Abstract
Background and Aims
The role of fire as a germination cue for Mediterranean Basin (MB) plants is still unclear. The current idea is that heat stimulates germination mainly in Cistaceae and Fabaceae and that smoke has a limited role as a post-fire germination cue, in comparison with other Mediterranean-type ecosystems (MTEs), suggesting that fire-stimulated germination is less relevant in the MB than in other MTEs. However, recent studies showed that the assembly of Mediterranean plant communities is strongly driven by post-fire germination, suggesting an important role for fire as a germination cue. We hypothesize that both heat and smoke have important effects on the different post-fire recruitment processes of MB species (e.g. level and rate of germination and initial seedling growth).
Methods
To ascertain the role of heat and smoke in the post-fire germination response of MB woody plants, a germination experiment was performed with seven heat and two smoke treatments on 30 MB woody species from seven different families, including species with water-permeable seeds and species with water-impermeable seeds.
Key Results
Heat stimulated the germination (probability and rate) of 21 species and smoke in eight species, out of the 30 species studied. In addition, six species showed enhanced initial seedling growth after the smoke treatments.
Conclusions
The results suggest that both heat and smoke are important germination cues in a wide range of MB woody species and that fire-cued germination in woody plants of the MB may be as important as in other MTEs.
Keywords: Post-fire germination, heat treatments, Mediterranean Basin, smoke treatments, seedling growth, Cistaceae, Fabaceae, Ericaceae, Lamiaceae, Linaceae, Scrophulariaceae, Primulaceae
INTRODUCTION
Fire-stimulated germination is found in many species from Mediterranean fire-prone ecosystems (Keeley, 1995). Post-fire germination may be triggered by different mechanisms, with heat and smoke being the main fire-related germination cues (Keeley and Fotheringham, 2000; van Staden et al., 2000). Heat shock can trigger germination by accelerating after-ripening in species with a water-permeable seed coat, or by rupturing the seed coat layer, allowing water uptake, in species with water-impermeable seeds (Baskin and Baskin, 1998). The smoke produced during fire may also stimulate germination and seedling growth (Blank and Young, 1998; Keeley and Fotheringham, 2000; Light et al., 2009). Smoke-stimulated germination has been attributed to the presence of butenolide molecules (karrikins; e.g. KAR1, 3-methyl-2H-furo[2,3-c]pyran-2-one), a family of structurally related plant growth regulators (Flematti et al., 2004; Nelson et al., 2009).
In the Mediterranean Basin (MB), heat-stimulated germination has been linked to the rupture of the seed coat in species with water-impermeable seeds, mostly Cistaceae and Fabaceae (González-Rabanal and Casal, 1995; Herranz et al., 1998, 1999; Paula and Pausas, 2008). The role of smoke is less clear as most studies performing germination experiments with smoke or charred wood on MB species suggest that smoke has a limited role as a post-fire germination cue (Keeley and Baer-Keeley, 1999; Buhk and Hensen, 2006; Reyes and Casal, 2006; Rivas et al., 2006; Reyes and Trabaud, 2009). However, a few studies have found some evidence of smoke-stimulated germination in MB woody plants, both in species with water-permeable seeds and in species typically having a high percentage of water-impermeable seeds (i.e. Cistaceae and Fabaceae; Pérez-Fernández and Rodríguez-Echevarría, 2003; Crosti et al., 2006). Smoke cannot stimulate germination of seeds that are physically dormant; therefore, smoke stimulation (i.e. increased germination percentage, germination rate or seedling growth) in species with water-impermeable seeds is likely to occur only if seeds are released from physical dormancy (become water permeable) by suitable environmental factors or if smoke acts over the fraction of water-permeable seeds, which in some cases (species or populations) may be high (Thanos et al., 1992).
This limited experimental evidence of fire-cued germination in the MB flora, particularly with regard to smoke, suggests that fire-stimulated germination is less relevant in the MB than in other Mediterranean-type ecosystems (MTEs). Indeed, it has been postulated that smoke-stimulated germination may be an example of the lack of convergent evolution between the MB and other MTEs (Keeley and Baer-Keeley, 1999). On the other hand, recent studies suggest that fire-stimulated germination is of paramount importance for understanding the evolution of MB flora (Pausas and Verdú, 2005) and the assembly processes of MB communities (Verdú and Pausas, 2007; Pausas and Verdú, 2008; Coca and Pausas, 2009). We suggest that our understanding of the role of fire in the recruitment process of MB species is limited by the availability of well-designed experiments, and we hypothesize that both heat and smoke play an important role in the different post-fire recruitment processes (e.g. level and rate of germination and initial seedling growth) of MB species.
To ascertain the role of fire in the post-fire recruitment processes of MB woody plants, a germination experiment was conducted with 30 MB woody species typical of Mediterranean shrublands, in which the same treatments were applied to each species, using a replicated design and subsequently testing the post-treatment seed viability. This experiment included a range of species, from different families, showing evidence of post-fire germination; it also included species both with and without water-impermeable seeds. This experiment allowed an accurate test of the effect of both heat and smoke on the different post-fire recruitment processes (i.e. seed resistance to heat shock, germination and seedling growth stimulation).
MATERIALS AND METHODS
Species and seed collection
Thirty woody species were selected (Table 1), including both shrubs (nanophanerophytes) and scrubs (chamaephytes), occurring in fire-prone areas of the SE Iberian Peninsula in which there was evidence of post-fire seedling emergence (Paula et al., 2009). All seeds were collected from ripe fruits in wild populations during 2007, coinciding with the dispersal period of each species, with the sole exception of the seeds of Genista umbellata, which were bought from an accredited commercial collector and collected from wild populations in June 2006. Seeds of each species (except G. umbellata) were collected from a minimum of 30 individuals (spatially dispersed) of the same population. Seeds were separated from the fruit tissues that would normally be lost during dispersal and cleaned of unwanted material such as fruit residues. Seeds were then stored in paper bags at controlled temperature (20 °C) and low humidity (40–50 %) until the start of the experiment, in November 2007.
Table 1.
