Abstract
Objective
To examine if reductions in added sugar intake or increases in fiber intake in response to a 16-week intervention were related to improvements in metabolic outcomes related to type 2 diabetes mellitus risk.
Design
Secondary analysis of a randomized control trial.
Setting
Intervention classes at a lifestyle laboratory and metabolic measures at the General Clinical Research Center.
Participants
Fifty-four overweight Latino adolescents (mean [SD] age, 15.5 [1] years).
Intervention
Sixteen-week study with 3 groups: control, nutrition, or nutrition plus strength training.
Main Outcome Measures
Body composition by dual-energy x-ray absorptiometry; visceral adipose tissue by magnetic resonance imaging; glucose and insulin incremental area under the curve by oral glucose tolerance test; insulin sensitivity, acute insulin response, and disposition index by intravenous glucose tolerance test; and dietary intake by 3-day records.
Results
Fifty-five percent of all participants decreased added sugar intake (mean decrease, 47 g/d) and 59% increased fiber intake (mean increase, 5 g/d), and percentages were similar in all intervention groups, including controls. Those who decreased added sugar intake had an improvement in glucose incremental area under the curve (−15% vs +3%; P=.049) and insulin incremental area under the curve (−33% vs −9%; P=.02). Those who increased fiber intake had an improvement in body mass index (−2% vs +2%; P=.01) and visceral adipose tissue (−10% vs no change; P=.03).
Conclusions
Individuals who reduced added sugar intake by the equivalent of 1 can of soda per day or increased fiber intake by the equivalent of a ½ cup of beans showed improvements in key risk factors for type 2 diabetes, specifically in insulin secretion and visceral fat. Improvements occurred independent of group assignment and were equally likely to occur in control group participants.
In 2003-2006, 38.9% of Mexican American adolescents aged 12 to 19 years were at risk of overweight or overweight, as compared with 33.1% of non-Hispanic white adolescents.1 In addition, independent of body composition, Latino children are more insulin resistant and thus more likely to develop obesity-related chronic diseases than their white counterparts.2In a convenience sample of overweight Latino children in Los Angeles, California, we previously showed that 30% had a clustering of diabetes mellitus and cardiovascular disease risk factors known as the metabolic syndrome and 32% had pre-diabetes (ie, impaired fasting or 2-hour glucose intolerance).3,4
Diet is one of the main modifiable risk factors for the development of type 2 diabetes. In previous cross-sectional analyses in overweight Latino youth, we showed that dietary fiber consumption is inversely associated with both waist circumference and the metabolic syndrome5 and that intake of total and added sugar is associated with poor beta-cell function, independent of adiposity.6 Additionally, we showed that in a 12-week pilot intervention study, overweight Latina girls with greater reductions in added sugar intake showed greater reductions in insulin secretion.7 To date, only a few studies have examined the effects of a high-fiber, low-sugar diet on metabolic health in overweight youth,8,9 and to our knowledge, none have tested the effects of this type of intervention in a mixed-sex group of Latino youth.
This article is a secondary data analysis from a 16-week randomized control trial. The original study assessed the incremental effects of the following 3 intervention groups on adiposity and risk factors for type 2 diabetes in overweight Latino adolescents: (1) control, (2) a nutrition education program designed to reduce sugar and increase fiber intake, and (3) same nutrition education program with twice per week strength training. The main outcomes analysis showed no significant overall effects of the intervention on body weight, body composition, or metabolic parameters related to risk for type 2 diabetes, with the exception of an improvement in oral glucose response (6% and 18% reductions in nutrition and combined groups, respectively, compared with a 32% increase in the control group).10 However, despite the overall lack of intervention effects, there was considerable individual variation in dietary changes and metabolic outcomes within each of the randomized groups. These results prompted the question of whether metabolic outcomes varied by achievement of the dietary goals, regardless of group assignment. The objective of this analysis was, therefore, to test if participants who reduce added sugar intake and/or increase fiber intake will have stronger metabolic improvements related to future diabetes risk, including improvements in insulin/glucose indexes and in adiposity parameters.
