Abstract
Neurogenesis is continually occurring in two regions within the mammalian central nervous system (CNS) and increasing evidence suggests that it is important for selective learning and memory. How this plasticity is maintained in isolated niches within mature networks has been extensively studied in recent years, and a large body of evidence has accumulated describing many different regulatory factors and points of regulation. In this review, we attempt to organize the current research by summarizing findings affecting early neurogenesis: during proliferation, fate commitment and migration, versus late neurogenesis: including dendritic development, synaptic integration and survival. We discuss the roles of three different classes of factors regulating early and late phases of neurogenesis: intrinsic factors, extrinsic factors and neurotransmitters. Finally, we suggest that neurotransmitters may act upstream from other extracellular factors and cell-intrinsic mechanisms by coupling network activity to the niche microenvironment and intracellular machinery to ultimately regulate neurogenesis.
Background
Historically, the production of new neurons within the central nervous system (CNS) was believed to be restricted to embryonic development (Cajal and May, 1928). In the 1960s and 1970s Altman and Das discovered newly born cells occurring postnatally in the mature CNS using radioanalog tracing (Altman and Das, 1965; Kaplan and Hinds, 1977). However, this work was largely ignored until postnatal neurogenesis was “rediscovered” in the 1990s when better tools became available to definitively establish those new cells as neurons and not glia (Alvarez-Buylla, 1990; Reynolds and Weiss, 1992). Since then a proliferation of research in this past decade has begun to unravel its mechanisms, functional roles and therapeutic potential. Neurogenesis continues to persist in two specific areas of the adult mammalian brain: the subventricular zone (SVZ) lining the lateral ventricles, and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus (Lois and Alvarez-Buylla, 1994; Kempermann et al., 1998; Lledo et al., 2006). In these two regions, resident pools of slow-dividing, glia-like stem cells generate highly proliferative transit amplifying progenitors that then give rise to fate-committed neuronal or glial precursors (Doetsch et al., 1999; Imura et al., 2003; Seri et al., 2001; Alvarez-Buylla and Lim, 2004). These precursors (called neuroblasts or glioblasts) then migrate to their final destinations and differentiate into postmitotic neurons, astroglia and oligodendrocytes. In the case of neurogenesis, newly born neurons undergo additional maturation to develop appropriate dendritic morphology, synaptically integrate into the preexisting circuitry and survive an activity-dependent weeding out process (Carleton et al., 2003). Thus neurogenesis may be broadly divided into two phases: an early phase that includes (1) stem cell proliferation, neuronal vs. glial fate commitment and migration; and a later phase that involves the (2) morphological and synaptic development and survival of newly born neurons. We will use this distinction to organize our discussion of the involvement of intracellular and extracellular factors as well as the neurotransmitters γ-aminobutyric acid (GABA) and glutamate that have been shown to regulate the different steps of postnatal neurogenesis. In this review, astrocytic stem cells are defined as the self-renewing, multipotent cells present in the SVZ and SGZ that express glial markers, including the glutamate aspartate transporter (GLAST), the intermediate filament proteins glial fibrillary acidic protein (GFAP) and nestin, and the carbohydrate Lewis X (Lex) (Doetsch et al., 1999; Capela and Temple, 2002). Intermediate progenitors, also called transit amplifying progenitors or cells, express epidermal growth factor receptor (EGFR), while neuroblasts, or neuronal precursors, are defined by the presence of immature neuronal markers, including doublecortin (DCX) and βIII-tubulin (Tuj1) (Platel et al., 2009a; Bordey, 2006).
In the SVZ, astrocytic stem cells reside in-between the striatal parenchyma and the ependymal cell layer that lines the lateral ventricles (Doetsch et al., 1997). These stem cells generate rapidly dividing transit amplifying progenitors, which give rise to neuroblasts that migrate by moving tangentially through the rostral migratory stream (RMS) and into the olfactory bulb (OB) (Altman, 1969; Lois and Alvarez-Buylla, 1994; De et al., 2001). During migration these proliferative cells travel in chains, and are ensheathed by specialized astrocytes (Lois et al., 1996). In the OB the neuroblasts exit the RMS, change direction and migrate radially outward to differentiate into GABAergic, dopaminergic and glutamatergic periglomerular and granule interneurons (Altman and Das, 1966; Hinds, 1968; Kishi, 1987; Brill et al., 2009; Kosaka et al., 1998). Granule cells form dendrodendritic reciprocal synapses with mitral and tufted neurons in the bulb and inhibit their activity to fine-tune their output, ultimately playing a role in olfactory discrimination and learning (Lledo et al., 2006). Periglomerular cells make one-way as well as reciprocal synapses with the apical dendrites of the mitral/tufted cells and the terminals of the olfactory nerves that converge into the glomeruli (Kosaka et al., 1985; Kosaka et al., 1998; Kosaka and Kosaka, 2005).
In the SGZ, astrocytic stem cells reside in the inner third of the granule cell layer (GCL) between the dentate gyrus and the hilus. These stem cells generate transit amplifying progenitors, which give rise to neuroblasts that migrate locally into the GCL to differentiate into granule neurons (Kuhn et al., 1996). From the GCL the newly generated granule neurons extend axonal projections called mossy fibers into the CA3 subfield, and extend dendritic branches toward the molecular layer (Stanfield and Trice, 1988; Markakis and Gage, 1999; Claiborne et al., 1986; Toni et al, 2008). Upon maturation these neurons receive glutamatergic inputs from the entorhinal cortex, GABAergic inputs from local interneurons and send glutamatergic output to the CA3 subfield (Toni et al, 2008). Hippocampal neurogenesis has been shown to be important in learning, memory, and emotionality using a variety of behavioral paradigms (Clelland et al., 2009).
The progression from astrocytic stem cell to neuronal progenitor to synaptic integration requires tightly coordinated, complex regulation by a multitude of factors. This review covers this progression in separate sections examining the impact of intracellular factors, extracellular factors, GABA and glutamate on neurogenesis. The last two neurotransmitters are given their own sections due to their potential function as coordinators of both early and late phases and their action upstream of a multitude of intracellular cascades. In addition, they provide the coupling of neurogenesis to neuronal activity in the SGZ (Cameron et al., 1995; Tozuka et al., 2005). In addition, unpublished data from our laboratory suggest that dendrites from striatal neurons impinge onto SVZ cells leading to intracellular calcium increases upon neuronal spiking (Young and Bordey, 2008). Each section is discussed chronologically by first considering the early phases of neurogenesis (stem cell proliferation, fate commitment and neuroblast migration), and then considering the late phases of neurogenesis (synaptic integration, survival, and morphology).
Intracellular factors regulating early phases of neurogenesis
Postnatal neurogenesis is subject to tight regulation, confined to isolated microenvironments and sensitive to neuronal activity, stress and aging. This control may be required to prevent network instability, maintain experience-driven memory and behavioral patterns, and prevent tumorigenesis. To this end, cell-intrinsic factors comprise a major component of this regulation and help coordinate neurogenesis by forming the bulwark of how cells make choices and adapt to changes in their environments.