List of tested species, their family, seed coat type (P, water-permeable, I, water-impermeable; based on the permeability tests described in Materials and methods) and the mean germination percentage in control conditions and after the heat treatments
| Species | Family | Seed coat | Control | 80 °C 5 min | 80 °C 10 min | 100 °C 5 min | 100 °C 10 min | 120 °C 5 min | 120 °C 10 min | 150 °C 5 min |
|---|---|---|---|---|---|---|---|---|---|---|
| Anthyllis cytisoides | Fabaceae | I | 1 | 2ns | 2ns | 3ns | 4ns | 1ns | 4ns | 9** |
| Anthyllis lagascana | Fabaceae | I | 5 | 5ns | 3ns | 5ns | 8ns | 7ns | 6ns | 0** |
| Cistus albidus | Cistaceae | I | 11 | 21* | 55**** | 60**** | 55**** | 61**** | 18ns | 0**** |
| Cistus monspeliensis | Cistaceae | I | 11 | 19ns | 22* | 42**** | 50**** | 73**** | 48**** | 51**** |
| Coris monspeliensis | Primulaceae | P | 2 | 1ns | 1ns | 4ns | 3ns | 3ns | 7ns | 1ns |
| Coronilla minima | Fabaceae | I | 5 | 12ns | 8ns | 6ns | 20*** | 20** | 0** | 0** |
| Digitalis obscura | Scrophulariaceae | P | 90 | 86ns | 83ns | 92ns | 88ns | 94ns | 89ns | 58**** |
| Dorycnium pentaphyllum | Fabaceae | I | 12 | 11ns | 12ns | 9ns | 17ns | 20ns | 0**** | 2*** |
| Erica multiflora | Ericaceae | P | 81 | 83ns | 79ns | 80ns | 90* | 87ns | 87ns | 93*** |
| Erica terminalis | Ericaceae | P | 22 | 25ns | 30ns | 38* | 30ns | 31ns | 21ns | 46*** |
| Erica umbellata | Ericaceae | P | 0 | 0ns | 0ns | 0ns | 0ns | 0ns | 1ns | 1ns |
| Fumana ericoides | Cistaceae | I | 0 | 2ns | 9*** | 11**** | 17**** | 17**** | 18**** | 0ns |
| Fumana thymifolia | Cistaceae | I | 8 | 19* | 27*** | 43**** | 57**** | 58**** | 1** | 0**** |
| Genista scorpius | Fabaceae | I | 17 | 10ns | 17ns | 22ns | 24ns | 23ns | 4** | 1*** |
| Genista triacanthos | Fabaceae | I | 4 | 84**** | 86**** | 91**** | 86**** | 82**** | 91**** | 67**** |
| Genista umbellata | Fabaceae | P | 78 | 76ns | 72ns | 86ns | 86ns | 92** | 83ns | 34**** |
| Helianthemum syriacum | Cistaceae | I | 12 | 16ns | 13ns | 8ns | 6ns | 6ns | 0**** | 0**** |
| Lavandula latifolia | Lamiaceae | P | 13 | 20** | 18ns | 32**** | 25** | 30*** | 2** | 1*** |
| Lavandula stoechas | Lamiaceae | P | 10 | 21** | 31**** | 41**** | 68**** | 72**** | 12ns | 0**** |
| Linum suffruticosum | Linaceae | P | 98 | 100ns | 95ns | 95ns | 98ns | 97ns | 94ns | 88** |
| Ononis minutissima | Fabaceae | I | 2 | 1ns | 3ns | 3ns | 29**** | 21**** | 1ns | 3ns |
| Rosmarinus officinalis | Lamiaceae | P | 19 | 27ns | 37** | 46**** | 26ns | 35** | 28ns | 14ns |
| Sideritis angustifolia | Lamiaceae | P | 87 | 84ns | 82ns | 73* | 30**** | 11**** | 9**** | 1**** |
| Teucrium capitatum | Lamiaceae | P | 17 | 22ns | 39**** | 33** | 25ns | 18ns | 20ns | 1**** |
| Teucrium ronnigeri | Lamiaceae | P | 35 | 36ns | 48* | 52** | 25ns | 26ns | 18** | 1**** |
| Thymus piperella | Lamiaceae | P | 78 | 83ns | 81ns | 95**** | 81ns | 54**** | 62** | 22**** |
| Thymus vulgaris | Lamiaceae | P | 86 | 93ns | 91ns | 93ns | 87ns | 95* | 90ns | 70** |
| Ulex borgiae | Fabaceae | I | 5 | 62**** | 72**** | 58**** | 90**** | 89**** | 75**** | 0** |
| Ulex parviflorus | Fabaceae | I | 4 | 40**** | 67**** | 59**** | 83**** | 92**** | 66**** | 1ns |
| Xolantha tuberaria | Cistaceae | I | 1 | 5ns | 2ns | 8* | 44**** | 66**** | 63**** | 34**** |
The significance of the pairwise comparison of each treatment with its corresponding control is also included (ns, not significant; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001).
The studied species belong to seven different families; two of them (Cistaceae and Fabaceae) are typically characterized by having water-impermeable seeds (Baskin and Baskin, 1998). Nevertheless, some species of these families may have a high percentage of water-permeable seeds (Baskin et al., 2000) and thus to verify seed permeability ten seeds of each species were weighed (individually), they were imbibed in water for 24 h and then they were re-weighed. Seeds with a significant increment in weight (>20 %) were considered to be water permeable. For Fumana species, the presence of mucilage in the seeds invalidated this test and, thus, water permeability was evaluated through a cut test, as water uptake softened the seeds whereas water-impermeable seeds remained hard. Species with at least 20 % permeable seeds were considered to have water-permeable seeds despite belonging to families typically with water-impermeable seeds (Table 1).
Treatments
Before applying any treatment, the viability of each seed lot was tested by the cut test method using a sample of 50 intact seeds of each species. Empty seeds or seeds with symptoms of decay were considered non-viable. Seven heat and two smoke treatments, plus two controls, were then performed.
