Abstract
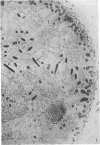
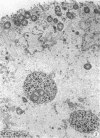
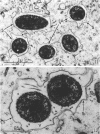
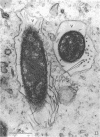
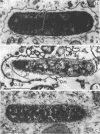
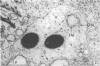
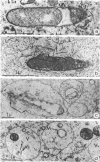
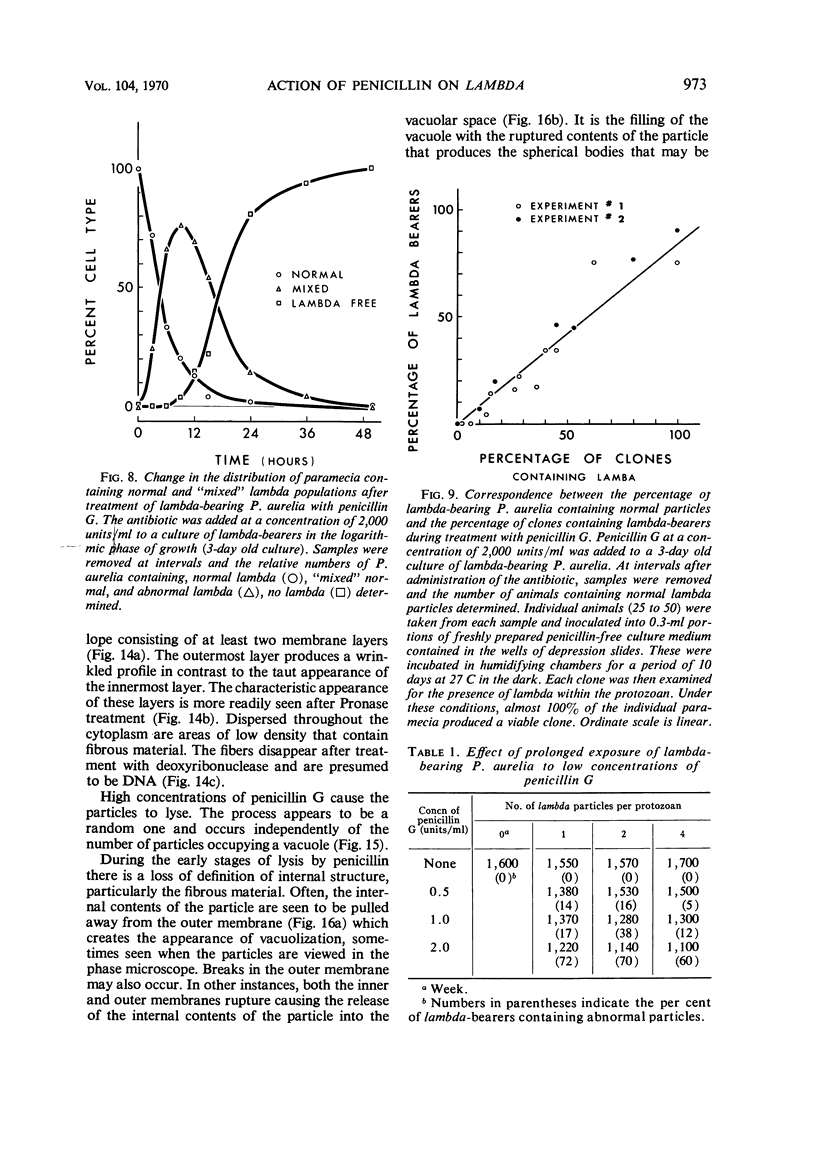
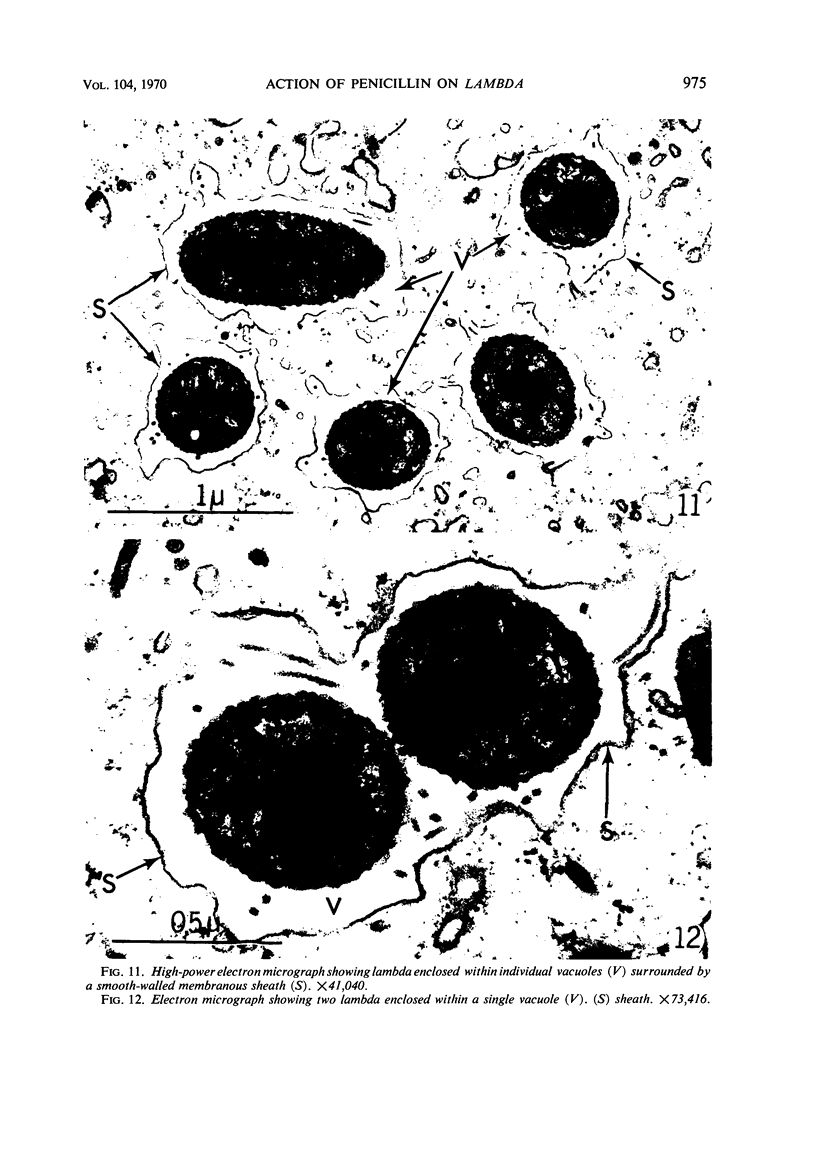
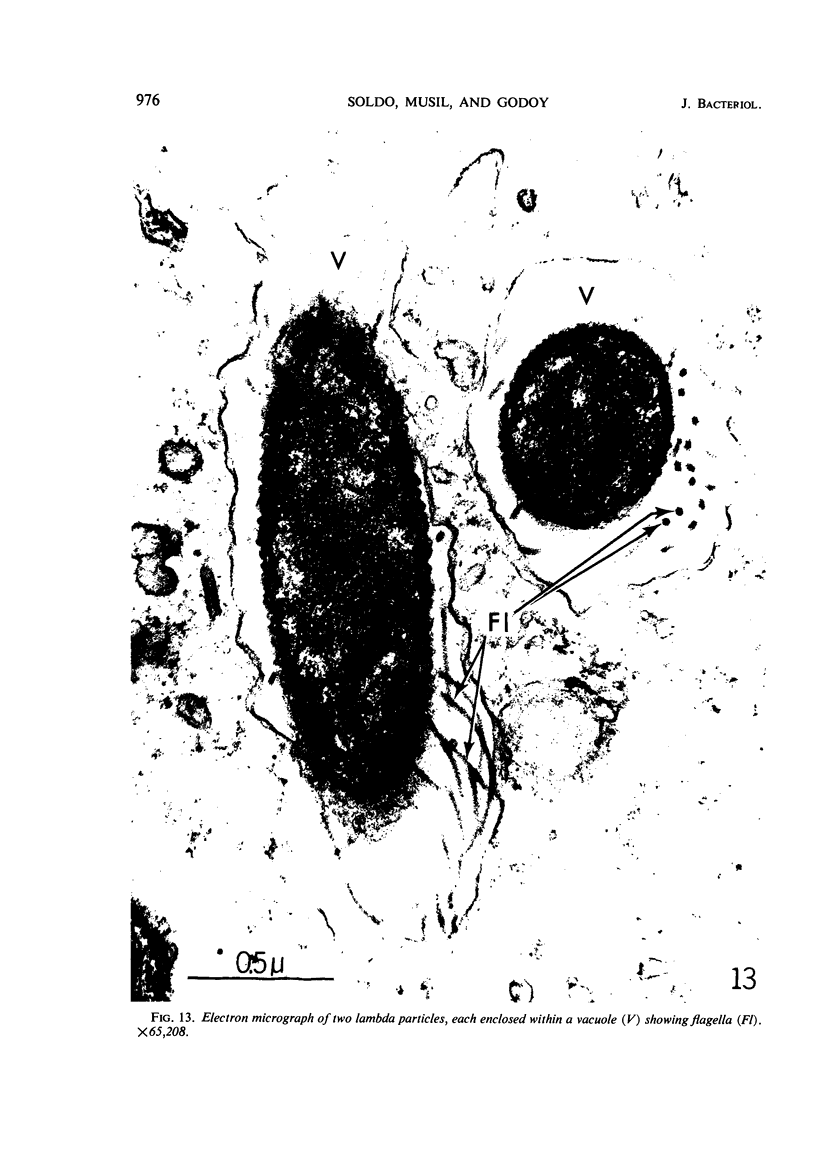
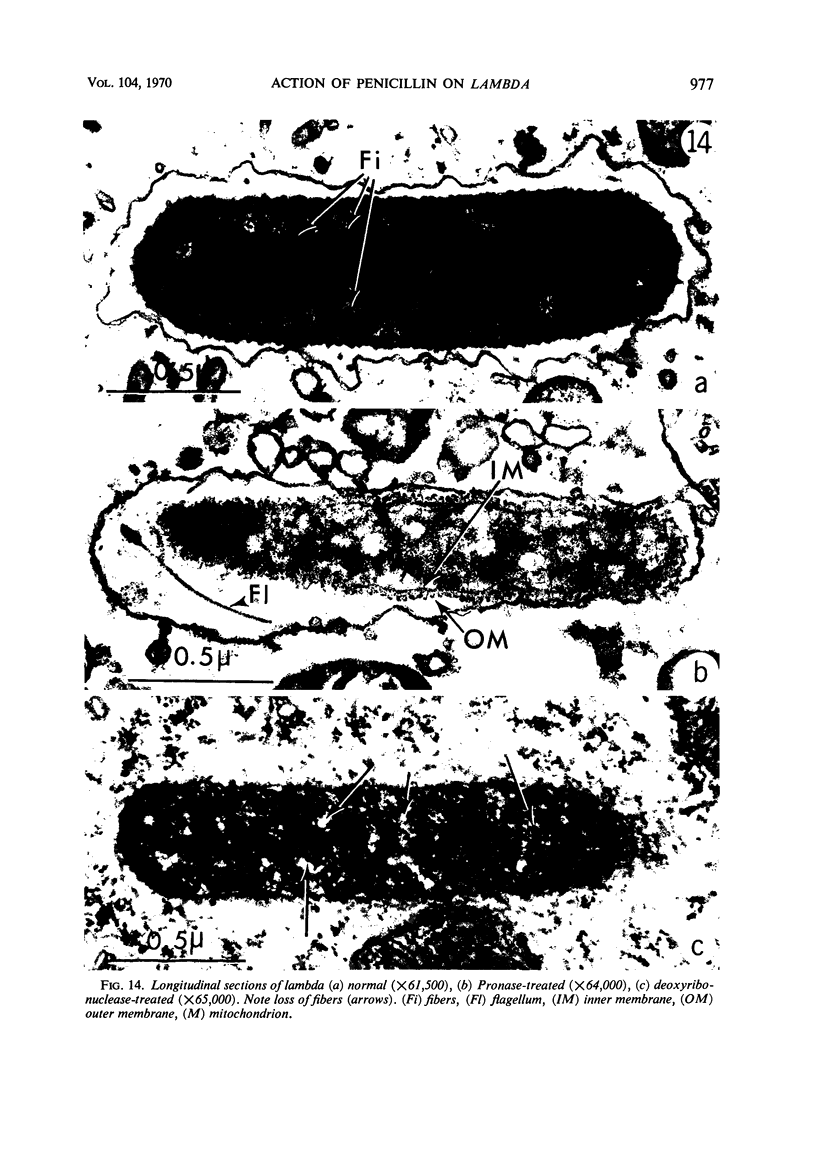
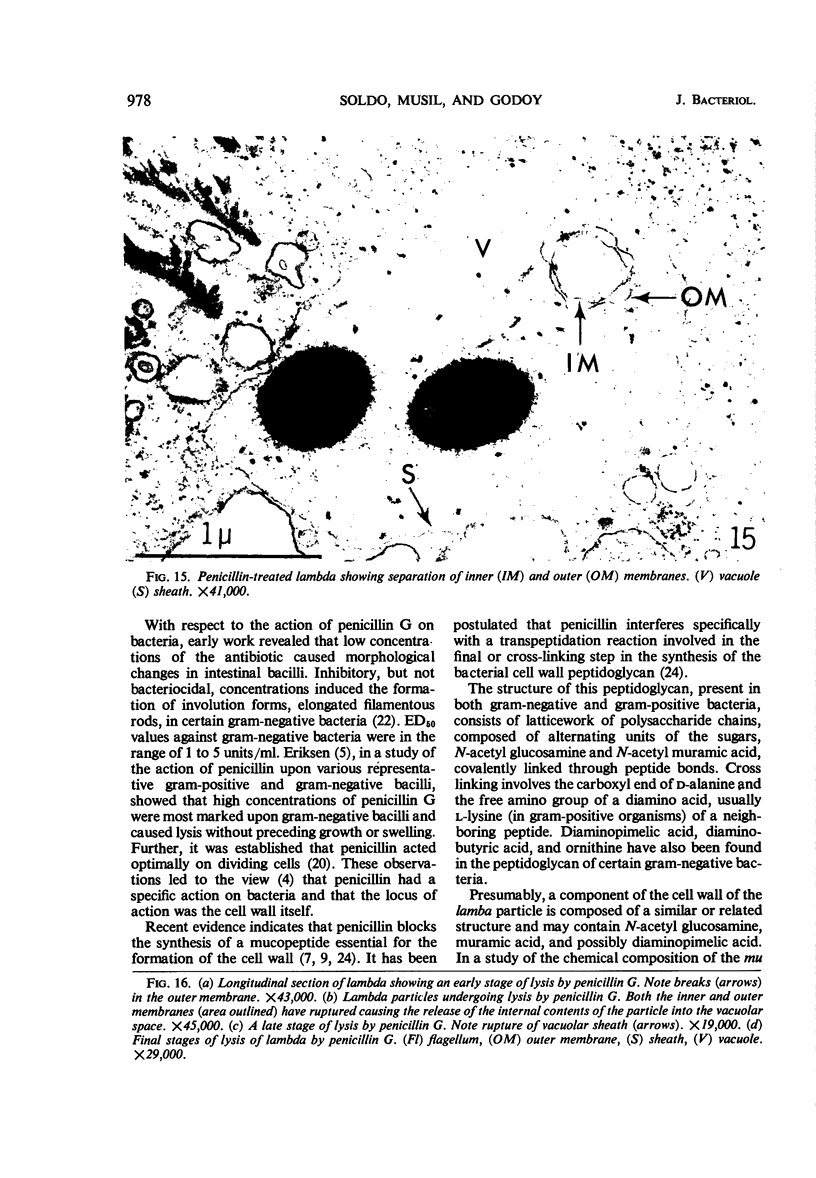
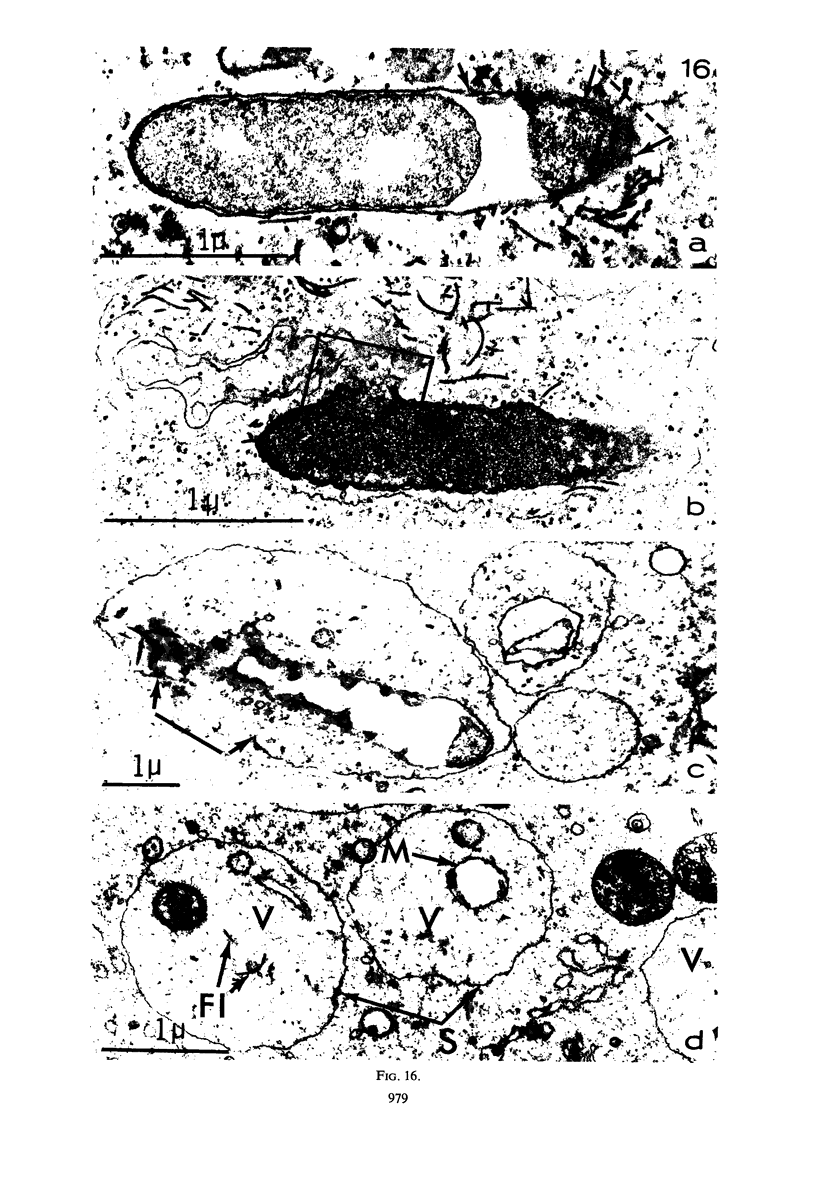
