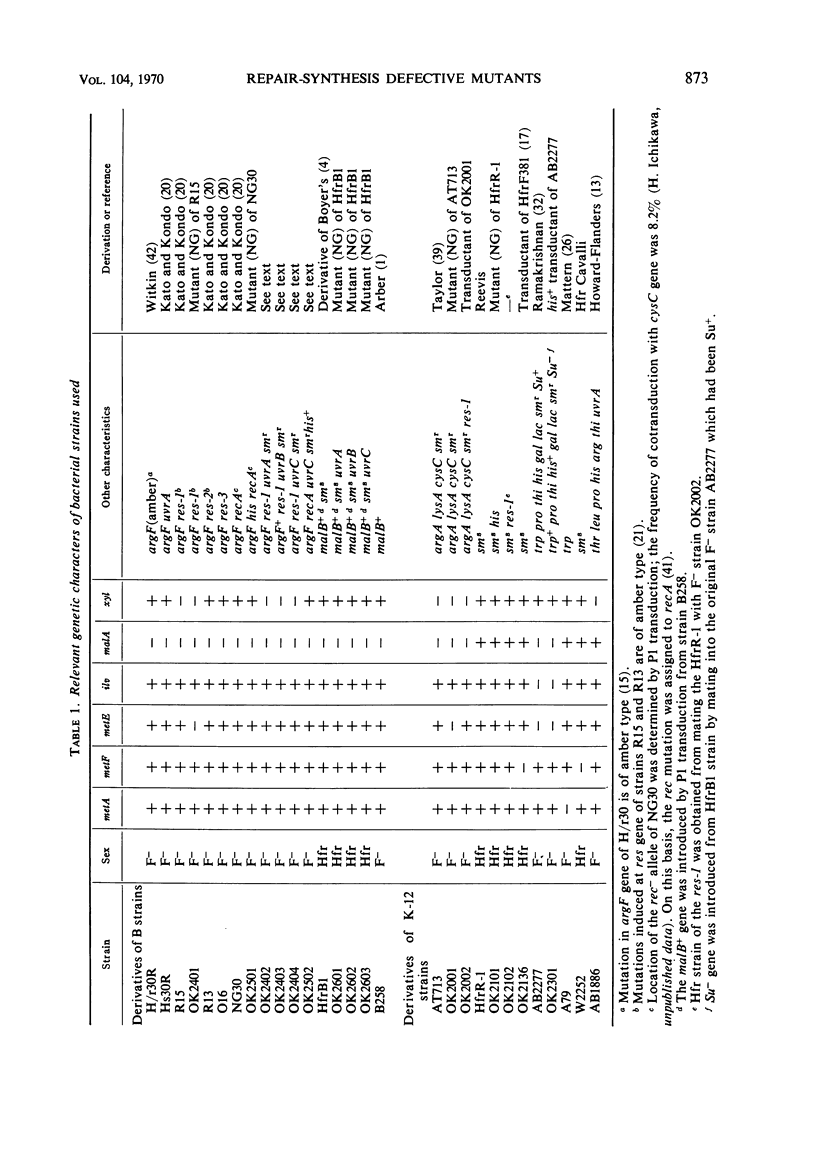
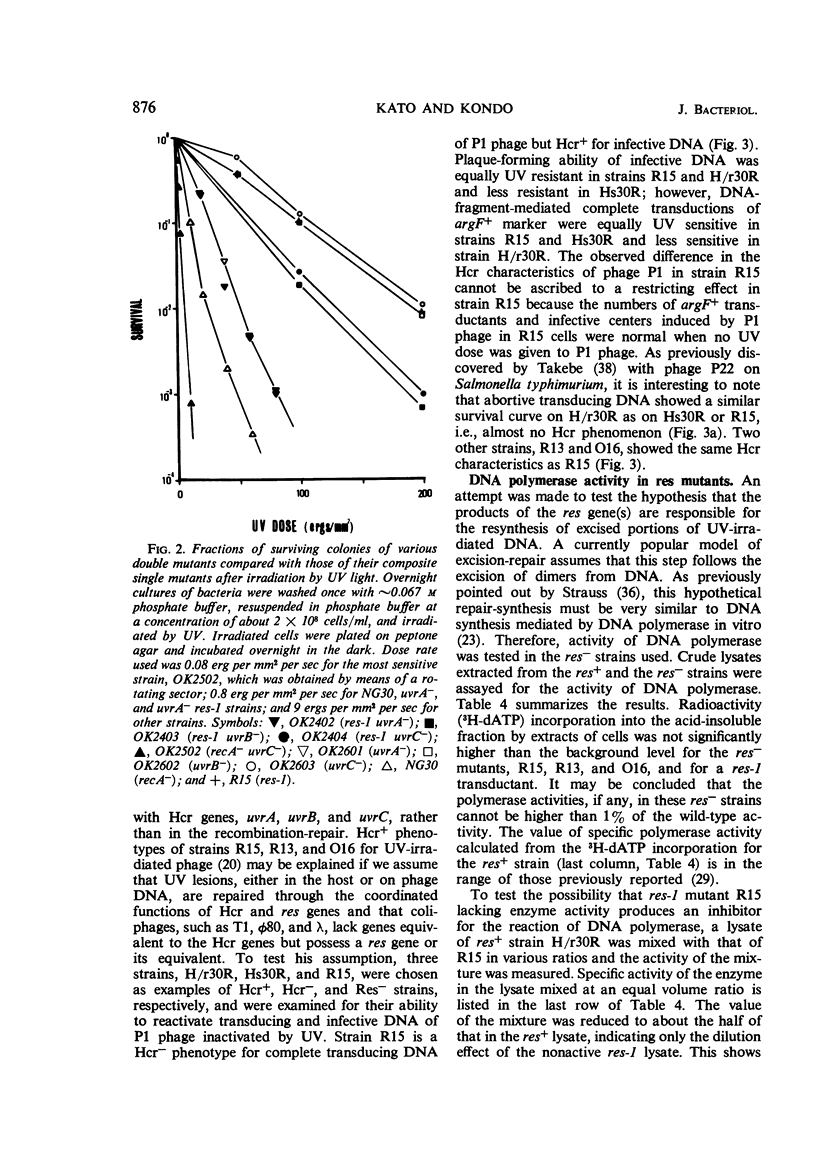
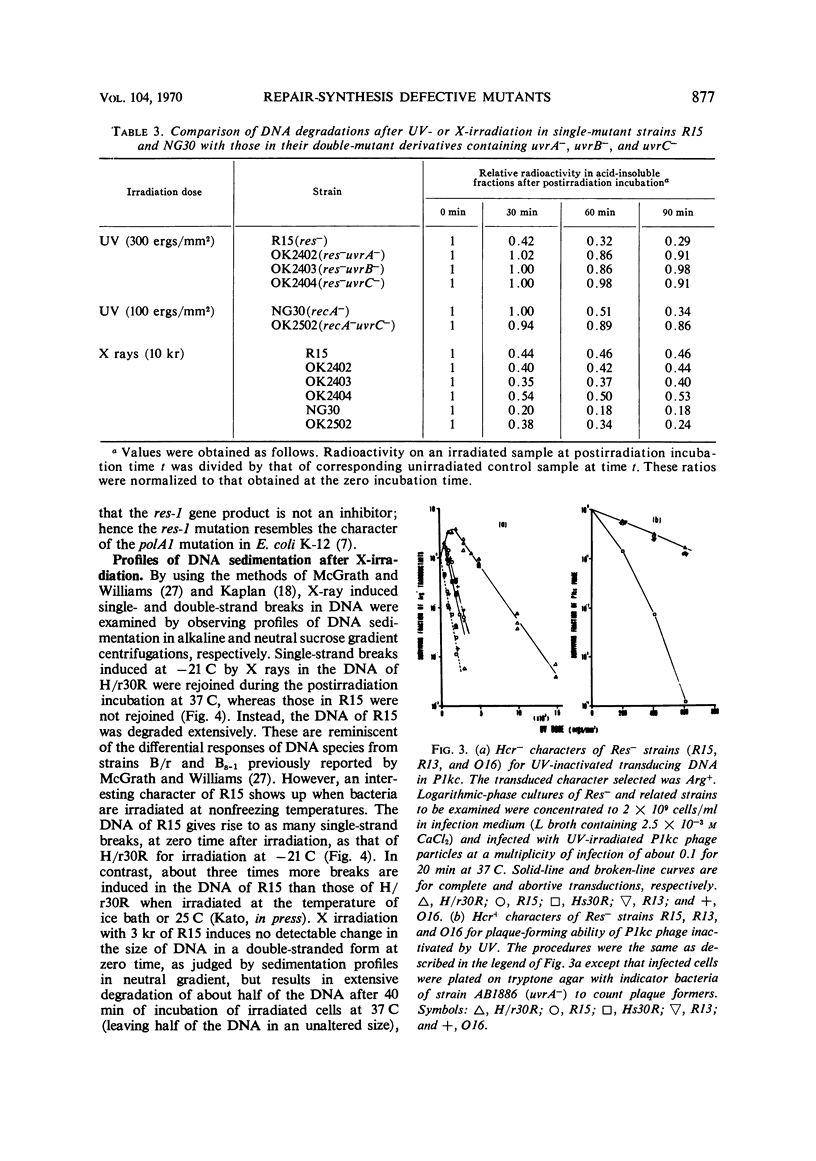
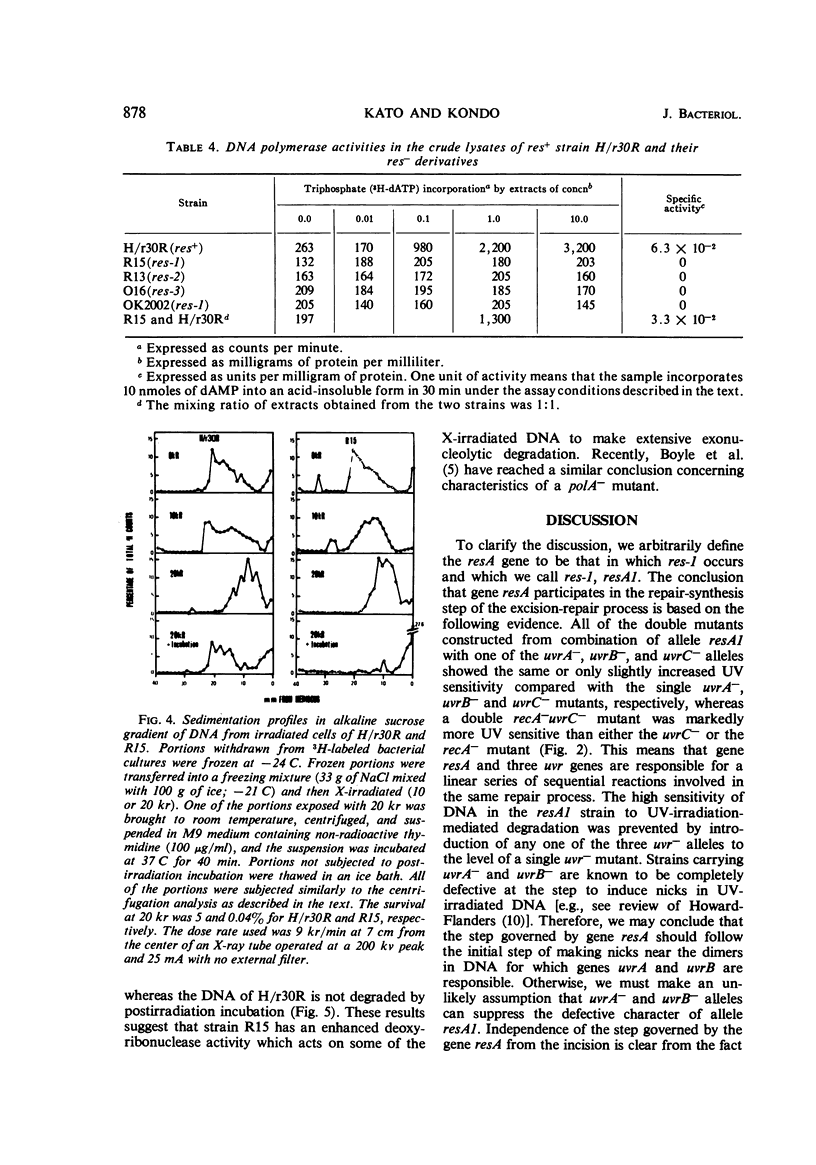
Abstract
Alleles responsible for X-ray-sensitive characteristics of three mutants of Escherichia coli B, which were also sensitive to ultraviolet (UV) irradiation, were mapped near metE locus, and named res-1, res-2, and res-3. All the res− mutants showed no host cell reactivability (Hcr−) for transducing deoxyribonucleic acid (DNA) of P1 phage irradiated by UV but they were Hcr+ for infective DNA of P1 phage. Furthermore, they showed no detectable activity of DNA polymerase. Characteristics of allele res-1 were studied in detail. The mutant res-1 uvr+ showed an extensive degradation of DNA after UV irradiation. Double mutants carrying res-1 uvrA−, res-1 uvrB−, and res-1 uvrC− showed no marked increase in UV sensitivity beyond that of the uvr− single mutants and only negligible UV-induced DNA degradation. The uvr− mutations showed no such suppressive effect on DNA degradation induced by X rays in these double mutants. It is concluded that res− mutants are defective in the second step (repair synthesis) of the excision repair process and that DNA polymerase is partly responsible for the assumed resynthesis step.