Abstract
Background and Aims
Genome size is a function, and the product, of cell volume. As such it is contingent on ecological circumstance. The nature of ‘this ecological circumstance’ is, however, hotly debated. Here, we investigate for angiosperms whether stomatal size may be this ‘missing link’: the primary determinant of genome size. Stomata are crucial for photosynthesis and their size affects functional efficiency.
Methods
Stomatal and leaf characteristics were measured for 1442 species from Argentina, Iran, Spain and the UK and, using PCA, some emergent ecological and taxonomic patterns identified. Subsequently, an assessment of the relationship between genome-size values obtained from the Plant DNA C-values database and measurements of stomatal size was carried out.
Key Results
Stomatal size is an ecologically important attribute. It varies with life-history (woody species < herbaceous species < vernal geophytes) and contributes to ecologically and physiologically important axes of leaf specialization. Moreover, it is positively correlated with genome size across a wide range of major taxa.
Conclusions
Stomatal size predicts genome size within angiosperms. Correlation is not, however, proof of causality and here our interpretation is hampered by unexpected deficiencies in the scientific literature. Firstly, there are discrepancies between our own observations and established ideas about the ecological significance of stomatal size; very large stomata, theoretically facilitating photosynthesis in deep shade, were, in this study (and in other studies), primarily associated with vernal geophytes of unshaded habitats. Secondly, the lower size limit at which stomata can function efficiently, and the ecological circumstances under which these minute stomata might occur, have not been satisfactorally resolved. Thus, our hypothesis, that the optimization of stomatal size for functional efficiency is a major ecological determinant of genome size, remains unproven.
Keywords: Stomatal size, genome size, seed size, life history, photosynthesis, allometry, ecology, evolution, SLA, leaf structure, CAM, C4
INTRODUCTION
Within flowering plants, nuclear DNA content, or genome size, varies almost 2000-fold (Bennett and Leitch, 2005; Greilhuber et al., 2006). The significance of this wide range of values remains uncertain (Knight et al., 2005). High DNA amount is not associated with evolutionary advancement or organizational complexity; much takes the form of highly repeated sequences of non-genic DNA (Davidson and Britten, 1973). Processes have been identified both for reducing genome size (Kirik et al., 2000; Orel et al., 2003; Bennettzen et al., 2005) and for its increase (Kidwell, 2002; Bennettzen et al., 2005). These mechanisms have doubtless contributed to the major increases and decreases in genome size reported within evolutionary lineages (Leitch et al., 1998; Soltis et al., 2003; Caetano-Anollés, 2005; Johnston et al., 2005; Leitch et al., 2005) and the large differences in nuclear DNA amount recorded between closely related species (Bennett and Leitch, 2005).
The exact role of this extra DNA is a matter of debate. Some have argued that the additional DNA generally has little impact on function and relates to the ‘selfish’ nature of most ‘junk’ DNA (Doolittle and Sapienza, 1980; Orgel and Crick, 1980). Others have contended that the extra DNA has functional importance (Bennett, 1971; Cavalier-Smith, 1985, 2005) and many evolutionary and ecological correlates with genome size have been identified (e.g. Bennett, 1976; Jones and Brown, 1976; Grime and Mowforth, 1982; Thompson, 1990; Vinogradov, 2003; Knight et al., 2005; Beaulieu et al., 2007).
Cells with a large genome exhibit disproportionately slow mitotic division (Darlington, 1965; Bennett, 1971, 1972; Cavalier-Smith, 2005), and this relationship between genome size and rates of cell division impacts upon phenology. Species whose growth peaks in summer generally have a small genome, whereas, because of differences in the sensitivity of cell division and cell expansion to low temperatures, high nuclear DNA amounts appear advantageous for species that grow mainly in spring (Grime and Mowforth, 1982; Grime et al., 1985). In addition, genome size and nuclear and cell volume are positively correlated for non-vacuolated cells (Bennett, 1972; Edwards and Endrizzi, 1975; Sugiyama, 2005; Cavalier-Smith, 2005; Jovtchev et al., 2006). Many ecological correlates with genome size appear to originate more directly from the controlling impacts of nuclear DNA amount on cell volume (see Cavalier-Smith, 2005).
One such relationship with genome size relates to seed mass. Despite ecologically important inverse correlations with both fecundity (number of seeds produced) and long-term persistence in the soil (Fenner and Thompson, 2005), seed size is a plant characteristic that also appears constrained by nuclear DNA amount. Genome size and seed mass are positively correlated (Jones and Brown, 1976; Thompson, 1990; Maranon and Grubb, 1993; Knight and Ackerly, 2002; Knight et al., 2005; Beaulieu et al., 2007). The relationship is, however, inexact, perhaps in part because of the developmental complexity and the nutritional and structural diversity of seeds (Martin, 1947; Johansen, 1950; Hodgson and Mackey, 1986; Raven, 1999). Species with small genomes may produce large seeds by, for example, producing a small embryo but an abundance of endosperm (e.g. Fraxinus excelsior). Moreover, the seeds of species with large genomes may be minute (e.g. Orchidaceae, with obligate mycotrophy and ripe seeds containing only a small undifferentiated embryo and no endosperm). Thus, in practice, genome size accounts for only a small amount (variously estimated at 3 % and 6 %) of the recorded variation in seed mass (Beaulieu et al., 2007).
Another relationship with genome size involves stomata. Stomata consist of small pores at the leaf surface, each bounded by two guard cells. They provide the primary mechanism controlling the exchange of gases, particularly the influx of carbon dioxide and the efflux of water vapour, between the interior of the leaf and the atmosphere (Woodward, 1998; Raven, 2002; Hetherington and Woodward, 2003). The efficiency with which carbon dioxide, a key raw material for photosynthesis, is taken up and water loss restricted appears to be in part a function of stomatal size (Allen and Pearcy, 2000; Aasamaa et al., 2001; Hetherington and Woodward, 2003). Gaseous exchange is regulated through changes in the size of the stomatal pore. Because of their more rapid opening and closure, small stomata afford greater water-use efficiency in dry habitats, whereas in cool, moist and shaded habitats large stomata may be advantageous.
A general requirement in autotrophic plants to combine high photosynthetic capacity and water-use efficiency represents a strong ecological driver for optimizing stomatal size. However, stomatal size is not simply an ecologically important characteristic. The maximum size of the stomatal aperture is primarily determined by the length of its associated guard cells. This length is, in turn, constrained by genome size. Because of their greater structural uniformity, stomata may be expected to show a more consistent allometric relationship with genome size than that observed for seed mass. Certainly, polyploidy in closely related lineages appears initially to cause a virtual doubling of genome size and a concomitant increase in guard-cell length (Speckman et al., 1965; Masterson, 1994; Joachimiak and Grabowska-Joachimiak, 2000; Bennett and Leitch, 2005). The generality of this positive correlation between genome and stomatal size, however, remained untested until two recent contemporaneous studies, each with a somewhat different focus, ours and that of Beaulieu et al. (2008).
Stomatal and leaf characteristics for 1442 species measured by the authors mainly in England, Iran, Spain and Argentina and published data on nuclear DNA amounts (Bennett and Leitch, 2005) have, therefore, been used to investigate correlates with genome size in angiosperms. First a preliminary analysis of geographical, taxonomic and ecological trends in stomatal size is provided in a dataset. Subsequently, the extent to which values for stomatal size correlate with the size of the angiosperm genome are identified and, by means of a literature review, the claims of stomatal size to be the ‘missing link’, the primary determinant of genome size in the angiosperms, is assessed for a first time.
MATERIALS AND METHODS
Study areas
The investigation centres on the floras of four climatically contrasted and geographically disparate areas: the Córdoba region of central western Argentina (55 species measured), the Sheffield region of central England (745 species), the Arazbaran Protected Area in northern upland Iran (463 species) and the Zaragoza region of north-east Spain (278 species). The climatic characteristics of each region are briefly outlined in Table 1 and the areas described more fully in Díaz et al. (2004). The species studied represent an ecologically balanced subset of their respective floras, except for England, where a disproportionately large number of species with large genomes have been included. Data for a further 161 species collected from south-east Europe and the Near East during other ecological projects were also incorporated into this study.