The kinetics of loss from the cytoplasm and changes in ultrastructure of symbiont lambda particles after treatment of axenically cultivated lambda-bearing Paramecium aurelia with penicillin G was investigated. Low concentrations (1 to 2 unit/ml) of the antibiotic caused many particles within the cell to become filamentous; high concentrations (2,000 unit/ml) caused lysis of the particles without noticeably affecting the protozoan. The ED50 value (2 to 3 unit/ml) was within the range of values found to cause lysis of many gram-negative bacteria. Rapidly dividing lambda were more vulnerable to the action of the antibiotic than slowly dividing particles. Nondividing particles were not affected by exposure to the antibiotic. Ultrastructural changes observed in lambda during lysis by penicillin G were consistent with the view that penicillin interferes with the synthesis of a vital component of the cell envelope of the particle, possibly a peptidoglycan similar to that found in the cell walls of bacteria. The deoxyribonucleic acid of lambda was dispersed throughout the particle as electron dense fibers enclosed within electron transparent areas. The cell envelope appeared to consist of at least two morphologically distinguishable layers, an inner layer homologous to the plasma membrane of bacteria and an outer layer homologous to the bacterial cell wall. Lambda may be regarded as a randomly distributed population of bacteria growing and dividing synchronously within the collective cytoplasm of its protozoan host.
Full text
PDF

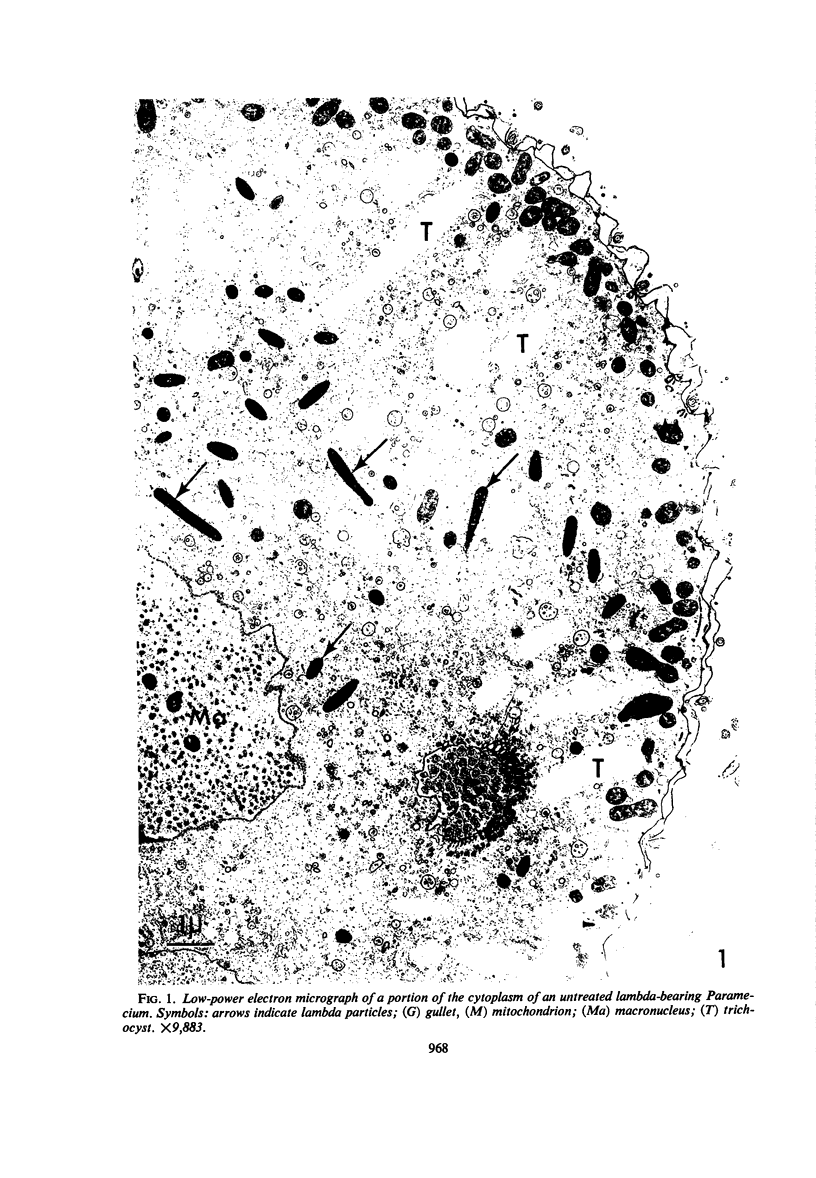
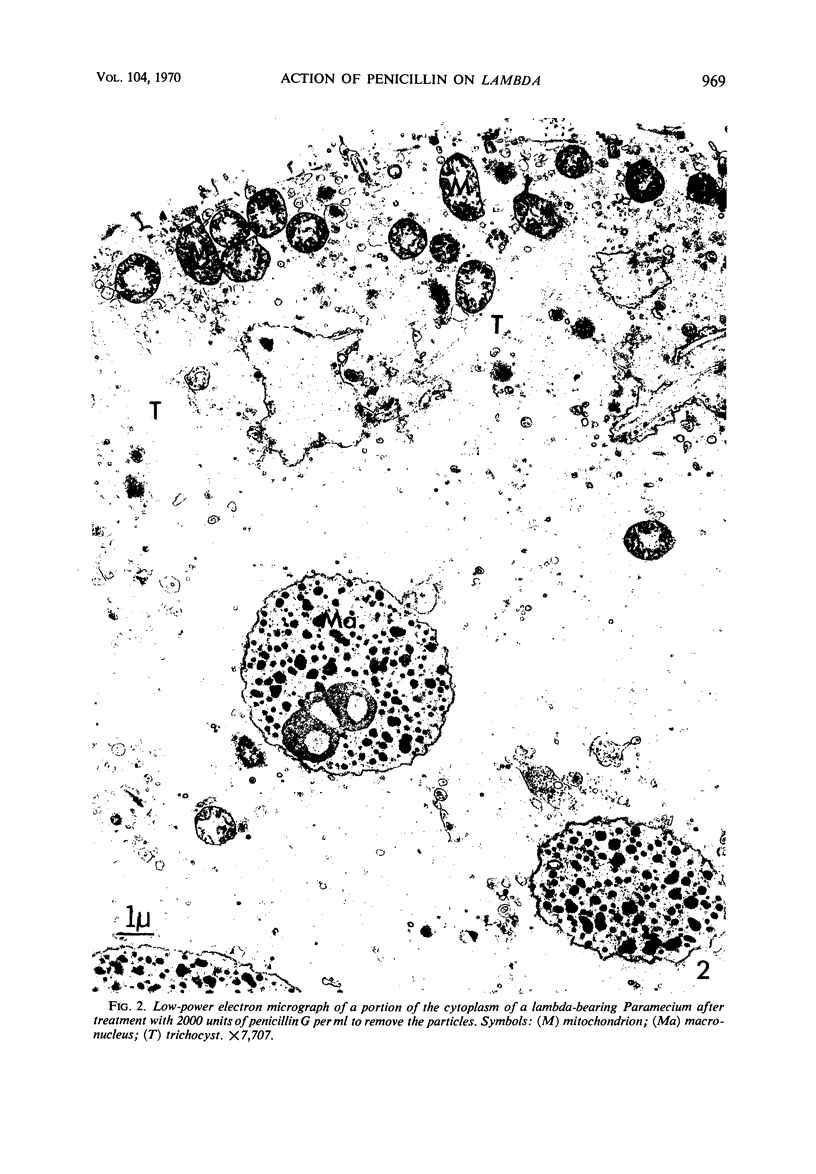
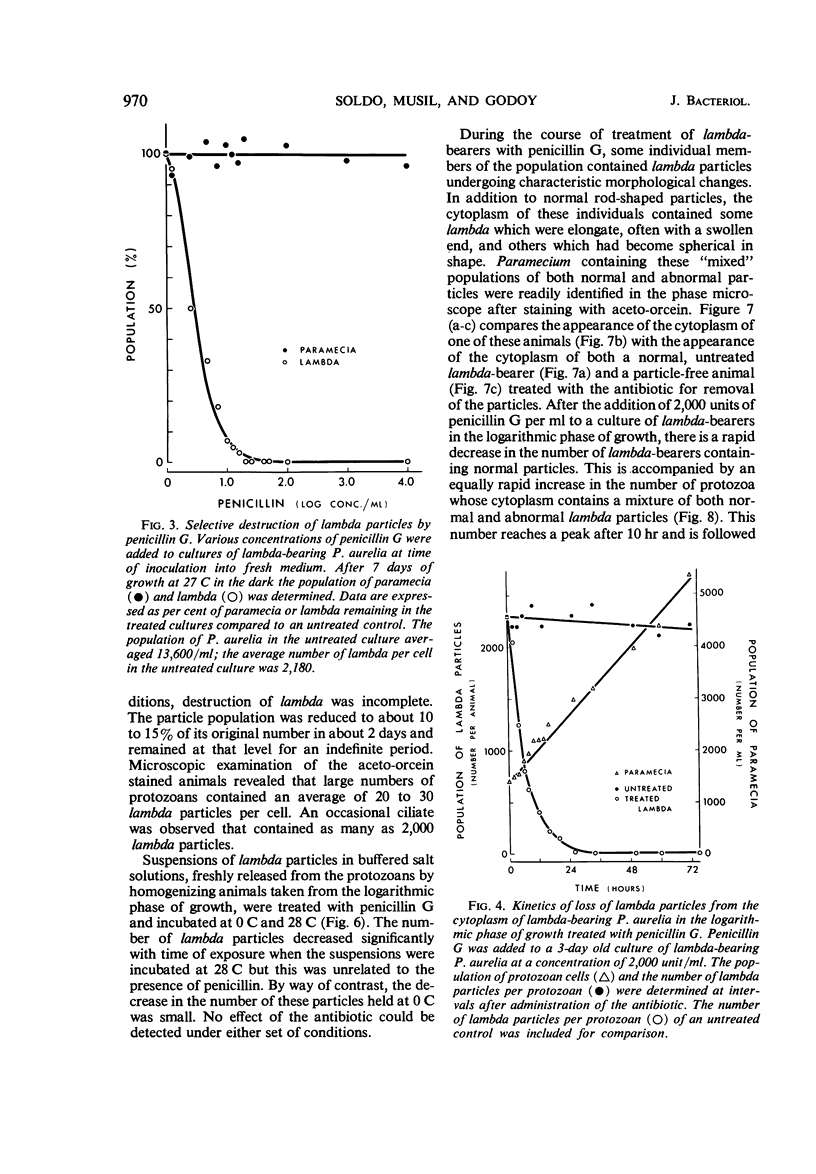
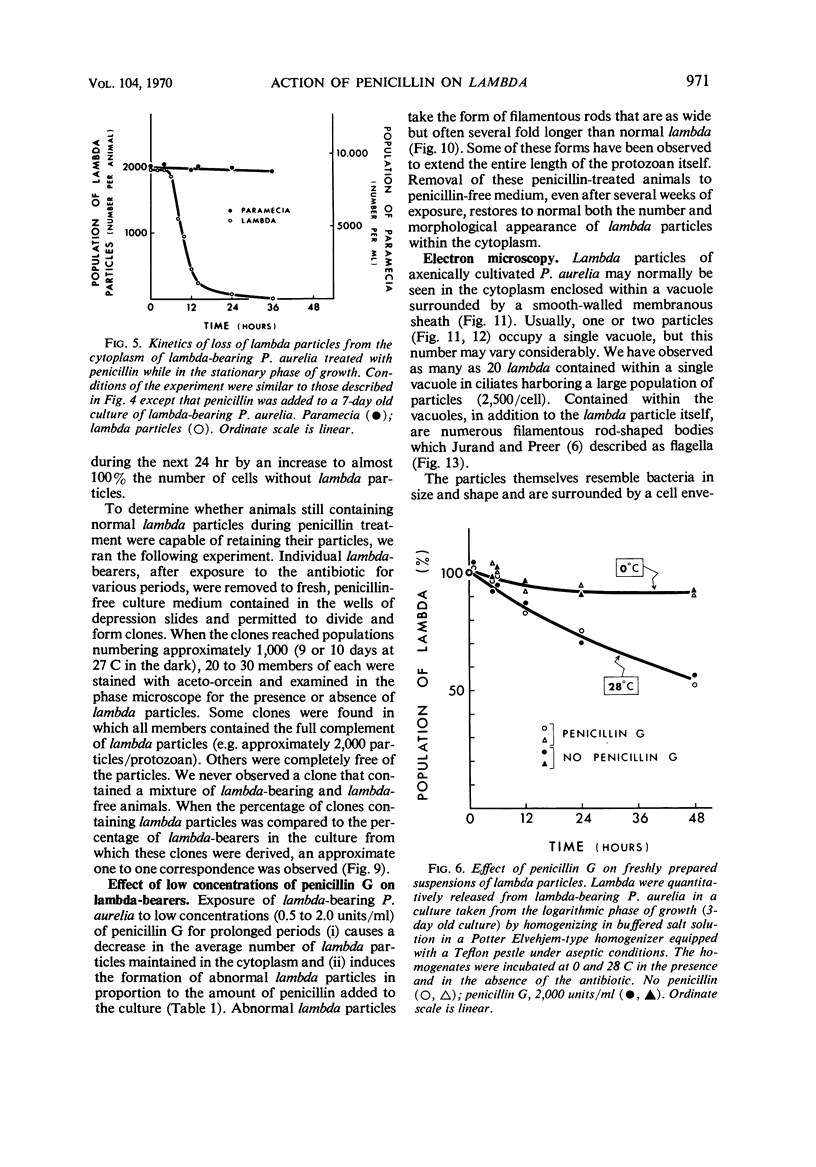



