Abstract
Sleep is important for memory consolidation and is responsive to waking experience. Clock circuitry is uniquely positioned to coordinate interactions between processes underlying memory and sleep need. Flies increase sleep both after exposure to an enriched social environment and after protocols that induce long-term memory. We found that flies mutant for rutabaga, period, and blistered were deficient for experience-dependent increases in sleep. Rescue of each of these genes within the ventral lateral neurons (LNVs) restores increased sleep after social enrichment. Social experiences that induce increased sleep were associated with an increase in the number of synaptic terminals in the LNV projections into the medulla. The number of synaptic terminals was reduced during sleep and this decline was prevented by sleep deprivation.
Although sleep is a process that is necessary for survival, the functions of sleep are unknown (1, 2). Sleep is regulated by circadian influences and is important for consolidation of long-term memory (LTM) (3–5). Additionally, LTM is modulated by circadian mechanisms (6, 7). Because the relationship between sleep, memory, and circadian rhythms seem to be phylogenetically conserved, Drosophila can be used to explain mechanisms that coordinate these processes. Drosophila show an increase in daytime sleep after exposure to socially enriched environments (5). Similarly, an increase in sleep after courtship conditioning is necessary for LTM (5).
Increased sleep after social enrichment is dependent upon genes that are required for learning and memory, including genes that alter cyclic adenosine monophosphate signaling (5). Although newly eclosed flies that are mutant for the adenylyl cyclase rutabaga (rut2080) show increased sleep after social enrichment, 3- 4-day-old adult rut mutants do not respond to changes in the social environment (fig. S1). Elevating wild-type rut in adult flies with an RU486-inducible driver rescued experience-dependent increases in sleep in adult rut mutants (fig. S1); vehicle-treated siblings showed no increase in sleep (fig. S1). To identify circuits that mediate experience-dependent increases in sleep, we used a series of GAL4 lines to drive wild-type rut expression in brain circuits (Fig. 1A). Figure S2 illustrates the data analysis used to quantify each GAL4 rescue. Expression of UAS-rut using pdf-GAL4 restored the increase in daytime sleep and daytime sleep-bout duration, although to a lesser extent than GSelav (Fig. 1, B to E). The expression pattern of pdf-GAL4 is limited to the ventral lateral neurons (LNVs), a group of clock neurons that express pigment-dispersing factor (pdf) (8, 9). Although pdf is the only known output from the LNVs, flies mutant for pdf show a wild-type increase in sleep (fig. S3).
Fig. 1.
Clock cells regulate experience-dependent increases in sleep. (A) Data for each GAL4 line (GAL4>/+:rut2080/+;;UAS-rut/+) is compared to its parental line (GAL4/+:rut2080/+). No increase in Δ daytime sleep is observed in the absence of GAL4 [(rut2080/+;;UAS-rut/+)-(rut2080/+); white bar]. The mean Δ daytime sleep for Gs-elav-GAL4 (RU+ versus RU−) is shown to facilitate comparisons (black). One-way analysis of variance (ANOVA) for genotype, F(33,903) =9.09.(*, P < .05 with correction for 34 comparisons; n ≥ 16 for all groups). (B and C) Sleep after social enrichment in mutant rut2080/+;pdf-GAL4/+ flies and rut2080/+;pdf-GAL4/+;UAS-rut/+ flies (n = 16 in each group). (D and E) Average daytime sleep and sleep-bout duration in rut2080/+;pdf-GAL4/+ mutant and rut2080/+;pdf-GAL4/+;UAS-rut/+ rescue flies. Two-way ANOVA reveals a genotype by condition interaction for sleep [F(1,57) = 4.44, P = 0.03]andbouts[F(1,57) = 6.59, P = 0.013](*, P < .05, planned pairwise comparisons with a Tukey correction; n = 14 to 16 for all groups).(F and G) PER immunohistochemistry of per01;per+7.2-2 and per01mutants. (H) Δ daytime sleep in per01 mutants and per01;per+7.2-2 flies (P = 0.0002; n = 30 to 32 in each group). (I) Male per01 mutants do not exhibit a reduction in courtship 48 hours after a spaced courtship conditioning (N, Naïve; T, Trained; n = 13 to 14).(J) per01 flies only exhibit a transient increase in sleep immediately after training. (K) per01;per+7.2-2 flies exhibit suppression of courtship 48 hours after training (P = 0.001; n = 13 in each group). (L) per01;per+7.2-2 males also show sustained increases in sleep for 2 days. (M) Δ daytime sleep is quantified for per01 and per01;per+7.2-2 males Two-way ANOVA reveals a genotype by day interaction F(1,39) = 7.48, P = 0.009(*, P < .05 planned pairwise comparisons with a Tukey correction; n = 13 to 14 each group).