METHODS
PARTICIPANTS
Participants were recruited from Los Angeles County and met the following inclusion criteria: body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) in the 85th percentile or higher,11 Latino ethnicity, and grades 9 through 12. Participants were excluded if they (1) were using medication or were diagnosed with any syndrome or disease that could influence dietary intake, exercise ability, body composition and fat distribution, or insulin action and secretion, (2) were previously diagnosed with any major illness, (3) met diagnostic criteria for diabetes, or (4) participated in a structured exercise, nutrition, or weight loss program in the past 6 months. Informed written consent from parents and assent from the children were obtained. This study was approved by the institutional review board of the University of Southern California, Health Sciences Campus.
RANDOMIZATION
Sixty-six participants were randomized to 1 of 3 groups and allocations were concealed from participants until after pretesting was complete. Of the 66 participants who were randomized, 54 completed the intervention. There were no statistically significant differences in baseline demographics, anthropometrics, or body composition measures between the 12 participants who dropped out of the program and the 54 participants who completed the program.
DESCRIPTION OF INTERVENTIONS
The nutrition-only group received 1 nutrition class per week for 16 weeks. The dietary intervention targeted 2 goals: a decrease in added sugar consumption and an increase in fiber consumption. Participants in the nutrition plus strength training group received the same weekly nutrition classes along with strength training 2 times per week for 16 weeks.
Participants randomized to the control group received no intervention between preintervention and postintervention data collection. Periodically through the 16-week intervention, participants received non–health-related incentives, such as T-shirts, and regular telephone calls to enhance retention. After posttesting, participants were offered a delayed intervention for 1 month.
PROTOCOL AND OUTCOME MEASURES
At both baseline and 16 weeks, participants had both an outpatient and inpatient clinic visit for assessment of insulin and glucose indexes, anthropormorphics, body composition, and dietary intake.
Outpatient Visit
Participants arrived at the University of Southern California General Clinical Research Center at approximately 7:30 am after an overnight fast. A licensed pediatric health care provider conducted a medical history examination and determined Tanner staging using established guidelines.12,13 Following the examination, a 3-hour oral glucose tolerance test (OGTT) was conducted. A flexible intravenous catheter was placed in an antecubital vein and subjects then ingested 1.75 g of oral glucose solution per kilogram of body weight (to a maximum 75 g). Blood samples were drawn at baseline and every 10 minutes for 3 hours and were assayed for glucose, insulin, and C peptide levels. Fasting and 2-hour glucose levels were used to determine normal glucose tolerance (2-hour glucose level, <140 mg/dL) or impaired glucose tolerance (2-hour glucose level, ≥140 and <200 mg/dL) as defined by the American Diabetes Association.14 Three-hour insulin and glucose area under the curve (AUC) and incremental area under the curve (IAUC) were calculated from the OGTT data, in milligrams per minute per deciliter for glucose and microunits per minute per milliliter for insulin. Glucose and insulin AUCs are the sum of the area of each time segment by insulin or glucose concentration and IAUCs are the sum of the same area adjusted for the starting point. Insulin AUC and IAUC are approximate measures of insulin secretion in response to a standard oral glucose load.
Anthropometry and Body Composition
Weight and height were measured to the nearest 0.1 kg and 0.1 cm. Body mass index and BMI percentiles for age and sex were determined using EpiInfo 2000, version 1.1 (Centers for Disease Control and Prevention, Atlanta, Georgia). Whole-body fat and soft lean tissue were measured by dual-energy x-ray absorptiometry (DEXA) using a Hologic QDR 4500W (Hologic, Bedford, Massachusetts).
