score at GA start | |||
| Mean ± SD | 2.8 ± 1.5 | 2.5 ± 1.3 | 3.0 ± 1.6 |
| Median | 2.5 | 2.2 | 3.0 |
| Range | 0-7.0 | 0-6.0 | 0.0-7.0 |
| EDSS at last observation | |||
| Mean ± SD | 3.6 ± 2.2 | 3.1 ± 2.1 | 4.0 ± 2.3 |
| Median | 3.5 | 2.8 | 3.5 |
| Range | 0-9.0 | 0-8.5 | 0.0-9.0 |
| EDSS change from GA start to last observation | |||
| Mean ± SD | 0.9 ± 1.8 | 0.6 ± 2.0 | l.0 ± 1.7 |
| Median | 0.5 | 0.5 | 0.5 |
| Range | -3.5-6.5 | —3.5-6.5 | —3.5-5.5 |
| Average EDSS change per year | |||
| Mean ± SD | 0.2+-0.6 | 0.1+-0.2 | 0.5+-0.8 |
| Median | 0.1 | 0.0 | 0.3 |
| Range | — 1.8—4.0 | —0.3-0.5 | — 1.8—4.0 |
EDSS, Expanded Disability Status Scale; GA, glatiramer acetate.
Figure 2.
Yearly mean Expanded Disability Status Scale (EDSS) scores for Ongoing patients (n ¼ 100) in the glatiramer acetate (GA) study at the 15-year analysis. The asterisk (∗) indicates that the yearly mean EDSS scores for years 14 and 15 are derived from the scores of patients who were originally randomized to GA in the placebo-controlled phase of the study (n ¼ 50).
Table 4.
Progression to secondary-progressive multiple sclerosis
| While on GA | Number of patients progressed to SPMS (%)∗ | EDSS Mean ± SD Median | Disease duration GA start (years) Mean ± SD Median | Disease duration total (years) Mean ± SD Median | Duration of follow-up (years on GA) Mean ± SD Median |
| mITT | 59 (25%)∗∗ | 5.4 ± 2.2 | 9.35 ± 4.9 | 20.68 ± 5.8 | 9.8 (3.5) |
| n = 231 | 6.0 | 9.0 | 19.7 | 12.9 | |
| Ongoing cohort | 35 (35%) | 4.9 ± 2.2 | 9.82 ± 5.0 | 23.3 ± 5.1 | 12.0 (0.2) |
| n = 100 | 6.0 | 9.0 | 22.3 | 12.0 | |
| Withdrawn cohort | 24 (18.3%)∗∗ | 6.2 ± 2.0 | 8.67 ± 4.7 | 16.8 ± 4.4 | 6.4 (3.4) |
| n = 131 | 6.3 | 8.5 | 16.9 | 6.0 |
∗Total number of patients who met the definition of SPMS, an increase of 1.0 EDSS points for 12 months with no relapses.
∗∗Two patients in the withdrawn cohort had an increase of 1.0 EDSS points and had a relapse within the last 12 months.
GA, glatiramer acetate; EDSS, Expanded Disability Status Scale; SPMS, secondary-progressive multiple sclerosis.
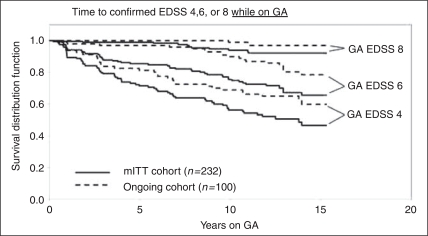
The proportions of patients reaching defined EDSS thresholds of 4, 6, and 8, while on GA therapy, are 39%, 23%, and 5% for mITT; 38%, 18%, and 3% for ongoing; and 40%, 27%, and 6% for withdrawn patients, respectively. The duration of follow-up for the mITT cohort was 5.6 years, the Ongoing cohort 13.6 years, and the Withdrawn cohort 4.8 years. The median times to EDSS 4, 6, and 8 were not reached (i.e. 50% of the cohort did not reach the endpoint). The estimated time for one quartile (25%) of patients to reach EDSS 4 was 4.0 years in the mITT cohort, 6.8 years in the Ongoing cohort, and 2.75 years in the Withdrawn cohort. The estimated time for one quartile (25%) of patients to reach EDSS 6 was 3.9 years for the Withdrawn cohort. Less than 25% of the mITT and Ongoing cohorts reached EDSS 6, and less than 25% of any cohort reached EDSS 8 by year 15 of the trial (Figure 3).
Figure 3.
Time to reach confirmed Expanded Disability Status Scale 4, 6, and 8 for the mITT and Ongoing cohorts while they were on glatiramer acetate therapy. The mean disease duration at glatiramer acetate start for the mITT cohort was 8.3 years and for the Ongoing cohort was 8.4 years.