In the postnatal SVZ, progenitor fate commitment is sequentially driven by a family of proneural proteins called the basic helix-loop-helix (bHLH) transcription factors, such as Ascl1, neurogenin2 (Ngn2), Neuro-D1, Neuro-D2, Tbr1 and Tbr2, in a pattern similar to cortical and hippocampal neurogenesis (Roybon et al., 2009a; Farah et al., 2000). This bHLH-driven fate commitment is thought to develop in a temporally successive fashion, from more broadly proneural proteins (Ascl1) to more neuronal and subtype-specifying (Neuro-D1) (Roybon et al., 2009b). Although more work needs to be done to fully flesh out the role of bHLH proteins in the postnatal SVZ, in vitro transient expression of bHLH proteins is sufficient to induce neuronal fate commitment. The bHLH proteins are thought to induce differentiation in part by activating cyclin-dependent kinase inhibitors that then induce cell cycle exit. This finding has been shown in culture and awaits confirmation in vivo (Bally-Cuif and Hammerschmidt, 2003). Cdk inhibitors p27KIP1 and p19INK4d have also been shown to modulate proliferation in the SVZ. Mice lacking p27KIP1 show increased progenitor proliferation and a reduction in neuroblast number (Doetsch et al., 2002b; Coskun and Luskin, 2001). Furthermore, mice deficient for both p27KIP1 and p19INK4d display renewed proliferation of post-mitotic neurons and increased cell death- causing seizures, movement disorders and death by postnatal day 18 (Zindy et al., 1999). This indicates that cdk inhibitors positively regulate cell cycle exit in SVZ progenitors and their absence prolongs or renews cell cycling. Cdk inhibitors mediate cell cycle exit by inhibiting phosphorylation of the retinoblastoma protein (Rb) (Weinberg, 1995). Dephosphorylated Rb binds to and sequesters the E2F transcription factor that normally functions in the nucleus to positively regulate cell cycle progression, thereby promoting cell cycle exit. In the SVZ, E2F-deficient mice show reduced progenitor proliferation and neuroblast numbers, suggesting that E2F transcriptional activity is required for cell cycle progression and the maintenance of neurogenic ability (Yoshikawa, 2000). Taken together, these results outline a stereotyped program of cell-intrinsic mechanisms at work within the SVZ to regulate the proliferation and fate commitment of SVZ progenitors in the early steps of postnatal neurogenesis.
MicroRNAs (miRs) are short, non-coding, single-stranded RNA molecules approximately 19–23 nucleotides in length that regulate gene expression by binding to complementary elements in the untranslated regions of target mRNAs and inhibiting protein synthesis. They exert epigenetic control either to maintain the status quo in a cell, (i.e. to maintain tissue identity), or they act in dynamic processes occurring within cells to refine and sharpen transitional states, (i.e. facilitating the switch in expression profile occurring at active synapses that were previously silent) (Hornstein and Shomron, 2006; Giraldez et al., 2005; Schratt et al., 2006). Their role in shaping the temporal dynamics and phenotypic outcome of gene regulatory networks means that the functions of particular miRs become especially resolvable in plastic processes like postnatal neurogenesis, and miRs have recently been implicated in regulating the fate commitment of SVZ progenitors (Cheng et al., 2009). Although as yet untested, one idea for how bHLH proteins are sequentially activated and repressed and the transitions from multipotent progenitor to post-mitotic neuron more sharply defined is the successive miR-mediated downregulation of targeted bHLH proteins. In the SVZ, microRNA-124 was shown to be upregulated upon neuronal differentiation in the SVZ, and overexpression increased the number of mature neurons at the cost of proliferative progenitors and neuroblasts, while knockdown decreased mature neuron production and increased the number of glia (Cheng et al., 2009). MiR-124 was shown to mediate these effects by antagonizing sox9, a transcription factor that directs glial differentiation. MiR-124 has also been shown to target SCP1, a component of the REST/NRSF complex that represses neuronal genes in non-neuronal cells and PTBP1, a repressor of neuron-specific alternative splicing (Visvanathan et al., 2007; Conaco et al., 2006; Makeyev et al., 2007; Boutz et al., 2007). Together these results indicate that miR-124 is emerging as a critical regulator of neuronal development and tissue identity, acting early on in the shift from transit amplifying cells to neuroblast.
In the postnatal DG, bHLH transcription factors have also been shown to induce neuronal differentiation, acting through Ascl1 and Ngn2 (Jessberger et al., 2008b; Ozen et al., 2007; Kim et al., 2007). E2F-deficiency also causes decreases in proliferation in the DG and decreases new neuron production, just as it does in the SVZ (Cooper-Kuhn et al., 2002). Furthermore, accumulating evidence in the hippocampus suggests a major role for the schizophrenia susceptibility gene Disrupted in Schizophrenia 1 (DISC1) in modulating both early and later phases of postnatal neurogenesis. A role for DISC1 in the SVZ has not been evaluated so far. Recently, DISC1 was shown to regulate the proliferative ability of progenitors in the SGZ by negatively regulating GSK3β-mediated inhibition of β-catenin (Mao et al., 2009).
Cell-intrinsic factors and their roles in the early phases of postnatal neurogenesis have only begun to become amenable to experimental dissection, and exciting developments are forthcoming. Some aspects of early phases of neurogenesis, in terms of fate commitment and migration, have not been studied very deeply in the SGZ, as most studies measure proliferation alone. With emerging techniques, such as conditional-inducible lines of mice and floxed-stop strategies to limit manipulations to the postnatal period, a more complete view of early neurogenesis in the SGZ may emerge.
Intracellular factors regulating later phases of neurogenesis
Later stages of neurogenesis include the survival, synaptic integration and dendritic elaboration of neuronal precursors within their target sites. CREB (cAMP response element binding) is a long-studied transcription factor known for underlying the later stages of synaptic plasticity and memory formation, as well as for linking neuronal activity to survival. In the postnatal SVZ-OB, CREB has been shown to be important in the survival and dendritic arborization of SVZ neuroblasts (Giachino et al., 2005). CREB phosphorylation is transient and parallels maturation, increasing during migration towards the OB and decreasing once radial migration and synaptic integration are completed. CREB-deficient mice show deficits in neuroblast survival in the OB, and CREB inhibition in vitro severely attenuates neurite outgrowth, suggesting that CREB positively modulates survival and dendritic elaboration in the OB and plays an important role in the later phases of SVZ neurogenesis. Unpublished results from our lab suggest that a CREB-regulated microRNA, miR-132, may be involved in mediating some of the effects seen by impairing CREB activity in the SVZ. miR-132 expression is upregulated along the migratory route of the SVZ neuroblasts, peaking in the OB, and miR-132 overexpression enhances morphological complexity in vivo (Pathania et al., 2009). These data suggest that CREB and a CREB-regulated miRNA may form the basis of a structural plasticity program seen in SVZ postnatal neurogenesis.