Subcutaneous and visceral fat volumes were obtained by magnetic resonance imaging, using a Siemens Magnetom 1.5-T Symphony Maestro Class Syngo 2004A (Siemens AG, Erlangen, Germany) with a Numaris/4 software at the University of Southern California–Health Consultation Center II imaging center. Patients were positioned supine, and 19 axial images of the abdomen with a thickness of 10 mm were taken. Visceral and subcutaneous abdominal tissue were calculated using image analysis software (SliceOmatic; Tomovision, Montreal, Quebec, Canada) at Image Reading Center (New York, New York).
Inpatient Visit
Approximately 7 to 14 days following the outpatient visit, participants were admitted to the General Clinical Research Center and served a standardized dinner and an evening snack, with only water permitted after 8 pm. At approximately 7:30 am the following day, an insulin-modified frequently sampled intravenous glucose tolerance test was performed. At time 0, glucose (25% dextrose, 0.3 g/kg of body weight) was administered intravenously. Blood samples were collected at points −15, −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 minutes. Insulin (0.02 U/kg of body weight, Humulin R [regular insulin for human injection]; Eli Lilly, Indianapolis, Indiana) was injected intravenously at 20 minutes. Glucose and insulin values were entered into the MINMOD Millennium 2003 computer program (version 5.16; Richard N. Bergman, PhD, University of Southern California) to determine insulin sensitivity (SI), acute insulin response (AIR) (ie, insulin AUC above basal for the first 8 minutes of the frequently sampled intravenous glucose tolerance test), and disposition index (DI) (an index of pancreatic beta-cell function calculated as the product of SI × AIR).
Dietary Intake
At both baseline and 16 weeks, participants were given 3-day diet records to complete. Participants were given a short lesson on how to estimate portion sizes and were given measuring cups and rulers to aid in accurate reporting. Research staff, trained and supervised by a registered dietitian, clarified all dietary records. Nutrition data were analyzed using the Nutrition Data System for Research (NDS-R version 5.0_35), a program developed by the University of Minnesota.
STATISTICAL ANALYSIS
Data Cleaning and Normalization
Of the 54 participants who completed the intervention, 49 had available dietary data. Five of the 49 were missing 1 of the 3 days of diet records for either pretesting or posttesting, and an average of 2 days was used. The DEXA measures were collected for 45 of the 49 subjects because 4 participants were over the 300-lb weight limit and magnetic resonance imaging data, for 40 of the 49 because of logistical problems.
The following outcome variables were nonnormally distributed and analyses were run on the log-transformed values: weight, DEXA fat and lean mass, 2-hour glucose level, glucose AUC and IAUC, insulin AUC and IAUC, fasting insulin level, SI, AIR, and DI. All transformations were log transformations with one exception; BMI percentile used the following transformation: yT=ln(highest value + 1)-y. Two outliers were identified and removed from models related to glucose and insulin indexes.
Definition of Sugar Intake Decrease and Fiber Intake Increase
Subjects were divided into categories based on whether they decreased sugar intake and/or increased fiber intake. A sugar intake decrease was defined as a decrease in added sugar intake of any magnitude (postintervention–preintervention < 0), as a percentage of total caloric intake, and fiber intake increase was defined as an increase of any magnitude in fiber intake (in grams) per 1000 calories of total energy intake (postintervention–preintervention > 0).
Baseline Comparisons
Baseline characteristics were compared between sugar and fiber intake change categories (decrease vs increase) using χ2 tests and independent t tests. Because there were no significant differences in sugar or fiber intake change by randomization group, all participants were combined for subsequent analyses, and randomization group was used as a covariate.