Given this role of clock cells, we examined the clock gene period (per), which is expressed in the LNVs and is required for LTM (6). Rescue of wild-type per using a 7.2-kb fragment of the per genomic sequence (per01; per+7.2-2) restored expression of PER at CT0 within the LNVsaswellasthedorsal lateral neurons, LNDs (Fig. 1F); mutant flies carrying a null mutation, per01, expressed no PER (Fig. 1G). Although per01 mutants showed no increase in sleep after social enrichment, per01;per+7.2-2 flies displayed normal experience-dependent increases in sleep (Fig. 1H). per01 mutants have no LTM when tested 48 hours after training and only show a transient increase in sleep (Fig. 1, I and J). per01;per+7.2-2 flies displayed LTM (Fig. 1K) and increases in sleep (Fig. 1, L and M). Although per levels are low in mutants for Clock and cycle, both acquire LTM (6) and increase sleep after social enrichment (5). Thus, only a very small amount of per may be required to support increased sleep and LTM.
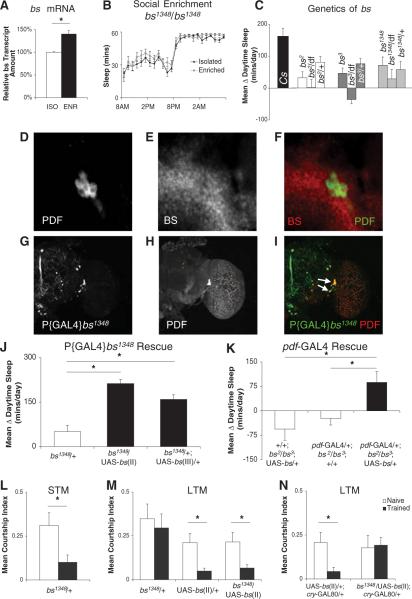
To further investigate the role of synaptic plasticity in clock cells, we used the Drosophila homolog for serum response factor (SRF), blistered (bs). In mice, SRF is essential for activity-induced gene expression and plays an important role in synaptic long-term potentiation (10) and in contextual habituation (11). bs retains a 93% identity with SRF within the DNA-binding MCM1-ARG80-Agamous-Deficiens-SRF (MADS) domain (12). Social enrichment elevated the transcription of bs in wild-type Canton-S (Cs) flies (Fig. 2A). Mutants carrying a P element inserted into the bs gene (P{GAL4}bs1348) do not increase sleep after social enrichment (Fig. 2B). This deficit was also found in flies carrying either of two other mutant alleles for bs (bs2 and bs3) and was present in flies that are homozygous for mutant bs alleles and flies that have been outcrossed to either Cs or to flies carrying the In(2LR)Px4 deficiency (Fig. 2C). The P-element insertion in bs1348 preserves the MADS domain; similar N-terminal truncated mutant SRF acts as dominant negative (13). BS is expressed throughout the brain, including pdf-expressing LNVs (Fig. 2, D to F). When UAS-egfp was driven by P{GAL4}bs1348, expression was restricted to a small number of neurons, including the LNVs (Fig. 2, G and I). Expression of bs using P{GAL4}bs1348 to drive either of two wild-type bs (UAS-bs) constructs rescued experience-dependent increases in sleep (Fig. 2J). Moreover, inducing bs expression within the LNVsusing pdf-GAL4 increased sleep after social enrichment (Fig. 2K).