In the postnatal DG, CREB activity was also recently shown to be important for dendritic development and neuron survival (Jagasia et al., 2009). GABA-induced depolarization is important for CREB phosphorylation in the SGZ: knocking down NKCC1, a Cl− importer, abolished GABAA-mediated depolarization in newborn cells and resulted in reduced CREB phoshphorylation, attenuated dendrite extension and decreased survival. Loss of GABA-depolarization also reduced numbers of newly born neurons, suggesting an effect on differentiation. These effects of losing GABA depolarization could be rescued by enhancing downstream CREB signaling, suggesting that GABA-depolarization in immature neuroblasts induces CREB activation and promotes survival and dendrite elaboration. Cyclin-dependent kinase 5 (Cdk5) has also been implicated in modulating some aspects of later phases in neurogenesis. Retroviral knockdown in a subset of postnatal SGZ cells resulted in abnormal migration and aberrant dendritic growth leading to ectopic and reduced synapse formation (Jessberger et al., 2008a). Another study found that conditional and inducible ablation of Cdk5 in the postnatal DG resulted in decreased neuroblast production, but no change in numbers of proliferating cells, suggesting that Cdk5 is needed for maturation and survival of immature neurons (Lagace et al., 2008). Neuro-D1, presumably downstream from the early phase-acting neurogenin2, was recently shown to regulate the late phase survival of newly born neurons in the dentate gyrus using inducible ablation (Gao et al., 2009). The use of conditional knockout approaches is fast becoming the gold standard for delineating the role of a molecule in postnatal neurogenesis. Another cell-intrinsic regulator important for proper positioning and morphological differentiation is DISC1. DISC1 knockdown has been shown to accelerate integration resulting in aberrant dendritic elaboration and mispositioning of the cell body to the outer granule cell layer and molecular layer (Duan et al., 2007). DISC1 knockdown also enhanced membrane excitability and synapse formation, suggesting that this protein is an important negative regulator of later phases of neurogenesis such as synaptic integration and dendrite development. Recently, another cell-intrinsic protein, Kruppel-like factor 9 (Klf-9) was shown to positively regulate neuroblast maturation and synaptic integration (Scobie et al., 2009). Klf-9 deletion spares proliferation and neuronal fate commitment in the postnatal SGZ, but leads to impaired morphological differentiation and decreased LTP, suggesting a requirement for Klf-9 in later stages of neurogenesis.
Intrinsic mechanisms regulating later stages of neurogenesis are some of the least elaborated aspects of postnatal neurogenesis. With the emergence of inducible and conditional manipulation techniques, it has become possible to discretely assay the roles of many factors within the context of postnatal neurogenesis. Work is also emerging that utilizes the stop-flox-mediated overexpression of factors in a conditional and inducible manner.
Extracellular cues regulate early phases of neurogenesis
Neurotrophic factors have long been implicated in the dynamic regulation of postnatal neurogenesis. In the SVZ, fibroblast growth factor 2 (FGF-2) has been shown to affect the early steps in neurogenesis, positively regulating progenitor proliferation and leading to an increase in the number of neurons migrating from the SVZ into the OB (Mudo et al., 2009; Wagner et al., 1999). Furthermore, FGF-2 is known to interact with epidermal growth factor (EGF) receptor signaling in neuroblasts, wherein prior FGF exposure is necessary for progenitors to respond to EGF or transforming growth factor α (TGFα), the endogenous ligand for EGF receptors (Kuhn et al., 1997; Jin et al., 2003a; Jin et al., 2003b; Lillien and Raphael, 2000; Burrows et al., 1997). EGF has been shown to also increase proliferation specifically in the SVZ transit amplifying progenitor population, resulting in fewer postmitotic neurons (Doetsch et al., 2002a). Vascular endothelial growth factor (VEGF), an angiogenic protein that can produce neurotrophic effects, has been shown to stimulate proliferation and neuroblast production in the SVZ, while insulin-like growth factor-I (IGF-I), a growth factor implicated in mediating the positive effects of exercise on adult neurogenesis, has been also implicated in enhancing proliferation and migration in the postnatal SVZ (Hurtado-Chong et al., 2009; Jin et al., 2002).
Apart from modulating proliferation, diffusible factors have also been shown to affect the initial establishment of the neurogenic niche itself, by influencing stem cell self-renewal and cell fate decisions. The factors that regulate such homeostatic effects within the niche may comprise a separate class of growth factors and unlike FGF-2, VEGF and IGF-I, are not circulating systemically in the bloodstream at appreciable levels nor produced in an acute, dynamically regulated manner. Based on this hypothesis, sonic hedgehog (Shh), wnts, bone morphogenic proteins (BMPs) and Notch/Delta are extracellular factors possibly acting in a more local fashion. Sonic hedgehog signaling is required for establishing and maintaining the quiescent pool of stem cells in the postnatal SVZ. Ablating Smoothened, the transmembrane protein required for hedgehog signaling, specifically in the SVZ results in the depletion of stem cells and proliferative transit amplifying progenitors by postnatal day 8 and the depletion of neuroblasts by postnatal day 30 (Balordi and Fishell, 2007). The Notch-DSL (Delta/Serrate/LAG-2) pathway is a very highly conserved cell-cell signaling system that acts through single-pass transmembrane proteins. Binding of Notch to its ligand causes cleavage of an intracellular domain that translocates to the nucleus where it interacts with transcriptional regulators to initiate expression of target genes like Hes1 and Hes5. In mammals, Notch ligands like Delta-like and Jagged bind Notch on stem and proliferative cells to maintain self-renewal and prevent terminal differentiation. In the neonatal SVZ, retrovirally delivered activated Notch enhances the numbers of quiescent SVZ progenitors at the cost of migratory neuroblasts (Chambers et al., 2001). Another study shows that conditional ablation of Notch signaling in the ependymal cells reprograms these cells and enables them to leave their position in the epithelium, take on SVZ stem cell characteristics and differentiate into granule and periglomerular neurons in the OB (Carlen et al., 2009). BMPs, a family of growth factors within the transforming growth factor β superfamily, have been shown to instruct a glial lineage in SVZ stem cells, and noggin, a BMP antagonist secreted by the adjacent ependymal cells, blocks glial differentiation of stem cells in favor of neurogenesis (Lim et al., 2000). However, deletion of Smad4, a downstream target of BMP signaling, instead of increasing neurogenesis has been shown to result in oligodendrocyte production and a neurogenic deficit (Colak et al., 2008). Therefore, it seems that BMP signaling can have divergent effects in the SVZ.
