Abstract
Social experience alters the expression of genes related to synaptic function and plasticity, induces elaborations in the morphology of neural structures throughout the brain (Volkmar and Greenough, 1972; Greenough et al., 1978; Technau, 2007), improves cognitive and behavioral performance (Pham et al., 1999a; Toscano et al., 2006) and alters subsequent sleep (Ganguly-Fitzgerald et al.,2006). In this review, we discuss the plastic mechanisms that are induced in response to social experience and how social enrichment can provide insight into the biological functions of sleep.
I. INTRODUCTION
Although research animals in laboratory environments are often housed individually in relatively simple enclosures, their wild counterparts must interact in more complex environments outside of a controlled setting. In nature, they must reliably find food, avoid enemies and predators, interact socially with conspecifics, and compete for potential mates. While standardized lab environments allow researchers to easily control environmental and social influences, manipulating the social environment of research animals can be a powerful experimental tool. Social experience alters the expression of genes related to synaptic function and plasticity, induces elaborations in the morphology of neural structures throughout the brain (Greenough et al., 1978; Technau, 2007; Volkmar and Greenough, 1972), improves cognitive and behavioral performance (Pham et al., 1999a; Toscano et al., 2006), and alters subsequent sleep (Ganguly-Fitzgerald et al., 2006). In this review, we discuss the use of social enrichment/isolation as an experimental paradigm to study plastic mechanisms in the brain and to investigate the relationship between sleep and synaptic plasticity.
Although specific details regarding the conditions of “enriched” environments may differ between studies (e.g., some enriched environments included novel objects that were periodically changed, some consisted of bare enclosures), all include enhanced social exposure. Thus, while nonsocial factors may contribute to some experimental results, it is likely that many of the experimental outcomes can be attributed to the enhanced social exposure. Greenough et al. (1978) compared the effect of two different social enrichment conditions on synaptic ultrastructure. In this study, the first enrichment condition consisted of a large cage in which 12 rats were housed and presented with toys that were changed daily as well as a 30-min opportunity to explore a different “toy-filled field.” In the second enrichment condition, two rats were housed together in a standard laboratory cage. After 30 days in these conditions, rats were sacrificed and tissue from the occipital cortex was inspected for postsynaptic densities containing subsynaptic plate perforations, a marker of increased synaptic strength. While tissue from both enriched groups exhibited approximately 25% more subsynaptic plate perforations than tissue from isolated siblings, there was no difference detected between enriched conditions indicating that social interaction alone, not exposure to novel objects or a spatially larger enclosure, was likely sufficient to induce the changes in synaptic structure. Similarly, studies of environmental enrichment using the fruit fly Drosophila melanogaster have found significant changes in neural structure and behavior that are associated with social interactions and cannot be attributed to differences in enclosure volume or other physical differences in the housing conditions (Ganguly-Fitzgerald et al., 2006; Heisenberg et al., 1995).
II. SOCIAL ENRICHMENT INDUCES PLASTIC MECHANISMS
Following a period of enriched social experience, elaborations in neural circuitry have been characterized in a number of vertebrate and invertebrate species. Neurons in the visual cortex of socially enriched rats have an increased number of dendritic branches (Volkmar and Greenough, 1972) and show ultrastructural evidence of strengthened synaptic connections (Greenough et al., 1978). Significant increases in the number of hippocampal synapses in socially enriched rats have also been reported (Briones et al., 2006). Structural elaboration of individual cerebellar Purkinje cells in monkeys housed in a social environment suggests that the plastic effects of social enrichment on neural structures are evolutionarily conserved from rodents to primates (Floeter and Greenough, 1979). Elevated levels of neuronal growth factors may promote the increased dendritic elaboration and synaptogenesis that have been observed in response to social enrichment; exposure to an enriched social environment has been associated with increased expression of a number of neurotrophic factors including nerve growth factor (NGF) (Ickes et al., 2000; Pham et al., 1999a; Torasdotter et al., 1998), brain-derived growth factor (BDNF) (Ickes et al., 2000; Young et al., 1999), and glial-cell-derived neurotrophic factor (GDNF) (Faherty et al., 2005; Young et al., 1999). Neurotrophic factors, particularly NGF and BDNF, play an important role in synaptic plasticity (Gómez-Palacio-Schjetnan and Escobar, 2008; Hennigan et al., 2009) and impaired neurotrophin signaling results in memory impairments (Bekinschtein et al., 2007; De Rosa et al., 2005; Heldt et al., 2007; Walz et al., 2005).
The observations of structural plasticity in response to social enrichment seem to be widely conserved across species. Structural plasticity in response to enriched environments has been observed not only in mammals but also in several invertebrate species. Honeybees progress through a series of social roles within the hive structure that are associated with experience-dependent changes in the structure of brain regions including the mushroom bodies (MBs) (Withers et al., 1993) and antennal lobes (Winnington et al., 1996). Although the mechanisms that control the transition between social roles are not well characterized, these transitions are associated with extensive changes in gene expression profiles, indicating a role for genetic regulation (Whitfield et al., 2003). While genetic studies are difficult to conduct using honeybees, the fruit fly D. melanogaster is a widely used genetic model for the study of behavioral genetics. Indeed, social experience does induce significant changes in neuropil structure throughout the Drosophila brain (Heisenberg et al., 1995; Technau, 2007). In these studies, the number of MB fibers were significantly elevated in socially enriched animals compared to wild-type siblings housed in isolation or deprived of visual or olfactory stimuli during social interactions. It should be noted that it is not yet clear whether the increased number of fibers results from increased branching of projections in existing neurons or the birth of new neurons. Interestingly, recent studies showed newly born Kenyon cells in the MBs of socially enriched wild-type flies (Technau, 2007). While little other evidence has been found for neurogenesis in the MBs of adult Drosophila, observations of newly born neurons have been made in the MBs of other insect species following social enrichment. BrdU labeling was used to reveal a significant elevation in the rate of neurogenesis in the MBs of adult crickets that were housed in a socially enriched environment for several days (Scotto-Lomassese et al., 2000, 2002) and subsequent investigations found this elevation to be mediated via a nitric oxide-dependent signaling mechanism (Cayre et al., 2005). Similar observations have been made in mice. Social enrichment resulted in an overall increase in the number of cells in the hippocampus including a significantly higher number of newly generated neurons and astroytes compared to socially isolated controls (Kempermann et al., 1997). Based on their measurements, Kempermann et al. (1997) estimated that, on average, socially enriched mice possessed approximately 40,000 more dentate gyrus granule cells per hemisphere than their isolated siblings. These data suggest not only that the morphology of existing neurons can be altered by social experience but also that the generation of new neurons can be enhanced in response to the social enrichment.