Table 1.
A climatic comparison of the four main study areas (data abstracted from Díaz et al., 2004)
| Argentina | Spain | Iran | England | |
|---|---|---|---|---|
| Mean annual rainfall (mm) range, distribution | 85–912, confined to warm season | 300–350, mainly in spring and autumn | 316–686, throughout the year with winter maximum | 565–1800, throughout the year with winter maximum |
| Mean annual temperature range (°C) | 8–20 | 6–24 | 5–14 | 9–11 |
| No. of months in which evaporation exceeds precipitation (range) | 1–12 | 6 | 2–4 | 0–2 |
| No. of frost-free months (range) | 0–8 | 3–5 | 6 | 3–6 |
Attributes measured
Stomatal size and distribution
Material was collected from unshaded habitats. The method of Beerling and Chaloner (1993) was used to take acetate impressions from the upper and lower surfaces of each of three replicate leaves for each species collection. For most UK and Spanish measurements the Aequitas Image Analysis program (Dynamic Data Links, 1993–1996) or a more modern version was used. In Argentina and Iran, more traditional microscopy, using an eye-piece graticule, was employed. Stomata were counted on each surface and, where possible, the lengths (micrometres) of at least three closed stomata measured from each leaf impression.
Genome size, cytology and taxonomy
Values for genome size were abstracted from Bennett and Leitch (2005) and other more recent publications. Chromosome numbers were collated from a range of sources, including Federov (1969), Gornall and Bailey (1998) and Bennett and Leitch (2005). Ploidy was subsequently assessed in relation to the base number for the family and that of the genus using a variety of publications including Federov (1969) and Raven (1975). Many species were, of necessity, left out of certain analyses through a lack of chromosomal and/or genome-size data. Worst affected was the Argentinian flora, with 90 % of species lacking genome-size measurements. The Angiosperm Phylogeny Group (APG) has redefined the species composition of a number of major angiosperm taxa. The classification of Angiosperm Phylogeny Group (APG III, 2009) has been followed and families have been ordered in the sequence set out by Haston et al. (2007, 2009).
Leaf traits
Values relate to healthy, sexually mature plants growing in unshaded habitats and are usually an average of at least six replicate measurements. Prior to measurement, leaves were enclosed within a moistened paper towel and kept refrigerated overnight in a sealed polythene bag to ensure that they are fully imbibed. Subsequently, the area of the leaf lamina (using a leaf area machine or scanner), leaf fresh weight, leaf dry weight and intervenal leaf thickness (to the nearest 0·01 mm, using a dial thickness gauge) were measured. Because of their ecological importance (Givnish, 1988; Reich et al., 1992; Bolhàr-Nordenkampf and Draxler, 1993; Garnier and Laurent, 1994; Díaz et al., 2004), the following leaf traits were assessed: maximum leaf size (mm2); leaf dry-matter content (100 × dry mass of leaf/saturated mass of leaf); leaf thickness (mm); specific leaf area [leaf area (mm2)/leaf mass (mg)]. The procedures used are described in detail in Charles et al. (1997). They conform to the general recommendations of Garnier et al. (2001) and Cornelissen et al. (2003).
Increasing efficiency of water use
The development of specializations for restricting water loss and for maximizing carbon gain is a recurrent theme in the adaptive radiation of angiosperms (Woodward, 1998; Raven, 2002; Hetherington and Woodward, 2003). Accordingly, the following functional groupings have been separated: C3 (the majority), C4 (specialized leaf anatomy and an extra biochemical pathway; mainly fast-growing tropical species) and crassulacean acid metabolism (CAM; nocturnal stomatal opening, when the air is cooler and more humid, i.e. temporal uncoupling of carbon dioxide uptake and its fixation by the Calvin cycle; succulents, etc.). Here, more controversially and in the light of our early results, vernal geophytes were also treated as a further ‘avoidance’ grouping. Vernals geophytes are, variously, woodland herbs that complete their annual life cycle before trees come into leaf and plants that similarly avoid drought in rocky or freely drained habitats. Their extremely large stomata are a consequence of the large genome necessary for a specialized type of growth in which cell division occurs during a period of dormancy (often late summer–autumn) and rapid vegetative ‘growth’ by cell expansion is delayed until early spring (Grime and Mowforth, 1982). We suggest that vernals can be additionally viewed as an ‘avoidance’ group because the low water-use efficiency inevitably associated with their exceptionally large stomata precludes an extension of the period of growth into hotter, drier summer conditions (see Hetherington and Woodward, 2003).
Ecological attributes
Hetherington and Woodward (2003) have suggested that selection for optimal stomatal size relates to survival in shaded and in droughted habitats, large stomata being favoured in the former and small in the latter. With a view to confirming these relationships, habitat type was included in the analyses. This was assessed from published sources, particularly Braun Blanquet and de Bolós (1953) and Grime et al. (2007), and from unpublished vegetation surveys and field observations. Some species from Iran were too ecologically wide-ranging for a confident assessment of habitat type and there were too few data to include Argentina in the analyses.
The following life-history classes were also separated: annual, monocarpic perennial (‘biennial’), herbaceous polycarpic perennial (excluding vernal geophytes), vernal geophytes and woody species (trees, shrubs and subshrubs).
Analyses
The statistical properties of guard-cell length, genome size, leaf size, leaf thickness, leaf dry-matter content and amphistomy were checked. It was found necessary to log10-transform the first four variables prior to statistical analysis and present leaf dry-matter content as its square-root. No satisfactory transformation for amphistomy was identified and species were, therefore, grouped into the following five subequal classes: 1, 0 %; 2, <25 %; 3, 25–40 %; 4, 40–45 %; 5, 45–50 % (% stomata on the surface with lower density). Except where otherwise stated, statistical tests were performed using SPSS for WindowsTM (Version 14·0).
Three sets of analyses were carried out. In the first, stomatal length values from different geographical regions, families, life-history groupings and habitats were compared using one- or two-way ANOVAs with differences between subsets assessed by post-hoc (Tukey) tests and t-tests. In the second, to detect general specialization trends in leaf structure, the data for leaf and stomatal characters were organized into a single 6 traits by 1186 species matrix and the matrix submitted to a Principal Component Analysis (PCA) based on the correlation matrix of variables, in which data are centred and standardized by standard deviation. In the third, the level of correlation between stomatal length and genome size was assessed for a wide range of major taxa. Excluded from these correlations were taxonomic groupings with few valid samples (n < 10) and those with an unusually narrow range of stomatal lengths (<2- and <1·5-fold, respectively, for families and infra-familial groupings).
RESULTS
Geographical and taxonomic variation in stomatal size
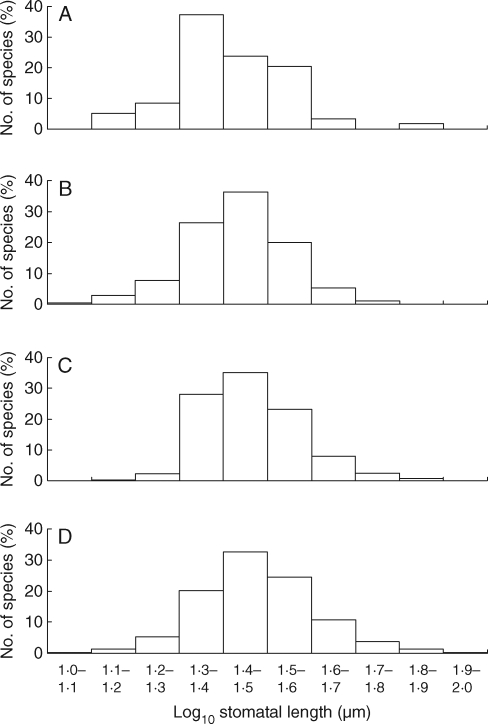
Mean stomata size differed significantly in the four study areas with the smallest average stomatal length associated with Argentina and the largest with England (Fig. 1). The range of guard-cell lengths, however, differed little between regions and was similar to the 10–80 µm range cited by Hetherington and Woodward (2003) for the world flora. Values ranged from 15·3 µm (Trichloris crinita, Poaceae) to 71·3 µm (Baccharis articulata, Asteraceae) in Argentina, from 12·5 µm (Medicago orbicularis, Fabaceae) to 61·0 µm (Adonis annua, Ranunculaceae) in Spain, from 14·9 µm (Punica granatum, Lythraceae, formerly Punicaceae) to 67·4 µm (Ophrys punctulata, Orchidaceae) in northern Iran and from 12·1 µm (Salix repens, Salicaceae) to 100·8 µm (Fritillaria meleagris, Liliaceae) in England.