In the SVZ, neuroblasts have to migrate a greater distance than do their analogues in the dentate gyrus, to arrive at their ultimate destinations in the OB. Furthermore, the migration behavior exhibited by SVZ neuroblasts has two phases; it is tangential from the SVZ to the RMS-OB, and then becomes radial-like as the neuroblasts exit the RMS and begin synaptic integration into the granule cell layer. For our purposes in this review both phases of this migration are being considered within the “early” phase of neurogenesis, prior to dendritic arborization, reception of synaptic inputs and survival. This feature of SVZ neurogenesis distinguishes it from hippocampal neurogenesis where the neuroblasts migrate very short distances, and has allowed for the isolation of a few important guidance molecules that enable this directed migration. Slit-1 and Slit-2 expression in the SVZ and septum is thought to repel neuroblasts away from the SVZ and towards the OB, while netrin expression in the mitral cells of the OB, and the coincident expression of netrin receptors neogenin and deleted in colorectal cancer (DCC) in neuroblasts may form a chemoattractive cue drawing neuroblasts towards the OB (Wu et al., 1999; Murase and Horwitz, 2002; Nguyen-Ba-Charvet et al., 2004). The secreted protein prokineticin2 (PK2) has also been shown to act as potent chemoattractant for SVZ neuronal progenitors. PK2 is expressed in the OB, and attracts SVZ cells to the OB through the two G-protein coupled prokineticin receptors (PRK1 and PRK2) (Ng et al., 2005). Both ephrins and their Eph tyrosine kinase receptors are expressed in the SVZ and have been shown to play a role in both regulation of progenitor proliferation and neuroblast migration. EphA7, EphB2 and ephrin-B2 are associated with astrocytic progenitors in the SVZ, while ephrin-A2 is expressed in the neuroblasts. Ephrin-B2 and EphB2 signaling seem to positively regulate progenitor proliferation while also disrupting neuroblast migration in the postnatal SVZ, while ephrin-A2 seemed to positively regulate progenitor proliferation (Conover et al., 2000; Holmberg et al., 2005). Tangential migration in the RMS has also been shown to involve the α6β1 integrin (Emsley and Hagg, 2003). Once in the OB, neuroblasts have to reorient from tangential to radial migration into the GCL. This process has been shown to involve the expression of tenascin-R, in both the GCL and internal plexiform layers of the OB, and reelin, expressed by the mitral cells (Saghatelyan et al., 2004; Hack et al., 2002; Hagg, 2005).
In the DG, the effects of trophic factors on early phases of neurogenesis are consistent for the most part with their effects in the SVZ. FGF-2 is also needed to positively regulate proliferation of the neuroblasts in the DG (Zhao et al., 2007). Both IGF-1 and VEGF infusion increase the number of new neurons in the DG (Aberg et al., 2000; Jin et al., 2002). One growth factor implicated in regulating early phases of neurogenesis in the DG and not in the SVZ is neurotrophin-3. NT-3 has been shown to enhance neuronal differentiation in the DG and NT-3 deficiency leads to the reduction in number of new neurons but not glia (Shimazu et al., 2006).
As in the SVZ mentioned above, loss of sonic hedgehog signaling in the postnatal SGZ also results in the near complete loss of hippocampal progenitor pools and severe hypotrophy (Han et al., 2008). Interestingly, this paper also reported the finding that hedgehog signaling in the SGZ requires primary cilia in order to maintain progenitor pool numbers within the neurogenic niche (Han et al., 2008). SVZ stem cells also possess a primary cilium that contacts the ventricle, and while the cerebrospinal fluid contains Shh and the beating cilia of the ependymal cells may set up gradients, there are as yet no published data for the requirement for cilia in Shh signaling in the SVZ. SGZ astrocytes secrete wnts that promote a neurogenic program in DG progenitors (Lie et al., 2005). In the postnatal DG conditional ablation of Notch in glial fibrillary acidic protein-positive (GFAP+) astrocytes and stem cells induced a reduction in proliferation and increased the numbers of doublecortin-positive (DCX+) neuroblasts, while constitutive expression of activated Notch had the opposite effect, increasing proliferation and decreasing levels of DCX+ neuroblasts (Breunig et al., 2007). BMP antagonists too are found in the DG: neurogenesin-1, a BMP antagonist, is secreted by astrocytes and granule cells in the DG to block gliogenesis (Ueki et al., 2003).
There is now an immensity of information being generated within the field regarding the effects that neurotrophic factors mediate in the early stages of neurogenesis. On the one hand, many exciting avenues are emerging for therapeutic intervention into neurodegenerative diseases and psychiatric illnesses and knowledge of how neurogenic niches are formed, maintained, and neuronal and glial programs directed is fundamental for devising a clinical paradigm of directed neurogenesis. On the other hand however, the volumes of data on extracellular factor-based modulation of postnatal neurogenesis needs more critical validation within the context of in vivo experiments or behavioral analyses.
Extracellular cues regulate later phases of neurogenesis
Later stages of postnatal neurogenesis have also been shown to be responsive to neurotrophic factor signaling. A single nucleotide polymorphism in the human brain derived neurotrophic factor (BDNF)-encoding gene (Val66Met) has been shown to correlate with mood disorders and memory deficits, and knock-in mice possessing the human SNP showed reduced activity-dependent BDNF secretion ultimately resulting in reduced survival of SVZ neuroblasts and impaired spontaneous olfactory discrimination (Bath et al., 2008). In this study, activity-dependent BDNF signaling in the SVZ was shown to exert its effects on survival and olfactory function through TrkB receptors on neuroblasts.
BDNF signaling has been shown to be important in postnatal hippocampal neurogenesis. Val66Met knock-in mice possess increased anxiety and memory deficits that coincide with decreased hippocampal volume (Chen et al., 2006). Conditional and inducible deletion of TrkB, the receptor with the greatest affinity for BDNF, in SGZ astrocytes reduced survival of newly born granule neurons and decreased their morphological complexity and dendritic length (Bergami et al., 2008). TrkB deletion also increased measures of anxiety-like behavior, and impaired late-phase LTP, implicating BDNF-signaling in the regulation of synaptic integration and plasticity of postnatally generated dentate granule cells. FGF-2 has also been shown to be important in later phases of neurogenesis in the OB. Specifically, FGFR1-deletion leads to severe impairment of LTP and leads to deficits in memory consolidation (Zhao et al., 2007). NT-3 deletion also results in decreased LTP, but only in lateral and not in medial perforant path-granule neuron synapses. Furthermore, NT-3 deficiency leads to decreased performance on spatial memory tasks (Shimazu et al., 2006). Conditional and inducible ablation of Notch signaling resulted in stunted dendritic arborization in newly born neurons in the postnatal DG, while expression of the constitutively active Notch enhanced morphological complexity (Breunig et al., 2007).
Later stages of neurogenesis are poorly studied in the SVZ. Knowledge of molecules regulating the survival, synaptic integration and morphogenesis of newly born cells is more limited in comparison to the literature covering the DG. However, BDNF-signaling is an example of emerging data within the field that unites hypotheses between the two neurogenic niches. It is also interesting to note the sustained differences between the two niche microenvironments. NT-3-signaling is exclusive to the DG and may promote excitatory versus inhibitory neurogenic potential. It has also been suggested that the convergence of dopaminergic and serotonergic fibers defines the SVZ, while convergence of noradrenergic and serotonergic projections may define the SGZ (Hagg, 2005).