Fig. 2.
blistered regulates experience-dependent increases in sleep. (A) bs transcripts are significantly elevated in socially enriched Cs flies compared with their isolated siblings (P = 0.03). (B) Flies homozygous for the P{GAL4}bs1348 insertion do not respond to social enrichment with an increase in sleep (n = 16 in each group). (C) Mean Δ daytime sleep is absent in bs2 and bs3 mutants and persists when each allele (bs2, bs3, or P{GAL4}bs1348) is outcrossed to Cs or with the Deficiency In(2LR)Px4. One-way ANOVA for genotype [F(9,124) = 2.73; P = 0.04; n = 32 in each group]. (D) Expression of UAS-egfp using pdf-GAL4 labels the cell bodies of LNVs. (E) Immunohistochemistry using mouse antibody to BS (diluted 1:1000). (F) Colocalization of BS and pdf-GAL4 indicates that BS is expressed in pdf-expressing LNVs. (G) P{GAL4}bs1348 was used to drive UAS-egfp, and the expression pattern was evaluated using confocal microscopy. (H) Brains of P{GAL4}bs1348/+;UAS-egfp flies were colabeled with guinea pig antibody to PDF (diluted 1:10,000) (I) Colocalization of GFP with PDF indicates that P{GAL4}bs1348 drives GAL4 expression in PDF-expressing LNVs (arrows). (J) The failure to respond to social enrichment with an increase in sleep can be rescued by combining P{GAL4}bs1348 with either of two separate UAS-bs alleles. One-way ANOVA for genotype [F(2,45) = 22.86; P = 1.4×10−;*, P < .05 Bonferroni post-hoc test; n = 16 in each group)].(K) Expressing wild-type bs using pdf-GAL4 in an otherwise bs mutant background (pdf-GAL4/+;bs2/bs3;UAS-bs) rescues increases in Δ daytime sleep); parental lines are shown in white. The lower Δsleep versus 2J may indicate a partial rescue. One-way ANOVA for genotype [F(2,45) = 6.30; P = 0.003; *, P < .05, Bonferroni post-hoc test; n = 16 in each group)]. (L) Courtship suppression in P{GAL4}bs1348 mutants tested 5 min after Courtship Conditioning White bars represent naïve males; dark bars represent trained males (P = 0.02; n =13 in each group). (M) LTM is disrupted in P{GAL4}bs1348/+ mutants tested 48 hours after training and rescued by expression of UAS-bs. Two-way ANOVA reveals a genotype by condition interaction [F(2,72) = 16.73; P = 0.0001] (*, P <.05, planned pairwise comparisons with a Tukey correction; n = 13 in each group).(N) Blocking GAL4 expression in clock cells using cry-GAL80 prevents rescue of LTM. Genotype by condition interaction [F(1,48) = 2.32; P = 0.14] (*, P <.05, planned pairwise comparisons with a Tukey correction; n = 13 in each group).
To establish whether expression of bs is required for LTM, we tested flies carrying the P{GAL4}bs1348 mutant allele using courtship conditioning. Although P{GAL4}bs1348/+ flies acquire short-term memory (Fig. 2L), LTM was impaired (Fig. 2M, left). Rescue of wild-type bs using P{GAL4}bs1348 restored LTM (Fig. 2M, right). Next, we used the GAL4 repressor cry-GAL80toblock UAS-bs expression within the LNs. Although UAS-bs/+;cry-gal80/+ control flies showed significant courtship suppression (Fig. 2N, left), P{GAL4}bs1348/UAS-bs;cry-GAL80/+ flies had no LTM (Fig. 2N, right), which suggests a role for the LNs, although we cannot exclude a role for the dorsal neurons (DNs). Although SRF deletion in mouse forebrain results in neurons with abnormal morphology (14), the morphology of LNVs in mutant P{GAL4}bs1348/+ flies (fig. S4A) did not differ from that of LNVsin P{GAL4}bs1348/UAS-bs rescue flies (fig. S4B). All three mutants for bs had intact circadian rhythms and showed anticipatory activity before light-dark transitions; only bs3 flies show an altered period under constant darkness (fig. S5, A to F). These findings suggest that there are no developmental abnormalities in the LNVsin bs mutants.