As mentioned above, an increase in the number of MB fibers were found in socially enriched flies of a number of different wild-type strains. Interestingly, no change in fiber number was found in flies with mutant alleles of the classical memory genes rutabaga or dunce (Balling et al., 2007). Together, these important studies suggest that complex sensory cues that accompany social interactions in the fly induce plastic mechanisms that alter the structure of the MBs, an important structure for associative memory in the fly. Further investigations have found that deprivation of visual stimuli by housing flies in complete darkness also alters the structure of several other neuropils involved in visual processing including the lamina, medulla, and lobula plate (Barth et al., 1997)as well as the volume of the central complex and MB calyces (Barth and Heisenberg, 1997). Interestingly, Barth et al. (1997) describe a critical window for the effects of visual stimuli on the volume of the early visual system that is restricted to the first 4-5 days after eclosion; housing flies in complete darkness starting 5 days after eclosion had no effect on the volume of the lamina. Conversely, another study showed that social experience can alter the volume of the MB calyces and medulla for at least 16 days after eclosion (Heisenberg et al., 1995). Together, these findings suggest that experience-dependent plasticity may be controlled by two separate mechanisms. First, the early visual system (e.g., photoreceptor projections into the lamina) may be shaped by visual experience in the first few days after eclosion to optimize the ability of the brain to receive visual stimuli. Second, another type of plasticity that can encode memories throughout adult life can exhibit experience-dependent morphological changes for a much longer period of time and may allow downstream associative centers (including the MB) which are influenced by more complex, multisensory stimuli (including social interactions). This kind of temporal division can also be observed in mammalian models. While ocular dominance plasticity in the visual cortex can be robustly observed during a critical period early in life (reviewed in Berardi et al.,2000), structural changes in other brain regions including the hippocampus can be observed much later in life (reviewed in Lee and Son, 2009).
III. FUNCTIONAL EFFECTS OF SOCIAL ENRICHMENT
Exposure to complex social environments not only alters neural circuitry at the structural level but also alters the physiological functioning of synapses in circuits throughout the brain. Hippocampal slices from rats housed in an enriched environment, for example, exhibit robust long-term potentiation (LTP) and long-term depression (LTD) in the Schaffer collateral pathway, while rats housed in social isolation demonstrate relative impairments for both LTP and LTD at this synapse (Artola et al., 2006; Duffy et al., 2001). Other studies, however, have found that hippocampal synapses in the medial perforant path can become significantly potentiated during social enrichment to the extent that LTP can be occluded (Foster et al., 1996; Green and Greenough, 1986). Similar studies have found that mice housed in social isolation in lab conditions exhibit impaired LTP in the cingulate cortex when compared to tissue from wild mice that had developed in a natural, socially complex environment (Zhao et al., 2009). Along with the physiological impairments observed in socially isolated animals, exposure to a socially enriched environment increases the expression of glutamatergic AMPA receptors throughout the hippocampus (Foster et al., 1996), providing molecular evidence that glutamatergic synapses in the hippocampus can become highly potentiated during exposure to complex social environments. Because technical limitations have prevented the wide-spread use of electrophysiology to characterize the activity patterns of Drosophila central neurons until recently, very little is known about whether social enrichment induces similar physiological changes in the adult fly. Recent studies have, however, found that social enrichment significantly alters the excitability of motorneurons in larvae (Ueda and Wu, 2009).
Given the structural elaborations and physiological enhancements induced by socially enriched environments, it might be expected that these conditions improve the behavioral and cognitive performance of animals exposed to complex social interactions. Indeed, wild-type animals housed in an enriched environment demonstrate significant improvements in hippocampus-dependent memory in both the Morris water maze (Briones et al., 2006; Pham et al., 1999b; Toscano et al., 2006) and contextual fear conditioning (Duffy et al., 2001). Social enrichment seems to not only improve spatial memory in healthy animals but may also aid recovery from brain damage following traumatic brain injury, neonatal hypoxia-ischemia, excitotoxic injury, and early exposure to lead poisoning; in all of these conditions, enriched animals exhibited significant improvements in behavioral assays and normalized physiological functioning compared to socially isolated controls (Cao et al., 2008; Hoffman et al., 2008; Kline et al., 2007; Koh et al., 2005; Pereira et al., 2008, 2009; Young et al., 1999). Additionally, many of the cognitive benefits of social enrichment have also been observed in rodent models of neurodegenerative disease. In a rodent model of Parkinsonism, social enrichment during adulthood prevents the death of dopaminergic neurons in the substantia nigra (Faherty et al., 2005). Similar studies using a transgenic mouse model for Alzheimer's disease found that socially isolated mice expressing a mutant form of the human amyloid precursor protein (APP) exhibited more rapid decline in memory in the fear conditioning paradigm as well as a dramatic acceleration in amyloid-beta plaque deposition relative to socially housed animals that expressed the same mutant transgene (Dong et al., 2004). Importantly, recent studies of human populations have indicated that increased cognitive activity, including social interaction, throughout life may reduce the risk of developing Alzheimer's disease later in life (Carlson et al., 2008). Together, these data suggest that exposure to social enrichment may induce neuroprotective mechanisms to function normally in the face of genetic defects that would otherwise induce impairment. While the mechanisms that mediate these effects are not well characterized, the same neurotrophic signals that are elevated in response to social enrichment have been found to have neuroprotective effects in disease models for neurodegeneration; infusion of BDNF into the entorhinal cortex of aged mice expressing transgenic APP restores performance in the Morris water maze assay and restored expression of synaptic markers in the hippocampus (Nagahara et al., 2009). Similar effects have been observed by increasing NGF signaling (De Rosa et al., 2005) and there is preliminary evidence that these pathways may be effective therapeutic targets for humans afflicted with Alzheimer's disease (Tuszynski et al., 2005). Although the consequences of social interactions on aging have not been well studied in Drosophila, recent experiments have suggested that social enrichment can delay premature death in flies mutant for Cu/Zn superoxide dismutase (CuZnSOD) (Ruan and Wu, 2008), suggesting a phylogenetically conserved role for the functional benefits of social interactions. It is important to note that lifespan extension was not observed in socially enriched wild-type flies indicating that flies lacking CuZnSOD, which has been implicated in mechanisms associated with a number of aging-related neurodegenerative disorders including Parkinson's, Huntington's, and Alzheimer's diseases, may be especially sensitive to beneficial effects of socially complex environments and further implicates social enrichment as a potential intervention for the alleviation of neurodegenerative disorders (Ruan and Wu, 2008).
