Abstract
Background and objectives
Diagnosis of multiple sclerosis (MS) requires exclusion of diseases that could better explain the clinical and paraclinical findings. A systematic process for exclusion of alternative diagnoses has not been defined. An International Panel of MS experts developed consensus perspectives on MS differential diagnosis.
Methods
Using available literature and consensus, we developed guidelines for MS differential diagnosis, focusing on exclusion of potential MS mimics, diagnosis of common initial isolated clinical syndromes, and differentiating between MS and non-MS idiopathic inflammatory demyelinating diseases.
Results
We present recommendations for 1) clinical and paraclinical red flags suggesting alternative diagnoses to MS; 2) more precise definition of “clinically isolated syndromes” (CIS), often the first presentations of MS or its alternatives; 3) algorithms for diagnosis of three common CISs related to MS in the optic nerves, brainstem, and spinal cord; and 4) a classification scheme and diagnosis criteria for idiopathic inflammatory demyelinating disorders of the central nervous system.
Conclusions
Differential diagnosis leading to MS or alternatives is complex and a strong evidence base is lacking. Consensus-determined guidelines provide a practical path for diagnosis and will be useful for the non-MS specialist neurologist. Recommendations are made for future research to validate and support these guidelines. Guidance on the differential diagnosis process when MS is under consideration will enhance diagnostic accuracy and precision.
Keywords: diagnosis, differential diagnosis, multiple sclerosis
Introduction
Diagnostic criteria for multiple sclerosis (MS) have evolved over the past 50 years. Although successive versions have differed in emphasis, all have required dissemination of disease in space and time documented by either clinical, paraclinical, or laboratory criteria. Additionally, MS diagnostic criteria have emphasized that alternative explanation for the clinical presentation must be considered and excluded before a diagnosis of MS can be made [1–4].
The most recent McDonald Criteria formally integrate data from magnetic resonance imaging (MRI) and focus on early diagnosis of patients presenting with a clinically isolated syndrome (CIS) suggestive of MS (e.g., unilateral optic neuritis, internuclear ophthalmoplegia, partial myelopathy) [3,4]. Because most such patients will develop a second event over months or years, these diagnostic criteria have been perceived as being prognostic for subsequent disease activity (whether a further relapse will occur), rather than diagnostic (an instrument to differentiate MS from other diseases).
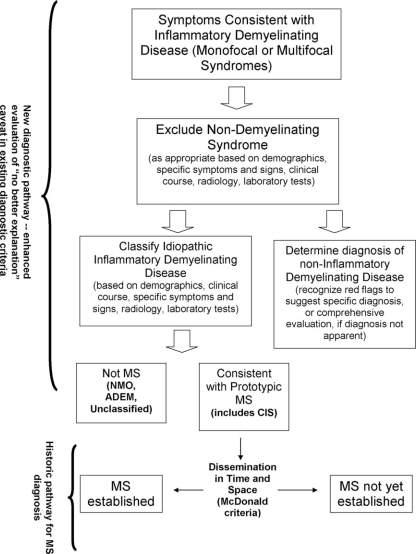
Patients suspected of having MS may have neurological syndromes upon initial examination that are clinically monofocal (no dissemination in space, for which a single CNS lesion can explain signs and symptoms), multifocal (dissemination in space, for which symptoms and signs can only be explained by at least two lesions in separate parts of the CNS) and that have been, over time, monophasic (a single occurrence), multiphasic (relapsing), or progressive in nature. Similar presentations can occur in patients who have an infectious, neoplastic, congenital, metabolic or vascular disease, or non-MS idiopathic inflammatory demyelinating disease (IIDD). Other IIDDs have symptoms that can be similar to those seen in MS (for instance, neuromyelitis optica [NMO], opticospinal MS in Asian populations [OSMS], acute disseminated encephalomyelitis [ADEM]), but differ in course, pathophysiology, treatment, and prognosis (see Figure 1). The ability to make an accurate diagnosis as early as possible is important for patient management, counseling, and optimal therapy.
Figure 1.
Steps in MS differential diagnosis.
A conceptual framework for differential diagnosis of MS does not exist. The European MAGNIMS group defined MRI red flags in the setting of clinically suspected MS, which suggest that an alternative diagnosis to MS is likely [5] but not in the context of other relevant clinical or laboratory examinations and findings. The current article describes an international consensus-based effort to guide the clinical, laboratory, and imaging assessment of patients with a possible diagnosis of MS, so as to help satisfy the requirement for “no better explanation” that is an integral component of all MS diagnostic criteria.
Methods
International Task Force composition and mission
In 2006, the International Advisory Committee on Clinical Trials in MS of the US National MS Society convened the Task Force on Differential Diagnosis in MS to develop a perspective that clinicians may use to address the principle of “no better explanation” for a suspected MS clinical presentation. The Task Force consisted of 18 international (United States, Canada, Europe, Japan) experts in the field of demyelinating disease with differing clinical and research expertise (neurology, ophthalmology, infectious disease, MRI).
The group's initial mission statement was: to provide a data-driven and consensus-based diagnostic approach for patients who present with symptoms and objective clinical evidence suggesting CNS white matter disease; to include guidance for appropriate clinical, radiological, and/or laboratory tests that should be done to exclude alternative diagnoses, especially those that are amenable to appropriate treatment; and to develop a practical tool for neurologists to facilitate accurate diagnosis and to guide management and which will complement the McDonald Diagnostic Criteria. The intent was not to present a comprehensive literature or conceptual review because the spectrum of differential diagnoses is enormous. We focused on patients who present with objective signs that are suggestive of CNS white matter disease and also considered apparently asymptomatic individuals or patients with other common clinically distinct neurological conditions (e.g., migraine) in whom lesions suggestive of white matter disease are showed through MRI.
Task Force work plan
To address these questions the Task Force formed into three working groups focused on exclusion of potential alternatives to an MS diagnosis, on diagnosis of common initial isolated syndromes when MS is in question, and on differentiating between MS and non-MS IIDDs. Consensus within subgroups and in the Task Force as a whole was reached and recommendations developed through a series of conference calls and subgroup meetings over a year and a meeting of the full Task Force in February 2007. Although informed by published evidence identified by Task Force members when available, the effort did not include a formal literature survey. Opinion-based consensus among the convened experts was developed for diagnostic approaches and classifications for which evidence-based data were lacking.
Exclusion of diagnostic alternatives
One subgroup focused on the exclusion of diagnostic alternatives to MS and generated a series of clinical and paraclinical “red flags” that are likely to point away from an MS diagnosis. The group reviewed selected literature relating to demographics, general clinical and neurological findings, paraclinical and laboratory findings (including various imaging techniques and laboratory tests including serum and cerebrospinal fluid [CSF] analyses and evoked potentials) of a spectrum of diseases that might be reasonably considered in the differential diagnoses for MS.
Recognizing that when confronted with a patient with suspected CNS white matter disease for diagnostic evaluation, the first step is to do a clinical exam and order imaging and other laboratory tests to help assess the condition, a table of 79 demographic, clinical, laboratory, and imaging features was prepared. The table was rated independently by the six subgroup members on a 1–5 scale to classify these characteristics as major red flags (rating 4 or 5) that point fairly definitively to a specific non-MS alternative diagnosis or as minor red flags (rating 1 or 2), which suggest that a disorder other than MS should be considered. An intermediate score (rating 3) indicated uncertainty. Scores on each finding from each rater were summed and standard deviations (SD) were calculated for each item (a high SD represents a low degree of concordance among raters). The potential 79 red flags were then classified into three groups according to the following criteria:
Major red flags: total score ≥24 or total score of 23 and no more than one individual score of 3 (SD ≤ 0.41).
Intermediate red flags, indicating a lack of agreement among the raters about the weighting: total score of ≥13 and ≤23 with more than one individual rating of 3 (SD ≥ 4.1).
Minor red flags: total score ≤12 or total score of 13 with not more than one individual score of 3 (SD ≥ 0.41).