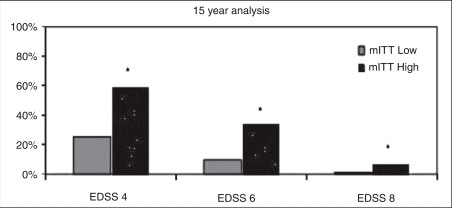
Low versus High EDSS strata. There were no significant differences between the baseline Low and High EDSS strata in the proportion of patients with stable/ improved EDSS scores at the 15-year analysis in the mITT cohort (54.4% versus 54.3%, respectively). There were significant differences in the proportion of patients in the Low and High strata of the mITT cohort reaching EDSS thresholds of 4, 6, and 8 (Figure 4). In the Low stratum of the mITT cohort, 25%, 9% and 1% reached EDSS thresholds of 4, 6, and 8, respectively, and in the High EDSS stratum, 61%, 34%, and 8% reached EDSS 4, 6, and 8, respectively.
Figure 4.
Proportion of Low and High strata mITT patients reaching Expanded Disability Status Scale (EDSS) 4, 6, and 8. The proportion of patients in the Low and High strata mITT cohorts reaching the EDSS thresholds of EDSS 4, 6, and 8 was significantly different (∗p < 0.02). Patients in the Low strata had EDSS scores of 0-2.0 at glatiramer acetate (GA) start (n= 103) and those in the High strata had EDSS scores >2.5 at GA start (n= 129).
Safety
The most commonly reported adverse events in patients receiving long-term GA, regardless of whether the investigator judged them to be related to GA therapy or not, were accidental injury, muscle weakness, back pain, dizziness, depression, hypoesthesia, paresthesia, insomnia, upper respiratory infections and urinary tract infections, headache, and pain. Adverse events thought to be related to GA therapy were consistent with known side effects of GA administration and included local injection-site reactions (e.g. erythema, pain, nodules, edema) and symptoms associated with an immediate post-injection reaction (IPIR), which may have included vasodilation, chest pain, palpitation, tachycardia, or dyspnea. No apparent time-dependent adverse events emerged. No evidence of hematologic, hepatic, or renal dysfunction; immunosuppression; emergence of malignancy; or development of other autoimmune disease was observed. There was one death due to respiratory failure caused by pneumonia that was not believed to be drug related.
Discussion
Results of this prospective, ongoing, open-label study indicate that long-term continuous GA use is safe and that the majority of the patients continuing on GA therapy in the study have had few relapses and minimal disease progression. With a mean disease duration of 22 years and mean patient age of 50 years, two-thirds of patients receiving continuous GA as sole immunomodulatory therapy for 15 years have not tran-sitioned to SPMS, 57% have retained stable or improved EDSS scores over the course of the study, and 82% remain ambulatory without mobility aids. No unforeseen adverse events to GA therapy occurred.
While receiving GA, the Withdrawn cohort, which included all patients who received at least one GA dose and subsequently left the study, and the Ongoing cohort patients appeared to do comparably well; however, it is important to remember that the mean disease durations in these cohorts were different (13 versus 22 years, respectively). Outcomes in the Withdrawn cohort reflect the last observed value carried forward, and patients who left the trial may have done so at any stage in their disease course. Although patients withdrew for many stated reasons, a significant proportion did so because they may have had more significant progression of clinical disability than those who stayed in the study. Approximately 41% of all patients who withdrew from the study indicated that they perceived that their disease was progressing or that they wanted to switch treatments. Of the withdrawal patients, those who progressed to SPMS had a mean EDSS of 6.2 with disease duration of 17 years. No follow-up neurologic assessment has been performed on the eight patients who have left the study since the 2003 assessment.
Our results are prospective and should not be compared directly with those of recent, retrospective investigations on the benefits of long-term DMT use. In other long-term follow-up studies, patient assessments have been relatively infrequent, patient populations are different or less well defined, disease durations and follow-up intervals vary, subjects are not on a single drug and the studies use diverse methodologies and definitions for data collection and reporting. This open-label prospective extension study of GA as single immunomodulatory therapy, with highly controlled, consistent follow-up, is the only long-term study in MS in which patients were required to remain on a single immunomodulator and not switch or add concomitant immunotherapy. The long-term extension studies of IFNß-1b (16-year follow-up visit) and IFNß-1a SC (PRISMS 8-year follow-up visit) included time periods in which patients may have used other immunomodulatory therapy or none at all. In the 16-year follow-up study of IFNß-1b, the mean time since diagnosis in the long-term follow-up (LTFU) cohort (260/372, 70%) was 20 years.23,24 Of patients who received IFNß-1b for more than 80% of the 16-year interval, approximately 44% reached EDSS ≥6 and 29% reached EDSS 8. The estimated median time to EDSS 6 in those patients was approximately 13 years. For those patients on IFNß-1b for less than 10% and between 10% and 80% of the 16-year interval, 44% and 31% reached EDSS 8, respectively. In the current study, only 18% of Ongoing patients, with slightly longer disease duration (22 years), reached EDSS 6 and 3% reached EDSS 8 while on GA. In addition, the proportion of patients who were judged to have converted to SPMS in the IFNß-1b study was 45% versus 35% of Ongoing patients and 25% of mITT patients in this GA study.
