Abstract
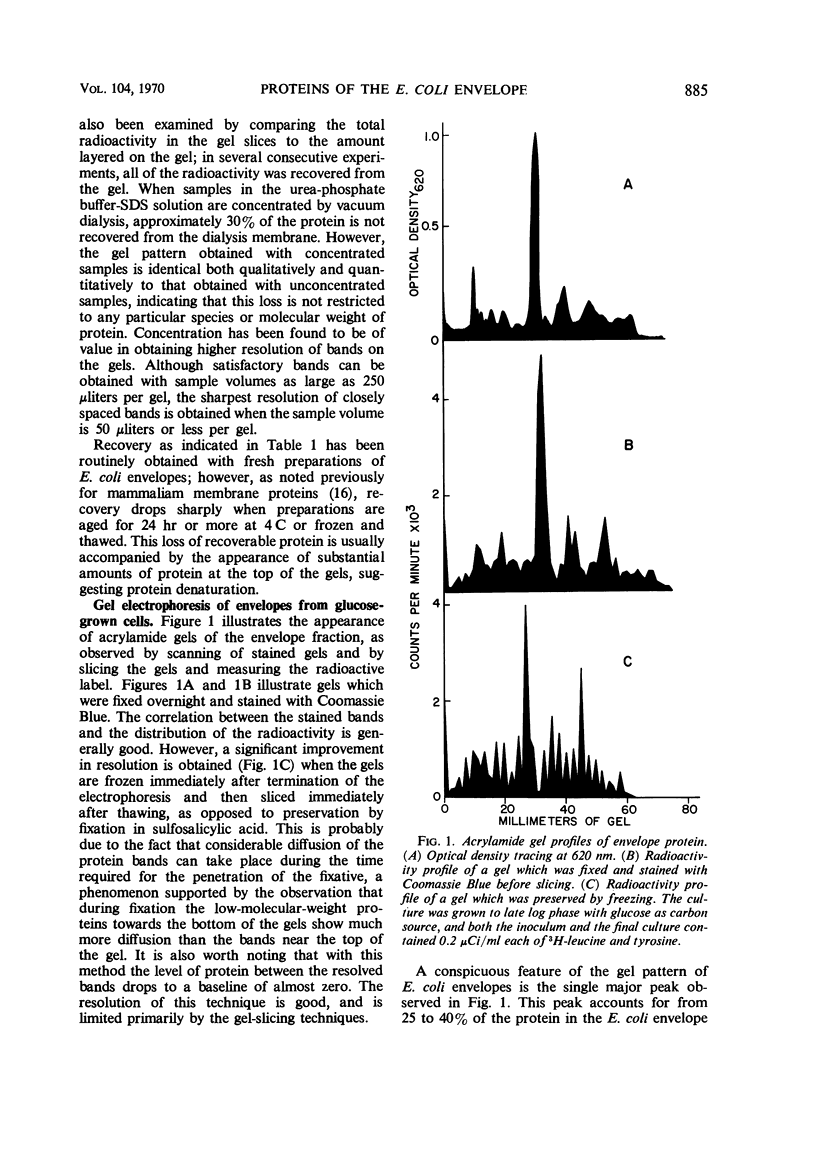
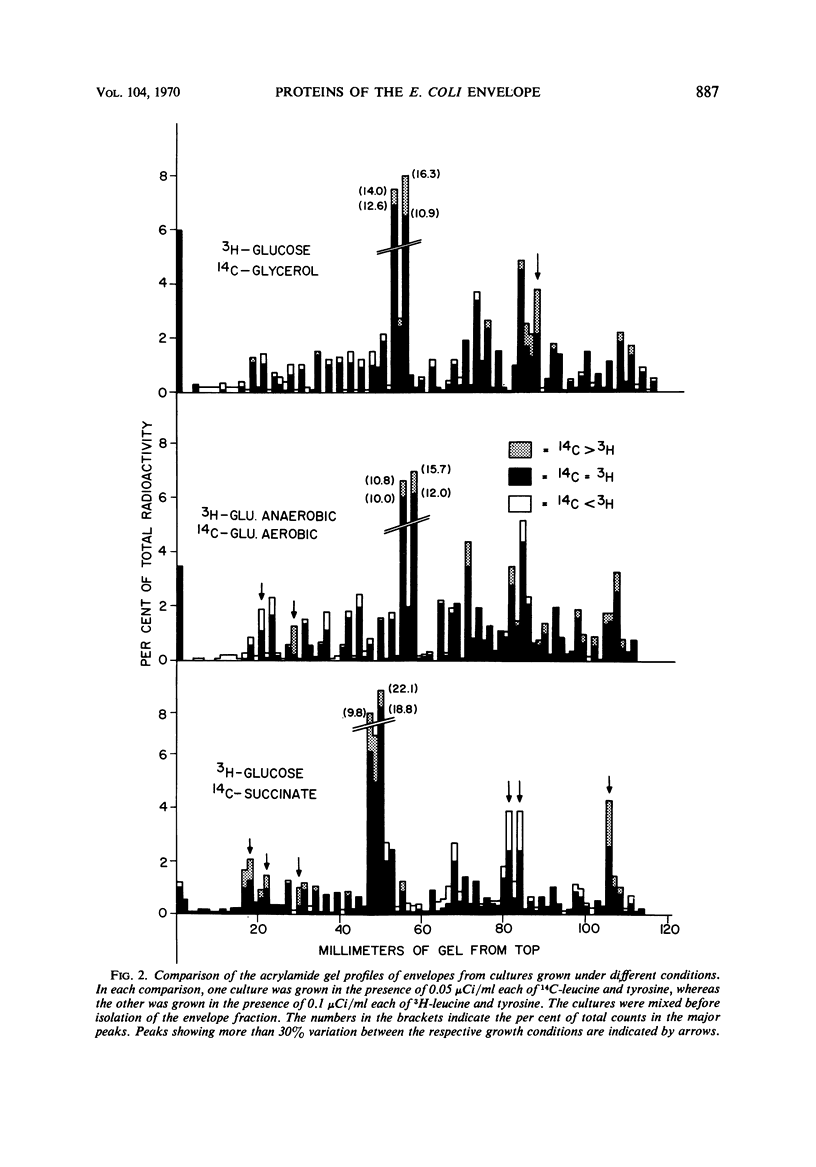
An envelope preparation containing the cell wall and cytoplasmic membrane of Escherichia coli was obtained by breaking the cells with a French pressure cell and sedimentating the envelope fraction by ultracentrifugation. This fraction was prepared for polyacrylamide gel electrophoresis by dissolving the protein in an acidified N,N′-dimethylformamide, removing lipids by gel filtration in the same organic solvent and removing the solvent by dialysis against aqueous urea solutions. More than 80% of the total protein of the envelope fraction was recovered in soluble form. Electrophoresis on sodium dodecyl sulfate-containing gels yielded from 20 to 30 well-resolved bands of protein. One major protein band was observed on the gels. This protein had a molecular weight of 44,000 and accounted for as much as 40% of the total protein of the envelope fraction. A double-labeling technique was used to examine the protein composition of the envelope fraction from cells grown under different sets of conditions which result in large changes in the levels of membrane-bound oxidative enzymes. These changes in growth conditions resulted in only minor alterations in the protein profiles observed on the gels, suggesting that this organism is able to adapt to changes in growth environment with only minor modifications of the major proteins of the cell envelope.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., TISDALE H. D., CRIDDLE R. S., CHEN P. Y., BOCK R. M. Isolation and properties of the structural protein of mitochondria. Biochem Biophys Res Commun. 1961 Jun 2;5:109–114. doi: 10.1016/0006-291x(61)90021-3. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- Green D. E., Haard N. F., Lenaz G., Silman H. I. On the noncatalytic proteins of membrane systems. Proc Natl Acad Sci U S A. 1968 May;60(1):277–284. doi: 10.1073/pnas.60.