IV. USING SOCIAL ENRICHMENT TO INVESTIGATE FUNCTIONS OF SLEEP
A. Sleep and plasticity
Although sleep is a biological process that is necessary for survival in vertebrates and invertebrates, the underlying biological functions of sleep are currently unknown. A growing body of literature, however, suggests an important and evolutionarily conserved role for sleep in the processing and consolidation of new memories. For example, hippocampal ensembles that were activated together when rats navigated a novel maze were reactivated in an identical manner when the animals slept later (Wilson and McNaughton, 1994). Further studies have found that similar reactivation can be observed in the visual cortex and that replay in these two regions is coordinated to replay the same experience (Euston et al., 2007; Ji and Wilson, 2007). There is also evidence for replay of waking experience during sleep in humans. Subjects were recruited to play the video game Tetris for several hours over the course of 3 days; over the course of the study, a majority of the subjects reported hypnogogic imagery associated with playing the game (Stickgold et al., 2000). This type of imagery seemed to be associated with the process of learning how to play the game because subjects who had less previous Tetris experience were the most likely to report Tetris-related imagery during dreams at night. This replay of newly formed associations during sleep seems to facilitate the processing and consolidation of those memories. In one recent study, subjects were taught to play a card game while being exposed to a specific odor cue. When subjects were reexposed to the same odor cue during slow-wave sleep that night, hippocampal activation was significantly elevated along with significantly improved hippocampus-dependent declarative memory of the card game the next day (Rasch et al., 2007). Additionally, human performance in motor learning tasks stabilized with repeated trials over the course of the day, then dramatically improved following a night of sleep (Stickgold and Walker, 2007; Walker et al., 2005) and sleep deprivation impaired consolidation of long-term memory in rodents and flies (Ganguly-Fitzgerald et al., 2006; Graves et al., 2003). Indeed, sleep is significantly altered by previous experience and is important for the consolidation of recently acquired memories.
In the decade since the establishment of Drosophila as a model system for the study of sleep (Hendricks et al., 2000; Shaw et al., 2000), the fly has been successfully used to identify a number of candidate genes and pathways that are involved in sleep regulation. Among these pathways is the cAMP/PKA signaling cascade, which has classically been associated with learning and memory in the fly (Dudaí et al., 1983). Several genetic mutants that induce a downregulation in cAMP signaling result in decreased sleep time and, conversely, manipulations that increase cAMP signaling yield elevated sleep compared to genetic controls (Hendricks et al., 2001). Although the circuitry that controls sleep in the fly is largely unknown, additional investigations have found that disruption of the cAMP/PKA signaling cascade within the MBs, a structure that is important for associative processing, can strongly modulate sleep time (Joiner et al., 2006).
Using a forward genetic screen to identify genes that alter sleep time, Cirelli et al. (2005) found that flies mutant for the voltage-dependent potassium channel Shaker (Sh) exhibit a robust decrease in sleep when compared to wild-type controls. Similarly, several mutants for the beta modulatory subunit Hyper-kinetic (Hk), which influences Sh conductance, also sleep less than background controls (Bushey et al., 2007). When these mutant flies were tested for memory using the heat-box assay (Putz and Heisenberg, 2002), flies with mutant alleles for Sh or Hk that resulted in decreased sleep time also exhibited memory impairments while alleles that did not change sleep also had no significant effect on memory performance (Bushey et al., 2007). Although these results are purely correlational, they provide evidence that genetic mechanisms that influence sleep in the fly may be tightly intertwined with pathways that are important for learning and memory.
B. Social enrichment increases sleep
Despite the evidence for a relationship between plasticity and sleep, little is known about the mechanisms by which sleep and plasticity interact. As described above, social enrichment is a simple experimental manipulation that induces robust plasticity in circuits throughout the brain. Given that the social environment can induce structural changes in the brain in both mammals and invertebrates, it may be possible to begin to elucidate the underlying molecular mechanisms linking sleep and plasticity using the power of Drosophila genetics.
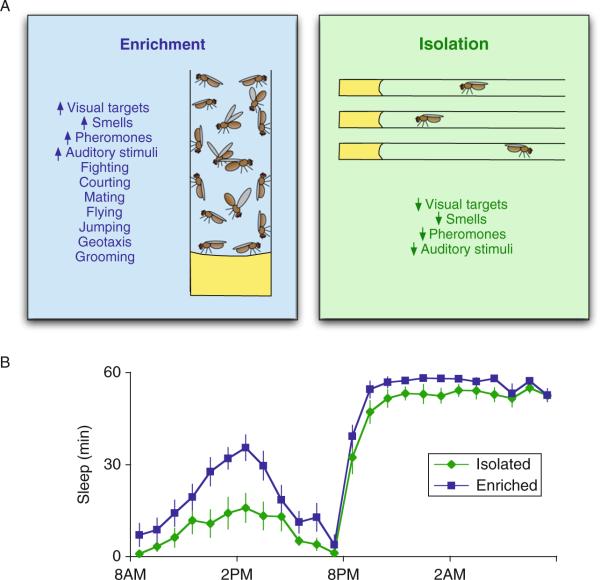
With that in mind, we evaluated flies that were housed in a socially enriched environment since they are likely to experience increased visual, olfactory phermonal, and auditory signals compared to siblings that are housed individually in social isolation. Moreover, interactions between individuals in a social environment are likely to increase the incidence of other behaviors including flying, jumping, geotaxis, and grooming, to name a few (Ganguly-Fitzgerald et al., 2006). We hypothesized that increased exposure to these events would likely produce changes in structural plasticity within the brain which would increase sleep need. To test this hypothesis, wild-type flies were housed in a socially enriched environment with ~35–40 siblings for 5 days. As seen in Fig. 3.1, socially enriched flies sleep approximately 2 h/day more than siblings that have been socially isolated for 5 days (Donlea et al., 2009; Ganguly-Fitzgerald et al., 2006)(Fig. 3.1). The increase is observed in both single-sex vials (male–male and female–female) and in mixed vials (male–female). Interestingly, social enrichment not only increases sleep time but, importantly, increases sleep consolidation as well. That is, isolated flies exhibit short-sleep bouts during the day which do not appear to be restorative (Seugnet et al., 2008). In contrast, socially enriched flies maintain sleep bout durations more typical of that seen during night-time sleep (Ganguly-Fitzgerald et al., 2006). Thus, social enrichment increases sleep consolidation sufficiently to permit the restorative properties of sleep.
Figure 3.1.