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEALE G. H., JURAND A. Structure of the mate-killer (mu) particles in Paramecium aurelia, stock 540. J Gen Microbiol. 1960 Oct;23:243–252. doi: 10.1099/00221287-23-2-243. [DOI] [PubMed] [Google Scholar]
- Beale G. N., Jurand A., Preer J. R. The classes of endosymbiont of Paramecium aurelia. J Cell Sci. 1969 Jul;5(1):65–91. doi: 10.1242/jcs.5.1.65. [DOI] [PubMed] [Google Scholar]
- Jurand A., Preer L. B. Ultrastructure of flagellated lambda symbionts in Paramecium aurelia. J Gen Microbiol. 1968 Dec;54(3):359–364. doi: 10.1099/00221287-54-3-359. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959 Aug;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREER J. R., Jr Microscopically visible bodies in the cytoplasm of the "killer" strains of Paramecium aurelia. Genetics. 1950 May;35(3):344–362. doi: 10.1093/genetics/35.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH-SONNEBORN J. E., VANWAGTENDONK W. J. PURIFICATION AND CHEMICAL CHARACTERIZATION OF KAPPA OF STOCK 51, PARAMECIUM AURELIA. Exp Cell Res. 1964 Jan;33:50–59. doi: 10.1016/s0014-4827(64)81011-9. [DOI] [PubMed] [Google Scholar]
