Abstract
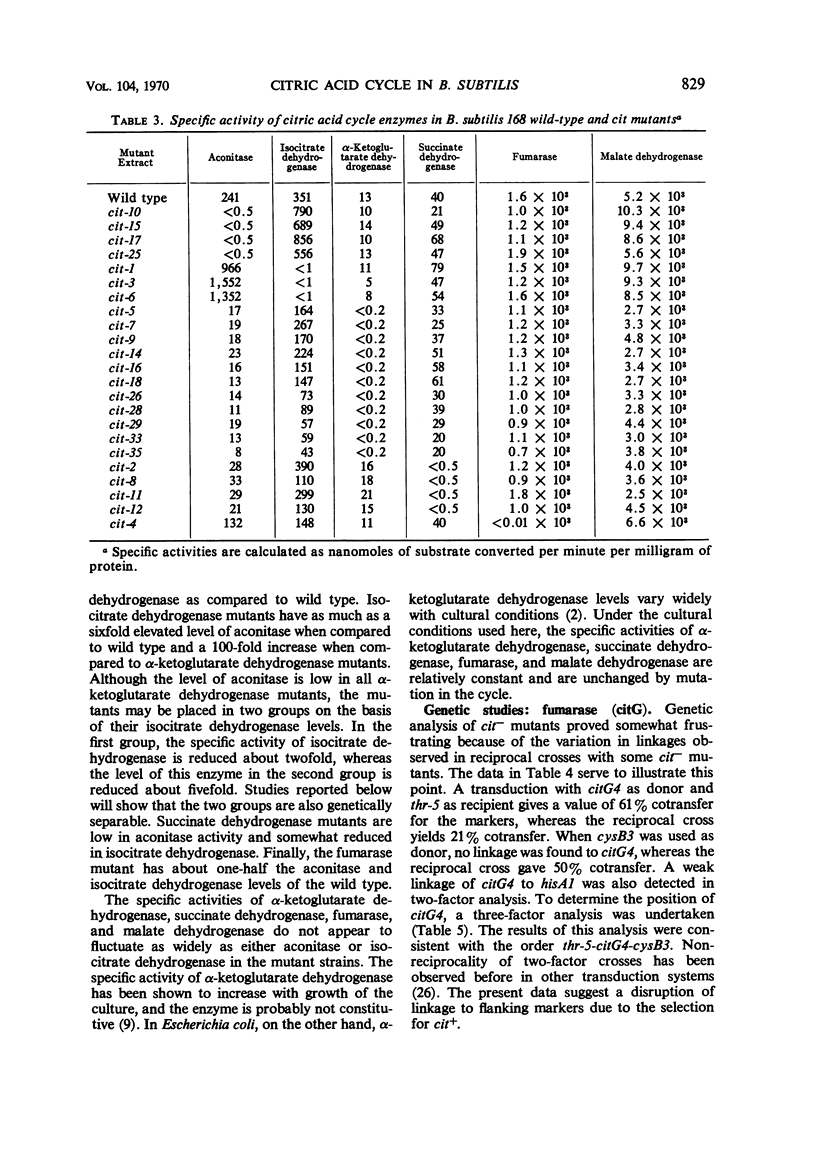
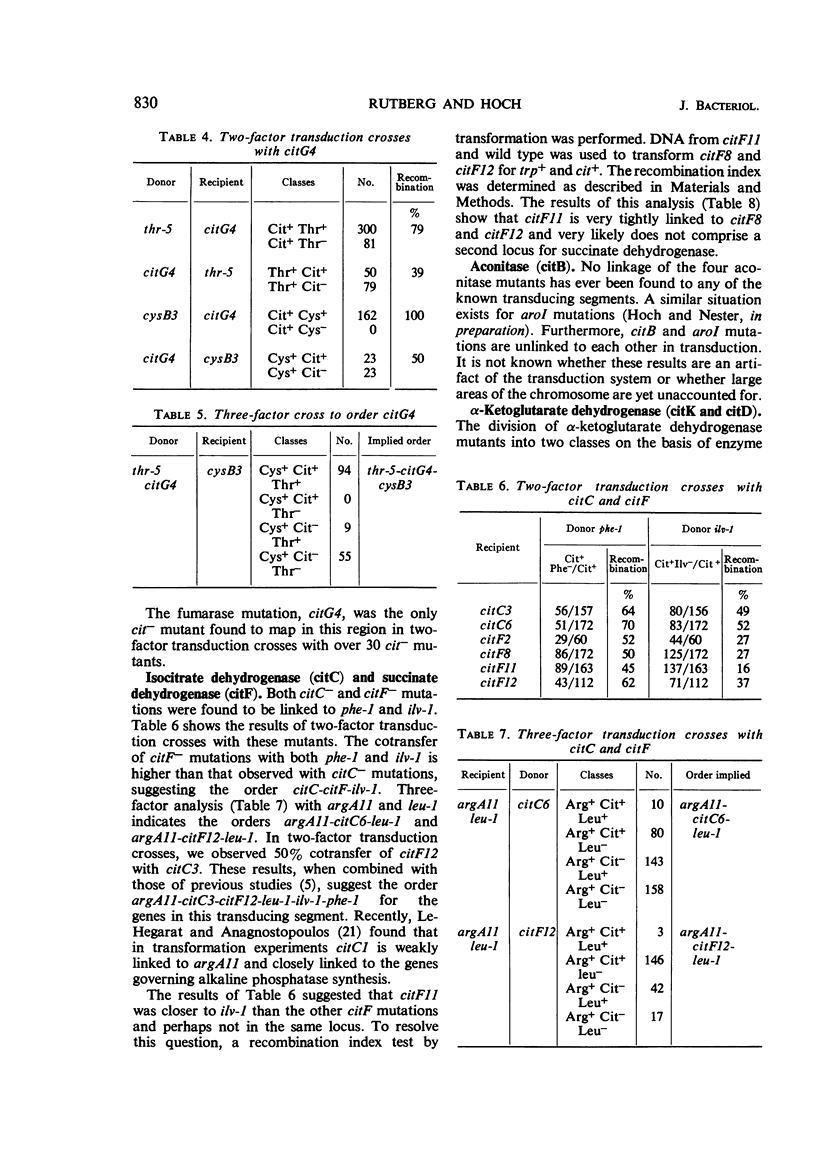
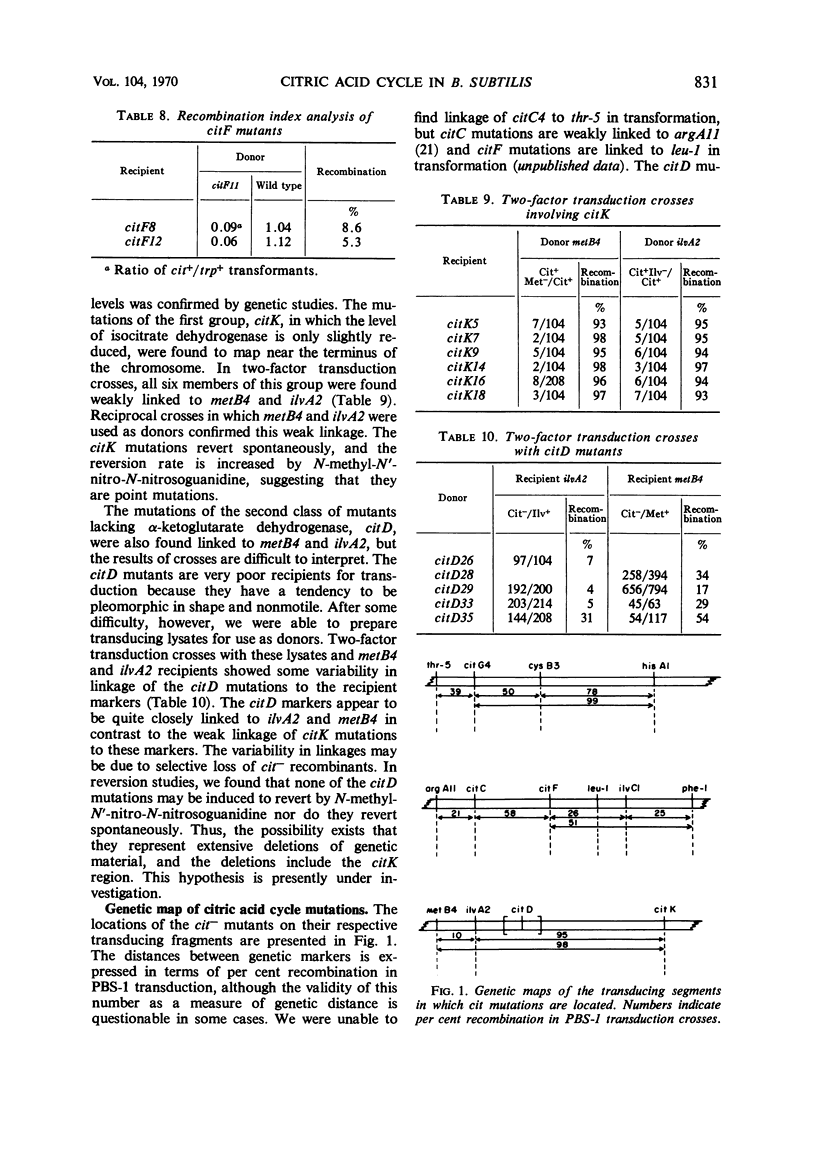
The genetic location of mutations affecting the citric acid cycle and the properties of mutants of Bacillus subtilis possessing these mutations have been examined. Genes coding for the component enzymes of the cycle were found to be unlinked to each other and thus do not form an operon. The mutational defect in a mutant lacking fumarase mapped between thr-5 and cysB3. Mutations causing inability to produce isocitrate dehydrogenase and succinate dehydrogenase were found to map between argA11 and leu-1. The α-ketoglutarate dehydrogenase mutations were mapped at the terminal end of the B. subtilis chromosome through a weak linkage in phage PBS-1 transduction of one class of these mutations of ilvA2 and metB4. A second class of α-ketoglutarate dehydrogenase mutations mapped closer to ilvA2 and metB4 but still terminal with respect to these markers. Aconitaseless mutants possessed mutations that could not be linked to any of the known transducing segments of the chromosome. An effect of mutation conferring loss of one enzyme of the cycle on the specific activity of the other enzymes