Fig. 1.
Stomatal length distribution within each of the four main study areas. (A) Argentinaa: log10(stomatal guard-cell length, μm) ± s.d. 1·42 ± 0·13, n = 59; (B) Spainab: 1·43 ± 0·11, n = 284; (C) Iranbc: 1·47 ± 0·11, n = 475; (D) Englandc: 1·47 ± 0·13, n = 745. ANOVA F3,1559 = 13·1, P < 0·001. Here and in the remaining figures and tables, groupings with the same suffix are not statistically significantly different at P < 0·05 in Tukey (post-hoc) tests.
Variation in stomatal size in relation to life history and habitat
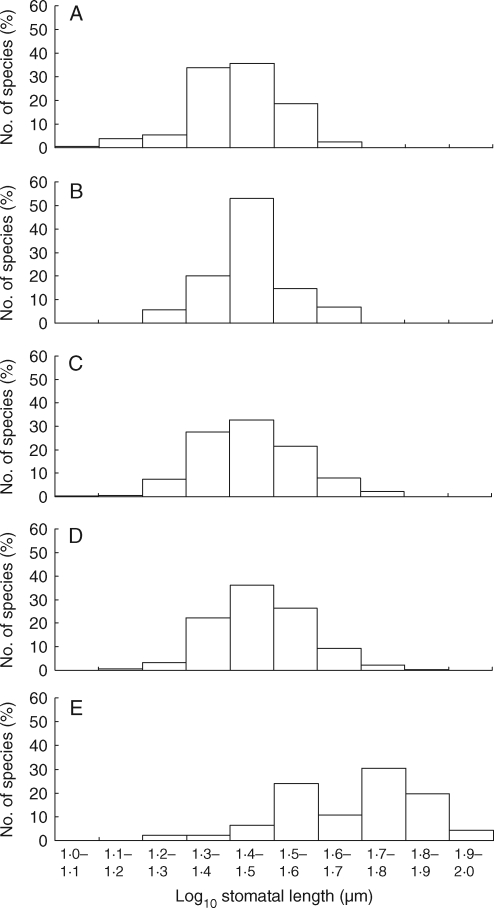
In each life-history class there was at least a 3-fold difference between the largest and smallest average stomatal sizes (Fig. 2). Nevertheless, despite this high level of variation within groupings, stomatal size appeared to be very much a function of life history (Fig. 2). Moreover, general trends appeared to be similarly expressed in different study areas and families (Table S1 in Supplementary data, available online). Species with the largest stomata were almost exclusively monocotyledonous vernal geophytes [Fritillaria meleagris (Liliaceae), 100·8 µm; Lilium martagon (Liliaceae), 83·4 µm; Gagea lutea (Liliaceae),76·3 µm; Orchis mascula (Orchidaceae), 75·4 µm; Orchis anthropophora (Orchidaceae), 70·9 µm; Ophrys apifera (Orchidaceae), 68·3 µm, Ophrys punctulata (Orchidaceae), 67·4 µm; Ophrys insectifera (Orchidaceae), 66·1 µm; Scilla mischtschenkoana (Asparagaceae), 65·3 µm; Dactylorhiza praetermissa (Orchidaceae), 64·2 µm]. The only exceptions were Baccharis articulata (Asteraceae), a stem succulent, 71·3 µm, and Caltha palustris (Ranunculaceae), an early-flowering, wetland herb, 65·6 µm. By contrast, and consistent with the findings of Beaulieu et al. (2008), species with the smallest stomata were predominately woody species [Salix repens (Salicaceae), 12·1 µm; Pistacia terebinthus (Anacardiaceae), 12·8 µm; Arthrocnemum macrostachyum (Amaranthaceae, formerly Chenopodiaceae), 13·2 µm; Salix caprea (Salicaceae), 14·3 µm; Punica granatum (Lythraceae), 14·9 µm; Ficus carica (Moraceae), 15·3 µm; Myrica gale (Myricaceae), 15·4 µm; Robinia pseudoacacia (Fabaceae), 15·4 µm]. Only four herbaceous species occurred in ‘the bottom twelve’: the perennial C4 grass, Trichloris crinita (15·3 µm), one herbaceous perennial (Trifolium fragiferum, 15·2 µm) and two annual legumes (Medicago orbicularis, 12·5 µm; Medicago radiata, 14·5 µm). More typically, representatives of these other life-history categories (annual, monocarpic perennial, herbaceous polycarpic perennial) were of intermediate stomatal size (Fig. 2).
Fig. 2.
Stomatal length distribution within different life-history classes: (A) woody polycarpic perennialsa [log10(stomatal guard-cell length, μm) ±s.d. 1·41 ± 0·10, n = 205]; (B) monocarpic perennialsab (1·45 ± 0·09, n = 89); (C) annualsab (1·45 ± 0·11, n = 451); (D) herbaceous polycarpic perennialsb (1·47 ± 0·11, n = 658); (E) vernal geophytes (1·68 ± 0·16, n = 46). ANOVA F4,1444 = 58·6, P < 0·001.
Stomatal size also varied according to habitat, but with lesser statistical significance (Table 2A). In part, this relates to the frequent co-existence within the same habitat of contrasted life-history types differing in stomatal size (e.g. woodland with trees with small stomata and small vernal geophytes with large stomata). When comparisons were focused more narrowly, a negative relationship between stomatal size and aridity could be consistently detected. For both shallow-rooted annuals and deep-rooted trees and tall shrubs, species from more droughted environments had smaller stomata than comparable ones from mesic habitats (Table 2Bi). It was not possible, however, to find any evidence supporting another suggestion of Hetherington and Woodward (2003), namely that the possession of large stomata is an important component of specialization for shade-tolerance. Summer-green species from the woodland floor (i.e. shade-tolerant species) have smaller stomata than woodland vernal geophytes, ‘shade-avoiders’, which exploit only the open phase of the woodland before canopy closure (Table 2Bii).
Table 2.