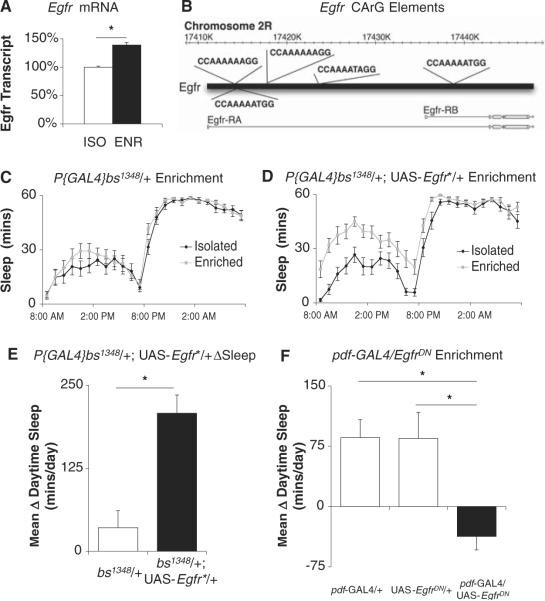
Hypomorphic alleles for bs prevent proper wing development through interactions with Epidermal growth factor receptor (Egfr) signaling (15–17). Because Egfr alters sleep in Drosophila (18), interactions between bs and Egfr may regulate responses to social experience. After social enrichment, transcription of Egfr was significantly elevated in Cs flies (Fig. 3A). The Egfr genomic sequence contains several CC(A/T)6GG CArG elements that can be bound by bs to promote transcription (Fig. 3B), and transcription of Egfr was significantly reduced in bs mutants (data not shown). Thus, we used P{GAL4}bs1348 to drive expression of a constitutively active Egfr construct (UAS-Egfr*). Although P{GAL4}bs1348/+ mutants showed no change in sleep after social enrichment (Fig. 3C), activation of Egfr in P{GAL4}bs1348/+;UAS-Egfr*/+ flies increased sleep (Fig. 3, D and E). Conversely, the expression of a dominant-negative construct for Egfr (UAS-EgfrDN) using pdf-GAL4 prevented increases in sleep after social enrichment, but parental controls (pdf-GAL4/+ and UAS-EgfrDN/+) were wild-type (Fig. 3F).
Fig. 3.
Egfr mediates experience-dependent sleep. (A) Transcription of Egfr is significantly elevated in socially enriched Cs flies compared with their isolated siblings, as measured by quantitative polymerase chain reaction. (B) Genomic Egfr sequence contains several SRF-binding CArG elements. (C and D) Driving the expression of UAS-Egfr* with P{GAL4}bs1348 restores ΔSleep after social enrichment. (E) Summary of the response for data shown in (C) and (D) (*, P = 7×10−5; n = 16 in each group). (F) No change in Δ sleep is observed in pdf-GAL4/UAS-EgfrDN. One-way ANOVA for genotype F(2,44) = 7.75, P =0.001 (*, P < .05, Bonferroni correction; n = 15 to 16 in each group).
A recent theory proposes that a function of sleep is to downscale synaptic connections (19). Moreover, structural plasticity can be induced by environmental manipulation in Drosophila (20). To quantify the effect of social enrichment on the number of post-synaptic terminals in LNV projections, we used pdf-GAL4 to drive expression of a green fluorescent protein (GFP)–tagged construct of the postsynaptic protein discs-large (UAS-dlgWT-gfp). After 5 days of social enrichment, LNV projections into the medulla of pdf-GAL4/+;;UAS-dlgWT-gfp/+ flies contained significantly more GFP-positive terminals (Fig. 4, A to C). Although we have not demonstrated that the labeled synaptic terminals are functional, these tools have been used to quantify synapses (21). The expression of the UAS-dlgWT-GFP marker did not alter synaptic function in a wild-type background (20) and did not prevent the increase in sleep when expressed using pdf-GAL4 after social enrichment (fig. S6). To determine the effect of waking on synapse number, socially isolated pdf-GAL4/+;;UAS-dlgWT-gfp/+ flies and their enriched siblings either were allowed to sleep ad libitum or were sleep deprived for 48 hours after social enrichment. Although the number of dlg-GFP positive terminals remained elevated in sleep-deprived socially enriched flies, terminal number was significantly reduced in siblings that were allowed to sleep (Fig. 4D). Similarly, the number of presynaptic terminals in LNV projections into the medulla using a GFP-tagged construct of the presynaptic protein synaptobrevin (UAS-VAMP-GFP) in pdf-GAL4/+;UAS-VAMP-GFP/+ flies was increased (Fig. 4, E to G). After 48 hours of recovery, socially enriched pdf-GAL4/+;UAS-VAMP-GFP/+ flies had a reduced number of VAMP-GFP–positive presynaptic terminals relative to their sleep-deprived siblings (Fig. 4H). A recent study has reported a clock-dependent remodeling in the axonal terminals of the PDF circuit that is highest during the day (22). Recent data indicates that hyperexcitation of a subset of the LNVs suppresses sleep in Drosophila (23–25). Together with our results, these data suggest that the PDF circuit is well suited to test the hypothesis that sleep acts to downscale synaptic connections that are potentiated during waking experience.