Diagnostic algorithms for common initial isolated syndromes suggestive of MS
A second subgroup focused on CISs that are frequently seen as a first presentation of disease that is eventually diagnosed as MS. The subgroup concluded that the term CIS is confusing in a diagnostic context because it is unclear if it refers to syndromes isolated in time, space, or both and because it lacks pathological specificity [6–9]. A more clear definition of CIS was developed. In addition, the subgroup developed diagnostic algorithms for three of the most typical CISs (optic neuropathy, brain stem, and spinal cord syndromes) and distinguished between features of CIS that commonly precede MS versus uncommon or atypical features that merit consideration of alternative diagnoses and an expanded evaluation.
Differentiating MS from non-MS IIDDs
A third subgroup evaluated clinical, demographic, and paraclinical factors that differentiate prototypic MS from “variants,” such as NMO and ADEM, and proposed consensus criteria for their diagnosis in light of recent data on specific imaging features and biomarkers. The subgroup also developed a working classification of IIDDs, recognizing that data on which to base such a classification was scant.
Consensus perspectives
The Panel agreed that in making a differential diagnosis, patients with presentations suggestive of MS should be evaluated using a sequential strategy and presented its findings accordingly:
The first approach helps to exclude diseases not likely to be MS or non-MS IIDD (for instance infectious, malignant, congenital, metabolic, vascular, and other diseases) and provides specific guidance for differential diagnosis of common initial presentations reflecting pathology in the optic nerve, brainstem, and spinal cord.
The second approach helps to differentiate prototypic MS from non-MS IIDDs and proposes a classification scheme and diagnostic criteria for non-MS IIDDs.
Eliminating likely alternatives to an MS diagnosis
Differential diagnostic schemes have devoted little attention to patients with clinical presentations of CNS disease similar to MS but which may not develop into MS. Such patients may include those who eventually are determined to have, for example, vascular or infectious disorders. Diagnostic evaluation strategies apply to individuals with:
-
1)
Clinical, laboratory, and imaging features that are “classic” for MS and where no features strongly suggest an alternative diagnosis. MS is likely. Additional examinations or tests beyond those that satisfy the McDonald criteria for MS are likely unnecessary.
-
2)
Features that are compatible with MS but occur in the presence of other features (red flags) that suggest a possible alternative diagnosis. MS can only be diagnosed after tests to exclude alternative diagnoses. In equivocal situations, repeated imaging and laboratory tests over a period of observation may be advisable before reaching a conclusive diagnosis.
-
3)
Clinical and/or paraclinical red flags that point to a non-MS diagnosis. MS is improbable. Efforts should be directed at defining the alternative condition, especially when treatable.
-
4)
Clinical and/or paraclinical findings that suggest the presence of MS with another superimposed disorder. Appropriate imaging and laboratory tests should be performed to confirm the coexistence of the two conditions.
Table 1 presents 79 clinical and paraclinical red flag findings of patients who present with CNS disease for which MS is being considered. Through a ballot process among the subgroup members, as described in the “Methods” section, 36 major red flags were identified that point fairly definitively to a non-MS alternative diagnosis, the majority of which are clinical in nature. Eleven minor red flags were identified that suggest while MS is a possible diagnosis, a disorder other than MS should be considered, and that a decision cannot be made simply based on the noted assessment alone. An additional 32 clinical, paraclinical, and laboratory assays, many of which are imaging findings, were determined to be of intermediate weight. These received a relatively high mean score for weighting in the consensus process, but with high SDs among the raters, indicating a lack of consensus on their weight and importance. Their value for differential diagnosis should be considered in the overall context in which they appear (i.e., additional informative clinical, laboratory, or paraclinical findings).
Table 1.
Red flags
| Red flag | Type Clinical | Total score | SD | Red flaga | Examples of alternative diagnosis |
| Bone lesions | Clinical | 30 | 0.00 | Major | Histiocytosis; Erdheim Chester disease |
| Lung involvement | Clinical | 30 | 0.00 | Major | Sarcoidosis; Lymphomatoid granulomatosis |
| Multiple cranial neuropathies or polyradiculopathy | Clinical | 30 | 0.00 | Major | Chronic meningitis, including sarcoidosis and tuberculosis; Lyme disease |
| Peripheral neuropathy | Clinical | 30 | 0.00 | Major | B12 deficiency; adrenoleukodystrophy; metachromatic leukodystrophy, Lyme disease |
| Tendon xanthomas | Clinical | 30 | 0.00 | Major | Cerebrotendinous xanthomatosis |
| Cerebral venous sinus thrombosis | MRI | 30 | 0.00 | Major | Behçet's disease; vasculitis; chronic meningitis, antiphospholipid or anticardiolipin antibody syndromes |
| Cardiac disease | Clinical | 29 | 0.41 | Major | Multiple cerebral infarcts; brain abscesses with endocarditis or right to left cardiac shunting |
| Myopathy | Clinical | 29 | 0.41 | Major | Mitochondrial encephalomyopathy (e.g., MELAS); Sjögren's syndrome |
| Renal involvement | Clinical | 29 | 0.41 | Major | Vasculitis; Fabry disease, systemic lupus erythematosus |
| Cortical infarcts | MRI | 29 | 0.41 | Major | Embolic disease; thrombotic thrombocytopenic purpura; vasculitis |
| Hemorrhages/microhemorrhages | MRI | 29 | 0.41 | Major | Amyloid angiopathy; Moya Moya disease; CADASIL; vasculitis |
| Meningeal enhancement | MRI | 29 | 0.41 | Major | Chronic meningitis; sarcoidosis; lymphomatosis; CNS vasculitis |
| Extrapyramidal features | Clinical | 28 | 0.52 | Major | Whipple's disease; multisystem atrophy; Wilson's disease |
| Livedo reticularis | Clinical | 28 | 0.52 | Major | Antiphospholipid antibody syndrome; systemic lupus erythematosus; Sneddon's syndrome |
| Retinopathy | Clinical | 28 | 0.52 | Major | Mitochondrial encephalomyopathy; Susac, and other vasculitides (retinal infarction); neuronal ceroid lipofuscinosis |
| Calcifications on CT scans | MRI | 28 | 0.52 | Major | Cysticercosis; toxoplasmosis, mitochondrial disorders |
| Diabetes insipidus | Clinical | 28 | 0.82 | Major | Sarcoidosis; histiocytosis; neuromyelitis optica |
| Increase serum lactate level | Clinical | 27 | 0.55 | Major | Mitochondrial disease |
| Selective involvement of the anterior temporal and inferior frontal lobe | MRI | 27 | 0.55 | Major | CADASIL |
| Hematological manifestations | Clinical | 27 | 0.84 | Major | Thrombotic thrombocytopenic purpura; vitamin B12 deficiency; Wilson's disease (hemolytic anemia); copper deficiency |
| Lacunar infarcts | MRI | 27 | 0.84 | Major | Hypertensive ischemic disease; CADASIL; Susac syndrome |
| Persistent Gd-enhancement and continued enlargement of lesions | MRI | 27 | 0.84 | Major | Lymphoma; glioma; vasculitis; sarcoidosis |
| Mucosal ulcers | Clinical | 27 | 1.22 | Major | Behçet's disease |
| Myorhythmia | Clinical | 27 | 1.22 | Major | Whipple's disease |
| Hypothalamic disturbance | Clinical | 26 | 0.52 | Major | Sarcoidosis; neuromyelitis optica; histiocytosis |
| Recurrent spontaneous abortion or thrombotic events | Clinical | 26 | 0.52 | Major | Antiphospholipid antibody syndrome; thrombotic thrombocytopenic purpura; metastatic cancer with hypercoagulable state |
| Simultaneous enhancement of all lesions | MRI | 26 | 0.52 | Major | Vasculitis; lymphoma; sarcoidosis |