Exposure to an enriched social environment increases sleep in Drosophila. (A) Flies are housed for 5 days either in a socially enriched vial with ~40 other siblings or in small vials in social isolation. During this time, flies in the socially enriched environment are exposed to complex visual, olfactory, pheromonal, and auditory stimuli that are generated by other flies and fight, court, and mate with conspecific flies. Socially isolated animals, however, are not exposed to these sensory cues and have no social interactions with other flies. (B) When transferred to sleep monitors after 5 days of enrichment/ isolation, socially enriched females sleep ~120 min/day more than their socially isolated sisters.
Although the number of interactions that can potentially occur in the socially enriched environment is difficult to quantify precisely, it is clear that compared to their isolated siblings the brains of socially enriched flies must adapt and respond to a larger variety of stimuli. In that regard, it is worth noting that the change in sleep does not appear to be induced by other factors such as the volume of the enclosure or abiotic factors indirectly caused by enrichment. For example, blind and olfactory defective flies do not respond to social enrichment with an increase in sleep indicating that being in close proximity with 35–40 siblings for 5 days is not sufficient to induce a sufficient change in neuronal plasticity as to induce an increase in sleep (Ganguly-Fitzgerald et al., 2006). Similarly, changes in sleep are not observed in enriched animals that are mutant for classical memory genes that also influence structural plasticity such as the adenylyl cyclase rutabaga or the cAMP phosphodiesterase dunce. Finally, the increase in sleep following social enrichment is directly proportional to the number of flies housed in the enriched environment (Ganguly-Fitzgerald et al., 2006). Together, these observations indicate that the increase in sleep is likely due to social interactions inducing changes in neuronal plasticity.
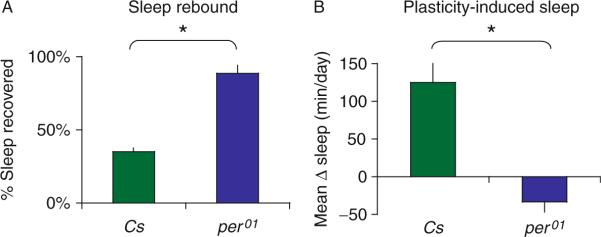
An alternative hypothesis is that sleep is disrupted during social enrichment such that the subsequent increase in sleep simply reflects enhanced homeostatic drive not changes in plasticity. However, as mentioned above, flies mutant for memory genes do not exhibit an increase in sleep even though one would expect that their sleep would be as disrupted during social enrichment as that hypothesized to occur in wild-type flies. Interestingly, flies mutant for the circadian clock gene period (per01) exhibit a sleep rebound that is approximately three times larger than wild-type flies, indicating that they are very responsive to even small changes in sleep (Fig. 3.2). Despite their sensitivity to sleep loss, however, per01 flies do not increase their sleep following social enrichment (Fig. 3.2). Thus, if social enrichment resulted in disrupted sleep as the alternative hypothesis suggests, per01 flies would show a larger increase in sleep than that seen in wild-type flies. Since per01 flies do not show an increase in sleep following social enrichment, we believe that the genetic mechanisms that control sleep homeostasis following sleep deprivation are dissociable from the mechanisms underlying increased sleep following social enrichment.
Figure 3.2.
Flies mutant for per01 are extremely sensitive to sleep loss but do not respond to social enrichment with an increase in sleep. (A) Following 12 h of sleep deprivation Cs flies recover ~30% of their lost sleep while per01 mutants recover >90%. % Sleep recovered is calculated for each individual as a ratio of the minutes of sleep gained above baseline during the 24 h of recovery divided by the total min of sleep lost during 12 h of sleep deprivation. (B) Following 5 days of social enrichment, Cs flies sleep significantly more than their siblings that were housed in social isolation while sleep is unaffected by social enrichment in per01 mutants. Increased sleep after social enrichment is shown as a difference in daytime sleep amount between socially enriched and socially isolated siblings.
C. Circuits and genes that influence the response to social enrichment
To determine whether social enrichment may be dependent upon plastic mechanisms that are invoked following social interactions, we conducted a minibrain screen to restore rutabaga functioning in specific circuits in an otherwise rutabaga mutant background. We used the bipartite yeast-GAL4 system to express rutabaga in ~35 circuits (Brand and Perrimon, 1993). Surprisingly, when rutabaga was rescued in ventral lateral neurons (LNVs), 16 cells that comprise the circadian clock, the increase in sleep following social enrichment was restored (Donlea et al., 2009). Recent studies from several independent groups have reported that the LNVs play an important role in sleep–wake regulation in the fly (Agosto et al., 2008; Parisky et al., 2008; Sheeba et al., 2008). That is, manipulations that increase the excitability of the LNVs result in large increases in waking behavior. A subset of the LNVs innervates the medulla where they are uniquely situated to modulate the processing of simple and complex visual information. Together, these data suggest that environmentally induced plasticity in the LNVs may be important for changes in sleep following social enrichment.
Having identified a circuit, the LNVs, that is required for the social environment to induce an increase in sleep, we began to evaluate candidate genes that coordinate the changes in neuronal plasticity that are induced by social experience within this circuit. Although the clock gene period is widely expressed throughout the fly brain, specific rescue of period within clock cells was able to restore plasticity induced sleep in an otherwise per01 mutant background (Donlea et al., 2009). This observation is particularly interesting given that the period gene has been shown to play a noncircadian role in memory consolidation (Sakai et al., 2004). Similarly, the transcription factor blistered (bs) is both transcriptionally elevated in the brain following social enrichment and is required in the LNVs for the social environment to modify sleep time. It is worth noting that the mammalian homolog of blistered, serum response factor induces transcription of a genetic program that mediates synaptic potentiation and is necessary both for in vitro assays of plasticity such as LTP and LTD as well as in vivo assays of behavioral plasticity (Etkin et al., 2006; Ramanan et al., 2005). To further explore the role of bs in coordinating social enrichment and sleep, we evaluated genes that are known to be regulated by bs. One such gene, Epidermal growth factor receptor (Egfr), is transcriptionally activated by social enrichment in wild-type flies. When Egfr was expressed in the LNVsofa bs mutant, the increase in sleep following social enrichment was rescued. Thus, we have identified four genes that operate within a specific circuit that is known to both influence sleep–wake regulation and that is able to coordinate the interaction between social environment and sleep.