Dopamine regulates early phases of neurogenesis
The SVZ is innervated by dopaminergic fibers originating in the substantia nigra, while the SGZ is innervated by dopaminergic fibers coming from the ventral tegmental area (VTA). Dopaminergic signaling has been shown to regulate progenitor proliferation through D2 receptors in both the SVZ and SGZ (Hoglinger et al., 2004; Miller and Gauthier-Fisher, 2009). In patients with Parkinson’s Disease, SVZ proliferation is markedly reduced. This effect on proliferation has been shown to be mediated through the induction of EGF and CNTF secretion from SVZ stem cells in response to dopaminergic activity (O'Keeffe et al., 2009; Yang et al., 2008). Dopaminergic deafferentiation reduces proliferation in the SVZ, and one study reports that this decrease in overall SVZ cell proliferation is nonetheless accompanied by an increase in numbers of cells expressing Pax6 in the dorsal SVZ. Pax6 is a transcription factor responsible for enabling a dopaminergic differentiation program in postnatally generated periglomerular neurons. Therefore, dopaminergic activity may not only affect proliferation but may also impact cell fate choice in the SVZ (Winner et al., 2006). More work is needed to verify these findings in the SGZ.
Dopaminergic regulation of postnatal neurogenesis is only beginning to be uncovered, and its role in later stages of neurogenesis has not been conclusively established as yet.
GABA regulates early phases of neurogenesis
GABA, a major inhibitory neurotransmitter in the mature CNS, has a well-established role in the development of neuronal circuits in both the embryo and adult (Bordey, 2006; Barker et al., 1998; Owens and Kriegstein, 2002; Ben Ari, 2002). Its ambient release in the form of spillover from synaptic and extra-synaptic sources has led researchers to its role in regulating the functional integration of new neurons into both immature and mature networks. Owing to the initial abundance of the Cl− importer NKCC1 and the low expression of the Cl− exporter KCC2, internal Cl− concentrations are higher in immature neurons than mature neurons (Owens and Kriegstein, 2002). The resultant high equilibrium potential for Cl− in neuroblasts causes GABA, acting through GABAA receptors, to depolarize immature cells in the first few weeks after fate determination. The depolarizing effect of GABA on young neurons and progenitors has been shown to regulate key stages of neurogenesis such as proliferation, migration and morphogenesis, in both the embryonic and adult neurogenic zones (Marty and Llano, 2005).
Adult neurogenesis in the SVZ and SGZ recapitulates the embryonic role for GABA (Ge et al., 2007a; Bordey, 2006; Esposito et al., 2005). In the SVZ, both astrocytic stem cells and their neuroblast progeny express GABAA receptors (Carleton et al., 2003; Wang et al., 2003a). Electrophysiological evidence indicates that neuroblasts release GABA in a non-synaptic and non-vesicular fashion, and that this tonically activates GABAA receptors on SVZ astrocytic stem cells (Wang et al., 2003b; Nguyen et al., 2003). The SVZ astrocytes also express GABA transporters that may further regulate levels of ambient GABA within the niche. Pharmacological inhibition of GABAA receptors in SVZ slice-culture preparations increases mitotic activity within the SVZ (Liu et al., 2005; Nguyen et al., 2003). Blocking GABA transporters or enhancing GABA release from neuroblasts slows the speed of their own migration, in a paracrine/autocrine fashion in the SVZ and RMS (Bolteus and Bordey, 2004). These data together suggest that GABA has a role as a negative regulator of early stages of neurogenesis in the SVZ, where it reduces neuroblasts and SVZ astrocyte proliferation and decreases the speed of neuroblast migration. This is analogous to the role of GABAA activation in the developing cortex, where it also serves to limit proliferation of ventricular zone progenitors and migration of postmitotic neuroblasts (LoTurco et al., 1995; Haydar et al., 2000; Behar et al., 1996).
In the postnatal hippocampus, electrophysiological characterization of newborn neurons, using retroviral labeling of dividing progenitors or developmentally regulated reporter mice like the POMC-EGFP line, indicate that functional GABAA receptors are expressed in both neural progenitors and their progeny (Wang et al., 2005; Tozuka et al., 2005; Overstreet et al., 2005). GABA, presumably from local interneurons, tonically activates the astrocytic stem cells and neuroblasts in the SGZ, and these cells first display tonic and then synaptic GABAergic transmission (Ge et al., 2007a). GABAergic activity in SGZ progenitors seems to promote proliferation and the expression of NeuroD, thereby suggesting that it positively regulates differentiation during postnatal hippocampal neurogenesis (Tozuka et al., 2005).
GABA’s role in regulating early phases of neurogenesis such as proliferation and migration has been examined more extensively in the SVZ where it has been shown to act as a negative regulator of early neurogenesis. Whether these effects are corroborated in the SGZ is as yet unknown. An attractive hypothesis explaining GABA’s disparate roles in development, postnatal neurogenesis, and at the synapse is that neurotransmitter-based signaling may serve as a bridge that brings an activity-dependence to cell-autonomous and locally present instructive signals that drive neurogenesis and network plasticity. In this way, neuronal and metabolic activity may loop back onto the SVZ and SGZ.
GABA regulates late phases of neurogenesis
GABA-mediated depolarization of immature neurons has been shown to be critical for synapse formation in the developing cortex (Wang and Kriegstein, 2008). Postnatally, following migration into the OB SVZ neuroblasts begin the process of integrating into the local circuitry by radially migrating out of the RMS core, elaborating a complex dendritic structure and establishing appropriate synapses (Toni et al, 2008; Carleton et al., 2003; Belluzzi et al., 2003). A role for GABAA signaling in the initiation, elongation and stabilization of dendritic structures in immature neurons has been established in the OB. Specifically, it was discovered that ambient GABA-induced depolarization and Ca2+-influx was necessary for the stabilization of emerging dendritic protrusions and enhanced the number and length of preexisting dendrites, in SVZ culture as well as OB slice preparations (Gascon et al., 2006). This effect was specific to the immature neuron population because six days following plating, KCC2 levels had increased sufficiently to block the depolarizing effects of GABA and the modulatory effects of GABA-depolarization on dendrite development. Furthermore, GABA activity promoted initiation and elongation of immature neuroblast dendrites in culture by stabilizing tubulin in its polymerized form.
In the postnatal DG, ambient GABA release from local interneurons depolarizes newly born neurons in the first few weeks after birth (Ge et al., 2006). Following this period, reversal of the chloride gradient is complete and GABA becomes hyperpolarizing. Perturbing this stereotyped developmental process by knocking down NKCC1 in hippocampal progenitors using lentivector-shRNAs results in prematurely low internal Cl− concentrations that cause GABA to hyperpolarize instead of depolarize immature neurons (Ge et al., 2006). This loss of GABAA-mediated depolarization prevents the growth and stability of developing dendritic segments and reduces the complexity of their arborizations. Furthermore, proper formation of GABAergic and glutamatergic synapses was affected, as assessed by decreases in the amplitude of evoked GABAergic and glutamatergic synaptic currents as well as decreased frequency of spontaneously generated GABAergic and glutamatergic currents.
This regulation by GABA in both the OB and DG helps shape neurogenesis as an activity-dependent process where GABA is involved in regulating later stages of postnatal neurogenesis orchestrating synapse formation and dendritic outgrowth. However, although GABA’s role in the synaptic integration of postnatally generated neurons is becoming clearer, more work is needed to fully flesh out the internal mechanisms by which GABA activity leads to modulation of actions as disparate as proliferation, migration, synaptic integration, and dendritogenesis. Do the region and subtype-dependent differential expression patterns of GABA receptors interact with the different intracellular and extracellular factors discussed above, like CREB and BDNF, to mediate these diverse functions?