| Rash | Clinical | 26 | 0.82 | Major | Systemic lupus erythematosus; T-cell lymphoma; Lyme disease, Fabry disease |
| T2-hyperintensity in the dentate nuclei | MRI | 26 | 0.82 | Major | Cerebrotendinous xanthomatosis |
| Arthritis, polyarthalgias, myalgias | Clinical | 26 | 1.63 | Major | Systemic lupus erythematosus; Lyme disease; fibromyalgia |
| Amyotrophy | Clinical | 25 | 0.75 | Major | Amyotrophic lateral sclerosis; syringomyelia; polyradiculpathy |
| Headache or meningismus | Clinical | 25 | 0.98 | Major | Venous sinus thrombosis; chronic meningitis; lymphoma or glioma, vasculitis, systemic lupus erythematosus |
| T1-hyperintensity of the pulvinar | MRI | 25 | 0.98 | Major | Fabry disease; hepatic encephalopathy; manganese toxicity |
| Persistently monofocal manifestations | Clinical | 24 | 0.63 | Major | Structural lesion (e.g., Chiari malformation); cerebal neoplasm |
| Large and infiltrating brainstem lesions | MRI | 24 | 1.10 | Major | Behçet's disease; pontine glioma |
| Predominance of lesions at the cortical/subcortical junction | MRI | 23 | 0.41 | Major | Embolic infarction; vasculitis; progressive multifocal leukoencephalopathy |
| Hydrocephalus | MRI | 23 | 0.98 | Intermediate | Sarcoidosis or other chronic meningitis; lymphoma or other CNS neoplasm |
| Punctiform parenchymal enhancement | MRI | 23 | 0.98 | Intermediate | Sarcoidosis; vasculitis |
| Sicca syndrome | Clinical | 23 | 1.33 | Intermediate | Sjögren's syndrome |
| T2-hyperintensities of U-fibers at the vertex, external capsule and insular regions | MRI | 22 | 1.37 | Intermediate | CADASIL |
| Gastrointestinal symptoms | Clinical | 22 | 1.51 | Intermediate | Whipple's disease; celiac disease and other malabsorptive states that lead to B12 or copper deficiency |
| Regional atrophy of the brainstem | MRI | 21 | 0.55 | Intermediate | Behçet's disease; adult onset Alexander's disease |
| Diffuse lactate increase on brain MRS | MRI | 21 | 0.84 | Intermediate | Mitochondrial disease |
| Marked hippocampal and amygdala atrophy | MRI | 21 | 0.84 | Intermediate | Hyperhomocystinemia |
| Loss of hearing | Clinical | 21 | 1.38 | Intermediate | Susac's syndrome; glioma; vertebrobasilar infarction |
| Fulminant course | Clinical | 20 | 0.82 | Intermediate | Thrombotic thrombocytopenic purpura; intravascular lymphoma; acute disseminated encephalomyelitis |
| Symmetrically distributed lesions | MRI | 20 | 0.82 | Intermediate | Leukodystrophy |
| T2-hyperintensities of the basal ganglia, thalamus and hypothalamus | MRI | 20 | 1.03 | Intermediate | Behçet's disease; mitochondrial encephalomyopathies; Susac's syndrome; acute disseminated encephalomyelitis |
| Diffuse abnormalities in the posterior columns of the cord | MRI | 20 | 1.37 | Intermediate | B12 deficiency; copper deficiency; paraneoplastic disorder |
| Increase serum ACE level | Clinical | 20 | 1.86 | Intermediate | Sarcoidosis; histiocytosis |
| Prominent family history | Clinical | 19 | 0.41 | Intermediate | Depending on pattern of inheritance suggested by family history: hereditary spastic paraparesis; leukodystrophy; Wilson's disease; mitochondrial disorder; CADASIL |
| Constitutional symptoms | Clinical | 19 | 1.17 | Intermediate | Sarcoidosis; Whipple's disease, vasculitis |
| Lesions across GM/WM boundaries | MRI | 19 | 1.1 7 | Intermediate | Hypoxic-ischemic conditions; vasculitis; systemic lupus erythematosus |
| T2-hyperintensities of the temporal pole | MRI | 19 | 1.17 | Intermediate | CADAS IL |
| Complete ring enhancement | MRI | 18 | 0.63 | Intermediate | Brain abscess; glioblastoma; metastatic cancer |
| Progressive ataxia alone | Clinical | 18 | 1.10 | Intermediate | Multisystem atrophy; hereditary spinocerebellar ataxia; paraneoplastic cerebellar syndrome |
| Central brainstem lesions | MRI | 17 | 0.75 | Intermediate | Central pontine myelinolysis; hypoxicischemic conditions; infarct |
| Predominant brainstem and cerebellar lesions | MRI | 1 7 | 0.75 | Intermediate | Behçet's disease; pontine glioma |
| Neuropsychiatric syndrome | Clinical | 1 7 | 1.33 | Intermediate | Susac's syndrome; systemic lupus erythematosus; Wilson's disease, GM2 gangliosidosis |
| Lesions in the center of CC, sparing the periphery | MRI | 1 7 | 1.33 | Intermediate | Susac's syndrome |
| Seizure | Clinical | 16 | 1.63 | Intermediate | Whipple's disease; vasculitis; metastases |
| Dilation of the Virchow-Robin spaces | M RI | 15 | 0.55 | Intermediate | Hyperhomocystinemia; primary CNS angiitis |
| Uveitis | Clinical | 15 | 0.84 | Intermediate | Sarcoidosis; lymphoma; Behcet's disease |
| Cortical/subcortical lesions crossing vascular territories | MRI | 14 | 1.21 | Intermediate | Ischemic leukoencephalopathy; CADASIL; vasculitis |
| Pyramidal motor involvement alone | Clinical | 13 | 0.75 | Intermediate | Primary lateral sclerosis variant of ALS; hereditary spastic paraparesis |
| Large lesions with absent or rare mass effect and enhancement | MRI | 1 3 | 0.98 | Intermediate | Progressive multifocal leukoencephalopathy |
| Gradually progressive course from onset | Clinical | 13 | 1.1 7 | Intermediate | HTLV-1 associated myelopathy; adrenomyeloneuropathy; adrenoleukodystrophy; metachromatic leukodystrophty, B12 deficiency |
| No “occult” changes in the NAWM | MRI | 1 3 | 1.33 | Intermediate | Lyme disease, isolated myelitis, CADASIL |
| Brainstem syndrome | Clinical | 7 | 0.41 | Minor | Pontine glioma; cavernous angioma; vertebrobasilar ischemia |
| No enhancement | MRI | 8 | 0.52 | Minor | Progressive multifocal leukoencephalopathy; ischemic lesions; metachromatic leukodystrophy |
| Myelopathy alone | Clinical | 9 | 0.55 | Minor | Chiari type 1 malformation; cord compression including cervical spondylosis; B12 or copper deficiency; HTLV1 |
| No optic nerve lesions | MRI | 9 | 0.55 | Minor | Metastatic carcinoma; gliomatosis cerebri; toxoplasmosis |
| Onset before age 20 | Clinical | 10 | 0.52 | Minor | Mitochondrial encephalomyopathy; leukodystrophy; Friedrich's ataxia |
| No spinal cord lesions | MRI | 10 | 0.52 | Minor | Multiple infarcts; vasculitis; progressive multifocal leukoencephalopathy |
| Abrupt onset | Clinical | 11 | 1.17 | Minor | Cerebral infarction; cerebral hemorrhage; cerebral venous sinus thrombosis |
| Large lesions | MRI | 11 | 0.75 | Minor | Glioblastoma; lymphoma; progressive multifocal leukoencephalopathy |
| No T1 hypointense lesions (black holes) | MRI | 11 | 0.75 | Minor | Ischemic degenerative leukoencephalopathy; progressive multifocal leukoencephalopathy |
| Onset after age 50 | Clinical | 12 | 0.89 | Minor | Cerebral infarction; amyloid angiopathy; lymphoma |
| Marked asymmetry of WM lesions | MRI | 12 | 0.89 | Minor | Glioblastoma; lymphoma; cerebral infarction |
aRed flags are ordered from the most “major” to the most “minor” as per subgroup rankings described in text. Major red flags point fairly definitively to a non-MS diagnosis; minor red flags may be consistent with MS or an alternative diagnosis. Intermediate red flags are those for which there was poor agreement and uncertainty among raters about the weighting of the flag for differential diagnosis in MS, especially in isolation of other informative symptoms, signs, and assays. Minor red flags suggest that a disease other than MS should be considered and fully explored, but an MS diagnosis is not excluded.
Differential diagnosis of initial isolated clinical presentations
At first presentation when MS is in question, there may already be a history of more than one relapse or of a progressive course from onset. However, most patients eventually diagnosed with MS present with an acute clinical episode reflecting CNS white matter pathology, generally called a CIS. Because this occurs frequently and because the absence of information indicating dissemination in time precludes an immediate diagnosis of MS, we focused on differential diagnosis of such isolated syndromes.