D. Social environment alters synaptic terminals
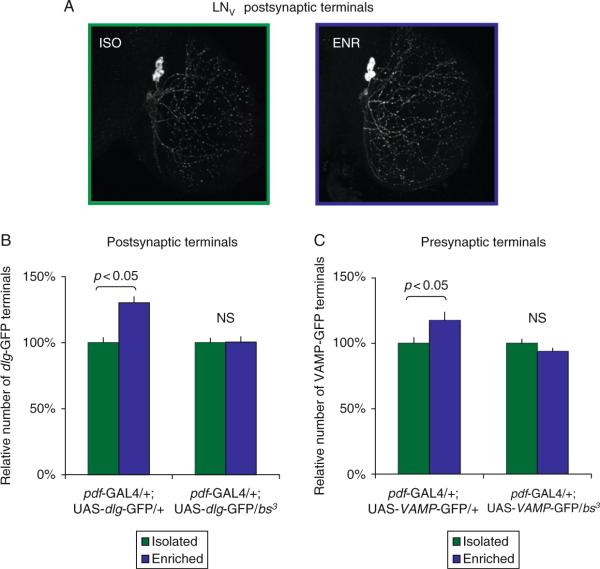
As mentioned above, Heisenberg and colleagues conducted a series of elegant studies demonstrating that it is possible to quantify environmentally induced structural changes in the brains of adult flies. To determine whether the social enrichment paradigm was able to induce structural changes in the brain, we expressed a GFP-tagged construct of the postsynaptic protein discs-large (UAS-dlgWT-gfp) in the LNVs. As seen in Fig. 3.3, the number of synaptic terminals was significantly increased after 5 days of social enrichment. Similar results were obtained using a GFP tagged presynaptic marker. These results compliment those reported by Heisenberg and colleagues and demonstrate that the social environment is able to induce quantifiable changes in brain structures within a circuit that is known to play a role in sleep regulation. Since we have not demonstrated that the labeled synaptic terminals are functional, it is possible that the increased number of GFP-tagged terminals is an artifact of the environmental manipulation and does not reflect functional changes that influence sleep time. To test this possibility, UAS-dlgWT-gfp was expressed in a bs mutant background and flies were exposed to social enrichment. As seen in Fig. 3.3, the number of synaptic terminals was not altered in socially enriched bs mutants. That is, flies that are not capable of responding to the social environment with an increase in sleep do not display structural changes in the number of synaptic terminals. These data suggest that in the absence of functional plasticity, the environment is not associated with changes in structural plasticity.
Figure 3.3.
Flies mutant for bs show no increase in LNV terminals following exposure to social enrichment. (A) LNV projections in socially isolated pdf-GAL4/+; UAS-dlg-GFP/+ flies (left, representative image) contain fewer GFP-positive terminals than those of socially enriched siblings (right, representative image). (B) Following 5 days of social enrichment, wild-type controls exhibit a significant increase in the number of LNV postsynaptic terminals labeled with dlg-GFP (left), but no change in the number of postsynaptic terminals can be detected in flies carrying a mutant allele for bs (right). (C) Similarly, an increased number of LNV presynaptic terminals labeled with VAMPGFP-GFP can be measured in wild-type control flies (left), but no change in presynaptic terminal number was observed in flies with the bs3 mutation (right).
E. Social enrichment versus long-term memory
Although structural plasticity and increased sleep following social enrichment require expression of several genes that are necessary for the formation and consolidation of associative memories, it is difficult to determine whether the increase in sleep is truly dependent upon plastic mechanisms. We hypothesized that if the increase in sleep following social enrichment is dependent upon plasticity-related processes then the circuits and genes that have been identified for social enrichment should also play a role in the consolidation of long-term memory. To test this hypothesis we utilized “courtship conditioning,” an associative assay in which male flies learn to alter their courtship behavior based on previous exposure to unreceptive courtship targets. In courtship conditioning training, male flies are paired with mated female flies that are unreceptive to further copulation attempts or with male flies that have been genetically altered to express aphrodisiac pheromonal cues. During this training period, the subject male will proceed through stereotypical courtship behaviors in an attempt to woo the unreceptive courtship trainer, but is ultimately unable to copulate and forms an operant association between courtship rejection and normally aphrodisiac pheromonal cues (Gailey et al., 1984). Following training, male subject flies are returned to individual tubes. Subsequent memory is probed by exposing trained males to a normally attractive courtship target; if a trained male retains memory of the training experience, he will spend less time courting during the test period than his naïve brothers. When wild-type male flies are subjected to a spaced training protocol consisting of three 1-h training periods with a pheromonally feminized Tai2 male fly, they exhibit robust reductions in courtship for at least 48 h (Ganguly-Fitzgerald et al., 2006). Although acquisition and consolidation of memory following courtship conditioning requires similar genetic and neural mechanisms as memories formed in other associative assays (Siwicki and Ladewski, 2003), courtship conditioning requires male flies to process complex, naturalistic visual, and pheromonal cues and to interpret social behaviors and postures and, thus, seems more directly comparable to the types of neural processing that might occur during social enrichment than occurs in many other assays.
Following a spaced training protocol that induces long-term memories, male flies exhibit a significant increase in sleep. The increase in sleep appears necessary for memory consolidation since 4-h of sleep deprivation eliminates subsequent LTM. Interestingly, sleep deprivation immediately following training not only eliminates LTM but it also blocks the increase in sleep typically observed following the spaced-training protocol. This observation suggests that the increase in sleep following training is likely due to molecular processes associated with memory consolidation; once the memory has been disrupted there is no need for more sleep. Consistent with their role in experience-dependent changes in sleep, flies mutant for period and bs do not show increases in sleep following training and show no evidence for LTM after 48 h. Importantly, expression of either wild-type per or wild-type bs within the subset of clock neurons that are required for social enrichment, restores both the increase in sleep and LTM consolidation following courtship conditioning in per and bs mutant backgrounds, respectively. Thus, the genes and circuits that have been identified as playing a role in mediating the effects of social enrichment on sleep are also required for the increase in sleep following the formation of LTM. Together with the data demonstrating that social enrichment results in an increase in the number of synaptic terminals, it appears as if the social environment alters sleep time by modifying molecular processes associated with synaptic plasticity.
F. Synaptic homeostasis and sleep
Although the direct effects of sleep at the synapse are largely unknown, a recent hypothesis has suggested that a function of sleep may be to downscale synaptic connections throughout the brain (Tononi, 2003; Tononi and Cirelli, 2005). According to this hypothesis, experiences during waking drive patterns of activity in neural circuits that potentiate synapses and increase the strength and number of neural connections. If this kind of potentiation were allowed to continue unchecked, we could ultimately run into some severe consequences; energy requirements for the brain would grow, space available for new synaptic connections would disappear, and the signal strength between synaptic connections would reach a saturation point. To prevent these problems, Tononi and Cirelli have proposed that synchronized patterns of activity during sleep facilitate global synaptic downscaling to allow for normal functioning each morning (Tononi, 2003; Tononi and Cirelli, 2005). In support of this hypothesis, they have found that the levels of proteins that are associated with synaptic potentiation are increased during waking and decreased during sleep in both flies and rats (Gilestro et al., 2009; Vyazovskiy et al., 2008). Although these studies provide molecular data that are consistent with the downscaling hypothesis, they do not address whether sleep acts to reduce synaptic connections at the level of individual terminals.