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., LATASTE-DOROLLE C. [Enlargement of the host area of bacteriophage lambda for Escherichia coli B]. Pathol Microbiol (Basel) 1961;24:1012–1018. [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W. Repair and recombination in phage T4. II. Genes affecting UV sensitivity. Cold Spring Harb Symp Quant Biol. 1968;33:333–338. doi: 10.1101/sqb.1968.033.01.038. [DOI] [PubMed] [Google Scholar]
- Boyer H. Conjugation in Escherichia coli. J Bacteriol. 1966 May;91(5):1767–1772. doi: 10.1128/jb.91.5.1767-1772.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Ashwood-Smith M. J., Munson R. J. Correlation of bacterial sensitivities to ionizing radiation and mild heating. J Gen Microbiol. 1969 Sep;58(1):115–124. doi: 10.1099/00221287-58-1-115. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- FRANKEL F. R. An unusual DNA extracted from bacteria infected with phage T2. Proc Natl Acad Sci U S A. 1963 Mar 15;49:366–372. doi: 10.1073/pnas.49.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., BOYCE R. P., SIMSON E., THERIOT L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., THERIOT L. A method for selecting radiation-sensitive mutants of Escherichia coli. Genetics. 1962 Sep;47:1219–1224. doi: 10.1093/genetics/47.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Kano Y., Matsushiro A., Shimura Y. Isolation of the novel regulatory mutants of the tryptophan biosynthetic system in Escherichia coli. Mol Gen Genet. 