Defining and classifying CIS
CIS presentations most commonly involve a single optic nerve, the spinal cord, or the brain stem, although other isolated syndromes may occur, such as ones affecting the cerebral hemispheres (e.g., hemianopia). Although widely used to signal the first presentation of demyelinating disease, the term CIS is inadequate: it has been variably used to group patients with: i) a single clinical event and signs that indicate a single lesion only (thus disease isolated in space and time); ii) recurrent episodes in a single location (thus with disease isolated in space but not in time, e.g. recurrent optic neuritis) [10]; and iii) a single clinical event where the symptoms and/or neurological examination findings suggest the presence of two or more lesions in separate locations (thus isolated in time but not in space).
Most clinical trials of patients with CIS aimed at determining the role of interferon-β in delaying time to clinically definite MS included patients whose clinical episode was monophasic but who were polysymptomatic and whose examination showed multifocal CNS signs (e.g., a patient with acute optic neuritis and Lhermitte's sign or a patient with optic neuritis who also has an extensor plantar response) [7,8]. In one CIS trial, 48% of patients with CIS had evidence for multifocal disease [11]. Strategies have been proposed to reduce variability in interpreting clinical trial outcomes by distinguishing initial monofocal from multifocal presentations and stratifying enrolment accordingly [8]. Furthermore, the term CIS ignores first presentations that may not be clinical but may be detected by paraclinical and laboratory findings [12], and does not discriminate between patients who have a single clinical presentation with or without additional symptomatic lesions on MRI, two entities that have different prognoses [13–16].
Because of these ambiguities of interpretation and the importance of CIS in the differential diagnosis process, the Panel felt it important to more closely define the term. We agreed that a CIS should be defined as a monophasic presentation with suspected underlying inflammatory demyelinating disease. “Monophasic presentation” implies a single clinical episode at first presentation that is of relatively rapid onset. Multiple simultaneous clinical/ paraclinical presentations (representing dissemination in space) are possible, although dissemination in time should not be evident. Four classes of CIS can thus be defined based on whether the monophasic clinical presentation has mono- or multifocal clinical or MRI features (Table 2; CIS types 1–4). Finally, there should be reasonable grounds for suspecting inflammatory demyelinating disease as the underlying pathology.
Table 2.
Clinically isolated syndromes (CIS) in the differential diagnosis of MS
| Type 1 CIS: clinically monofocal, at least one asymptomatic MRI lesion |
| Type 2 CIS: clinically multifocal, at least one asymptomatic MRI lesion |
| Type 3 CIS: clinically monofocal, MRI may appear normal; no asymptomatic MRI lesions |
| Type 4 CIS: clinically multifocal, MRI may appear normal; no asymptomatic MRI lesions |
| Type 5 CIS: no clinical presentation to suggest demyelinating disease, but MRI is suggestive |
Note: symptomatic lesions should appear typical for demyelination; they may be located in the brain or cord, although more often occur in the brain; current evidence on the prognostic value of asymptomatic lesions comes mainly from brain imaging.
MRI can influence a decision as to whether a presentation is due to monofocal or multifocal lesions and the likelihood for an ultimate diagnosis of MS (Table 2). Patients with at least one asymptomatic MRI lesion characteristic of demyelination have a high probability of later meeting criteria for MS (CIS Types 1 and 2); the prognosis varies but is not strongly based on the number and location of lesions [13,14]. Patients with monofocal clinical presentations and no asymptomatic lesions characteristic of demyelination have a relatively low risk for later meeting criteria for MS (CIS Type 3) [16]. A multifocal clinical presentation without MRI-detected asymptomatic lesions is likely rare (CIS Type 4) and such patients require further follow-up to determine if they have MS or another condition.
Although symptoms and signs of a monophasic illness have been an essential prerequisite for diagnosis of CIS, there is an exceptional scenario that the Panel feels warrants inclusion as CIS Type 5 (Table 2): patients who have no symptoms or only non-specific symptoms (e.g., headache, dizziness), but have MRI evidence for multifocal abnormalities typical for demyelination. Such patients are increasingly identified using MRI for incidental indications (e.g., headache) especially with high-field strength magnets with greater sensitivity for such lesions [17]. Current criteria preclude a diagnosis of MS without objective clinical evidence for CNS abnormality and the ability to establish a confident diagnosis of MS in such individuals and their natural history should be addressed through prospective studies [18].
Differential diagnosis of CIS affecting the optic nerves, brainstem, and spinal cord
A wide array of isolated CNS syndromes encountered in a diagnostic work-up for possible MS can be included in the CIS definition. As noted, some are more strongly predictive of an eventual MS diagnosis than others, depending on imaging characteristics but their clinical appearance at initial presentation can also provide clues to the likelihood of an eventual MS diagnosis. Table 3 categorizes CIS presentations of patients eventually diagnosed as having MS into i) those that are typical of patients eventually diagnosed as having MS; (ii) those that are less common but nevertheless may be an initial presentation in patients eventually diagnosed with MS, or may signal another disease; and (iii) those that are atypical and suggest an alternative diagnosis.
Table 3.
CIS clinical features and likelihood of signaling an MS diagnosis
| CIS features typically seen in MS | Less common CIS features which may be seen in MS | Atypical CIS features not expected in MS |
| Optic nerve | ||
| Unilateral optic neuritis | Bilateral simultaneous optic neuritis | Progressive optic neuropathy |
| Pain on eye movement | No pain | Severe, continuous orbital pain |
| Partial and mainly central visual blurring | No light perception | Persistent complete loss of vision |
| Normal disc or mild disc swelling | Moderate to severe disc swelling with no hemorrhages | Neuroretinitis (optic disc swelling with macular star) |
| Uveitis (mild, posterior) | Uveitis (severe, anterior) | |
| Brain stem/cerebellum | ||
| Bilateral internuclear ophthalmoplegia | Unilateral internuclear ophthalmoplegia, facial palsy, facial myokymia | Complete external ophthalmoplegia; vertical gaze palsies |
| Ataxia and multidirectional nystagmus | Deafness | Vascular territory syndrome, e.g., lateral medullary |
| Sixth nerve palsy | One-and-a-half syndrome | Third nerve palsy |
| Facial numbness | Trigeminal neuralgia | Progressive trigeminal sensory neuropathy |
| Paroxysmal tonic spasms | Focal dystonia, torticollis | |
| Spinal cord | ||
| Partial myelopathy | Complete transverse myelitis | Anterior spinal artery territory lesion (sparing posterior columns only) |
| Lhermitte's symptom | Radiculopathy, areflexia | Cauda equina syndrome |
| Deafferented hand | Segmental loss of pain and temperature sensation | Sharp sensory level to all modalities and localized spinal pain |
| Numbness | Partial Brown-Sequard syndrome (sparing posterior columns) | Complete Brown-Sequard syndrome |
| Urinary urgency, incontinence, erectile dysfunction | Faecal incontinence | Acute urinary retention |
| Progressive spastic paraplegia (asymmetrical) | Progressive spastic paraplegia (symmetrical) | Progressive sensory ataxia (posterior columns) |
| Cerebral hemispheres | ||
| Mild subcortical cognitive impairment | Epilepsy | Encephalopathy (obtundation, confusion, drowsiness)a |
| Hemiparesis | Hemianopia | Cortical blindness |
aAlthough encephalopathy is required for ADEM, it may also be seen at presentation and/or during the course of MS.