in the cycle was observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann E., Allmann D. W., Green D. E. The membrane systems of the mitochondrion. I. The S fraction of the outer membrane of beef heart mitochondria. Arch Biochem Biophys. 1966 Jul;115(1):153–164. doi: 10.1016/s0003-9861(66)81051-2. [DOI] [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Coarse and fine control of citrate synthase from Bacillus subtilis. Biochim Biophys Acta. 1969 Jul 30;184(2):252–262. doi: 10.1016/0304-4165(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Inhibition of aconitase by chelation of transition metals causing inhibition of sporulation in Bacillus subtilis. J Biol Chem. 1968 Oct 25;243(20):5289–5295. [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., MII S., KOHOUT P. M. Studies on the terminal electron transport system. I. Succinic dehydrogenase. J Biol Chem. 1955 Dec;217(2):551–567. [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Studies with alpha-ketoglutarate dehydrogenase mutants of Escherichia coli. Mol Gen Genet. 1969 Oct 13;105(2):182–190. doi: 10.1007/BF00445687. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Hegarat J. C., Anagnostopoulos C. Localisation chromosomique d'un gène gouvernant la synthèse d'une phosphatase alcaline chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 17;269(20):2048–2050. [PubMed] [Google Scholar]
- MAHLER H. R., WITTENBERGER M. H., BRAND L. Biochemical studies of the developing avian embryo. II. Enzymes of the citric acid cycle. J Biol Chem. 1958 Oct;233(4):770–782. [PubMed] [Google Scholar]
- Myers W. F., Huang K. Y. Separation of intermediates of the citric acid cycle and related compounds by thin-layer chromatography. Anal Biochem. 1966 Nov;17(2):210–213. doi: 10.1016/0003-2697(66)90199-0. [DOI] [PubMed] [Google Scholar]
- Ozeki H. Chromosome Fragments Participating in Transduction in Salmonella Typhimurium. Genetics. 1959 May;44(3):457–470. doi: 10.1093/genetics/44.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSERA C., PEDROTTI A., FERRARI G. THIN-LAYER CHROMATOGRAPHY OF CARBOXYLIC ACIDS AND KETO ACIDS OF BIOLOGICAL INTEREST. J Chromatogr. 1964 Apr;14:289–291. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Schmitt R., Freese E. Curing of a sporulation mutant and antibiotic activity of Bacillus subtilis. J Bacteriol. 1968 Oct;96(4):1255–1265. doi: 10.1128/jb.96.4.1255-1265.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]