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E. C., Mayer R. M., Edstrom R. D., Beaudreau C. A. Structure and biosynthesis of the cell wall lipopolysaccharide of Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):315–333. doi: 10.1111/j.1749-6632.1966.tb52374.x. [DOI] [PubMed] [Google Scholar]
- Jones T. H., Kennedy E. P. Characterization of the membrane protein component of the lactose transport system of Escherichia coli. J Biol Chem. 1969 Nov 10;244(21):5981–5987. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. Inhibitory effect of glucose on enzyme formation. Nature. 1956 Oct 13;178(4537):801–802. doi: 10.1038/178801b0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- NOLTMANN E. A., GUBLER C. J., KUBY S. A. Glucose 6-phosphate dehydrogenase (Zwischenferment). I. Isolation of the crystalline enzyme from yeast. J Biol Chem. 1961 May;236:1225–1230. [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Alteration of membrane proteins in a chlorate-resistant mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):1–5. doi: 10.1016/0006-291x(69)90871-7. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of rat liver mitochondrial and microsomal membrane proteins. Proc Natl Acad Sci U S A. 1969 Jun;63(2):412–419. doi: 10.1073/pnas.63.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E. The influence of the environment on acetate metabolism in Escherichia coli. J Bacteriol. 1954 Aug;68(2):140–145. doi: 10.1128/jb.68.2.140-145.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler P. Blood group antigens in relation to chemical and structural properties of the red cell membrane. Vox Sang. 1968;15(2):81–101. doi: 10.1111/j.1423-0410.1968.tb03414.x. [DOI] [PubMed] [Google Scholar]
- Zahler P. The structure of the erythrocyte membrane. Experientia. 1969 May 15;25(5):449–456. doi: 10.1007/BF01900755. [DOI] [PubMed] [Google Scholar]