The heat treatments consisted of the following combinations of temperatures and exposure times (heat dose): ‘80 °C for 5 min’ (lowest heat dose), ‘80 °C for 10 min’, ‘100 °C for 5 min’, ‘100 °C for 10 min’, ‘120 °C for 5 min’, ‘120 °C for 10 min’ and ‘150 °C for 5 min’ (highest heat dose). A control (untreated seeds) was also included. The heat treatments simulate the range of temperatures and exposure times in the top layer of soil during a fire (Trabaud, 1979) and were carried out using an electric oven. For each heat treatment, we proceeded as follows: seeds were placed in aluminium dishes (one for each species), which were arranged randomly in the centre of the oven tray to avoid the extremes and thus minimize the variation in temperature, and the tray was quickly introduced into the oven. This operation was repeated four times for each treatment and species (four independent replicates). Air temperature inside the oven was monitored using a thermocouple and took between 30 and 65 s to reach the desired temperature. The seeds were then held at this temperature (±1 °C) for the corresponding treatment time (5 or 10 min).
Smoke treatments corresponded to two different dilutions of liquid smoke solution: ‘smoke 1 : 1’ and ‘smoke 1 : 10’. To eliminate any effect on the germination caused by the seeds being imbibed in a liquid for 24 h before sowing, we used a ‘water control’ (pH 5·53; seeds immersed in distilled water for 24 h before sowing) and not the dry control used for the heat treatments. Smoke treatments were not performed for Genista triacanthos due to the limited availability of seeds. To produce the smoke solution, dry leaves and thin twigs of Quercus coccifera were ground in an electric grinder. Four batches (replicates) were separately heated in an oven for 30 min at 198 °C (±1 °C) and processed as described in Jager et al. (1996a), to obtain the concentrated smoke solution (smoke 1 : 1; pH 4·78). For each replicate of the 1 : 1 smoke solution, a sample was taken to obtain four replicates of diluted smoke solution (smoke 1 : 10; pH 4·90). Seeds were then incubated in the respective smoke solution for 24 h.
After the respective treatments, seeds were sown in Petri dishes with agar (0·7 %) as substrate and incubated at 20 °C (±1 °C) in darkness, being exposed to light only during the monitoring periods (see below). Incubation was performed under dark conditions to better ensure homogeneous conditions, given the magnitude of the germination experiment (1320 Petri dishes). The number of seeds sown was adjusted based on the estimation of the viability of the lots for each species (see above), in order to have a minimum of 100 viable seeds per species and treatment, distributed in the four replicates. Seed germination was monitored every 2 d during the first 2 weeks and then once a week until the end of the experiment, 2 months later. At each count, the germinated seeds (those with radicle emergence >1 mm, determined under a magnifying lens) were scored and removed from the Petri dishes. At the end of the experiment, the initial number of seeds sown was corrected by discarding empty seeds and anomalous germinations detected during the experiment.
Post-treatment seed viability
At the end of the experiment, the viability of the non-germinated seeds was checked by using the tetrazolium chloride test. A small incision was made in the seed coat of each non-germinated seed in order to facilitate uptake of the stain. Seeds were incubated in a 1 % solution of 2,3,5-triphenyl tetrazolium chloride for 24 h at 30 °C in dark conditions (International Seed Testing Association, 1999). Seeds were then dissected to check for intact and not discoloured embryo, and classified as viable (most of the embryo stained) or not viable (embryo either barely stained or unstained). A preliminary test with tetrazolium demonstrated that in fresh seeds of Sideritis angustifolia, Lavandula latifolia and Lavandula stoechas, only the radicle became stained; thus, this was the criterion used to classify viability in these species. In the same preliminary test, it was also verified that seeds of Erica umbellata and Erica terminalis did not become stained with tetrazolium. Therefore, for these species, the viability after treatment was verified through the cut test, and their seeds were classified as viable or non-viable depending on the consistency of the seed.
Initial seedling growth
To test the effect of smoke on the initial seedling growth, germinated seeds from the smoke treatments and water control were transferred to 150 mm diameter Petri dishes with agar (1·5 %) as substrate and placed in a germination chamber at 20 °C with a 12/12 h photoperiod. Petri dishes were placed vertically so that seedlings were able to grow, and exposed to similar irradiances. During this period, seedlings were not exposed to smoke. After the seedlings had grown in these conditions for 7–10 d (depending on the species) a digital photo was taken. Shoot and root length was measured with an image analysis program (ImageJ: Rasband, 2005). Only seeds germinated in the first 2 weeks and with radicle emergence <5 mm were used. The number of seeds placed in each of the vertically disposed Petri dishes depended on the number of seeds that fulfilled these requisites. Seedlings that died before accomplishing the monitoring time (7–10 d) were excluded from the analysis.
Statistical analysis
At each count, for each replicate and in all species, seeds were classified as germinated or not germinated following the criterion mentioned above (radicle emergence). For each species, the final germination of each treatment was compared with its respective control using an analysis of deviance (GLM) and assuming a binomial error distribution. Based on the results of post-treatment viability, the seed mortality of each heat treatment was compared with the respective control, and it was ascertained whether mortality was significantly higher than the control for each treatment. Germination rate was calculated by comparing the time to 50 % germination (T50) for each heat treatment with the respective control. Due to the large number of pairwise comparisons in all these analyses (germination percentage, seed mortality and germination rate), the significant level considered was P <0·01, which is a less conservative criterion than the Bonferroni correction (Moran, 2003).
To test the effect of smoke on the initial seedling growth, the final length (shoot, root and total) of seedlings growing from smoke-treated seeds was compared with the final length of seedlings growing from water-treated seeds. Preliminary analysis showed no differences in initial growth between the two smoke treatments and thus they were considered together in the analysis. This analysis of seedling growth was not performed in species with low germination in the smoke treatments or in the distilled water control. Thus, only 18 species were analysed for seedling growth.
RESULTS
Germination percentage
Most species had low germination values (<20 %) in the control, suggesting a high degree of dormancy (Tables 1 and 2). However, some species such as Digitalis obscura, Erica multiflora, G. umbellata, Linum suffruticosum, S. angustifolia, Thymus piperella and Thymus vulgaris had high germination values (>75 %) in control conditions, suggesting low dormancy (Tables 1 and 2). Note that the only Fabaceae that had high germination in the control (G. umbellata) was the one in which we detected that the seeds were water permeable (Table 1).
Table 2.