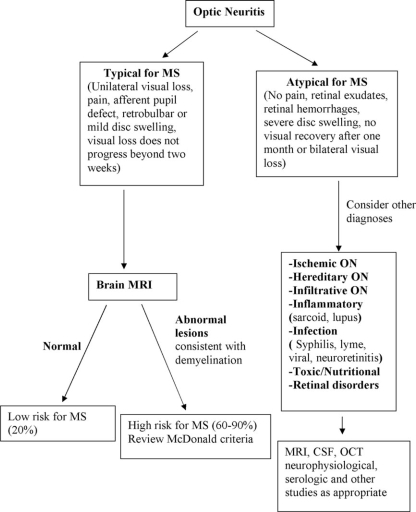
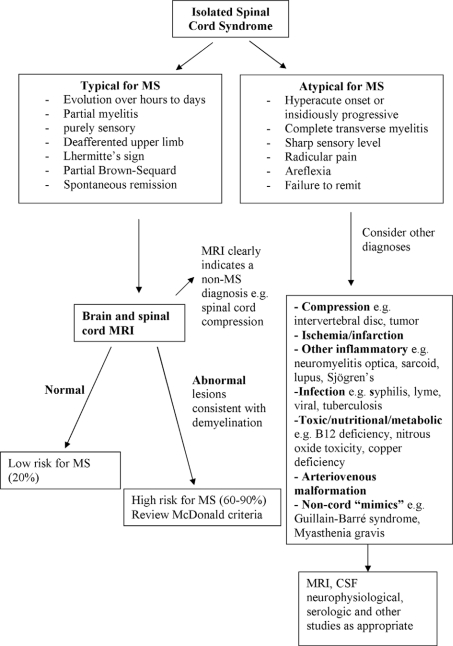
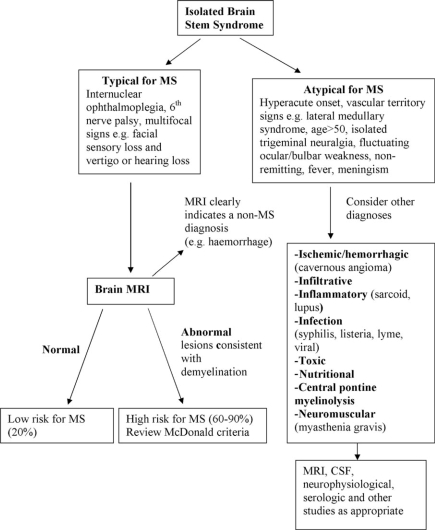
Perhaps the three most common CIS syndromes seen at presentation in the MS diagnosis process include those affecting the optic nerve, brain stem, and spinal cord. Flow diagrams illustrating an approach to their differential diagnosis are presented in Figures 2–4. The flow diagrams are presented to illustrate some principles of the clinical and laboratory evaluation and do not aim to be comprehensive; they emphasize typical and readily available investigations and outcomes.
Figure 2.
Differential diagnosis upon presentation with demyelinating optic neuritis.
Figure 4.
Differential diagnosis upon presentation with demyelinating spinal cord syndrome.
Figure 3.
Differential diagnosis upon presentation with demyelinating brain stem syndrome.
Differential diagnosis of MS and IIDD: nosology and classification
Although MS may be the most frequent ultimate diagnosis of a case presenting with IIDD, clinical, radiological, and immunological biomarkers that persist over time may help distinguish and define subgroups of IIDD from MS. We focused on two of the most common differential diagnoses for IIDD: NMO and ADEM. The Panel built upon recently proposed criteria for NMO that are 90% sensitive and specific in differentiating NMO from MS [19,20]. However, criteria for diagnosis of ADEM are not well validated and about 30% of patients who meet the ADEM criteria at initial presentation will later receive a diagnosis of MS [21–24]. The rate of conversion from ADEM to MS will be higher in adults (in whom ADEM is less common) than in children, in whom about 20% of those initially diagnosed with ADEM will ultimately be diagnosed with MS.
Definition and differential diagnosis of NMO
NMO is perhaps the most commonly seen non-MS IIDD [25,26]. It has been distinguished in the past from MS because of largely restricted manifestations of optic neuritis and myelitis and by its monophasic, not relapsing, course. However, more contemporary studies suggest that NMO is usually a relapsing IIDD, blurring the distinction between NMO and MS [27–31]. Normal brain imaging and longitudinally extensive cord lesions in the context of acute myelitis have helped to distinguish NMO from MS. A recently discovered, highly specific, and moderately sensitive serum biomarker, NMO-IgG, is useful for diagnosis of NMO [32,33].
In distinguishing NMO from MS, the subgroup agreed that:
NMO should be distinguished from MS because of its different course and prognosis [28,31] and because of putative differences in response to immunomodulatory therapy [34–36].
NMO is most commonly a relapsing disorder, and hence that characteristic is not useful to distinguish it from MS.
The key clinical characteristics that distinguish NMO from MS are the predilection in NMO for severe episodes of myelitis often, but not always, manifest as a complete transverse myelitis, and for severe episodes of optic neuritis, often but not always with incomplete recovery. The myelitis, unlike that which occurs in MS, is usually accompanied in the acute phase by a T2-weighted spinal cord lesion extending over three or more spinal segments (longitudinally extensive transverse myelitis, LETM) which may be hypointense on T1-weighted MRI and also associated with varying degrees of gadolinium enhancement.
Brain involvement in NMO is uncommon clinically and brain MRI is often normal [27,37] particularly early in the disease [38,39]. When present, brain lesions generally do not fulfill typical Barkhof/Tintoré criteria for dissemination in space [40,41]. Brain lesions in NMO may have a predilection for regions with high expression of aquaporin 4, including the hypothalamus, medulla, and other brainstem areas [41,42].
Oligoclonal bands or elevated IgG index in CSF are detected in 10–20% of patients with NMO compared with 70–90% of patients with MS (Table 4) [28,43].
Table 4.
Diagnostic criteria for neuromyelitis optica (NMO)a
| Major criteria: (all required, but may be separated by unspecified interval) |
| Optic neuritis in one or more eyes |
| Transverse myelitis, clinically complete or incomplete, but associated with radiological evidence of spinal cord lesion extending over three or more spinal segments on T2-weighted MRI images and hypointensity on T1-weighted images when obtained during acute episode of myelitis |
| No evidence for sarcoidosis, vasculitis, clinically manifest systemic lupus erythematosus or Sjögren's syndrome, or other explanation for the syndrome. |
| Minor criteria: (at least one must be satisfied) |
| Most recent brain MRI scan of the head must be normal or may show abnormalities not fulfilling Barkhof criteria used for McDonald diagnostic criteria, includingb: |
| Non-specific brain T2 signal abnormalities not satisfying Barkhof criteria as outlined in McDonald criteria |
| Lesions in the dorsal medulla, either in contiguity or not in contiguity with a spinal cord lesion |
| Hypothalamic and/or brainstem lesions |
| “Linear” periventricular/corpus callosum signal abnormality, but not ovoid, and not extending into the parenchyma of the cerebral hemispheres in Dawson finger configuration |
| Positive test in serum or CSF for NMO-IgG/aquaporin-4 antibodies |
aThese criteria exclude limited or inaugural syndromes that may be NMO, such as recurrent transverse myelitis with longitudinally extensive spinal cord lesions or recurrent optic neuritis; further study is warranted to clarify their relationship to NMO, especially in the setting of seropositivity for NMO-IgG/aquaporin-4 antibodies.
bPeriodic surveillance with brain MRI scanning is necessary to monitor for emergence of new lesions that may lead to a revised diagnosis.
Some subjects presenting with IIDD have recurrent transverse myelitis alone accompanied by long spinal cord lesions or recurrent optic neuritis alone and are seropositive for NMO-IgG. These may represent limited or inaugural syndromes of NMO. Although many clinicians believe that such patients should be treated as if they have NMO [26,44,45], until the relationship between isolated recurrent transverse myelitis or isolated recurrent optic neuritis and NMO is better established, the Panel concluded that these spatially limited syndromes should not qualify as NMO, even in the presence of a positive NMO-IgG serum assay. The subsequent development of optic neuritis in a patient with myelitis or vice versa may permit a later diagnosis of NMO.
Biopsy evidence of sarcoidosis or vasculitis, which can occasionally cause optic neuritis and myelitis, excludes NMO. Patients with optic neuritis, myelitis, or both occurring with preexisting or subsequently developing systemic lupus erythema-tosus or Sjögren's syndrome, a not infrequent clinical occurrence, are seropositive for NMO-IgG at the same frequency as in “uncomplicated NMO” and may have both NMO and organ-specific or non-organ specific autoimmunity [46]. However, the Panel concluded (conservatively) that pending further study, clinical evidence of systemic lupus erythematosus or Sjögren's should exclude a diagnosis of NMO. Seropositivity for antinuclear antibodies (ANA) or Sjögren's (SSA/SSB), which is commonly uncovered in the evaluation of patients for NMO, does not exclude the diagnosis of NMO when clinical evidence for systemic lupus erythe-matosus or Sjögren's syndrome is lacking.