Glutamate regulates early phases of neurogenesis
During embryonic neurogenesis, glutamate signaling has been shown to influence proliferation, fate commitment and migration of newly born neurons (LoTurco et al., 1995; Haydar et al., 2000; Cameron et al., 1998; Schlett, 2006; Komuro and Rakic, 1993). During postnatal neurogenesis, in the SVZ neuroblasts have been shown to express functional AMPA receptors as well as functional mGluR5 and GLUk5-containing kainate receptors, using both electrophysiology and calcium imaging (Platel et al., 2008a; Di, V et al., 2004). Unpublished evidence from our lab suggests glutamate released spontaneously from SVZ-RMS astrocytes generates phasic AMPA receptor activity in neuroblasts migrating towards the OB. Both mGluR5 and GLUk5 activation have also been shown to mediate increases in intracellular Ca2+ transients in SVZ neuroblasts (Platel et al., 2008a; Platel et al., 2008b). A mosaic of GABAA, NMDA, mGluR5 and GLUk5 (now known as GluK2) receptor-expressing cells reside in the SVZ, where most cells express GABAA receptors in caudal SVZ and moving rostrally, a greater proportion of cells begin to express a combination of receptors. Ultimately, nearly half of all cells in the rostral RMS express all four types of receptors, indicating the continuing maturation of newly born cells along the SVZ-OB neurogenic axis. Mice lacking mGluR5, or in which mGluR5 was pharmacologically blocked, displayed a marked decrease in the number of proliferating cells in the SVZ (Giorgi-Gerevini et al., 2005). This indicates a role for glutamate - acting tonically through metabotropic receptors- in positively regulating SVZ progenitor proliferation and antagonizing GABA’s previously established anti-mitotic activity; perhaps acting as a positive regulator of early neurogenic processes. Blocking GLUk5 in the RMS on the other hand, increased the speed of neuroblast migration, suggesting that tonic GLUk5-mediated glutamatergic transmission decreases neuroblast clearance from the SVZ and acts in concert with GABA’s effect on migration in the SVZ (Platel et al., 2008b). mGluR5 activity however, does not influence migration speed. It could be that GLUk5-mediated signaling activated different Ca2+-dependent intracellular cascades than mGluR5 signaling. It remains to be seen whether AMPA/kainate or NMDA receptor activity can have a positive effect on migration in the SVZ/RMS. These data together suggest that although glutamate receptor heterogeneity and the multiple intracellular pathways they may activate introduce ambiguity into what role glutamate may play in early neurogenesis in the SVZ, metabotropic glutamate receptor signaling enhances proliferation, while AMPA/kainate receptor signaling acts together with GABA to decrease migration of neuroblasts. More work is needed to fully flesh out the roles that the three different glutamate receptor families (NMDA, mGluR and AMPA/kainate) have in the SVZ. Work also is needed to elucidate how glutamate receptor heterogeneity parses among the different SVZ sublineages (Emx-1, Gsh2, Nkx2.1).
In the hippocampus, induction of long-term potentiation at the glutamatergic synapses between medial perforant pathway axons and granule cell dendrites promotes progenitor proliferation in the SGZ in an NMDAR-dependent manner (Bruel-Jungerman et al., 2006; Chun et al., 2006). However, other studies involving the infusion of NMDA into the hippocampal formation showed a decrease in proliferation, while NMDAR-antagonist infusion increased proliferation (Cameron et al., 1995; Nacher et al., 2001). These diverging results indicate that perhaps different mechanisms are at play, as synaptic versus extra-synaptic NMDA receptors are known to activate antagonizing pathways. Additionally, different ages and experimental paradigms often yield divergent results and standardization is necessary for interpretation of results. However, what may be gleaned from these results is that NMDA receptor activity can modulate progenitor proliferation in the SGZ.
The diversity of glutamate receptors, the myriad intracellular pathways that they may activate and the many mechanisms by which levels of ambient glutamate are regulated suggests that glutamate, despite being nearly ubiquitously present, can have very specific and differential effects on cells. The data so far suggests glutamate may regulate the early phases of neurogenesis in manner that reflects this complexity. However, further work will involve clarifying some of the associated ambiguity surrounding glutamate availability, the receptor complement, the different intracellular pathways and their effects on neurogenesis.
Glutamate regulates late phases of neurogenesis
Glutamate has been shown to be important for neuroblast survival, dendritic development and synaptogenesis in the developing CNS (Nguyen et al., 2001). In the postnatal SVZ, spontaneous glutamate release from astrocytes onto neuroblasts results in phasic NMDAR activation that increases in frequency and amplitude upon migration towards the bulb. Genetic ablation of the NR1 subunit in migrating neuroblasts results in 60% of these NR1-deficient newly born neurons entering apoptosis, suggesting that NMDAR-dependent glutamatergic signaling is an important factor in regulating neuroblast survival and numbers of new neurons in the OB (Platel et al., 2009b). Once in the bulb, newly generated neurons begin to integrate synaptically into the local circuitry, generate action potentials and first establish GABAergic inputs followed by glutamatergic inputs ~4 weeks after birth (Belluzzi et al., 2003). Recently, newly born granule cells in the OB were shown to express a transient form of LTP in response to focal glutamatergic stimulation in the granule cell layer. This type of LTP was not present in mature granule cells and was observed in cells between 2 and 8 weeks old, implying that new neurons have a capacity for synaptic plasticity that is different from their mature counterparts (Nissant et al., 2009). Perhaps this sort of synaptic enhancement can help explain the positive effects olfactory learning has been shown to have on SVZ neuroblast survival, as well as the negative effects anti-mitotic activity in the SVZ has on olfactory discrimination.
In the DG on the other hand, electrophysiological studies show that immature neuroblasts possess ionotropic glutamate receptors as well, and immunological evidence indicates that these might contain NR1 and NR2B subunits (Ambrogini et al., 2004; Overstreet et al., 2005; Nacher et al., 2007). Single-cell gene knock-out of the NR1 subunit in proliferating dentate progenitors reduced survival of NR1-deficient cells 2–3 weeks after birth in the adult(Tashiro et al., 2006). This reduction in survival is relative to NMDAR activity, as injection of the NMDAR antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) begins to normalize the survival of NR1-deficient cells. This suggests a glutamate-dependent critical period during the maturation of these cells, where NMDAR activity mediates a “competitive survival”: enabling cells that receive NMDAR-dependent input to survive, and cells that do not to die. Later, 4–6 weeks after birth, a second glutamate-mediated critical period involves NR2B-containing NMDARs on immature neurons in the DG. Here, electrophysiology revealed a reduced induction threshold and enhanced LTP in newly born cells (Ge et al., 2007b; Schmidt-Hieber et al., 2004). Pharmacological blockade of NR2B-containing NMDARs abolished LTP induction in immature cells, but not in mature cells (Ge et al., 2007b). Furthermore, LTP in mature cells is sensitive to bicuculline, a GABAA receptor antagonist, but the LTP induced in immature cells is insensitive to GABAA antagonists and suggests immunity from GABA-mediated attenuation (Ge et al., 2008). Thus, between 4–6 weeks after birth newly born cells display enhanced synaptic plasticity that is immune from GABA regulation, putting them in a position to make a major contribution to the network dynamics of the hippocampal pathway. This enhancement eventually diminishes, presumably as perisomatic GABAergic inputs converge onto maturing granule cells at 8 weeks and beyond.