Mean germination percentage, mean germination rate (T50 in days) and mean initial seedling growth (root + shoot length in mm) in the control conditions and after the smoke treatments
| Germination percentage |
Germination rate |
Total seedling growth |
||||||
|---|---|---|---|---|---|---|---|---|
| Species | Control | Smoke 1 : 1 | Smoke 1 : 10 | Control | Smoke 1 : 1 | Smoke 1 : 10 | Control | Smoke |
| Anthyllis cytisoides | 1 | 0ns | 2ns | 264 | – | 154ns | – | – |
| Anthyllis lagascana | 4 | 3ns | 2ns | 321 | 96ns | 69ns | – | – |
| Cistus albidus | 9 | 12ns | 6ns | 194 | 137ns | 136ns | 39·52 | 44·01ns |
| Cistus monspeliensis | 18 | 18ns | 14ns | 105 | 112ns | 303ns | 27·01 | 35·05ns |
| Coris monspeliensis | 9 | 43**** | 24** | 172 | 60**** | 113**** | 14·36 | 36·23* |
| Coronilla minima | 11 | 11ns | 12ns | 146 | 125ns | 140ns | 42·85 | 63·53* |
| Digitalis obscura | 94 | 89ns | 96ns | 6 | 6ns | 4* | 25·73 | 26·04ns |
| Dorycnium pentaphyllum | 9 | 7ns | 7ns | 216 | 129* | 162ns | 50·04 | 45·88ns |
| Erica multiflora | 82 | 94** | 83ns | 16 | 11** | 15ns | 21·63 | 20·70ns |
| Erica terminalis | 33 | 60**** | 53*** | 76 | 44*** | 54** | – | – |
| Erica umbellata | 2 | 56**** | 7* | 195 | 43*** | 177ns | – | – |
| Fumana ericoides | 0 | 2ns | 4* | – | 173 | 131 | – | – |
| Fumana thymifolia | 5 | 11ns | 11ns | 133 | 128ns | 114ns | – | – |
| Genista scorpius | 17 | 14ns | 17ns | 161 | 154ns | 169ns | 44·87 | 58·79ns |
| Genista umbellata | 70 | 74ns | 77ns | 21 | 15ns | 11ns | 44·51 | 55·69ns |
| Helianthemum syriacum | 8 | 8ns | 11ns | 189 | 232ns | 153ns | – | – |
| Lavandula latifolia | 29 | 59**** | 47** | 85 | 35** | 53* | 26·98 | 48·86*** |
| Lavandula stoechas | 50 | 100**** | 96**** | 47 | 4**** | 5**** | 33·22 | 34·72ns |
| Linum suffruticosum | 95 | 99ns | 98ns | 3 | 2ns | 2ns | 49·02 | 51·87ns |
| Ononis minutissima | 0 | 2ns | 3* | – | 132 | 496 | – | – |
| Rosmarinus officinalis | 26 | 40* | 42** | 88 | 70ns | 66ns | 43·88 | 36·24ns |
| Sideritis angustifolia | 85 | 90ns | 90ns | 5 | 6ns | 6ns | 47·36 | 54·34ns |
| Teucrium capitatum | 30 | 34ns | 30ns | 75 | 75ns | 87ns | 23·42 | 18·35ns |
| Teucrium ronnigeri | 42 | 55* | 58* | 60 | 46ns | 45ns | 14·88 | 26·07** |
| Thymus piperella | 96 | 95ns | 83** | 2 | 2ns | 2ns | 37·12 | 45·33** |
| Thymus vulgaris | 88 | 100**** | 96ns | 4 | 2** | 2** | 31·59 | 35·64* |
| Ulex borgiae | 9 | 3ns | 3* | 180 | 319ns | 170ns | – | – |
| Ulex parviflorus | 2 | 7ns | 2ns | 171 | 167ns | 170ns | – | – |
| Xolantha tuberaria | 4 | 6ns | 4ns | 462 | 89ns | 176ns | – | – |
The significance of the pairwise comparison of each treatment with its corresponding control is also included (ns, not significant; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001).
–, not available.
Twenty species were stimulated by at least one of the heat treatments (Table 1) and eight species were stimulated by at least one smoke treatment (Table 2). Fifteen species, mainly Cistaceae or Fabaceae, were stimulated by heat and not by smoke treatments (smoke was not tested in G. triacanthos). Three species were stimulated strictly by the smoke treatments and five species by both heat and smoke treatments. Thus, 23 of 30 species showed a significant (P < 0·01) increase in germination percentage as a result of at least one of the germination treatments studied (Tables 1 and 2). Smoke stimulation was observed in Ericaceae, Lamiaceae and Primulaceae; all species with water-permeable seeds. In some of the species showing low germination in the control (Anthyllis lagascana, Dorycnium pentaphyllum, Genista scorpius and Helianthemum syriacum), all treatments failed to trigger germination, which remained at low values. Germination inhibition by heat treatments was observed mainly for the highest heat doses (150 °C 5 min).
Germination rate
Both heat and smoke treatments affected the germination rate (Figure 1). Cistus albidus, Cistus monspeliensis, Fumana thymifolia, Xolantha tuberaria, Anthyllis cytisoides, G. triacanthos, Ononis minutissima, Ulex borgiae, Ulex parviflorus, Rosmarinus officinalis and Teucrium capitatum had significantly faster germination in the heat treatments than in the control (Table 3). Erica umbellata and T. vulgaris had significantly faster germination in the smoke treatments than in the control (Table 2). Coris monspeliensis, E. multiflora, E. terminalis, L. latifolia, L. stoechas and T. vulgaris had significantly faster germination in both smoke and heat treatments (Tables 2 and 3). Significantly slower germination, in relation to control, occurred mainly for the highest heat doses in D. obscura, G. umbellata, L. suffruticosum, S. angustifolia, T. vulgaris and T. piperella.
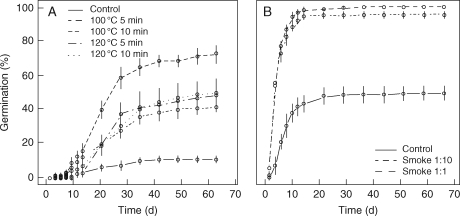
Fig. 1.