Asian optic-spinal forms of MS (OSMS) may be confused with NMO. It is unclear whether differences between OSMS and NMO in Asia and Western countries are due to biological differences or to differences in nomenclature. In Asia, patients with optic neuritis and myelitis, regardless of the length of their spinal cord lesion, are classified as OSMS, whereas such patients without LETM lesions in most instances would be classified as having typical MS in Western countries. Furthermore, whenever clinical or radiological involvement of the brain is detected (except when confined to the brainstem or hypothalamus), MS is diagnosed in Asia [47,48], whereas such patients who fulfill other criteria for NMO in Western countries are typically diagnosed with NMO. Continued natural history studies of such patients will be necessary to determine if clinical predictions about their phenotypes and responses to therapy are adequately predicted by the diagnosis assigned using Asian and Western approaches.
Definition and differential diagnosis of ADEM
ADEM has been historically recognized as distinct from MS based on its monophasic course and encephalopathy or coma in combination with mul-tifocal symptoms (e.g., cerebellar signs, cerebral motor or sensory features, optic neuritis or myelitis) characteristic of an IIDD, often following an infectious illness. MRI typically shows usually symmetrical multifocal or diffuse brain lesions [49]. Even when conservatively defined by requiring encepha-lopathy, an initial diagnosis of ADEM is often revised to prototypic MS after evidence emerges for continuing clinical activity consistent with MS [50]. It recently has been argued that the traditional requirement of a monophasic course for ADEM might be too strict and that some patients experience recurrence of their initial ADEM symptoms with re-emergence of the same MRI lesions as were present at the time of their initial illness (recurrent ADEM) [21–24]. Although characteristics such as encephalopathy with multifocal symptoms typical of IIDD may make ADEM more likely than MS, no clinical, paraclinical, or imaging criteria reliably distinguish fulminant initial episodes of MS from ADEM (Table 5) [51].
Table 5.
Criteria for diagnosis of acute disseminated encephalomyelitis (ADEM)
| Subacute encephalopathy (altered level of consciousness, behavior, or cognitive function) |
| Evolution over 1 week to 3 months; new symptoms, including focal/multifocal demyelinating syndromes, such as optic neuritis or myelitis within the first 3 months from onset are allowed, as long as they are not separated by a period of complete remission from the initial symptoms (in which case the diagnosis is MS) |
| Accompanied by improvement or recovery although residual neurological deficits may be present |
| MRI shows predominantly symptomatic white matter lesions that |
| Are acute (remote lesions accompanied by encephalomalacia cast doubt on the diagnosis if there is no previous explanation for them other than remote demyelinating disease) |
| Are multiple but rarely a single large lesion |
| Are supra- or infra-tentorial or both |
| Generally include at least one large (1–2 cm diameter) lesion |
| Variably enhance with gadolinium (gadolinium enhancement is not required)a |
| May be accompanied by basal ganglia lesions, but their presence is not required |
aSimultaneous enhancing lesions may occur but are not required; when present, this MRI finding may increase suspicion of ADEM, but should also lead to suspicion of other causes (e.g., vasculitis or lymphoma).
Recently proposed definitions for pediatric MS and ADEM were designed to minimize overlap between the two disorders, by requiring encepha-lopathy as an essential component of the ADEM definition [52]. Although these criteria are consensus-driven and based on pediatric cohorts, it is reasonable to propose their use in adult populations as well, pending further evaluation of differences between ADEM in children and adults. These criteria emphasize the relative specificity of encephalopathy in ADEM although they do not distinguish between degrees of encephalopathy, which can vary from irritability to coma; severe encepha-lopathy may be more specific for ADEM than milder encephalopathy.
The Panel proposed that a diagnosis of ADEM should be made in patients with a first event compatible with demyelinating disease that is acute or sub-acute in onset (over days to weeks), with a stable or stuttering course, but only when additional characteristics are present. Encephalopathy, manifest either as altered level of consciousness, behavioral change, or altered cognitive function, should be present. New symptoms may emerge over intervals up to 3 months from onset without intervening remission (but not beyond 3 months). However, if a remission of the initial symptoms occurs followed by new symptoms after an interval of 1 month, MS is more likely than ADEM. Although MRI is non-specific in ADEM, the presence of multiple supra- or infra-tentorial lesions in combination with lesions of deep grey nuclei and at least one lesion greater than 1–2 cm in diameter are characteristic. Spinal cord lesions may or may not be present, but when present, tend to be longitudinally extensive (Table 5).
Very rare ADEM patients may experience recurrence of initial symptoms and signs after 3 months without development of new lesions although existing lesions may enlarge or re-enhance with gadolinium [52,53], in other words a recurrent
course as distinct from a multiphasic course which might include new symptoms and new lesions. If no other diagnosis is apparent after appropriate evaluation, the diagnosis “recurrent ADEM” is warranted. However, the emergence of new lesions with different symptoms beyond a 3-month interval from onset likely signals MS regardless of the specific clinical characteristics. The Panel did not endorse a diagnosis of “multiphasic ADEM” when new lesions and different symptoms emerge over time [52,53] because of the inability to distinguish such presentations from MS and because such a diagnosis could result in a delay in use of MS-specific immunotherapy. The Panel concluded that most such patients will continue to have inflammatory disease activity that is characteristic of MS.
A classification of idiopathic inflammatory disease
Recognizing the differences between MS, NMO, ADEM, and their variants, a classification for IIDDs was developed, which should be considered whenever IIDD is the working diagnosis (Table 6). MS is diagnosed when McDonald criteria for dissemination of symptoms/signs in space and time are reported [3,4] and includes relapsing-remitting, secondary progressive, primary progressive, and progressive-relapsing forms [54]. Subjects who present with atypical IIDD syndromes, such as Marburg's variant of MS [55,56], Balo's concentric sclerosis [57], and other tumefactive forms of demyelinating disease [58,59] are considered to be “unclassifiable” in terms of a possible MS diagnosis at first event, but if and when criteria for dissemination in space and time are satisfied, a diagnosis of MS might be considered appropriate in spite of an atypical initial or subsequent presentation. Alternative diagnoses, such as cerebral lymphoma, glioma-tosis cerebri, or vasculitis should strongly be considered as well. Some multi-episode relapsing presentations, such as relapsing transverse myelitis, may also be unclassifiable in terms of potential for an MS diagnosis at presentation when there is neither clinical nor MRI evidence for dissemination in space. NMO and ADEM are separate conditions in the category of IIDDs and occur in both monophasic and recurrent forms. Some initial presentations of CIS are truly monophasic inflammatory demyelinating conditions, but many patients with CIS syndromes, if not most, will develop MS.
Table 6.
Classification of idiopathic inflammatory demyelinating diseases
| At first event |
| CIS |
| ADEM |
| Monophasic NMO |
| Unclassifiable (unless/until further disease evolution) monophasic diseases, including fulminant (Marburg's variant), Balo's concentric sclerosis and tumefactive presentations |
| After subsequent clinical or radiological events |
| MSa |
| Relapsing NMO |
| Recurrent ADEM |
| Unclassified (unless/until further disease evolution); for example, recurrent optic neuritis or transverse myelitis without dissemination in space; or clinically monofocal presentation without asymptomatic MRI (MRI may appear normal) plus a previous history suggesting a separate CNS event without objective signs |
aMS includes any IIDD eventually meeting criteria for dissemination in time and space in the McDonald criteria, including initial presentations of CIS and ADEM that may evolve into MS, but not NMO and rare recurrent ADEM, also includes tumefactive demyelinating disease, Marburg's variant of MS when criteria for dissemination in time and space are met.
Discussion/conclusions
The range of diseases that must be excluded to make a diagnosis of MS is wide. Although the exclusion of more likely alternatives is a critical aspect of MS diagnosis, little attention has been paid to providing guidance for clinicians. The Panel has delineated two broad categories of diseases that should be considered in the differential diagnosis work-up of CNS disease suggestive of MS: non-IIDDs with symptoms and signs that may mimic IIDDs (including MS) and non-MS IIDDs, some of which are also relapsing and remitting. We have suggested a classification of clinical presentations into those that are typical and suggestive for MS and those that are atypical for MS, and have described a series of red flags that might signal a more likely alternative diagnosis. Our conclusions and recommendations are largely consensus driven and should be assessed in prospective clinical studies, preferably in large multicenter efforts that include both specialized MS referral centers and non-specialized clinical settings to test their applicability in both environments. Such studies could establish diagnostic algorithms that provide an accurate diagnosis for CNS syndromes suggestive of MS.