A regional comparison of stomatal length for major habitats: (A) compares all habitats and (B) ecological species groupings subject to differing levels of drought and shade
| Habitat | n | Mean log10 guard-cell length ± s.d. (μm) |
|---|---|---|
| (A) All habitats | ||
| England | ||
| Skeletal | 79 | 1·43 ± 0·11a |
| Wasteland | 152 | 1·45 ± 0·12ab |
| Arable | 109 | 1·47 ± 0·10ab |
| Maritime | 23 | 1·47 ± 0·10ab |
| Woodland | 148 | 1·48 ± 0·14ab |
| Wetland | 167 | 1·49 ± 0·12b |
| Pasture | 98 | 1·49 ± 0·14b |
| F6,769 = 3·5 P < 0·01 | ||
| (R2 = 0·23; F31,744 = 7·0, P < 0·001; | ||
| life history F4,744 = 28·7, P < 0·001) | ||
| Iran | ||
| Secondary woodland (altitudinal zone 2) | 100 | 1·43 ± 0·10a |
| Dry pasture (altitudinal zone 4) | 69 | 1·45 ± 0·10ab |
| Primary woodland (altitudinal zone 1) | 125 | 1·47 ± 0·11b |
| Pasture (altitudinal zone 3) | 55 | 1·51 ± 0·09 |
| F3,345 = 5·9 P < 0·001 | ||
| (R2 = 0·19; F18,320 = 4·2, P < 0·001; | ||
| life history F4,320 = 5·6, P < 0·001) | ||
| Spain | ||
| Woodland | 31 | 1·37 ± 0·13a |
| Dry pasture and wasteland | 67 | 1·41 ± 0·10ab |
| ‘Saline’ (on gypsum soils) | 33 | 1·42 ± 0·12ab |
| Pasture and wasteland | 45 | 1·43 ± 0·13ab |
| Wetland | 18 | 1·45 ± 0·11ab |
| Arable | 84 | 1·47 ± 0·10b |
| F5,272 = 3·8 P < 0·01 | ||
| [R2 = 0·14; F21,256 = 2·0. P < 0·01; | ||
| life history F4,256 = 3·6. P < 0·01] | ||
| (B) Drought and shade | ||
| (i) Drought | ||
| Annuals: arid vs. ‘mesic’ habitats | ||
| England | ||
| Skeletal habitats | 46 | 1·40 ± 0·12 |
| Arable | 99 | 1·46 ± 0·10 |
| t = 3·5, P < 0·001 | ||
| Spain | ||
| Dry pasture | 26 | 1·38 ± 0·11 |
| Arable | 75 | 1·46 ± 0·10 |
| t = 3·8, P < 0·001 | ||
| Tall woody species (canopy height >3 m): arid vs. temperate climates | ||
| England | ||
| Argentina (arid) | 10 | 1·34 ± 0·11b |
| Spain | 26 | 1·36 ± 0·12b |
| Iran | 30 | 1·42 ± 0·08ab |
| England (temperate) | 47 | 1·45 ± 0·11a |
| F3,109 = 6·3, P < 0·001 | ||
| (ii) Shade | ||
| England: woodland ground-floor vegetation | ||
| Summer-green perennial herbs | 64 | 1·46 ± 0·11 |
| ‘Shade-avoiding’ vernal geophytes | 16 | 1·69 ± 0·16 |
| t = 6·9, P < 0·001 | ||
Species groupings with the same letters are not statistically significantly different at P < 0·05 in Tukey (post-hoc) tests.
In (A) statistical analyses in parenthesis relate to two-way ANOVAs where additionally life-history attributes are included; all statistically significant treatment effects of habitat or life history are appended.
Stomatal size and leaf structure
The three PCA axes identified, which account for approx. 73 % of the total variance in the data matrix, effectively separate species with small stomata from those with large ones (Fig. 3A and B). The species with the smallest stomata in the present study (Salix repens, 12·1 µm) had values 21 %, 19 % and 38 % of the maximum for PCA axes 1–3, respectively, whereas, for the species with the largest stomata (Fritillaria meleagris, 100·8 µm), the values were 68 %, 83 % and 71 %. The three specialist groups, C4 species, species with CAM and ‘cool season’ vernal geophytes also occupy different positions on the three PCA axes (Fig. 3C and D). Only C3 species were widely scattered.
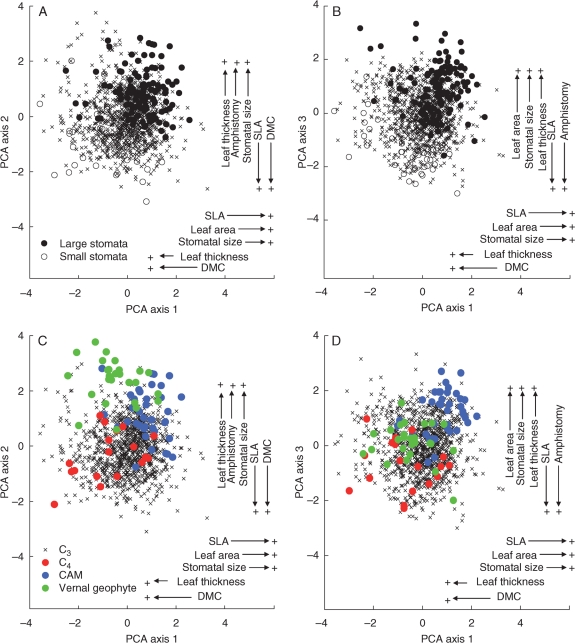
Fig. 3.
PCA ordination of 1186 angiosperm species from Argentina, England, Iran and Spain, on the basis of six leaf traits. Labels display traits with the highest eigenvector scores on PCA axes 1, 2 and 3, with the label with the highest score presented nearest the axis. In (A) and (B), the distribution of species with large stomata (>40 µm) and those with small stomata (<20 µm) is shown, as indicated in (A), and in (C) and (D) the distribution of C3, C4, CAM and vernal geophyte species is shown as indicated in (C).
PCA axis 1, explaining approx. 28 % of the variance, was a ‘xeromorphic-mesomorphic axis’ of leaf structure and has some ecological equivalence to the dry-shaded axis relating to stomatal size in Hetherington and Woodward (2003). At its lower end, were species, mainly from arid habitats in Argentina [e.g. Aspidosperma quebracho-blanco (Apocynaceae), Larrea divaricata (Zygophyllaceae) and Lithraea ternifolia (Anacardiaceae)], with small, thick leaves, high dry matter content, low specific leaf area and small stomata. At the higher extreme were species from mesic and from shaded, habitats, [e.g. Anthriscus cerefolium (Apiaceae) and Valeriana officinalis (Caprifoliaceae)], with large, thin leaves, a low dry matter content, high specific leaf area and larger stomata. Fast-growing species of productive habitats (see Grime and Hunt, 1975) occupied an intermediate position along PCA axis 1 [Chenopodium album (Amaranthaceae), value 51 % of maximum; Urtica dioica (Urticaceae), 56 %; Holcus lanatus (Poaceae), 65 %].
PCA axis 2, the ‘succulence axis’ [highest scores: Grahamia bracteata (Portulacaceae) and Sedum rupestre (Crassulaceae)], accounted for a further approx. 26 % of the variance. Species with high values had thick, amphistomatous leaves with a low dry matter content and moderately large stomata. As is characteristic of succulents (Vendramini et al., 2002), specific leaf area was relatively high. The stem succulents, Baccharis articulata and Cereus validus (Cactaceae), not included in the analysis, also have large stomata.
PCA axis 3, which explained a further approx. 19 % of the variance, was a ‘size axis’ relating to the dimensions of both the leaf and its component parts. At the upper end of PCA axis 3 were species with large, relatively thick, amphistomatous leaves and large stomata [e.g. Petasites hybridus (Asteraceae)] and at the lower end, with small, thin, hypostomatous leaves, were Aphanes arvensis (Rosaceae), Callitriche stagnalis (Plantaginaceae), and other similarly small and/or short-lived species.
Taxonomic variation in stomatal size
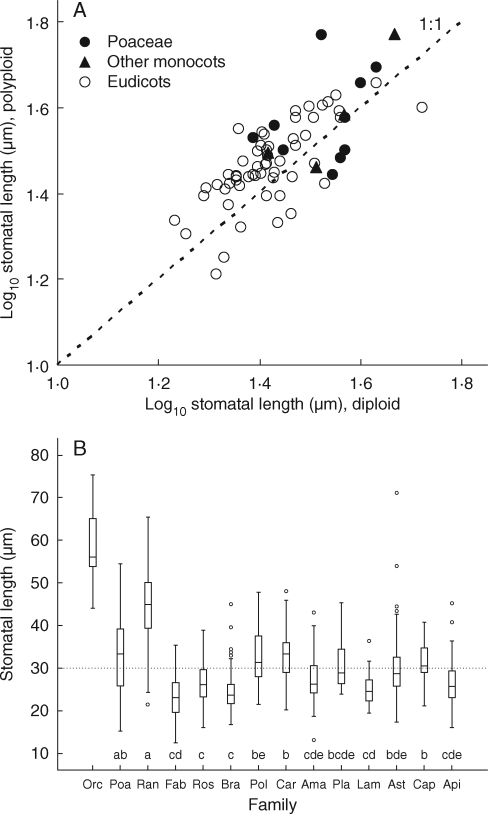
Stomatal size further relates to both cytological status and phylogeny. Intrageneric polyploids tended to have larger stomata than their close diploid relatives (Fig. 4A). Nevertheless, even when this increase in size through polyploidy is factored out by including only familial or ‘clade’ diploids, families showed significant differences in stomatal size (Fig. 4B). Within the present dataset, some, particularly Brassicaceae, Fabaceae and Rosaceae, had small stomata whereas others (e.g. Orchidaceae and Ranunculaceae) had consistently large stomata. A familial summary of stomatal size for the 1442 species measured is presented as Table S2 in Supplementary data, available online. These familial averages must, however, be treated with caution. For example in Orchidaceae, the values, originating only from England and Iran [range (mean) 44·1 − 75·4 (57·9) μm; n = 20], are considerably higher than, and statistically different from, those from the less geographically and phylogenetically restricted subset examined in Beaulieu et al. (2008) [27·4 − 62·7 (39·5) μm; n = 9; t = 4·6, P < 001]. In contrast, for species common to both studies there was broad correspondence in measured values for stomatal length between the two studies (r = 0·61, n = 24, P < 0·01; paired t = 0·7, n.s.).