In the 7- to 8-year follow-up to the PRISMS study of IFNß-1a SC, 382 out of 560 patients returned for a single follow-up assessment (approximately 13 years from disease diagnosis for the ITT population).9 Of the original PRISMS ITT cohort, 20% (110/557) had reached EDSS ≥6 (regardless of treatment), roughly the same proportion of Ongoing patients in the current study who had a disease duration of 22 years. In PRISMS LTFU patients, mean increase from baseline EDSS score at 8 years from study start was 1.1 point and approximately 60% of patients had a 1.0-point EDSS progression. In the current study, Ongoing patients had a mean increase of 0.6 EDSS points at the 15-year follow-up and 57% had progressed ≥0.5 EDSS points.
In long-term studies such as this, there are inherent limitations that make it inappropriate to draw definitive conclusions about the clinical efficacy of a therapy. Weaknesses of the current study include lack of a concurrent randomized control group and the potential for selection bias when patients with more aggressive disease drop out over time. At an observational level, the results of this study show that, for the patients with RRMS taking continuous daily GA for up to 15 years, there has been a consistent low relapse rate and slow progression of disability. It is also possible that these patients were destined to have relatively mild MS and have therefore chosen to remain on the medication longer than those with more severe disease. The reader will need to consider the implications of these opposing conclusions in interpreting the results presented.
The safety of GA therapy in these patients has not changed from what has been reported previously.15–19 The commitment of patients in the study to take daily SC injections for 15 years underscores the long-term tolerability and patient acceptance of GA. The study will continue for 20 years of prospective followup.
Acknowledgments
The authors acknowledge the assistance of Dottie Beerkircher, CCRP, Clinical Operations Teva Neuroscience, Horsham PA; and The Copaxone® Study Group in conducting this study. They acknowledge the assistance of Pippa Loupe, PhD, Teva Neuroscience, Kansas City, Missouri; Sivan Weiss, BS, Teva Pharmaceutical Industries Ltd., Petah Tiqva, Israel; Sheila Owens, BS, Medical Communication Co., Inc., Sarasota, Florida; and The Copaxone® Study Group in the development of this manuscript. The Copaxone® Study Group includes the following investigative teams: University of Pennsylvania Medical Center, Clyde Markowitz, MD, Dorothea Pfohl, RN, BS, MSCN; University of New Mexico School of Medicine, Gary A Rosenberg, MD, Elida Greinel, RN; Wayne State University School of Medicine, Omar A Khan, MD, Deena Lisak, BS, MA, RN, Alexandros Tselis, MD, PhD, John Kamholz, MD, PhD, Christina Caon, MSN, RN; UCLA School of Medicine, R Baumhefner, MD, Ricki Klutch, RN; University of Maryland School of Medicine, Christopher Bever, MD, Eleanor Katz, RN; VASLCHCS/ University of Utah, James B Burns, MD, Connie Kawai, RN; University of Rochester, Steven R Schwid, MD (Deceased), Cynthia Irish, Mary D Petrie, RN; Yale University School of Medicine, MD, Silva Markovic Plese, MD, George Blanco; University of Southern California School of Medicine, Norman Kachuck, MD; University of Texas Health Science Center at Houston, Staley Brod, MD, Myrna Koh, RN, patients at the University of Texas-Houston were seen in the Clinical Research Unit, supported by NIH grant UL1 RR024148; University of Wisconsin Hospital and Clinic, Benjamin Brooks, MD, Jennifer Parnell, BA, Kathy Roelke, RN. Teva Pharmaceuticals Industries Ltd, David Ladkani, PhD, Shaul Kadosh, MS, Yafit Stark, PhD. The funding for this study was provided by Teva Pharmaceuticals Industries Ltd, Petah Tiqva Israel and Teva Neuroscience, Kansas City, MO, USA.