Prospective studies should include all CIS syndromes as redefined here, including those with normal MRI findings and those with asymptomatic lesions highly suggestive of MS that are detected incidentally. A first descriptive retrospective study of patients with incidentally detected asymptomatic lesions indicated that a subset of such patients frequently shows rapid early conversion to early clinically defined MS [18]. Prospective long-term follow-up is needed to assess the natural history of such individuals. About 30% of patients with an otherwise typical isolated CNS syndrome suggestive of demyelination have completely normal MRI apart from the symptomatic lesion(s) [16,40,60]. Ten to 14-year follow-up has shown that about 20% of such patients develop clinically definite MS [13,15]. Although some MRI-negative CIS patients have CSF oligoclonal bands typically seen in patients with MS, others do not [61]. Such patients may have a truly isolated syndrome in space and time and CIS may be the only diagnosis possible [62].
Both clinical and paraclinical assessments inform the differential diagnosis and evaluation of a patient. We have presented a series of consensus-based red flag criteria that the Panel agrees signal a high likelihood that a disease at initial presentation is not MS. The sensitivity, specificity, and accuracy of these red flags have not been investigated. Each red flag was rated by the Panel in isolation and not in a larger clinical and/paraclinical context; this is rarely the situation confronting a clinician making a diagnosis. The differential diagnosis may be different if a given red flag is the sole finding or appears in conjunction with other relevant symptoms, signs, and paraclinical and laboratory abnormalities. The value and meaning of an isolated red flag can often be resolved in context of demographic, clinical, and paraclinical findings. For instance, onset under age 20 is listed as a minor red flag. However, in the setting of diffuse and symmetrical white matter changes suggestive of leukodystrophy, young onset would be a major red flag suggesting a different diagnosis than MS. However, in the setting of facial numbness or optic neuritis, it is not a red flag at all. Defining sets of symptoms/signs and red flags that would increase the diagnostic confidence in making a diagnosis of MS versus other conditions is a high research priority.
The most common alternative conditions that mimic MS may be the most difficult to eliminate in differential diagnosis. The relative prevalence of disorders in a specific geographic area or population should be considered in reaching a diagnosis. However, the lack of accurate prevalence data for many alternative diagnoses (and their associated red flags) relative to MS currently makes it difficult to weight diagnoses based on prevalence.
Although our discussions included a detailed evaluation of MS in Asian populations and the characteristics of Asian OSMS versus NMO and MS, most data related to the differential diagnosis of IIDD arise from studies conducted in Western populations. The frameworks proposed here may be less applicable to non-Western populations, and this issue should be addressed prospectively. Regional ethnically based differences in disease definition continue to complicate the determination of similarities and differences between NMO and Asian OSMS and confound the interpretation of data on seropositivity rates for NMO-IgG in NMO and in Asian OSMS [32,33,48]. A worldwide consensus definition would facilitate further clinical and biomarker research.
Disease biomarkers will aid enormously in differential diagnosis. Accurate and sensitive disease markers – imaging or laboratory based – may provide non-invasive aids to differential diagnosis. The discovery of NMO-IgG that helps to distinguish NMO from MS is a good example. Relevant imaging advances may include non-conventional MR techniques to quantitate change in normal appearing white matter that may be relatively specific for MS, and high-field MRI to better visualize Dawson's fingers [17] or cortical lesions that may be specific to MS.
The Panel adopted diagnostic criteria for NMO that were modified from previously presented criteria [19], which must be considered tentative and subject to further international study and validation. Other groups have reported similar sensitivity and specificity to those originally reported and thereby provide some independent validation [20]. The criteria may require revision pending further investigation into the relationship between symptoms of NMO and systemic autoimmune diseases such as systemic lupus erythematosus [46]. It is unclear if limited presentations of NMO (e.g., recurrent optic neuritis; recurrent transverse myelitis with longitudinally extensive spinal cord lesions) in patients seropositive for aquaporin-4 autoantibodies are NMO spectrum diseases [44]. Although NMO is usually a relapsing disease, it may also be monophasic; detailed long-term follow-up of such cases may provide insights into pathological differences between the two.
The recommended criteria for ADEM (requiring encephalopathy and a limited time frame for recurrent ADEM events) and MS [4] should facilitate prompt differential diagnosis and consequently different recommendations for counseling and treatment based on the prognosis. Prospective follow-up studies of cases of ADEM are recommended to investigate the performance of these criteria.
This detailed consideration to differential diagnosis at first clinical presentation when MS is being considered should help to refine a key provision of the McDonald diagnostic criteria – the need to exclude other more likely explanations than MS for the clinical presentation. Our recommendations are largely consensus-based and evidence to support many of the recommendations is currently lacking. Algorithms and other recommendations for diagnostic procedures need to be tested prospectively in a relevant spectrum of clinical settings internationally to confirm or refine their utility.
Acknowledgements
The authors gratefully acknowledge constructive criticism provided in review of an earlier version of the manuscript by Drs Sten Fredrikson (Copenhagen), Andrew Goodman (Rochester, NY), Catherine Lubetzki (Paris), John Newsom-Davis (Oxford, deceased), Paul O'Connor (Toronto), Richard Rudick (Cleveland), and Jack Simon (Portland, OR). The work of the Task Force was supported by the National Multiple Sclerosis Society (US).
References
- 1.Schumacher FA, Beeve GW, Kibler FR, et al. Problems of experimental trials of therapy in multiple sclerosis. Ann NY Acad Sci 1965;122:552–568 [DOI] [PubMed] [Google Scholar]
- 2.Poser CM, Paty DW, Scheinberg LC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231 [DOI] [PubMed] [Google Scholar]
- 3.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–846 [DOI] [PubMed] [Google Scholar]
- 5.Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol 2006;5:841–852 [DOI] [PubMed] [Google Scholar]
- 6.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demye-linating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000;343:898–904 [DOI] [PubMed] [Google Scholar]
- 7.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomized study. Lancet 2001;357:1576–1582 [DOI] [PubMed] [Google Scholar]
- 8.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;10:1242–1249 [DOI] [PubMed] [Google Scholar]
- 9.Swanton JK, Rovira A, Tintoré M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol 2007;6:677–686 [DOI] [PubMed] [Google Scholar]
- 10.Ormerod IE, Miller DH, McDonald WI, et al. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain 1987;110:1579–1616 [DOI] [PubMed] [Google Scholar]
- 11.Uitdehaag BMJ, Kappos L, Bauer L, et al. Discrepancies in the interpretation of clinical symptoms and signs in the diagnosis of MS: a proposal for standardization. Mult Scler 2005;11:227–231 [DOI] [PubMed] [Google Scholar]
- 12.Mastronardo G, Rocca MA, Iannucci G, Pereira C, Filippi M. A longitudinal MR study of the presymptomatic phase in a patient with clinically definite multiple sclerosis. Am J Neuroradiol 1999;20:1268–1272 [PMC free article] [PubMed] [Google Scholar]