Given the ability of the environment to increase synaptic terminals in the LNVs, social enrichment is uniquely suited to test the hypothesis that synaptic homeostasis is major role of sleep. If the synaptic homeostasis model is correct, then flies that were exposed to social enrichment for 5 days but are prevented from sleeping should show a persistence in the number of synaptic terminals. However, if consolidation is accomplished through synaptic potentiation then flies that are sleep deprived following social enrichment should display a decrease in the number terminals. Following social enrichment, flies were allowed to either sleep ad libitum for 48 h or were sleep deprived for 48 h. The number of synaptic terminals in projections from the large LNVs (l-LNVs) was then quantified. We found that the sleep-deprived flies retained an elevated number of l-LNV terminals following social enrichment, but those flies who could sleep ad libidum no longer had any significant change in terminal number when compared to their socially isolated siblings (Donlea et al., 2009). These data are consistent with the downscaling hypothesis and indicate that flies sleep more when LNV terminal number has been elevated by plastic mechanisms and that this increased sleep acts to reduce the number of terminals back to a baseline level.
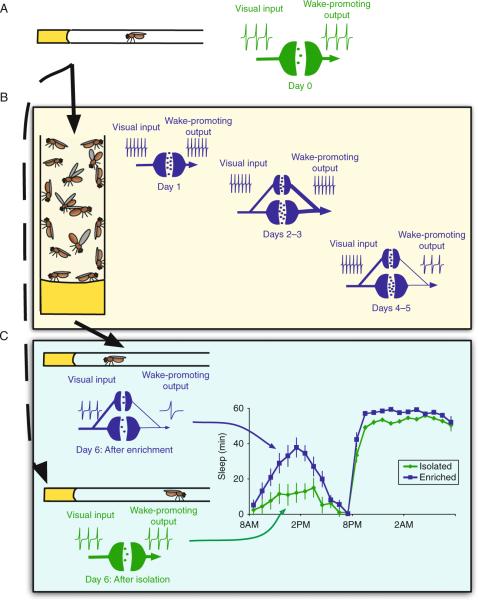
At first glance, the increase in sleep following social enrichment appears somewhat paradoxical. That is, social enrichment increases the number of synaptic terminals in the LNVs while the activation of the LNVs strongly promotes waking (Sheeba et al., 2008). How can these two observations be reconciled? During social enrichment, we hypothesize that complex sensory signals induce a prolonged elevation of LNV activity (Fig. 3.4). The strength of these sensory signals results in an increase in the strength of synaptic connections between visual input circuits and the LNVs that is mediated by Hebbian mechanisms (Fig. 3.4B). The experience-dependent increase in synaptic strength seems to be implemented as an increase in the number of synaptic terminals in the LNVs over the course of social enrichment. After LNV activity is elevated by several days of enriched social experience, it is possible that homeostatic mechanisms decrease the overall excitability of the LNVs to prevent chronic hyperexcitation and hold firing rate around a baseline set-point; similar homeostatic functioning has been previously described in Drosophila central synapses (Kazama and Wilson, 2008). Once enriched flies are moved into sleep monitors following social enrichment, complex sensory stimuli are removed and the lowered excitability of the LNVs reduces the firing rate below that of previously socially isolated controls (Fig. 3.4C). This lowered firing rate reduces the wake-promoting signal from these neurons and results in increased sleep for several days until LNV excitability can be homeostatically elevated to restore a normalized firing rate. Coincidentally, this model could also account for the downscaling of synaptic terminal number following social enrichment; if overall excitability of the LNVs is reduced when flies are removed from social enrichment and complex sensory stimuli are removed, input to each individual terminal could become less likely to drive action potentials in the LNVs and, as a result, Hebbian mechanisms might weaken the synaptic connections by decreasing terminal numbers.
Figure 3.4.
Proposed model for homeostatic regulation of synaptic terminals during social enrichment. (A) Under baseline conditions, wake-promoting output from the LNVs is modulated by signals originating from visual circuits in the medulla. (B) When exposed to a socially enriched environment, complex sensory stimuli likely drives increased activity in primary visual circuits that provide input to the LNVs. As a result, activity in the LNVs would become more strongly correlated with input signals from sensory circuits and synaptic connections to the LNVs become potentiated as new terminals are constructed. Following several days of hyperactivity and increased potentiation, homeostatic mechanisms may be induced in the LNVs to reduce overall firing rate and prevent chronic hyperexcitation. (C) Upon transfer to sleep monitors, flies are withdrawn from the complex visual stimuli that are associated with a socially enriched environment. As a result, wake-promoting output from the LNVs is decreased and socially enriched flies sleep more than their siblings that had been housed in social isolation.
V. FUTURE DIRECTIONS
A. Local versus global regulation of experience-dependent sleep
Although sleep has been classically characterized as a unitary behavioral state that alters global physiology, processes that are associated with sleep are regulated on a local, circuit-dependent basis in the brain. Several species of marine mammals, for instance, can exhibit electrophysiological patterns of sleep in one brain hemisphere while the other hemisphere appears to be awake (reviewed in Lyamin et al., 2008). Recent studies have also found that sleep-associated patterns of activity can be significantly altered in local circuits by previous activity. That is, if a circuit is stimulated locally during waking, then the intensity of slow-wave activity (SWA) during subsequent sleep is elevated in that circuit (Cottone et al., 2004; Huber et al., 2004; Iwasaki et al., 2004; Kattler et al., 1994; Miyamoto et al., 2003; Vyazovskiy et al., 2000; Yasuda et al., 2005). Conversely, if afferent activity to a cortical region is suppressed during the day, then SWA is locally reduced during sleep (Huber et al., 2007). In all, these studies indicate that although sleep is a global behavioral state, specific circuits in the brain may “sleep” differently depending on their specific need and previous activity.