References
- 1.Kremenchutzky M, Rice GPA, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 9: Observations on the progressive phase of the disease. Brain 2006;129:584–594 [DOI] [PubMed] [Google Scholar]
- 2.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989;112:133–146 [DOI] [PubMed] [Google Scholar]
- 3.Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 2001;56:1496–1504 [DOI] [PubMed] [Google Scholar]
- 4.European Study Group on Interferon b-1b in Secondary Progressive MS. Placebo-controlled multicentre randomized trial of interferon b-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998;352:1491–1497 [PubMed] [Google Scholar]
- 5.Panitch H, Miller A, Paty D, Weinshenker B. North American Study Group on interferon beta-1b in secondary progressive MS. Neurology 2004;63:1788–1795 [DOI] [PubMed] [Google Scholar]
- 6.Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon b-1a on MSFC progression in secondary progressive MS. Neurology 2002;59:679–687 [DOI] [PubMed] [Google Scholar]
- 7.Bornstein MB, Miller A, Slagle S, et al. A placebo-controlled, double-blind, randomized, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology 1991;41:533–539 [DOI] [PubMed] [Google Scholar]
- 8.Wolinsky JS, Narayana PA, O'Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 2007;61:14–24 [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing–remitting MS. Neurology 2006;67:944–953 [DOI] [PubMed] [Google Scholar]
- 10.Ebers G, Traboulsee A, Langdon D, Goodin D, Konieczny A, for the Betaseron/Betaferon LTF Study Group The interferon beta-1b 16-year long-term follow-up study: the results. Presented at the 58th Annual meeting of the American Academy of Neurology, San Diego, CA, 2006: PO1.079. [Google Scholar]
- 11.Kinkel RP, Kollman C, Glassman A, et al. Interferon beta-1a (Avonex) delays the onset of clinically definite MS over 5 years of treatment: results from the CHAMPIONS study. Presented at the 56th Annual Meeting of the American Academy of Neurology, San Francisco, CA, USA, 2004:D29.006 [Google Scholar]
- 12.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002;59:1412–1420 [DOI] [PubMed] [Google Scholar]
- 13.Trojano M, Pellegrini F, Fuiani A, et al. New natural history of interferon-b-treated relapsing multiple sclerosis. Ann Neurol 2007;61:300–306 [DOI] [PubMed] [Google Scholar]
- 14.Brown MG, Kirby S, Skedgel C, et al. How effective are disease-modifying drugs in delaying progression in relapsing-onset MS? Neurology 2007;69:1498–1507 [DOI] [PubMed] [Google Scholar]
- 15.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing– remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268–1276 [DOI] [PubMed] [Google Scholar]
- 16.Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Neurology 1998;50:701–708 [DOI] [PubMed] [Google Scholar]
- 17.Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing remitting multiple sclerosis patients observed for 6 years. Mult Scler 2000;6:255–266 [DOI] [PubMed] [Google Scholar]
- 18.Johnson KP, Ford CC, Lisak RP, Wolinsky JS. Glatiramer Acetate (Copaxone®): neurologic consequence of delaying glatiramer acetate therapy for multiple sclerosis: 8-year data. Acta Neurol Scand 2005;111:42–47 [DOI] [PubMed] [Google Scholar]
- 19.Ford CC, Johnson KP, Lisak RP, Panitch HS, Shifroni G, Wolinsky JS. A prospective open-label study of glatiramer acetate: Over a decade of continuous use in MS patients. Mult Scler 2006;12:309–320 [DOI] [PubMed] [Google Scholar]
- 20.Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J Neurol Sci 2003;206:135–137 [DOI] [PubMed] [Google Scholar]
- 21.D'Agostino R. Tutorial in biostatistics propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statist Med 1998;17:2265–2281 [DOI] [PubMed] [Google Scholar]
- 22.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Statist Med 2004;23:2937–2960 [DOI] [PubMed] [Google Scholar]
- 23.Ebers G, Traboulsee A, Landon D, Goodin D, Reder A, Konieczny A, for the Betaseron/Betaferon LTF Study Group Results from the Interferon Beta-1b 16-year long-term follow-up study. Presented at the 22nd Meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Madrid, Spain, September 27–30, 2006: P666. [Google Scholar]
- 24.Ebers G, Traboulsee A, Langdon D, Goodin D, Konieczny A, for the Betaseron/Betaferon LTF Study Group The interferon beta-1b 16-year long-term follow-up study: the results. Presented at 58th Annual meeting of the American Academy of Neurology, San Diego, CA, 2006: PO1.079. [Google Scholar]
- 25.Goodin DS, Ebers G, Traboulsee A, Konieczny A, for the Betaseron LTF Study Group The interferon beta-1b 16-year long-term follow-up study: clinical outcomes. Presented at the 131st Annual Meeting of the American Neurological Association, Chicago, IL, October 8–11, 2006: M-3. [Google Scholar]