- 13.Brex PA, Ciccarelli O, O'Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002;246:158–164 [DOI] [PubMed] [Google Scholar]
- 14.Minneboo A, Barkhof F, Polman CH, Uitdehaag BM, Knol DL, Castelijns JA. Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Arch Neurol 2004;61:217–221 [DOI] [PubMed] [Google Scholar]
- 15.Optic Neuritis Study Group. Long-term brain magnetic resonance imaging changes after optic neuritis in patients without clinically definite multiple sclerosis. Arch Neurol 2004;61:1538–1541 [DOI] [PubMed] [Google Scholar]
- 16.Tintoré M, Rovira A, Rio J, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology 2006;67:968–972 [DOI] [PubMed] [Google Scholar]
- 17.Kangarlu A, Bourekas EC, Ray-Chaudhury A, Rammohan KW. Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla Am J Neuroradiol 2007;28:262–266 [PMC free article] [PubMed] [Google Scholar]
- 18.Lebrun C, Bensa C, Debouverie M, et al. Unexpected multiple sclerosis: follow-up of 30 patients with magnetic resonance imaging and clinical conversion profile J Neurol Neurosurg Psychiatry 2008;79:195–198 [DOI] [PubMed] [Google Scholar]
- 19.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 20.Saiz A, Zuliani L, Blanco Y, et al. Revised diagnostic criteria for neuromyelitis optica: application in a series of suspected patients J Neurol 2007;254:1233–1237 [DOI] [PubMed] [Google Scholar]
- 21.Cohen O, Steiner-Birmanns B, Biran I, Abramsky O, Honigman S, Steiner I. Recurrence of acute disseminated encephalomyelitis at the previously affected brain site Arch Neurol 2001;58:797–701 [DOI] [PubMed] [Google Scholar]
- 22.Hartung HP, Grossman RI. ADEM: distinct disease or part of the MS spectrum Neurology 2001;56:1257–1260 [DOI] [PubMed] [Google Scholar]
- 23.Anlar B, Basaran C, Kose G, et al. Acute disseminated encephalomyelitis in children: outcome and prognosis Neuropediatrics 2003;24:194–199 [DOI] [PubMed] [Google Scholar]
- 24.Morimatsu M. Recurrent ADEM or MS Int Med 2004;43:647–648 [DOI] [PubMed] [Google Scholar]
- 25.Jacob A, Matiello M, Wingerchuk DM, Lucchinetti CF, Pittock SJ, Weinshenker BG. Neuromyelitis optica: changing concepts J Neuroimmunol 2007;187:126–138 [DOI] [PubMed] [Google Scholar]
- 26.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 27.O'Riordan JI, Gallagher HL, Thompson AJ, et al. Clinical, CSF and MRI findings in Devic's neuromyelitis optica J Neurol Neurosurg Psychiatry 1996;60:382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology 1999;53:1107–1114 [DOI] [PubMed] [Google Scholar]
- 29.de Seze J. Neuromyelitis optica Arch Neurol 2003;60:1336–1338 [DOI] [PubMed] [Google Scholar]
- 30.de Seze J, Lebrun C, Stojkovic T, Ferriby D, Chatel M, Vermersch P. Is Devic's neuromyelitis optica a separate disease? A comparative study with multiple sclerosis Mult Scler 2003;9:521–525 [DOI] [PubMed] [Google Scholar]
- 31.Ghezzi A, Bergamaschi R, Martinelli V, et al. Clinical characteristics, course and prognosis of relapsing Devic's Neuromyelitis Optica J Neurol 2004;251:47–52 [DOI] [PubMed] [Google Scholar]
- 32.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112 [DOI] [PubMed] [Google Scholar]
- 33.Jarius S, Franciotta D, Bergamaschi R, et al. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology 2007;68:1076–1077 [DOI] [PubMed] [Google Scholar]
- 34.Keegan M, Pineada AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 2003;58:143–146 [DOI] [PubMed] [Google Scholar]
- 35.Papeix C, Vidal JS, de Seze J, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256–259 [DOI] [PubMed] [Google Scholar]
- 36.Warabi Y, Matsumoto Y, Hayashi H. Interferon beta-1b exacerbates multiple sclerosis with severe optic nerve and spinal cord demyelination. J Neurol Sci 2007;252:257–261 [DOI] [PubMed] [Google Scholar]
- 37.Mandler RN, Davis LE, Jeffery DR, Kornfeld M. Devic's neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol 1993;34:162–168 [DOI] [PubMed] [Google Scholar]
- 38.Filippi M, Rocca MA, Moiola L, et al. MRI and magnetization transfer imaging changes in the brain and cervical cord of patients with Devic's neuromyelitis optica. Neurology 1999;53:1705–1710 [DOI] [PubMed] [Google Scholar]
- 39.Rocca MA, Agosta F, Mezzapesa DM, et al. Magnetization transfer and diffusion tensor MRI show gray matter damage in neuromyelitis optica. Neurology 2004;10:476–478 [DOI] [PubMed] [Google Scholar]
- 40.Barkhof F, Filippi M, Miller DH, et al. Comparison of MR imaging criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120:2059–2069 [DOI] [PubMed] [Google Scholar]
- 41.Pittock SJ, Lennon VA, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390–396 [DOI] [PubMed] [Google Scholar]
- 42.Pittock SJ, Weinshenker GB, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–968 [DOI] [PubMed] [Google Scholar]
- 43.Bergamaschi R, Tonietti S, Franciotta D, et al. Oligoclonal bands in Devic's neuromyelitis optica and multiple sclerosis. Differences in repeated cerebrospinal fluid examinations. Mult Scler 2004;10:2–4 [DOI] [PubMed] [Google Scholar]
- 44.Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinal extensive transverse myelitis. Ann Neurol 2006;59:566–569 [DOI] [PubMed] [Google Scholar]
- 45.Matiello M, Lennon VA, Jacopb A, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 2008;70:2197–2220 [DOI] [PubMed] [Google Scholar]
- 46.Pittock SJ, Lennon VA, de Seze J, et al. Neuromyelitis optica and non-organ-specific autoimmunity. Arch Neurol 2008;65:78–83 [DOI] [PubMed] [Google Scholar]
- 47.Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol 2003;2:117–127 [DOI] [PubMed] [Google Scholar]
- 48.Matsuoka T, Matsushita T, Kawano Y, et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain 2007;130:1206–1223 [DOI] [PubMed] [Google Scholar]
- 49.Kesselring J, Miller DH, Robb SA, et al. Acute disseminated encephalomyelitis. MRI findings and the distinction from multiple sclerosis. Brain 1990;113:291–302 [DOI] [PubMed] [Google Scholar]
- 50.de Seze J, Debouverie M, Zephir H, et al. Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol 2007;64:1426–1432 [DOI] [PubMed] [Google Scholar]
- 51.Banwell B, Shroff M, Ness JM, et al. MRI features of pediatric multiple sclerosis. Neurology 2007;68(Suppl. 2):S46–S53 [DOI] [PubMed] [Google Scholar]
- 52.Krupp LB, Banwell B, Tenembaum S. for the International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007;68(Suppl. 2):S7–S12 [DOI] [PubMed] [Google Scholar]
- 53.Brinar VV, Poser CM. The spectrum of disseminated encephalomyelitis. Clin Neurol Neurosurg 2006;108:2295–2310 [DOI] [PubMed] [Google Scholar]
- 54.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;40:469–479 [DOI] [PubMed] [Google Scholar]
- 55.Mendez MF, Pogacar S. Malignant monophasic multiple sclerosis or “Marburg's disease”. Neurology 1988;38:1153–1155 [DOI] [PubMed] [Google Scholar]
- 56.Johnson MD, Lavin P, Whetsell WO. Fuminant monophasic multiple sclerosis, Marburg's type. J Neurol Neurosurg Psychiatry 1990;53:918–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore GR, Neumann PE, Suzuki K, Lijtmaer HN, Traugott U, Raine CS. Balo's concentric sclerosis: new observations on lesion development. Ann Neurol 1985;17:604–611 [DOI] [PubMed] [Google Scholar]
- 58.Hunter SB, Ballinger WE, Jr., Rubin JJ. Multiple sclerosis mimicking primary brain tumor. Arch Pathol Lab Med 1987;111:464–468 [PubMed] [Google Scholar]
- 59.Kepes J. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis: a study of 31 patients. Ann Neurol 1993;22:18–27 [DOI] [PubMed] [Google Scholar]
- 60.Morrissey SP, Miller DH, Kendall BE, et al. The significance of brain magnetic resonance imaging abnormalities at presentation with clinically isolated syndromes suggestive of multiple sclerosis. A 5-year follow-up study. Brain 1993;116:135–146 [DOI] [PubMed] [Google Scholar]
- 61.Söderström M, Ya-Ping J, Hillert J, Link H. Optic neuritis. Prognosis for multiple sclerosis from MRI, CSF and HLA findings. Neurology 1998;50:708–714 [DOI] [PubMed] [Google Scholar]
- 62.Weinshenker BG, Gilbert JJ, Ebers GC. Some clinical and pathologic observations on chronic myelopathy: a variant of multiple sclerosis. J Neurol Neurosurg Psychiatry 1990;53:146–149 [DOI] [PMC free article] [PubMed] [Google Scholar]