Germination percentage (mean ± s.e.) in relation to time since sowing (days) for Cistus monspeliensis after different heat treatments (A), and for Lavandula stoechas after different smoke treatments (B). See text for details of treatments.
Table 3.
Mean germination rate (T50 in days) in the control conditions and after the heat treatments
| Species | Control | 80 °C 5 min | 80 °C 10 min | 100 °C 5 min | 100 °C 10 min | 120 °C 5 min | 120 °C 10 min | 150 °C 5 min |
|---|---|---|---|---|---|---|---|---|
| Anthyllis cytisoides | 266 | 177* | 187 * | 163** | 163** | 207ns | 183* | 144** |
| Anthyllis lagascana | 158 | 110ns | 217ns | 154ns | 178ns | 154ns | 168ns | – |
| Cistus albidus | 171 | 92* | 54** | 48*** | 50*** | 47** | 95* | – |
| Cistus monspeliensis | 135 | 107ns | 92* | 63*** | 52*** | 33**** | 52**** | 52*** |
| Coris monspeliensis | 264 | 283ns | 245ns | 211ns | 177* | 165* | 134** | 154ns |
| Coronilla minima | 154 | 179ns | 133ns | 181ns | 111ns | 109ns | – | – |
| Digitalis obscura | 7 | 7ns | 6ns | 6ns | 7ns | 10ns | 11ns | 25**** |
| Dorycnium pentaphyllum | 197 | 251ns | 168ns | 182ns | 133ns | 128ns | – | 216ns |
| Erica multiflora | 20 | 17ns | 18ns | 23ns | 14** | 14** | 14** | 13** |
| Erica terminalis | 96 | 82ns | 81ns | 59*** | 74* | 77* | 89ns | 63*** |
| Erica umbellata | – | – | – | – | – | – | 279 | 179 |
| Fumana ericoides | – | – | 161 | 261 | 125 | 92 | 130 | – |
| Fumana thymifolia | 146 | 114ns | 89** | 61*** | 46**** | 45**** | 165ns | – |
| Genista scorpius | 154 | 237ns | 156ns | 152ns | 110ns | 121ns | 170ns | 242ns |
| Genista triacanthos | 127 | 34*** | 27*** | 28*** | 28*** | 31*** | 25**** | 42*** |
| Genista umbellata | 9 | 13ns | 18ns | 8ns | 8ns | 7ns | 13ns | 81**** |
| Helianthemum syriacum | 103 | 122ns | 117ns | 253ns | 223ns | 241ns | – | – |
| Lavandula latifolia | 113 | 64* | 109ns | 80ns | 95ns | 85ns | 159ns | 177ns |
| Lavandula stoechas | 156 | 113* | 94** | 66**** | 24**** | 22**** | 145ns | – |
| Linum suffruticosum | 4 | 4ns | 4ns | 4ns | 4ns | 4ns | 6*** | 9*** |
| Ononis minutissima | 251 | – | 189* | 207ns | 87**** | 106*** | 213ns | – |
| Rosmarinus officinalis | 130 | 89** | 74*** | 56**** | 92* | 76*** | 99ns | 116ns |
| Sideritis angustifolia | 6 | 7ns | 7ns | 10ns | 52**** | 64**** | 65**** | 83**** |
| Teucrium capitatum | 129 | 103ns | 65** | 77** | 91* | 99ns | 118ns | 172ns |
| Teucrium ronnigeri | 71 | 69ns | 56ns | 50ns | 87ns | 84ns | 98* | – |
| Thymus piperella | 3 | 3ns | 5ns | 2ns | 4ns | 23** | 18* | 54**** |
| Thymus vulgaris | 6 | 6ns | 5ns | 5ns | 5ns | 4ns | 4ns | 16**** |
| Ulex borgiae | 198 | 53*** | 35**** | 40**** | 25**** | 29**** | 30**** | – |
| Ulex parviflorus | 143 | 70*** | 45**** | 55**** | 36**** | 30**** | 44**** | 156ns |
| Xolantha tuberaria | 211 | 191ns | 239ns | 119* | 59*** | 36*** | 33*** | 91** |
The significance of the pairwise comparison of each treatment with its corresponding control is also included (ns, not significant; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001).
–, not available.
Seed mortality after heat treatments
For low-moderate heat doses, seed mortality of most species did not change significantly with respect to the control. The heat treatments of 120 °C 10 min and 150 °C 5 min produced high mortality in most species. However, some species proved to be highly resistant to heat shock and their mortality did not increase significantly even at the highest heat dose (e.g. E. multiflora or L. suffruticosum).
Effect of smoke in the initial growth of the seedlings
Exposing seeds to smoke significantly increased total initial seedling growth compared with seedlings germinating from untreated seeds in six species out of 18: Coronilla minima (Fabaceae), C. monspeliensis (Primulaceae), L. latifolia, T. piperella, T. vulgaris and Teucrium ronnigeri (Lamiaceae, Table 2). For all these species, differences in seedling growth were seen to be greater in the roots than the shoots. None of the species showed any reduction in total seedling length following the smoke treatments, and four species (D. obscura, E. multiflora, L. stoechas and S. angustifolia) showed changes in root and/or shoot lengths without significantly changing total length.
DISCUSSION
Effect of heat
All species with water-impermeable seeds and thus physical dormancy (Cistaceae and most Fabaceae) showed low germination in control conditions. Germination was triggered in most species by one or several heat treatments. Nevertheless, stimulation by heat was not exclusive to Cistaceae and Fabaceae, for which heat-stimulated germination is well known (Herranz et al., 1998, 1999; Paula and Pausas, 2008). Most Ericaceae and Lamiaceae species, having water-permeable seeds, were also stimulated by heat (Table 4). Maximum stimulation by heat, in terms of both the number of species stimulated and the magnitude of the stimulation, was observed at 120 °C 5 min. The highest heat doses (treatments of 120 °C 10 min and 150 °C 5 min) were lethal for many species, and thus the stimulation of germination was reduced.
Table 4.