Our data suggest that plasticity in the LNVs can induce a robust increased sleep in response to social enrichment while other circuits that are known to play a role in learning and memory are much less effective (Donlea et al., 2009). Interestingly, Gilestro et al. (2009) have reported that levels of the synaptic active zone protein Bruchpilot are dramatically elevated throughout much of the brain following 16 h of waking. These data suggest that synapses in a wide variety of circuits in the fly brain may become potentiated during waking. Indeed, rescue of rutabaga in all neurons results in the strongest increase in sleep following social enrichment consistent with the observation that waking influences many circuits besides just the LNVs. Thus, while the morphology of the LNVs and their projections make them well suited for quantifying synaptic terminals, the relationship between synaptic homeostasis and sleep will be enhanced through the identification of additional circuits with discrete expression patterns that can be modulated by experimental interventions. That is, while we favor the use of social environment to alter neuronal plasticity, other interventions may be useful for identifying additional circuits that can then be used to determine whether they are downscaled during sleep to the same degree as reported with this paradigm. Based on the vertebrate literature described above, it is likely that the degree of downscaling that is observed in each region will be proportional to the amount of local stimulation during waking. Further examination of synaptic homeostasis will be necessary to identify the mechanisms that mediate synaptic downscaling during sleep and to determine whether homeostatic downscaling might occur on a circuit-dependent basis during sleep.
B. What social behaviors are happening during social enrichment?
Despite the use of Drosophila as a model system for behavioral genetics over the past several decades, relatively little is known about the naturalistic social behavior of the fly. Courtship and aggression behaviors that are used by male flies have been studied in detail and thoroughly reviewed elsewhere (Robin et al., 2007; Villella and Hall, 2008), but social enrichment using only females induces a robust plastic response. The types of social interactions that occur in a female-only environment are not well characterized and the behavioral consequences of these plastic responses are entirely unknown. In order to better understand which neural circuits are likely to be altered by social enrichment as well as their functional roles, it would be helpful to better describe the types of social interactions that might occur during social enrichment. Two recent studies (Branson et al., 2009; Dankert et al., 2009) have utilized automated tracking algorithms to identify and score the social behavior of flies either in pairs or in a large group. The “ethomics” approach that these groups have begun to utilize might allow for rapid large-scale quantification of social behavior and to identify possible genetic and neural mechanisms that underlie these behaviors. As currently designed, the algorithms used in these two studies complement each other fairly well; the Ctrax software designed by Branson and colleagues is capable of tracking the movements of individual flies within larger groups over long periods of time while the CADABRA software suite utilized by Dankert and colleagues provides more detailed analysis of individual actions related to known courtship or aggression behaviors. These algorithms, however, are not optimally designed for the identification of novel types of interactions between individual animals; Ctrax does not provide detailed analysis of behavior and CADABRA is only capable of identifying predefined behavioral characteristics. Ultimately, these software tools may provide efficient tools for dissecting the mechanisms controlling known types of behavior, but only careful observation by human investigators is likely to allow for the identification and characterization of currently undescribed social interactions within groups of flies.
Although these interactions have not yet been characterized, it is likely that they are mediated, at least in part, through pheromonal communication. These chemical cues consist of a number of hydrocarbon compounds that are embedded in the waxy surface of the abdominal cuticle and are known to be crucial for the initiation and regulation of courtship behavior (Ferveur, 2005). Recent studies indicate that social context can significantly alter the composition of these hydrocarbon cues in male flies (Kent et al., 2008; Krupp et al., 2008). Interestingly, these studies found that altered pheromone production in response to social experience may have important consequences for future behavior; exposure to a genetically heterogeneous group induces wild-type males to alter the expression of courtship-related pheromonal cues and also results in increased mating frequency (Krupp et al., 2008). Given the important role of chemosensation during enrichment in the induction of subsequent plasticity, it is possible that interactions via chemical cues comprise an important component of the social interactions that induce plastic changes in the brain. Further studies are needed to better characterize the neural circuits that are affected by pheromonal communication and the behavioral consequences of these signals on sleep. In conclusion, we expect that by examining how social interactions alter neuronal plasticity, we will identify novel roles of genes for sleep regulation as well as to better understand the role that these neural circuits play in modulating both daily sleep quotas and adaptive behavior.
References
- Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABA(A) receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat. Neurosci. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. NeuroSci. 2006;23:261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J. Neurogenet. 2007;21:209–217. doi: 10.1080/01677060701695383. [DOI] [PubMed] [Google Scholar]
- Barth M, Heisenberg M. Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn. Mem. 1997;4:219–229. doi: 10.1101/lm.4.2.219. [DOI] [PubMed] [Google Scholar]
- Barth M, Hirsch HV, Meinertzhagen IA, Heisenberg M. Experience-dependent developmental plasticity in the optic lobe of Drosophila melanogaster. J. Neurosci. 1997;17:1493–1504. doi: 10.1523/JNEUROSCI.17-04-01493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr. Opin. Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat. Methods. 2009;6:413–414. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Suh E, Jozsa L, Woods J. Behaviorally induced synaptogenesis and dendritic growth in the hippocampal region following transient global cerebral ischemia are accompanied by improvement in spatial learning. Exp. Neurol. 2006;198:530–538. doi: 10.1016/j.expneurol.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J. Neurosci. 2007;27:5384–5393. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Huang S, Ruan D. Enriched environment restores impaired hippocampal long-term potentiation and water maze performance induced by developmental lead exposure in rats. Dev. Psychobiol. 2008;50:307–313. doi: 10.1002/dev.20287. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimer's Dementia: J. Alzheimer's Assoc. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Holstein GR, Martinelli GP, Forni C, Nicolas S, Aouane A, Strambi C, Strambi A. A role for nitric oxide in sensory-induced neurogenesis in an adult insect brain. Eur. J. NeuroSci. 2005;21:2893–2902. doi: 10.1111/j.1460-9568.2005.04153.x. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill SL, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Cottone LA, Adamo D, Squires NK. The effect of unilateral somatosensory stimulation on hemispheric asymmetries during slow wave sleep. Sleep. 2004;27:63–68. doi: 10.1093/sleep/27.1.63. [DOI] [PubMed] [Google Scholar]
