Abstract
IL-17 is proinflammatory cytokine secreted by a unique CD4+ T (Th17) cell subset and proposed to play a role in host defense. We hypothesized that Th17 cells are lost in HIV-1 infection. HIV-1-infected children with plasma viremia below 50 copies/ml had IL-17 production, whereas those with detectable viremia had minimal secretion. These results imply viral-mediated destruction or impairment of Th17 cells and argue for complete suppression of viremia for reconstitution of Th17 cells.
IL-17 is a proinflammatory cytokine [1], unique in that it is produced by a distinct subset of human CD4+ (Th17) cells [2]. Recent studies [3-9] highlight the influence of IL-17 as a mediator of tissue inflammation in several autoimmune disorders and host defense. IL-17 may have a direct role in Candida or Mycobacterial infections, and a loss of Th17 cells could potentially lead to vulnerability to opportunistic infections [10-13]. In this study, we assessed the impact of HIV-1 infection on Th17 cells in a cohort of HIV-1-infected children [14].
First, we stimulated peripheral blood mononuclear cells (PBMCs) from healthy subjects with various monoclonal antibodies (mAb) and mitogenic stimuli for detection of IL-17 production in vitro. We observed that stimulation with anti-CD3/anti-CD28 mAbs induced IL-17 secretion, primarily by CD4+ T cells (data not shown). In response to anti-CD3/anti-CD28 stimulation, the majority of the Th17 cells did not coexpress IFN-γ. In contrast, stimulation with phorbol myristate acetate/ionomycin induced IL-17 secretion from both CD4+ and CD8+ T cells, as well as dual secreting IL-17/IFN-γ T cell subsets (data not shown). These results suggest that anti-CD3/anti-CD28 stimulation is an effective method to induce IL-17 secretion from CD4+ T cells in vitro and represents a more physiological stimulus than with mitogens.
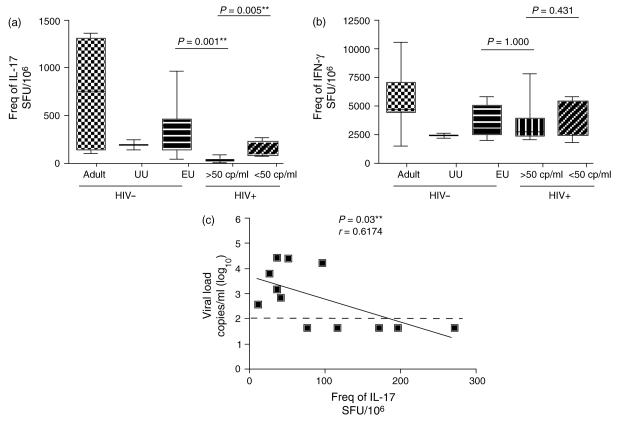
We next tested for the frequency of Th17 cells in PBMCs using an anti-CD3/anti-CD28 stimulation IL-17 enzyme-linked immunosorbent spot assay in 12 HIV-1-infected children with differing levels of plasma viremia and compared this to a group of HIV-1-uninfected subjects who served as controls. The median number of IL-17-specific spot forming units (SFU) in healthy pediatric and adult subjects was 300 SFU/106 (range: 85–970 SFU/106; n = 12) and 760 SFU/106 (range: 110–1370 SFU/106; n = 7), respectively (Fig. 1a). A marked reduction in Th17 cells was observed in the HIV-1-infected children (median: 62 SFU/106; range: 10–270 SFU/106; n = 12). Strikingly, the infected children with HIV-1 plasma viral loads exceeding 50 copies/ml had minimal numbers of Th17 cells (median: 35 SFU/106; range: 10–95 SFU/106; n = 7), whereas those with suppressed viral loads had higher levels of Th17 cells (median: 170 SFU/106; range: 75–270 SFU/106; n = 5). Interestingly, all groups had robust IFN-γ secretion (Fig. 1b). Flow cytometry assessment confirmed that CD4+ T cells were the primary source of IL-17 following anti-CD3/anti-CD28 mAb stimulation (data not shown). We carried out a univariate logistic regression analysis to test for an association between HIV-1 plasma viral load and Th17 cells. We observed a statistically significant correlation between the frequency of Th17 cells and HIV-1 plasma viremia (Fig. 1c). It remains unclear whether the loss of IL-17 production is a cause or an effect of the increasing HIV-1 viremia. Although it is possible that this loss may reflect the depletion of CD4+ T cells, we observed no expression of CCR5 on IL-17 secreting cells (data not shown), suggesting these cells may not be targeted by the virus directly but instead be in a milieu that will not sustain Th17 cells differentiation in vivo.
Fig. 1. Detection and comparison of the frequencies of IL-17 and IFN-γ secreting cells in peripheral blood mononuclear cells (PBMCs) derived from HIV-1-infected and uninfected subjects.
Graphs depict the frequency of (a) IL-17 and (b) IFN-γ secreting PMBCs in spot forming units (SFU)/106 PBMCs from HIV-1-uninfected adults, unexposed uninfected children, exposed but uninfected controls and infected pediatric subjects in response to anti-CD3/anti-CD28 stimulation. HIV-1-infected pediatric subjects were segregated depending on the level of viremia to more than 50 copies/ml or less than 50 copies/ml. The **P-value was derived from the Mann–Whitney test and considered statistically significant if the value was less than 0.05. (c) The degree of correlation was assessed by using Spearman's rank correlation coefficient for nonparametric data between HIV-1 plasma viremia and the frequency of IL-17 SFU. EU, exposed uninfected; UU, unexposed uninfected.
In summary, we show that HIV-1-infected children with a plasma viremia below 50 copies/ml had detectable IL-17 production in contrast to those with detectable viremia. These findings are different from a previous report [15] and argue for complete suppression of HIV-1 viral replication to below 50 copies/ml as a strategy for Th17 cell preservation.
Acknowledgements
The present research was supported by grants from the National Institutes of Health (NIH), University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI27763), the Fogarty International Center of the National Institutes of Health, University of California, Berkeley, School of Public Health, Division of Epidemiology, Berkeley, California 94720-7360, the UCSF AIDS Biology Program of the AIDS Research Institute (ARI), NIH grants (AI060379 and AI068498) and Center for HIV-AIDS Vaccine Immunology (CHAVI) U01-AI-067854. L.C.N. is supported by the Irvington Institute Fellowship Program of the Cancer Research Institute. We would like to thank Douglas Wachter for editorial assistance.
References
- 1.Kennedy J, Rossi DL, Zurawski SM, Vega F, Jr, Kastelein RA, Wagner JL, et al. Mouse IL-17: a cytokine preferentially expressed by alpha beta TCR R CD4-CD8-T cells. J Interferon Cytokine Res. 1996;16:611–617. doi: 10.1089/jir.1996.16.611. [DOI] [PubMed] [Google Scholar]
- 2.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 3.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T Cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 5.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibundgut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 9.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4(R) T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 11.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Myco-bacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 12.Lagrelius M, Jones P, Franck K, Gaines H. Cytokine detection by multiplex technology useful for assessing antigen specific cytokine profiles and kinetics in whole blood cultured up to seven days. Cytokine. 2006;33:156–165. doi: 10.1016/j.cyto.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 14.Ballan WM, Vu BA, Long BR, Loo CP, Michaelsson J, Barbour JD, et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J Immunol. 2007;179:3362–3370. doi: 10.4049/jimmunol.179.5.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maek ANW, Buranapraditkun S, Klaewsongkram J, Ruxrungthum K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007;20:66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]