Species whose germination (percentage and/or rate) and initial seedling growth is stimulated by heat and/or smoke
| Family | Species | Germination percentage | Germination rate | Smoke-stimulated growth |
|---|---|---|---|---|
| Cistaceae | Cistus albidus | Heat | Heat | No |
| Cistus monspeliensis | Heat | Heat | No | |
| Fumana ericoides | Heat | – | ||
| Fumana thymifolia | Heat | Heat | – | |
| Xolantha tuberaria | Heat | Heat | – | |
| Ericaceae | Erica umbellata | Smoke | Smoke | – |
| Erica multiflora | Heat, smoke | Heat, smoke | No | |
| Erica terminalis | Heat, smoke | Heat, smoke | – | |
| Fabaceae | Anthyllis cytisoides | Heat | Heat | – |
| Coronilla minima | Heat | Yes | ||
| Genista triacanthos | Heat | Heat | – | |
| Genista umbellata | Heat | No | ||
| Ononis minutissima | Heat | Heat | – | |
| Ulex borgiae | Heat | Heat | – | |
| Ulex parviflorus | Heat | Heat | – | |
| Lamiaceae | Teucrium capitatum | Heat | Heat | No |
| Teucrium ronnigeri | Heat | Yes | ||
| Thymus piperella | Heat | Heat | Yes | |
| Thymus vulgaris | Smoke | Smoke | Yes | |
| Lavandula stoechas | Heat, smoke | Heat, smoke | No | |
| Lavandula latifolia | Heat, smoke | Heat, smoke | Yes | |
| Rosmarinus officinalis | Heat, smoke | Heat | – | |
| Primulaceae | Coris monspeliensis | Smoke | Heat, smoke | Yes |
Stimulation is considered when the there was a significant difference from the control at P < 0·01 (from Tables 1–3).
In species with a low dormancy level (i.e. species with high germination in the control), germination remained high for almost the full range of heat treatments and was only inhibited in a few species and at high heat doses (heat doses higher than 100 °C 10 min in S. angustifolia; 120 °C 5 min in T. piperella; and 150 °C 5 min in D. obscura and L. suffruticosum). Thus, these species with low dormancy resist heat shock, suggesting that they may also germinate and recruit after fire, although with less intensity than the species with higher seed dormancy.
Effect of smoke
Previous studies on germination in MB flora had so far found a limited response to smoke or charred wood (Keeley, 1995; Keeley and Baer-Keeley, 1999; Pérez-Fernández and Rodríguez-Echeverría, 2003; Buhk and Hensen, 2006; Reyes and Casal, 2006; Rivas et al., 2006). These results could suggest that MB flora, in contrast to other MTEs (Dixon et al. 1995; Keeley and Bond, 1997; Keeley and Fotheringham, 1998; Brown et al., 2003), has not acquired the capacity to respond to this fire-related germination cue. Indeed, this difference has been interpreted as a lack of convergence between the MB and other MTEs where smoke stimulation is present, as the stimulation by heat presented by Cistaceae and Fabaceae can be considered a homologous character (Keeley and Baer-Keeley, 1999). However, our results suggest that smoke may indeed play an important role in the germination and growth of MB species, especially in species that are not stimulated by heat or in which the effect of heat on germination is limited. Smoke-stimulated germination was verified in Ericaceae (E. umbellata, E. terminalis and E. multiflora), Lamiaceae (L. latifolia, L. stoechas, R. officinalis and T. vulgaris) and Primulaceae (Coris monspeliensis; Table 2). The wide taxonomic range of smoke-stimulated species supports the possible ancient origin of this trait (Pausas and Keeley, 2009) rather than the convergent evolution (Keeley and Bond, 1997; Keeley and Baer-Keeley, 1999).
All three of the Erica species studied were stimulated by smoke. In E. umbellata, germination was triggered exclusively by smoke and not by heat, whereas the germination of E. multiflora and E. terminalis was stimulated by both smoke and heat. Smoke plays an important role in the germination of this genus in South Africa (Brown, 1993), and our results suggest that it is also an important germination cue for the Erica species of the MB.
With respect to the Lamiaceae, L. stoechas was highly stimulated by smoke, achieving very high germination values (95–100 %). Keeley and Baer-Keeley (1999) previously observed stimulation by charred wood in this species, although with a lower final germination percentage. Smoke also stimulated germination in three species, L. latifolia, R. officinalis and T. vulgaris, in which smoke and heat produced similar percentages of stimulation. As far as we know, this is the first time that stimulation by smoke has been demonstrated for these species, thus reinforcing the idea of the importance of smoke-cued germination in Lamiaceae. This family has also been shown to respond positively to smoke in MTEs of California, where of the 25 species stimulated by smoke in Keeley and Fotheringham (1998), the only shrubs (three species) were Lamiaceae.
Coris monspeliensis (Primulaceae) germinated exclusively after smoke treatments. Both the control and the heat treatments produced very low germination, while smoke greatly triggered the germination. As far as we know, this is the first time that a fire cue, specifically smoke, has been shown to stimulate germination in this species. Previous observations of smoke stimulation in Primulaceae were from Australia (Read et al., 2000).
Our results, along with those of other recent studies (Crosti et al., 2006), confirm that, at least for woody species with a water-permeable seed coat, smoke stimulates germination in several important MB species. The idea that germination response to smoke in the MB is very limited probably reflects the scarcity of studies on the effect of smoke in this flora. Most of the studies on post-fire germination in the MB have focused on shrub species of Cistaceae and Fabaceae (see the compilation by Paula and Pausas, 2008), whereas the majority of the species stimulated by smoke in other MTEs, such as California or South Africa, are annuals (Keeley, 1991; Keeley and Bond, 1997; Keeley and Fotheringham, 1998). In addition, some species may respond to the smoke stimulus only after being in the seed bank for a period of time (Keeley and Fotheringham, 1998; Baker et al., 2005a; Keeley et al., 2005) or after the heat shock caused by fire (Keith, 1997; Tieu et al., 2001). Moreover, stimulation by smoke may depend on factors such as seed age, temperature, light levels, hydration (Brown and van Staden, 1997; van Staden et al., 2000) or dormancy cycling during seed burial (Baker et al., 2005b). None of these possibilities has yet been studied for MB flora. Thus, although herein clear evidence of smoke as an important germination cue in woody plants of MB is provided, our knowledge of smoke-stimulated germination in this region is conservative, and future studies will probably demonstrate that smoke plays an even more prominent role in the germination of MB flora. For instance, MB flora lacks evidence of short-lived fire-dependent flora only found after fire, although this strategy is well represented in other Mediterranean ecosystems (e.g. California, South Africa and Australia) where it is largely stimulated by smoke. It remains unknown whether the absence of post-fire specialization by annual plants is due to different historical fire regimes, to different adaptive strategies, to different sorting processes, to the different historical human impacts or to the lack of studies (Pausas et al. 2006). Further studies on post-fire germination of annuals and other short-lived herbaceous plants may provide relevant insights on this topic.