Fig. 4.
LNV synapse number in medulla. (A and B) LNV projections in socially enriched pdf-GAL4/+;;UAS-dlgWT-gfp/+ flies contain more GFP-positive terminals than their socially isolated siblings when evaluated using confocal microscopy. (C) Relative quantification of dlg-GFP-immunopositive terminals in socially isolated pdf-GAL4/+;;UAS-dlgWT-gfp/+ flies versus socially enriched siblings (P = 1.5×10−; n =33 to 34 in each group). (D) dlg-GFP positive terminals 48 hours after social enrichment in sleep-deprived flies and their normally sleeping siblings. (P = 0.003; n = 15 to 18 in each group). (E and F) Social enrichment of pdf-GAL4/+;UAS-VAMP-GFP/+ flies induces a modest increase of LNV terminals relative to isolated siblings. (G) Relative quantification of VAMP-GFP-positive terminals in socially isolated pdf-GAL4/+;UAS-VAMP-gfp/+ flies and their socially enriched siblings (P = 0.03; n =16 to 18 in each group).(H) UAS-VAMP-gfp-positive terminals 48 hours after social enrichment in sleep-deprived flies and their normally sleeping siblings (P = 0.01; n = 18 in each group).
Supplementary Material
Acknowledgments
We thank M. Thimgan, W. Vanderheyden, P. Gray, and P. Taghert for comments. This study was funded by NIH grants R01-NS051305-01A1 and T32-GM008151.
Footnotes
References and Notes
- 1.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Science. 1983;221:182. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 2.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Nature. 2002;417:287. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Neuron. 2002;35:205. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 4.Graves LA, Heller EA, Pack AI, Abel T. Learn. Mem. 2003;10:168. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Science. 2006;313:1775. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 6.Sakai T, Tamura T, Kitamoto T, Kidokoro Y. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16058. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker S, McConnaughey S, Page TL. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15905. doi: 10.1073/pnas.0702082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helfrich-Forster C, Homberg U. J. Comp. Neurol. 1993;337:177. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 9.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. Cell. 1999;99:791. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 10.Ramanan N, et al. Nat. Neurosci. 2005;8:759. doi: 10.1038/nn1462. [DOI] [PubMed] [Google Scholar]
- 11.Etkin A, et al. Neuron. 2006;50:127. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Affolter M, et al. Development. 1994;120:743. doi: 10.1242/dev.120.4.743. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier-Rouviere C, et al. Exp. Cell Res. 1993;209:208. doi: 10.1006/excr.1993.1303. [DOI] [PubMed] [Google Scholar]
- 14.Knoll B, et al. Nat. Neurosci. 2006;9:195. doi: 10.1038/nn1627. [DOI] [PubMed] [Google Scholar]
- 15.Fristrom D, et al. Development. 1994;120:2661. doi: 10.1242/dev.120.9.2661. [DOI] [PubMed] [Google Scholar]
- 16.Montagne J, et al. Development. 1996;122:2589. doi: 10.1242/dev.122.9.2589. [DOI] [PubMed] [Google Scholar]
- 17.Roch F, Baonza A, Martin-Blanco E, Garcia-Bellido A. Development. 1998;125:1823. doi: 10.1242/dev.125.10.1823. [DOI] [PubMed] [Google Scholar]
- 18.Foltenyi K, Greenspan RJ, Newport JW. Nat. Neurosci. 2007;10:1160. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 19.Tononi G, Cirelli C. Brain Res. Bull. 2003;62:143. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Barth M, Heisenberg M. Learn. Mem. 1997;4:219. doi: 10.1101/lm.4.2.219. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, et al. Neuron. 2007;53:201. doi: 10.1016/j.neuron.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández MP, Berni J, Ceriani MF. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheeba V, et al. Curr. Biol. 2008;18:1537. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parisky KM, et al. Neuron. 2008;60:672. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang Y, Griffith LC, Rosbash M. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19587. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.