Fig. 4.
(A) Diploids tend to have smaller stomata than related polyploids. Each datum point is an intrageneric diploid–polyploid pair (monocots, excluding Poaceae; Poaceae; and eudicots, as indicated). Paired t = 5·1, n = 70, P < 0·001; r = 0·74, P < 0·001. Here, and in the remaining tables and figures, an ‘international’ average stomatal size value is used for more widely distributed species. (B) Stomatal length for diploid species differs between families. The box plots include the median (central line), the first and third quarters (box) and outliers. The 14 families illustrated (Amaranthaceae, Apiaceae, Asteraceae, Brassicaceae, Caprifoliaceae, Caryophyllaceae, Fabaceae, Lamiaceae, Orchidaceae, Plantaginaceae, Poaceae, Polygonaceae, Ranunculaceae and Rosaceae) are identified by their first three letters and phylogenetically ordered as recommended by Haston et al. (2007). ANOVA F13,305 = 29·43, P < 0·001. Here ‘diploidy’ relates to the familial base chromosome number as given in Raven (1975) except for Orchidaceae, where, using Bateman et al. (2003), ploidy was assessed in relation to clade base number. Families with the same letters are not statistically significantly different at P < 0·05 in Tukey (post-hoc) tests. The mean value for stomatal size for all diploid species measured is identified by a broken line.
Notwithstanding the results for Orchidaceae, and consistent with the results in Fig. 4B, stomatal size does appear conservatively expressed within major taxa (Fig. S1 in Supplementary Data, available online). Similar values were recorded for familial subsets differing in life-history. Superimposed on this a further ecological effect was noted for woody species (Fig. S1A). These tended to have smaller stomata than related herbaceous species (excluding vernal geophytes). For the much smaller vernal geophytes dataset, no statistically significant difference was observed (Fig. S1B). Vernal geophytes were primarily restricted to families in which all or, at least most, species had large stomata.
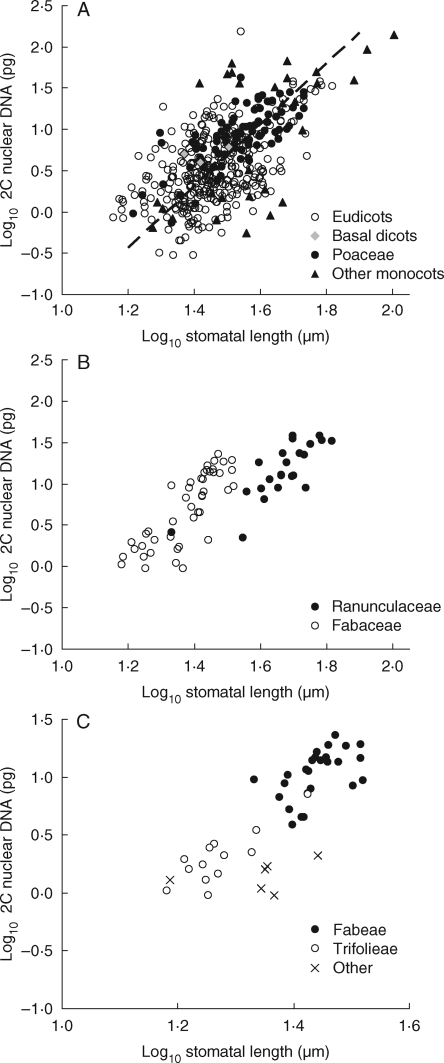
Stomatal and genome size
Stomatal length and genome size are positively correlated (Fig. 5). This relationship appears to be a general feature within the eudicots, Poaceae and the remaining monocots. Only in tribe Poeae (Poaceae), with a high incidence of intraspecific polyploidy, was the relationship not detected.
Fig. 5.
Examples of the relationship between stomatal and genome size. (A) All species: r2 = 0·36, P < 0·001, n = 446. Eudicots (r2 = 0·26, P < 0·001, n = 326); basal dicots (n = 3); monocots, excluding Poaceae (r2 = 0·39, P < 0·001, n = 30); and Poaceae (r2 = 0·53, P < 0·001, n = 87), as indicated. (B) Contrasted families: Ranunculaceae (r2 = 0·64, P < 0·001, n = 23), and Fabaceae (r2 = 0·64, P < 0·001, n = 46), as indicated. Other families: Asteraceae (r2 = 0·08, P < 0·05, n = 51; minus Chrysanthemum segetum, r2 = 0·08); Caryophyllaceae (r2 = 0·23, P < 0·1, n = 15); Polygonaceae (r2 = 0·32, P < 0·05, n = 13). (C) Contrasted tribes (Fabaceae): Fabeae (r2 = 0·29, P < 0·01, n = 26); Trifolieae (r2 = 0·67, P < 0·001, n = 13); and other (n = 7), as indicated. Tribes in other families: Asteraceae, Anthemideae (r2 = 0·13, n.s., n = 17; minus Chrysanthemum segetum, r2 = 0·30, P < 0·05, n = 16); Lactuceae (r2 = 0·50, P < 0·001, n = 18); Poaceae, Agrostideae (r2 = 0·49, P < 0·01, n = 13); Aveneae (r2 = 0·72, P < 0·001, n = 11); Poeae (r2 = 0·05, n.s., n = 19).
DISCUSSION
Problems and conclusions
Before any interpretation of the results, the basic difficulties in acquiring and analysing data for a broad study of this type should be considered. These include the following.
Intraspecific variation in stomatal size
Plants grow in heterogeneous environments under seasonally fluctuating light intensity, temperature, atmospheric humidity and water availability. As a result, phenotypic plasticity is an important component of leaf structure and functioning (Lechowicz, 1984; Givnish, 1988; Bolhàr-Nordenkampf and Draxler, 1993; Allen and Pearcy, 2000; Aronne and De Micco, 2001). This plasticity will have inevitably impacted upon the arithmetic precision and statistical strength of the results, but only up to a point. The intrapopulation range of stomatal size was typically approx. 40 % of the mean in the samples (data not shown) and intraspecific comparisons between countries indicated a broad correspondence (Fig. S2 in Supplementary data). Particularly encouraging were values for Trifolium repens, the only species common to all study areas. Its mean stomatal length in Spain, Argentina, England and Iran was 15·3, 16·2, 16·4 and 17·3 µm, respectively.
Classification of leaf structure
Information on certain important structural leaf characters and all of the important biochemical characters was lacking. With regard to structural characters, data on whether stomata were sunken and the extent to which leaves were inrolled or dissected would probably have been useful contributors to the ‘xeromorphic-mesomorphic axis’ (PCA axis 1), and the amount of non-photosynthetic water-storage tissue would have enhanced the ‘succulence axis’ (PCA axis 2).