- SOLDO A. T. Axenic culture of Paramecium-some observations on the growth behavior and nutritional requirements of a particle-bearing strain of Paramecium aurelia 299 lambda. Ann N Y Acad Sci. 1963 Jun 29;108:380–388. doi: 10.1111/j.1749-6632.1963.tb13392.x. [DOI] [PubMed] [Google Scholar]
- Stevenson I. Diaminopimelic acid in the Mu particles of Paramecium aurelia. Nature. 1967 Jul 22;215(5099):434–435. doi: 10.1038/215434a0. [DOI] [PubMed] [Google Scholar]
- Stevenson I. The biochemical status of mu particles in Paramecium aurelia. J Gen Microbiol. 1969 Jul;57(1):61–75. doi: 10.1099/00221287-57-1-61. [DOI] [PubMed] [Google Scholar]
- Thomas A. R., Levine M. Some Effects of Penicillin on Intestinal Bacteria. J Bacteriol. 1945 Jun;49(6):623–627. doi: 10.1128/jb.49.6.623-627.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANWAGTENDONK W. J., CLARK J. A., GODOY G. A. THE BIOLOGICAL STATUS OF LAMBDA AND RELATED PARTICLES IN PARAMECIUM AURELIA. Proc Natl Acad Sci U S A. 1963 Nov;50:835–838. doi: 10.1073/pnas.50.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]