Methodological issues may also lead to confusing results on the response of MB flora to smoke. For instance, in studies where no significant results were obtained using liquid smoke (e.g. Buhk and Hensen, 2006), it is difficult to know whether the concentration of liquid smoke used was adequate. Jager et al. (1996b), using commercial concentrated smoke, obtained maximum germination at 1 : 105 dilution (0·001 %), which decreased at 1 : 104 dilution (0·01 %) and was totally inhibited at 1 : 103 dilution (0·1 %). The lowest concentration of commercial liquid smoke used by Buhk and Hensen (2006) was 0·01 %. Without at least one positive response to validate the effectiveness of the smoke concentration used, it is hard to reach any conclusion.
The role of smoke on MB flora is also supported by our results concerning seedling growth. Exposing seeds to smoke significantly increased initial seedling growth in six out of 18 species tested. This higher development in initial seedling growth was more notable in the roots (seven of the 18 species). In some cases, root growth with the smoke treatment was about twice (C. minima and L. latifolia) or even three times (C, monspeliensis) higher than in untreated seeds. The fact that C. minima presented increased seedling growth, despite having mainly water-impermeable seeds, suggests that smoke can act over the proportion of water-permeable seeds. Indeed, we found that approx. 10 % of the seeds of this species were water permeable. This study represents the first time that smoke-stimulated seedling growth has been reported in woody species of MB flora [see Daws et al. (2007) for temperate and sub-tropical arable weeds that include some species also occurring in the MB]. Initial seedling growth is an important factor in the post-fire environment, as seedlings that grow faster and have a more robust root system will have a competitive advantage in the post-fire environment.
In conclusion, our results suggest that both heat and smoke are important germination cues for many MB woody species. The successful post-fire recruitment of these species is due not only to seed resistance to heat shock, but also to the enhanced germination by heat and/or smoke (in both germination percentage and germination rate). Furthermore, seedlings of some species develop faster and thus with greater fitness under post-fire conditions than after other disturbances. To understand fully the role of fire in the germination of MB flora, further studies will need to consider annuals and short-lived herbaceous species (as they are very important in the post-fire dynamics of other MTEs), as well as the interaction between heat and smoke. In any case, our results point towards fire-cued germination being an evolutionary response to the frequent fires in the MB as in the other MTEs, and this needs to be considered when addressing evolutionary questions on plant traits at global scale.
ACKNOWLEDGEMENTS
We thank J. Prieto, F. Marco-Rubio and B. Gamiz for their collaboration in the germination experiment, S. Paula for her comments on an early draft, and C. Thanos for insights on seed coat type and permeability. The work was supported by the Spanish projects PERSIST (CGL2006-07126/BOS) and GRACCIE (SCD2007-00067, Consolider-Ingenio 2010, CEAM). B.M. is supported by a grant from the Fundação para a Ciência e a Tecnologia (SFRH/BD/41343/2007). CEAM is supported by the Generalitat Valenciana and Bancaixa; CIDE is supported by the Generalitat Valenciana and the University of Valencia.
LITERATURE CITED
- Baker KS, Steadman KJ, Plummer JA, Merritt DJ, Dixon KW. Dormancy release in Australian fire ephemeral seeds during burial increases germination response to smoke water or heat. Seed Science Research. 2005a;15:339–348. [Google Scholar]
- Baker KS, Steadman KJ, Plummer JA, Merritt DJ, Dixon KW. The changing window of conditions that promotes germination of two fire ephemerals, Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae) Annals of Botany. 2005b;96:1225–1236. doi: 10.1093/aob/mci274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin C, Baskin J. Seeds, ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Blank R, Young J. Heated substrate and smoke: influence on seed emergence and plant growth. Journal of Range Management. 1998;51:577–583. [Google Scholar]
- Brown N. Promotion of germination of fynbos seeds by plant-derived smoke. New Phytologist. 1993;123:575–583. doi: 10.1111/j.1469-8137.1993.tb03770.x. [DOI] [PubMed] [Google Scholar]
- Brown NAC, van Staden J. Smoke as a germination cue: a review. Plant Growth Regulation. 1997;22:115–124. [Google Scholar]
- Brown N, van Staden J, Daws M, Johnson T. Patterns in the seed germination response to smoke in plants from the Cape Floristic Region, South Africa. South African Journal of Botany. 2003;69:514–525. [Google Scholar]
- Buhk C, Hensen I. ‘Fire seeders’ during early post-fire succession and their quantitative importance in south-eastern Spain. Journal of Arid Environments. 2006;66:193–209. [Google Scholar]
- Coca M, Pausas JG. Regeneration traits are structuring phylogenetic diversity in cork oak (Quercus suber) woodlands. Journal of Vegetation Science. 2009;20:1009–1015. [Google Scholar]
- Crosti R, Ladd P, Dixon K, Piotto B. Post-fire germination: the effect of smoke on seeds of selected species from the central Mediterranean basin. Forest Ecology and Management. 2006;221:306–312. [Google Scholar]
- Daws M, Davies J, Pritchard H, Brown N, Van Staden J. Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regulation. 2007;51:73–82. [Google Scholar]
- Dixon KW, Roche S, Pate JS. The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia. 1995;101:185–192. doi: 10.1007/BF00317282. [DOI] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. A compound from smoke that promotes seed germination. Science. 2004;305:977. doi: 10.1126/science.1099944. [DOI] [PubMed] [Google Scholar]