Vacuolization of the guard cell
Genome size and nuclear and cell volume are positively correlated for non-vacuolated cells (Bennett, 1972; Edwards and Endrizzi, 1975; Cavalier-Smith, 2005; Sugiyama, 2005; Jovtchev et al., 2006). However, the additional presence of a vacuole is essential for stomatal functioning: the opening and closure of the stomatal pore involves potentially large changes in vacuolar and cellular volume (Willmer and Fricker, 1995). A deterministic impact of vacuolar size on stomatal dimensions (i.e. the absence of a close allometric relationship between guard cell size and the size of its cytoplasmic component) would seriously undermine our rationale for examining the relationship between stomatal and genome size. Doubtless, there are differences in vacuolization both between species and between broader taxonomic groupings, and these will contribute to variability within the dataset. Nevertheless, available data indicate that cytoplasm occupies a sizeable proportion of guard cell volume [e.g. 31–41 % for Arabidopsis thaliana (Brassicaceae); Tanaka et al., 2007; see also, Fricker and White, 1990; Willmer and Fricker, 1995; Merkel et al., 2007] and provides no evidence that the fraction of guard cell volume at a given proportion of the maximum aperture is significantly greater in genotypically large as opposed to genotypically small guard cells.
Genome size and chromosome number
Two problems restrict the usefulness of published data. The first is accuracy, both in measurement and taxonomy. The second relates to cytology. A significant minority of species have cytotypes differing in ploidy and genome size (for examples, see Bennett and Leitch, 2005). Where necessary, taxa have been identified to their subspecies and known relationships between ploidy and geographical distribution assessed. However, some groups [e.g. tribe Poeae (Poaceae), with a high incidence of intraspecific polyploidy and, to a lesser extent, aneuploidy] are particularly problematic. We suspect that, despite our best efforts, some stomatal and genome values are cytologically mismatched. This may explain, for example, the lack of a statistically significant correlation between stomatal and genome size for tribe Poeae (Poaceae) in Fig. 5C.
Sampling bias
Although for each of the four floras studied, an ecologically balanced subset of species was chosen, there is not the same phylogenetic balance. For example, only three early diverging dicots were included. Moreover, there are almost no data for the species-rich tropics. The use of such a limited subset of the world flora can easily lead to misleading average values of stomatal size for major taxa [see above, the comparison of our results for Orchidaceae and those from Beaulieu et al. (2008)]. Representativeness is further reduced in comparisons between stomatal and genome size. The Plant DNA C-values Database at Kew (Bennett and Leitch, 2005) is a monumental achievement. Nevertheless, it includes <2 % of angiosperms (Gregory et al., 2007) and genome size is unknown for, respectively, 90 %, 77 %, 54 % and 53 % of the species featured in the present study from Argentina, Iran, Spain and England.
Insufficient sampling of vernal geophytes
Vernal geophytes are poorly represented within the four floras studied and constitute only a minor component of the present database. Moreover, in only nine families were there data for both vernal and non-vernal species. This is unfortunate. If we are to understand the ecological significance of large stomata, vernal geophytes are a key ecological grouping. Nevertheless, at present the data are lacking to separate the phylogenetic and ecological determinants of their stomatal size satisfactorily.
Nevertheless, despite these problems, the following conclusions can be drawn.
As with genome size (see Leitch et al., 1998), there are clear lineage-specific differences in stomatal size. These interfamilial differences appear to dwarf those found within polyploid series (Fig. 4; Table S1 in Supplementary Data, available online).
Variation was detected in stomatal size along three important ecological axes of leaf specialization: xeromorphic (stomata small) vs. mesomorphic (large), non-succulent (small) vs. succulent (large) and ‘small’ (small) vs. ‘large lamina’ (large). Stomatal size is an important component of leaf specialization and patterns with respect to C4, CAM and life history (woody species < herbaceous species < vernal geophytes).
Because of its relatively consistent and strong correlations with 2C DNA amount and its ecological importance, the possibility that stomatal size is a key determinant of genome size in angiosperms deserves consideration.
Stomatal size, a key determinant of genome size in angiosperms?
Beaulieu et al. (2008) argue that stomatal size is a consequence of genome size. However, since they do not identify what actually determines genome size, we find their arguments unconvincing. Instead, we favour the more mechanistic rationale of Cavalier-Smith (2005) who stated ‘Whatever group one examines reveals sensible adaptive reasons for the observed spectrum of cell size that account for the correlated genome size spectrum. To understand these one has to be familiar with the developmental biology and ecology of the group; a purely genetic or purely biochemical approach gets nowhere.’
Small stomata tend to show greater water-use efficiency (Aasamaa et al., 2001; Hetherington and Woodward, 2003) and in this study also (Table 2Bi) species from dry habitats tend to have smaller stomata than similar species from more mesic environments. Moreover, the development of mechanisms to reduce transpirational losses, such as C4 photosynthesis and CAM, has been a recurrent theme within the adaptive radiation of angiosperms (Woodward, 1998; Raven, 2002; Hetherington and Woodward, 2003). Efficient stomatal function is an important prerequisite of the photosynthesis on which autotrophic angiosperms depend. Thus, water-use efficiency matters and so too does stomatal size. Is stomatal size sufficiently important to regulate genome size? Correlation does not indicate causality. Moreover, species survival requires the integration of a diverse range of physiological functions of which photosynthesis is only one. Stomatal size is not an ecologically and physiologically ‘stand alone’ character. It will be subject to trade-offs with other ecologically important plant attributes and may often have, at best, a subordinate impact upon genome size.
Take, for example, vernal geophytes, a grouping in which, we suspect, endopolyploidy is uncommon (see Barrow and Meister, 2003). Here, a large genome facilitates a form of ‘cool season growth’ in which cell division and cell expansion are uncoupled (see Grime and Mowforth, 1982). These vernal geophytes have large, ‘conductively inefficient’ stomata but the cell size issues relevant to vernal growth appear to represent the main ecological determinant of genome size.
Depending on their level of endopolyploidy, succulent CAM species may have similar ‘design conflicts’. Genome size potentially represents a simple trade-off between selection for large cells with large vacuoles (to store water and the organic acids accumulated nocturnally) and that for small stomata (to restrict transpiration). However, metabolic adjustments resulting in predominantly nocturnal stomatal opening have reduced the importance of stomatal size as a regulator of water-use efficiency and even in arid habitats CAM species may have relatively large stomata.
C4 species generally have smaller stomata reflecting daytime opening in arid habitats (Fig. 3), but the importance of size, both here and in C3 species, may be similarly complicated by their mode of function: e.g. the ‘dumb-bell’ shape of stomata in Poaceae is a reflection of a mechanism by which subsidiary cells also change turgor, effectively relaxing as guard cells inflate, offering less resistance to guard cell movement and allowing stomatal responses an order of magnitude faster than species with ‘kidney’ type stomata (Franks and Farquhar, 2007).
Nonetheless, there remains a fundamental requirement within the angiosperms for efficient stomatal conduction, and size undoubtedly plays a large part in this (‘throughout biology size matters’; Cavalier-Smith, 2005). Genome size could potentially be an ultimate consequence of stomatal size simply because guard cell osmoregulation is dependent on endogenous protein synthesis (Thimann and Tan, 1988), particularly the enzymes of the malate synthesis pathway that regulate the accumulation of osmotica (Lawlor, 1993). Larger guard cells require more of this metabolic machinery and more copies of the ‘rDNA’ gene sequences coding for ribosomes (and thus greater protein assembly capacity) are indeed associated with larger eukaryotic genomes (Prokopowich et al., 2003). Cytoplasmic protein synthesis must be supported by sufficient nuclear RNA synthesis, resulting in a universal ‘karyoplasmic ratio’ whereby cytoplasmic volume determines nuclear volume (Cavalier-Smith, 2005). Larger nuclear envelopes are thought to require the physical support of larger amounts of non-genic skeletal DNA, ultimately imposing greater genome size (Cavalier-Smith, 2005). Conversely, extensive protein synthesis would be redundant and uneconomic for small guard cells, selecting for smaller genomes. Thus size constraints to stomatal function may favour smaller genomes. Minimum genome size in angiosperms may be expected to relate to the smallest dimensions of a guard cell at which stomata operate efficiently, potentially when the collision of gas molecules with the guard cell walls dominates diffusion through the aperture (such ‘Knudsen diffusion’ becomes important for apertures <0·5–1 µm; Leuning, 1983). Similarly, maximum genome size should be constrained by the speed of opening and closure of very large stomata.