The synaptic integration of hippocampal neuroblasts has been studied in greater detail than that of the SVZ neuroblasts. In the DG, the regulation of this process by glutamate reveals effects on survival and synaptic plasticity. In the SVZ, emerging evidence also suggests an NMDA receptor-dependent effect on survival; however, the role NMDA receptors play in synaptic plasticity in the OB remains to be elucidated. Furthermore, the role that the other families of glutamate receptors play in these later phases in postnatal neurogenesis also remains to be uncovered.
Conclusion
New neurons continue to be produced throughout life in two regions of the mammalian CNS and a plethora of research has accumulated demonstrating how this amazing propensity for plasticity is orchestrated and regulated. Postnatal neurogenesis has been shown to make important contributions to coordinated network activity in the OB and the DG, playing a role in learning, memory and synaptic plasticity. The mechanisms by which neurogenesis is permitted in these discrete regions offers the tantalizing possibility that postnatal neurogenesis could hold therapeutic potential for the treatment of a variety of neurological disorders. If the process by which this phenomenon occurs is dissected sufficiently well, perhaps it could be appropriated and redirected to sites of neuronal injury and loss, and repair from one’s own stem cell reserve could be undertaken. Research within the field, however, often reports results on different aspects of a process that is highly dynamic, temporally and spatially distinct, and subject to regulation by many different categories of factors.
While GABA and glutamate may not control the fate commitment of stem cells or progenitor cells, they act at every other level of cell development from proliferation to synaptic integration. GABA and glutamate may control the synthesis and release of other diffusible molecules such as growth factors through intracellular Ca2+ regulation. In addition, they can control the expression of transcription factors and other intracellular molecules such as microRNAs. GABA and glutamate are thus critical for homeostatic control over neurogenesis and form the link between network activity and neurogenesis; regulating synaptic differentiation and integration of adult born neurons. We propose that neurotransmitter-mediated network activity acting through the considerable receptor heterogeneity and divergent intracellular signaling mechanisms initiates growth factor secretion and transcription factor activation. This in turn may enable the stereotyped progression of neurogenesis in the SVZ and SGZ. Furthermore, GABA and glutamate signaling are the most amenable to pharmacological manipulation and their considerable involvement in the process of postnatal neurogenesis justifies their study. More work is needed to elaborate the interconnections between these three broad classes of regulatory factors, and how they converge to enable neurogenesis within the mature mammalian CNS.
Supplementary Material
Figure 1.
Neurogenesis persists in two regions of the postnatal brain: the subventricular zone (SVZ) lining the lateral ventricles (orange) and the subgranular zone (SGZ) of the dentate gyrus. Stem cell-like astrocytes in both regions are positive for glial-fibrillary acidic protein (GFAP) and divide to produce doublecortin-positive (DCX) neuroblasts. Once the neuroblasts arrive at their target destinations, in the granule or periglomerular layers in the olfactory bulb, or within the granule cell layer in the dentate gyrus, they elaborate their dendritic arbors, form synapses, and mature into neurons capable of producing action potentials. In the olfactory bulb the granule neurons (red) form GABAergic synapses onto mitral and tufted cells, while periglomerular neurons (green) form dopaminergic synapses on mitral and tufted cells and olfactory receptor neurons within and between glomeruli. In the dentate gyrus, granule neurons receive synaptic inputs onto their dendrites in the molecular layer from the entorhinal cortex, and send axons called mossy fibers that form glutamatergic synapses with neurons in CA3. EPL: external plexiform layer; GCL: granule cell layer ; LV: lateral ventricle; ML: molecular layer; OB: olfactory bulb; PG cell: periglomerular cell; TAC: Transit amplifying cell.
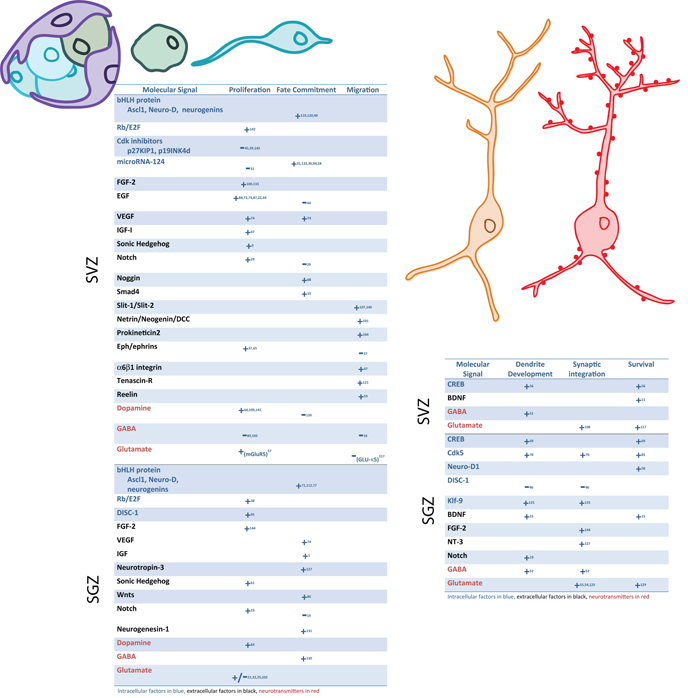
Figure 2.