- González-Rabanal F, Casal M. Effect of high temperatures and ash on germination of ten species from gorse shrubland. Plant Ecology. 1995;116:123–131. [Google Scholar]
- Herranz JM, Ferrandis P, Martínez-Sánchez JJ. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecology. 1998;136:95–103. [Google Scholar]
- Herranz JM, Ferrandis P, Martinez-Sánchez J. Influence of heat on seed germination of nine woody Cistaceae species. International Journal of Wildland Fire. 1999;9:173–182. [Google Scholar]
- International Seed Testing Association. International rules for seed testing. Seed Science and Technology. 1999;27 supplement. [Google Scholar]
- Jager AK, Light ME, van Staden J. Effects of source of plant material and temperature on the production of smoke extracts that promote germination of light-sensitive lettuce seeds. Environmental and Experimental Botany. 1996a;36:421–429. [Google Scholar]
- Jager A, Rabe T, Van Staden J. Food-flavouring smoke extracts promote seed germination. South African Journal of Botany. 1996b;62:282–284. [Google Scholar]
- Keeley JE. Seed germination and life history syndromes in the California chaparral. Botanical Review. 1991;57:81–116. [Google Scholar]
- Keeley JE. Seed germination patterns in fire-prone Mediterranean-climate regions. In: Arroyo MTK, Zedler PH, Fox MD, editors. Ecology and biogeography of Mediterranean ecosystems in Chile, California and Australia. San Diego: Academic Press; 1995. pp. 239–273. [Google Scholar]
- Keeley JE, Baer-Keeley M. Role of charred wood, heat-shock, and light in germination of postfire phrygana species from the eastern Mediterranean basin. Israel Journal of Plant Sciences. 1999;47:11–16. [Google Scholar]
- Keeley JE, Bond WJ. Convergent seed germination in South African fynbos and Californian chaparral. Plant Ecology. 1997;133:153–167. [Google Scholar]
- Keeley JE, Fotheringham CJ. Smoke-induced seed germination in California chaparral. Ecology. 1998;79:2320–2336. [Google Scholar]
- Keeley JE, Fotheringham CJ. Role of fire in regeneration from seed. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. vol 13. Oxon, UK: CAB International; 2000. pp. 311–330. [Google Scholar]
- Keeley JE, McGinnis TW, Bollens KA. Seed hermination of Sierra Nevada postfire chaparral species. Madroño. 2005;52:175–181. [Google Scholar]
- Keith DA. Combined effects of heat shock, smoke and darkness on germination of Epacris stuartii Stapf., an endangered fire-prone Australian shrub. Oecologia. 1997;112:340–344. doi: 10.1007/s004420050318. [DOI] [PubMed] [Google Scholar]
- Light ME, Daws MI, Van Staden J. Smoke-derived butenolide: towards understanding its biological effects. South African Journal of Botany. 2009;75:1–7. [Google Scholar]
- Moran MD. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos. 2003;100:403–405. [Google Scholar]
- Nelson DC, Riseborough J-A, Flematti GR, et al. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiology. 2009;149:863–873. doi: 10.1104/pp.108.131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S, Pausas JG. Burning seeds: germinative response to heat treatments in relation to resprouting ability. Journal of Ecology. 2008;96:543–552. [Google Scholar]
- Paula S, Arianoutsou M, Kazanis D, et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology. 2009;90:1420. [Google Scholar]
- Pausas JG, Keeley JE. A burning story: the role of fire in the history of life. BioScience. 2009;59:593–601. [Google Scholar]
- Pausas JG, Verdú M. Plant persistence traits in fire-prone ecosystems of the Mediterranean basin: a phylogenetic approach. Oikos. 2005;109:196–202. [Google Scholar]
- Pausas JG, Verdú M. Fire reduces morphospace occupation in plant communities. Ecology. 2008;89:2181–2186. doi: 10.1890/07-1737.1. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Keeley JE, Verdú M. Inferring differential evolutionary processes of plant persistence traits in Northern Hemisphere Mediterranean fire-prone ecosystems. Journal of Ecology. 2006;94:31–39. [Google Scholar]
- Pérez-Fernández MA, Rodríguez-Echeverría S. Effect of smoke, charred wood, and nitrogenous compounds on seed germination of ten species from woodland in Central-Western Spain. Journal of Chemical Ecology. 2003;29:237–251. doi: 10.1023/a:1021997118146. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Bethesda, MD, USA: US. National Institutes of Health; 1997–2005. (http://rsb.info.nih.gv/ij/ ) [Google Scholar]
- Read TR, Bellairs SM, Mulligan DR, Lamb D. Smoke and heat effects on soil seed bank germination for the re-establishment of a native forest community in New South Wales. Austral Ecology. 2000;25:48–57. [Google Scholar]
- Reyes O, Casal M. Seed germination of Quercus robur, Q. pyrenaica and Q. ilex and the effects of smoke, heat, ash and charcoal. Annals of Forest Science. 2006;63:205–212. [Google Scholar]
- Reyes O, Trabaud L. Germination behaviour of 14 Mediterranean species in relation to fire factors: smoke and heat. Plant Ecology. 2009;202:113–121. [Google Scholar]
- Rivas M, Reyes O, Casal M. Influence of heat and smoke treatments on the germination of six leguminous shrubby species. International Journal of Wildland Fire. 2006;15:73–80. [Google Scholar]
- van Staden J, Brown NAC, Jager AK, Johnson TA. Smoke as a germination cue. Plant Species Biology. 2000;15:167–178. [Google Scholar]
- Thanos C, Georghiou K, Kadis C, Pantazi C. Cistaceae: a plant family with hard seeds. Israel Journal of Botany. 1992;41:251–263. [Google Scholar]
- Tieu A, Dixon KW, Meney KA, Sivasithamparam K. The interaction of heat and smoke in the release of seed dormancy in seven species from Southwestern Western Australia. Annals of Botany. 2001;88:259–265. [Google Scholar]
- Trabaud L. Etude du comportement du feu dans la garrigue de Chêne Kermès à partir des témperatures et des vitesses de propagation. Annals of Forest Science. 1979;36:13–18. [Google Scholar]
- Verdú M, Pausas JG. Fire drives phylogenetic clustering in Mediterranean Basin woody plant communities. Journal of Ecology. 2007;95:1316–1323. [Google Scholar]