Do such limits operate in practice? Unfortunately, it is not possible to be sure. For example, there are discrepancies between our own observations and established ideas on the physiological and ecological significance of large stomata. It had been suggested that very large stomata facilitate photosynthesis in deep shade (Hetherington and Woodward, 2003). In this study, very large stomata were primarily associated with vernal geophytes, which exploit either unshaded habitats or the ‘light phase’ of deciduous woodland before closure of the canopy rather than with shade-tolerant, summer-green woodland herbs (Table 2Bii). A revised assessment of where and why large stomata occur is urgently required and, critically, it needs to take into account the findings of Grime and Mowforth (1982) – see above.
There is also uncertainty with respect to small stomata. It has been convincingly argued that the requirement for water-use efficiency has been an important ecological driver leading to the miniaturization of stomata (Aasamaa et al., 2001; Hetherington and Woodward, 2003) and the data in Table 2Bi further support this view. How small can efficiently functioning stomata be? There is the discovery by Greilhuber et al. (2006) that a few angiosperms have exceptionally small genomes. Previously, Arabidopsis thaliana was thought to have one of the smallest genome among angiosperms. Now, it is known that Genlisea margaretae and a few similar carnivorous species in Lentibulariaceae have a genome less than half this size. Much of the leaf of Genlisea takes the form of a below-ground trap, but the upper portion is green and photosynthetic. Are the stomata significantly smaller than those of other angiosperms? Do they function efficiently and provide sufficient photosynthate to support the whole plant? To date, heterotrophic carbon nutrition has not been unequivocally demonstrated for carnivorous plants, although it has been suggested that such a mechanism could exist for Drosera, and that the provenance of the carbon should be evident from stable isotope (13C) signatures (e.g. Millet et al., 2003).
A more adequate description is urgently needed of the ecological circumstances under which very large and very small stomata occur and a more exact definition of the lower and the upper size limit for efficiently functioning stomata. Only then can our hypothesis that photosynthetic processes, in general, and stomatal size, in particular, are the ‘missing link’, the primary determinants of genome size in angiosperms and other vascular plants, be adequately tested.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
A considerable quantity of the data used in this project was collected during projects funded by respectively NERC (UK), the Research Institute of Forests and Rangelands (RIFR, Iran), Universidad Nacional de Córdoba, Comisión Interministerial de Ciencia y Tecnología (Spain) and the Darwin Initiative for the Survival of Species (DEFRA, UK). We thank Jason Freidley, Ken Thompson and three anonymous referees for constructive criticism of an earlier version of this manuscript.
LITERATURE CITED
- Aasamaa K, Sober A, Rahi M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Australian Journal of Plant Physiology. 2001;28:765–774. [Google Scholar]
- Allen MT, Pearcy RW. Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia. 2000;122:470–478. doi: 10.1007/s004420050968. [DOI] [PubMed] [Google Scholar]
- APG III. An update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Aronne G, De Micco V. Seasonal dimorphism in the Mediterranean Cistus incanus. Annals of Botany. 2001;87:789–794. [Google Scholar]
- Barrow M, Meister A. Endopolyploidy in seed plants is differently correlated to systematic, organ, life strategy and genome size. Plant, Cell & Environment. 2003;26:571–584. [Google Scholar]
- Bateman RM, Hollingsworth PM, Preston J, Yi-Bo L, Pridgeon AM, Chase MW. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Botanical Journal of the Linnean Society. 2003;142:1–40. [Google Scholar]
- Beaulieu JM, Moles AT, Leitch IJ, Bennett MD, Dickie JB, Knight CA. Correlated evolution of genome size and seed mass. New Phytologist. 2007;173:422–437. doi: 10.1111/j.1469-8137.2006.01919.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytologist. 2008;179:975–986. doi: 10.1111/j.1469-8137.2008.02528.x. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Chaloner WG. Stomatal density as a measure of atmospheric CO2 concentration. The Holocene. 1993;2:71–78. [Google Scholar]
- Bennett MD. The duration of meiosis. Proceedings of the Royal Society of London, Series B. 1971;178:277–299. [Google Scholar]
- Bennett MD. Nuclear DNA content and minimum generation time in herbaceous plants. Proceedings of the Royal Society of London, Series B. 1972;181:109–135. doi: 10.1098/rspb.1972.0042. [DOI] [PubMed] [Google Scholar]
- Bennett MD. DNA amount, latitude, and crop plant distribution. Environmental and Experimental Botany. 1976;5:93–108. [Google Scholar]
- Bennett MD, Leitch IJ. Plant DNA C-values database (release 4·0, October 2005) 2005. http://www.kew.org/cval/homepage.html .
- Bennettzen J, Ma J, Devos KM. Mechanisms of recent genome size variation in flowering plants. Annals of Botany. 2005;95:127–132. doi: 10.1093/aob/mci008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhàr-Nordenkampf HR, Draxler G. Functional leaf anatomy. In: Hall DO, Scurlock JMO, Bolhàr-Nordenkampf HR, Leegood RC, Long SP., editors. Photosynthesis and production in a changing environment: a field and laboratory manual. London: Chapman and Hall; 1993. pp. 91–112. [Google Scholar]
- Braun Blanquet J, de Bolós O. La comunidades vegetales de la Depresion del Ebro y su dinamismo. Anales de la Estación Experimental de Aula Dei. 1953;5 [Google Scholar]
- Caetano-Anollés G. Evolution of genome size in grasses. Crop Science. 2005;45:1809–1816. [Google Scholar]
- Cavalier-Smith T. The evolution of genome size. Chichester: John Wiley & Sons; 1985. [Google Scholar]
- Cavalier-Smith T. Economy, speed and size matter: evolutionary forces driving nuclear genome miniaturisation and expansion. Annals of Botany. 2005;95:147–175. doi: 10.1093/aob/mci010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M, Jones G, Hodgson JG. FIBS in archaeobotany: functional interpretation of weed floras in relation to husbandary practices. Journal of Archaeological Science. 1997;24:1151–1161. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardized and easy measurement of plant functionl traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Darlington CD. Cytology. London: Churchill; 1965. [Google Scholar]
- Davidson EH, Britten RJ. Organisation, transcription and regulation in the animal genome. Quarterly Review of Biology. 1973;48:565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Díaz S, Hodgson JG, Thompson K, et al. The plant traits that drive ecosystems: evidence from three continents. Journal of Vegetation Science. 2004;15:295–304. [Google Scholar]
- Doolittle WF, Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980;284:601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Dynamic Data. Aequitas IDA: Image Database and Image Archive Management System. Cambridge: Dynamic Data Links; 1993–1996. ver. 1·5x. [Google Scholar]
- Edwards GA, Endrizzi JL. Cell size nuclear size and DNA content relationships in Gossypium. Canadian Journal of Genetics and Cytology. 1975;17:181–186. [Google Scholar]
- Federov A, editor. Chromosome numbers in flowering plants. Leningrad: Academy of Sciences, USSR; 1969. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Franks PJ, Farquhar GD. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiology. 2007;143:78–87. doi: 10.1104/pp.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker MD, White N. Volume measurements of guard cell vacuoles during stomatal movements using confocal microscopy. Transactions of the Royal Microscopical Society. 1990;1:345–358. [Google Scholar]
- Garnier E, Laurent G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytologist. 1994;128:725–736. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001;15:688–695. [Google Scholar]
- Givnish TJ. Adaptation to sun and shade: a whole-plant perspective. Australian Journal of Plant Physiology. 1988;15:63–92. [Google Scholar]