Table summarizing the affects of the different intracellular factors, extracellular factors and neurotransmitters on early phases of neurogenesis (proliferation, fate commitment, migration) versus late phases of neurogenesis (dendrite development, synaptic integration and survival) in the SVZ and SGZ. The numbers refer to references that are provided as Supplemental Material.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 4.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A. Mechanism of neurogenesis in adult avian brain. Experientia. 1990;46:948–955. doi: 10.1007/BF01939388. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 7.Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 8.Bally-Cuif L, Hammerschmidt M. Induction and patterning of neuronal development, and its connection to cell cycle control. Curr Opin Neurobiol. 2003;13:16–25. doi: 10.1016/s0959-4388(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 9.Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker JL, Behar T, Li YX, Liu QY, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen XL, Xian H. GABAergic cells and signals in CNS development. Perspect Dev Neurobiol. 1998;5:305–322. [PubMed] [Google Scholar]
- 11.Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 15.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordey A. Adult neurogenesis: basic concepts of signaling. Cell Cycle. 2006;5:722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- 18.Boutz PL, Stoilov P, Li Q, Lin CH, Chawla G, Ostrow K, Shiue L, Ares M, Jr, Black DL. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 2007;21:1636–1652. doi: 10.1101/gad.1558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascon S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Gotz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009 doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 23.Cajal S, May RM. Degeneration and regeneration of the nervous system. Oxford: Oxford University Press; 1928. [Google Scholar]
- 24.Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 25.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 27.Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisen J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 28.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 29.Chambers CB, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye JS. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128:689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun SK, Sun W, Park JJ, Jung MW. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- 34.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Gotz M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 38.Cooper-Kuhn CM, Vroemen M, Brown J, Ye H, Thompson MA, Winkler J, Kuhn HG. Impaired adult neurogenesis in mice lacking the transcription factor E2F1. Mol Cell Neurosci. 2002;21:312–323. doi: 10.1006/mcne.2002.1176. [DOI] [PubMed] [Google Scholar]
- 39.Coskun V, Luskin MB. The expression pattern of the cell cycle inhibitor p19(INK4d) by progenitor cells of the rat embryonic telencephalon and neonatal anterior subventricular zone. J Neurosci. 2001;21:3092–3103. doi: 10.1523/JNEUROSCI.21-09-03092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De MS, Fasolo A, Shipley M, Puche A. Unique neuronal tracers show migration and differentiation of SVZ progenitors in organotypic slices. J Neurobiol. 2001;49:326–338. doi: 10.1002/neu.10012. [DOI] [PubMed] [Google Scholar]
- 41.Di GGV, Caruso A, Cappuccio I, Ricci VL, Romeo S, Della RC, Gradini R, Melchiorri D, Nicoletti F. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 43.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002a;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 45.Doetsch F, Verdugo JM, Caille I, Alvarez-Buylla A, Chao MV, Casaccia-Bonnefil P. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J Neurosci. 2002b;22:2255–2264. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emsley JG, Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- 48.Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- 50.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007a;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007b;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, De Maria R, Nicoletti F. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- 58.Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP, Schier AF. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 59.Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 2002;5:939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- 60.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 62.Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968;134:287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- 64.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 65.Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;38 Suppl:S20–S24. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- 67.Hurtado-Chong A, Yusta-Boyo MJ, Vergano-Vera E, Bulfone A, de PF, Vicario-Abejon C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur J Neurosci. 2009;30:742–755. doi: 10.1111/j.1460-9568.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 68.Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jessberger S, Aigner S, Clemenson GD, Jr, Toni N, Lie DC, Karalay O, Overall R, Kempermann G, Gage FH. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008a;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008b;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003a;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 73.Jin K, Xie L, Childs J, Sun Y, Mao XO, Logvinova A, Greenberg DA. Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann Neurol. 2003b;53:405–409. doi: 10.1002/ana.10506. [DOI] [PubMed] [Google Scholar]
- 74.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 76.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kishi K. Golgi studies on the development of granule cells of the rat olfactory bulb with reference to migration in the subependymal layer. J Comp Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- 79.Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 80.Kosaka K, Kosaka T. synaptic organization of the glomerulus in the main olfactory bulb: compartments of the glomerulus and heterogeneity of the periglomerular cells. Anat Sci Int. 2005;80:80–90. doi: 10.1111/j.1447-073x.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 81.Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30:101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 82.Kosaka T, Hataguchi Y, Hama K, Nagatsu I, Wu JY. Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gamma-aminobutyric acid (GABA) and dopamine. Brain Res. 1985;343:166–171. doi: 10.1016/0006-8993(85)91172-2. [DOI] [PubMed] [Google Scholar]
- 83.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 87.Lillien L, Raphael H. BMP and FGF regulate the development of EGF-responsive neural progenitor cells. Development. 2000;127:4993–5005. doi: 10.1242/dev.127.22.4993. [DOI] [PubMed] [Google Scholar]
- 88.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 89.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 91.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 92.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 93.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 94.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 97.Marty A, Llano I. Excitatory effects of GABA in established brain networks. Trends Neurosci. 2005;28:284–289. doi: 10.1016/j.tins.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008 Aug;11(8):901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mudo G, Bonomo A, Di L, V, Frinchi M, Fuxe K, Belluardo N. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm. 2009;116:995–1005. doi: 10.1007/s00702-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 101.Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- 103.Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 104.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 105.Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 107.Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van EA, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24:1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 109.O'Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Overstreet WL, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 111.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 112.Ozen I, Galichet C, Watts C, Parras C, Guillemot F, Raineteau O. Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2. Eur J Neurosci. 2007;25:2591–2603. doi: 10.1111/j.1460-9568.2007.05541.x. [DOI] [PubMed] [Google Scholar]
- 113.Pathania M, Platel J-CJ, Teng Z-Q, Zhao X, Bordey A. A role for microRNA-132 in the postnatal subventricular zone and neurogenesis. Program No.531.2009 International Society for Stem Cell Research 7th Annual Meeting Poster Abstracts; Barcelona, Spain: International Society for Stem Cell Research; 2009. Online. 2009. Ref Type: Abstract. [Google Scholar]
- 114.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008a;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2009a;57:66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- 116.Platel JC, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in whole-mounts of the subventricular zone. J Physiol. 2008b;586:3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Platel J-CJ, Dave K, Gordon V, Lacar B, Bordey A. NMDA-receptors are critical for SVZ postnatal neurogenesis. Program No.30.16/B21.2009 Neuroscience Meeting Planner; Chicago, IL: Society for Neuroscience; 2009b. 2009.Online. Ref Type: Abstract. [Google Scholar]
- 118.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 119.Roybon L, Deierborg T, Brundin P, Li JY. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci. 2009a;29:232–243. doi: 10.1111/j.1460-9568.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- 120.Roybon L, Mastracci TL, Ribeiro D, Sussel L, Brundin P, Li JY. GABAergic Differentiation Induced by Mash1 Is Compromised by the bHLH Proteins Neurogenin2, NeuroD1, and NeuroD2. Cereb Cortex. 2009b doi: 10.1093/cercor/bhp187. [DOI] [PubMed] [Google Scholar]
- 121.Saghatelyan A, de CA, Schachner M, Lledo PM. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 2004;7:347–356. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- 122.Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006;6:949–960. doi: 10.2174/156802606777323665. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 124.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 125.Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 129.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 130.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 131.Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z, Maragakis NJ, Hevner RF, Miura N, Sugimura H, Sato K. A novel secretory factor, Neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J Neurosci. 2003;23:11732–11740. doi: 10.1523/JNEUROSCI.23-37-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang DD, Krueger DD, Bordey A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J Neurophysiol. 2003a;90:2291–2302. doi: 10.1152/jn.01116.2002. [DOI] [PubMed] [Google Scholar]
- 136.Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003b;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 138.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 139.Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 140.Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28:2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshikawa K. Cell cycle regulators in neural stem cells and postmitotic neurons. Neurosci Res. 2000;37:1–14. doi: 10.1016/s0168-0102(00)00101-2. [DOI] [PubMed] [Google Scholar]
- 143.Young S, Bordey A. Striatal neurons provide a relay for olfaction and SVZ neurogenesis. Program No.229.4/A4.2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. Online. 11-16-2008. Ref Type: Abstract. [Google Scholar]
- 144.Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 145.Zindy F, Cunningham JJ, Sherr CJ, Jogal S, Smeyne RJ, Roussel MF. Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc Natl Acad Sci U S A. 1999;96:13462–13467. doi: 10.1073/pnas.96.23.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.