- Dankert H, Wang L, Hoopfer E, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat. Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc. Natl. Acad. Sci. USA. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudaí Y, Uzzan A, Zvi S. Abnormal activity of adenylate cyclase in the Drosophila memory mutant rutabaga. Neurosci. Lett. 1983;42:207–212. doi: 10.1016/0304-3940(83)90408-1. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn. Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Alarcón JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. A role in learning for SRF: Deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res. Mol. Brain Res. 2005;134:170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ferveur JF. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Greenough WT. Cerebellar plasticity: Modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979;206:227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: Relation to long-term potentiation. Brain Res. 1996;736:243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Jackson FR, Siegel RW. Conditioning mutations in Drosophila melanogaster affect an experience-dependent behavioral modification in courting males. Genetics. 1984;106:613–623. doi: 10.1093/genetics/106.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I, Donlea JM, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Palacio-Schjetnan A, Escobar ML. In vivo BDNF modulation of adult functional and morphological synaptic plasticity at hippocampal mossy fibers. Neurosci. Lett. 2008;445:62–67. doi: 10.1016/j.neulet.2008.08.069. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EJ, Greenough WT. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: Evidence from hippocampal slices maintained in vitro. J. Neurophysiol. 1986;55:739–750. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: Changes with age and experience in the rat. Science. 1978;202:1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Heisenberg M, Heusipp M, Wanke C. Structural plasticity in the Drosophila brain. J. Neurosci. 1995;15:1951–1960. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Williams JA, Panckeri KA, Kirk D, Tello MK, Yin JC, Sehgal A. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat. Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- Hennigan A, Callaghan CK, Kealy J, Rouine J, Kelly AM. Deficits in LTP and recognition memory in the genetically hypertensive rat are associated with decreased expression of neurotrophic factors and their receptors in the dentate gyrus. Behav. Brain Res. 2009;197:371–377. doi: 10.1016/j.bbr.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Malena RR, Westergom BP, Luthra P, Cheng JP, Aslam HA, Zafonte RD, Kline AE. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci. Lett. 2008;431:226–230. doi: 10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, Karashima A, Tamakawa Y, Katayama N, Nakao M. Sleep EEG dynamics in rat barrel cortex associated with sensory deprivation. NeuroReport. 2004;15:2681–2684. doi: 10.1097/00001756-200412030-00026. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Kattler H, Dijk DJ, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kent C, Azanchi R, Smith B, Formosa A, Levine JD. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 2008;18:1384–1389. doi: 10.1016/j.cub.2008.07.088. [DOI] [PubMed] [Google Scholar]
- Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT(1A) receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Chung H, Xia H, Mahadevia A, Song Y. Environmental enrichment reverses the impaired exploratory behavior and altered gene expression induced by early-life seizures. J. Child. Neurol. 2005;20:796–802. doi: 10.1177/08830738050200100301. [DOI] [PubMed] [Google Scholar]
- Krupp J, Kent C, Billeter J, Azanchi R, So A, Schonfeld J, Smith B, Lucas C, Levine JD. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009;42:239–244. doi: 10.5483/bmbrep.2009.42.5.239. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: An unusual form of mammalian sleep. Neurosci. Biobehav. Rev. 2008;32:1451–1484. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat. Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat. Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LO, Strapasson AC, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but not in male, rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–266. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Pereira LO, Nabinger PM, Strapasson AC, Nardin P, Gonçalves CA, Siqueira IR, Netto CA. Long-term effects of environmental stimulation following hypoxia-ischemia on the oxidative state and BDNF levels in rat hippocampus and frontal cortex. Brain Res. 2009;1247:188–195. doi: 10.1016/j.brainres.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Pham TM, Söderström S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav. Brain Res. 1999a;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Söderström S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999b;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Putz G, Heisenberg M. Memories in Drosophila heat-box learning. Learn. Mem. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan N, Shen Y, Sarsfield S, Lemberger T, Schütz G, Linden DJ, Ginty DD. SRF mediates activity-induced gene expression and synaptic plasticity but not neuronal viability. Nat. Neurosci. 2005;8:759–767. doi: 10.1038/nn1462. [DOI] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Robin C, Daborn PJ, Hoffmann AA. Fighting fly genes. Trends Genet. 2007;23:51–54. doi: 10.1016/j.tig.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ruan H, Wu CF. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proc. Natl. Acad. Sci. USA. 2008;105:7506–7510. doi: 10.1073/pnas.0711127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc. Natl. Acad. Sci. USA. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Strambi A, Charpin P, Augier R, Aouane A, Cayre M. Influence of environmental stimulation on neurogenesis in the adult insect brain. J. Neurobiol. 2000;45:162–171. [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Aouane A, Strambi A, Cayre M. Sensory inputs stimulate progenitor cell proliferation in an adult insect brain. Curr. Biol. 2002;12:1001–1005. doi: 10.1016/s0960-9822(02)00889-8. [DOI] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr. Biol. 2008;18:1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki KK, Ladewski L. Associative learning and memory in Drosophila: Beyond olfactory conditioning. Behav. Processes. 2003;64:225–238. doi: 10.1016/s0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Stickgold RJ, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold RJ, Malia A, Maguire D, Roddenberry D, O'Connor M. Replaying the game: Hypnagogic images in normals and amnesics. Science. 2000;290:350–353. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- Technau GM. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J. Neurogenet. 2007;21:183–196. doi: 10.1080/01677060701695359. [DOI] [PubMed] [Google Scholar]
- Tononi G. Sleep and synaptic homeostasis: A hypothesis. Brain Res. Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2005;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 1998;93:83–90. doi: 10.1016/s0166-4328(97)00142-3. [DOI] [PubMed] [Google Scholar]
- Toscano CD, McGlothan JL, Guilarte TR. Experience-dependent regulation of zif268 gene expression and spatial learning. Exp. Neurol. 2006;200:209–215. doi: 10.1016/j.expneurol.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, Hoi Sang U, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Effects of social isolation on neuromuscular excitability and aggressive behaviors in Drosophila: Altered responses by Hk and gsts1, two mutations implicated in redox regulation. J. Neurogenet. 2009 Jun;30:1–17. doi: 10.3109/01677060903063026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Borbély AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J. Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Walz R, Roesler R, Reinke A, Martins MR, Quevedo J, Izquierdo I. Short- and long-term memory are differentialy modulated by hippocampal nerve growth factor and fibroblast growth factor. Neurochem. Res. 2005;30:185–190. doi: 10.1007/s11064-004-2440-z. [DOI] [PubMed] [Google Scholar]
- Whitfield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Winnington AP, Napper RM, Mercer AR. Structural plasticity of identified glomeruli in the antennal lobes of the adult worker honey bee. J. Comp. Neurol. 1996;365:479–490. doi: 10.1002/(SICI)1096-9861(19960212)365:3<479::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Yasuda K, Brown RA, Krueger JM. State-dependent effects of light-dark cycle on somatosensory and visual cortex EEG in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1083–R1089. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat. Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Wang YK, Zhuo M. Enhanced synaptic long-term potentiation in the anterior cingulate cortex of adult wild mice as compared with that in laboratory mice. Mol. Brain. 2009;2:11. doi: 10.1186/1756-6606-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]