- Gornall RJ, Bailey JP. Cytological catalogue of the British & Irish flora. 1998. BSBI database, http://rbg-web2.rbge.org.uk/BSBI/ . [Google Scholar]
- Gregory TR, Nicol JA, Tamm H, et al. Eukaryotic genome size databases. Nucleic Acid Research. 2007;35:D332–338. doi: 10.1093/nar/gkl828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Borsch T, Mueller K, Worberg A, Porembski S, Barthlott W. Smallest angiosperm genomes found in Lentibulariaceae, with chromosomes of bacterial size. Plant Biology. 2006;8:770–777. doi: 10.1055/s-2006-924101. [DOI] [PubMed] [Google Scholar]
- Grime JP, Hunt R. Relative growth-rate: its range and adaptive significance in a local flora. Journal of Ecology. 1975;63:393–422. [Google Scholar]
- Grime JP, Mowforth MA. Variation in genome size: an ecological interpretation. Nature. 1982;5:151–153. [Google Scholar]
- Grime JP, Shacklock JML, Band SR. Nuclear DNA contents, shoot phenology and species co-existence in a limestone grassland community. New Phytologist. 1985;100:435–445. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. Comparative plant ecology. 2nd edn. Dalbeattie: Castlepoint Press; 2007. [Google Scholar]
- Haston E, Richardson JE, Stevens PF, Chase MW, Harris DJ. A linear sequence of Angiosperm Phylogeny Group II families. Taxon. 2007;56:7–12. [Google Scholar]
- Haston E, Richardson JE, Stevens PF, Chase MW, Harris D. The Linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III. Botanical Journal of the Linnean Society. 2009;161:128–131. [Google Scholar]
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Mackey JML. The ecological specialization of dicotyledonous families within a local flora: some factors contraining optimization of seed size and their possible evolutionary significance. New Phytologist. 1986;104:479–515. [Google Scholar]
- Joachimiak A, Grabowska-Joachimiak A. Stomatal cell length and ploidy level in four taxa belonging to the Phleum sect. Phleum. Acta Biologica Cracoviensia Series Botanica. 2000;42:103–107. [Google Scholar]
- Johansen DA. Plant embryology. Waltham, MA: Chronica Botanica; 1950. [Google Scholar]
- Johnston JS, Pepper AE, Hall AE, et al. Evolution of genome size in Brassicaceae. Annals of Botany. 2005;95:229–235. doi: 10.1093/aob/mci016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Brown LM. Chromosome evolution and DNA variation in Crepis. Heredity. 1976;36:91–104. [Google Scholar]
- Jovtchev G, Schuber V, Meister A, Barrow M, Schubert I. Nuclear DNA content and nuclear and cell volume are positively correlated in angiosperms. Cytogenetic and Genome Research. 2006;114:77–82. doi: 10.1159/000091932. [DOI] [PubMed] [Google Scholar]
- Kidwell MJ. Transposable elements and the evolution of genome size in eukaryotes. Genetica. 2002;115:49–63. doi: 10.1023/a:1016072014259. [DOI] [PubMed] [Google Scholar]
- Kirik A, Salomon S, Puchta H. Species-specific double-strand break repair and genome evolution in plants. EMBO Journal. 2000;19:5562–5566. doi: 10.1093/emboj/19.20.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CA, Ackerly DD. Variation in nuclear DNA content across environmental gradients: a quantile regression analysis. Ecology Letters: 2002;5:66–76. [Google Scholar]
- Knight CA, Molinari NA, Petrov DA. The large genome constraint hypothesis: evolution, ecology and phenotype. Annals of Botany. 2005;95:177–190. doi: 10.1093/aob/mci011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW. Photosynthesis: molecular, physiological and environmental processes. 2nd edn. Harlow: Longman Scientific and Technical; 1993. [Google Scholar]
- Lechowicz MJ. Why do temperate deciduous trees leaf out at different times: adaptation and ecology of forest communities? American Naturalist. 1984;124:821–842. [Google Scholar]
- Leitch IJ, Chase MW, Bennett MD. Phylogenetic analysis of DNA C-values provides evidence for a small ancestral genome size in flowering plants. Annals of Botany. 1998;82:85–94. (Suppl. A) [Google Scholar]
- Leitch IJ, Soltis DE, Soltis PS, Bennett MD. Evolution of DNA amounts across land plants (Embryophyta) Annals of Botany. 2005;95:207–217. doi: 10.1093/aob/mci014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuning R. Transport of gases into leaves. Plant, Cell & Environment. 1983;6:181–194. [Google Scholar]
- Maranon T, Grubb PJ. Physiological basis and ecological significance of the seed size and relative growth rate relationship in Mediterranean annuals. Functional Ecology. 1993;7:591–599. [Google Scholar]
- Martin AC. The comparative internal anatomy of seeds. American Midland Naturalist. 1947;36:513–660. [Google Scholar]
- Masterson J. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science. 1994;264:421–424. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- Merkel T, Gal L, Semrau S, Homan U, Thiel G. Guard cells elongate: relationship of volume and surface area during stomatal movement. Biophysical Journal. 2007;92:1072–1080. doi: 10.1529/biophysj.106.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett J, Jones RI, Waldron S. The contribution of insect prey to the total nitrogen content of sundews (Drosera spp.) determined in situ by stable isotope analysis. New Phytologist. 2003;158:527–534. doi: 10.1046/j.1469-8137.2003.00763.x. [DOI] [PubMed] [Google Scholar]
- Orel N, Kyryk A, Puchta H. Different pathways of homologous recombination are used in the repair of double-strand breaks within tandemly arranged sequences in the plant genome. The Plant Journal. 2003;35:604–612. doi: 10.1046/j.1365-313x.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- Orgel LE, Crick FH. Selfish DNA: the ultimate parasite. Nature. 1980;284:604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Prokopowich CD, Gregory TR, Crease TJ. The correlation between rDNA copy number and genome size in eukaryotes. Genome. 2003;46:48–50. doi: 10.1139/g02-103. [DOI] [PubMed] [Google Scholar]
- Raven JA. The minimum size of seeds and spores in relation to the ontogeny of homoiohydric plants. Functional Ecology. 1999;13:5–14. [Google Scholar]
- Raven JA. Selection pressures on stomatal evolution. New Phytologist. 2002;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Raven PH. The bases of angiosperm phylogeny: cytology. Annals of the Missouri Botanical Garden. 1975;62:724–764. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf, life-span in relation to leaf plant and stand characteristics among diverse ecosystems. Ecological Monographs. 1992;62:365–392. [Google Scholar]
- Soltis DE, Soltis PS, Bennett MD, Leitch IJ. Evolution of genome size in the angiosperms. American Journal of Botany. 2003;89:1670–1681. doi: 10.3732/ajb.90.11.1596. [DOI] [PubMed] [Google Scholar]
- Speckman GJ, Post J, Dijkstra H. Length of stomata as an indicator for polyploidy in rye-grasses. Euphytica. 1965;14:225–228. [Google Scholar]
- Sugiyama S. Developmental basis of interspecific differences in leaf size and specific leaf area among C3 grass species. Functional Ecology. 2005;19:916–924. [Google Scholar]
- Tanaka Y, Kutsana N, Kanzawa Y, Kondo N, Hasezawa S, Sano T. Intra-vacuolar reserves of membranes during stomatal closure: the possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant and Cell Physiology. 2007;48:1159–1169. doi: 10.1093/pcp/pcm085. [DOI] [PubMed] [Google Scholar]
- Thimann KV, Tan Z-Y. The dependence of stomatal closure on protein synthesis. Plant Physiology. 1988;86:341–343. doi: 10.1104/pp.86.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. Genome size, seed size and germination temperature in herbaceous angiosperms. Evolutionary Trends in Plants. 1990;4:113–116. [Google Scholar]
- Vendramini F, Díaz S, Gurvich DE, Wilson PJ, Thompson K, Hodgson JG. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytologist. 2002;154:147–157. [Google Scholar]
- Vinogradov AE. Selfish DNA is maladaptive: evidence from the plant Red List. Trends in Genetics. 2003;19:609–614. doi: 10.1016/j.tig.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker MD. Stomata. London: Chapman and Hall; 1995. [Google Scholar]
- Woodward FI. Do plants really need stomata? Journal of Experimental Botany. 1998;49:471–480. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.