1968;102(1):15–26. doi: 10.1007/BF00341866. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. DNA-strand scission and loss of viability after x irradiation of normal and sensitized bacterial cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1442–1446. doi: 10.1073/pnas.55.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA, 1. Purification of two enzymes involved in the excision of thymine dimers from ultraviolet-irradiated DNA. Proc Natl Acad Sci U S A. 1969 May;63(1):144–151. doi: 10.1073/pnas.63.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Kondo S. Two types of x-ray-sensitive mutants of Escherichia coli B: their phenotypic characters compared with UV-sensitive mutants. Mutat Res. 1967 May-Jun;4(3):253–263. doi: 10.1016/0027-5107(67)90020-6. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XV. PURIFICATION AND PROPERTIES OF A POLYMERASE FROM BACILLUS SUBTILIS. J Biol Chem. 1964 Jan;239:259–268. [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Yasuda S., Okubo S., Nakayama H., Shimada K., Takagi Y. Mechanism of repair of DNA in bacteriophage. I. Excision of pyrimidine dimers from ultraviolet-irradiated DNA by an extract of T4-infected cells. J Mol Biol. 1970 Jan 28;47(2):231–242. doi: 10.1016/0022-2836(70)90342-6. [DOI] [PubMed] [Google Scholar]
- Strauss B. S. DNA repair mechanisms and their relation to mutation and recombination. Curr Top Microbiol Immunol. 1968;44:1–85. [PubMed] [Google Scholar]
- Takagi Y., Sekiguchi M., Okubo S., Nakayama H., Shimada K., Yasuda S., Nishimoto T., Yoshihara H. Nucleases specific for ultraviolet light-irradiated DNA and their possible role in dark repair. Cold Spring Harb Symp Quant Biol. 1968;33:219–227. doi: 10.1101/sqb.1968.033.01.025. [DOI] [PubMed] [Google Scholar]
- Takebe H. Role of host-cell reactivation in the enhancement of complete transduction after UV irradiation of P22 phage. Biochem Biophys Res Commun. 1968 Jun 28;31(6):938–944. doi: 10.1016/0006-291x(68)90543-3. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITKIN E. M. PHOTOREVERSAL AND "DARK REPAIR" OF MUTATIONS TO PROTOTROPHY INDUCED BY ULTRAVIOLET LIGHT IN PHOTOREACTIVABLE AND NON-PHOTOREACTIVABLE STRAINS OF ESCHERICHIA COLI. Mutat Res. 1964 May;106:22–36. doi: 10.1016/0027-5107(64)90049-1. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. Mechanism of repair of DNA in bacteriophage. II. Inability of ultraviolet-sensitive strains of bacteriophage in inducing an enzyme activity to excise pyrimidine dimers. J Mol Biol. 1970 Jan 28;47(2):243–255. doi: 10.1016/0022-2836(70)90343-8. [DOI] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]