Comparison of Metabolic Change by Sugar and Fiber Intake Change Categories
Preintervention to postintervention changes in adiposity as well as insulin and glucose indexes were analyzed in 2 steps. First, preliminary analysis of raw change scores (postintervention–preintervention) for metabolic outcomes were tested for significance against zero with independent t tests. The grouping variables were sugar intake decrease (yes/no) and fiber intake increase (yes/no). In the second step, repeated-measures analysis of covariance (ANCOVA) was conducted for variables, controlling for covariates with biological significance. The between-subjects factor was sugar or fiber intake change category (increase vs decrease) and the time variable was weeks (0 vs 16). First, all models were run separately with 1 dichotomous between-subjects factor variable (either sugar intake decrease, yes/no, or fiber intake increase, yes or no). Subsequently, a 2-factor model was used by including both between-subjects factors to test for interactions in sugar and fiber intake categories (ie, decreased sugar intake only, increased fiber intake only, both, or neither). In all repeated-measures models, the following a priori covariates were included: sex, randomization group, and baseline sugar and/or fiber intake. Pretest and posttest total fat mass and total lean tissue mass, as well as age, were evaluated as covariates in each model and included only when significant. Pretest and posttest subcutaneous fat was included a priori in all visceral adipose tissue models. Data were analyzed with SPSS version 13.0 (SPSS Inc, Chicago, Illinois), and type I error was set at α<.05.
RESULTS
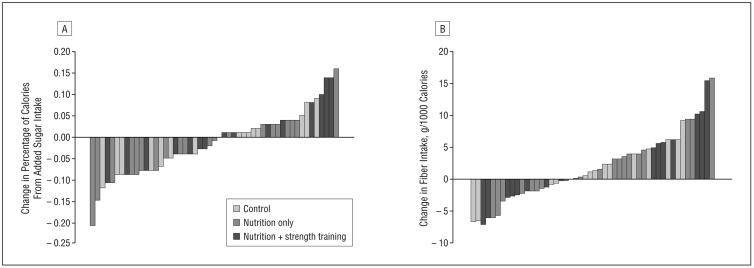
Participants whose sugar and/or fiber intake improved were randomly spread across intervention groups, as is illustrated in Figure 1. There were no significant differences in sugar or fiber intake change categories (increase vs decrease) across the 3 different intervention groups (Table 1 and Table 2; P>.05). Of the 49 total participants, 55% (n=27) reduced added sugar intake and 59% (n=20) increased fiber intake, and these values were similar across intervention groups.
Figure 1.
Changes in added sugar (A) and fiber (B) intake displayed by subject, coded by randomization group.
Table 1.
Baseline Characteristics in Those Participants Who Decreased or Increased Their Percentage of Calories From Added Sugar Intakea
| Mean (SD) |
|||
|---|---|---|---|
| Decreased Added Sugar Intake (n=27) |
Increased Added Sugar Intake (n=22) |
P Value | |
| Sex, M/F, % | 52/48 | 50/50 | .90 |
| Randomization group, control/nutrition/combo, % | 30/44/26 | 27/36/36 | .72 |
| Age, y | 15.6 (1.0) | 15.2 (1.1) | .20 |
| Height, cm | 165.7 (8.2) | 165.3 (7.7) | .84 |
| Weight, kgb | 98.7 (26.9) | 87.0 (15.1) | .11 |
| BMI | 35.6 (7.6) | 32.0 (6.0) | .08 |
| BMI percentileb | 97.3 (3.7) | 95.8 (4.2) | .11 |
| Total fat mass, kgb | 34.7 (12.3) | 31.2 (11.4) | .31 |
| Visceral fat, L | 1.7 (0.8) | 1.6 (0.8) | .68 |
| Subcutaneous fat, Lb | 10.1 (4.2) | 8.0 (3.8) | .12 |
| Total lean tissue mass, kgb | 55.3 (10.9) | 53.3 (7.1) | .63 |
| Fasting glucose level, mg/dL | 92.2 (5.8) | 92.3 (8.4) | .93 |
| 2-h Glucose level, mg/dLb | 125.9 (24.3) | 132.6 (27.0) | .37 |
| Glucose IAUC, mg/min/dLb | 101.1 (49.6) | 103.2 (56.6) | .56 |
| Fasting insulin level, μU/mLb | 28.8 (15.7) | 26.7 (15.4) | .68 |
| 2-h Insulin level, μU/mLb | 190.4 (150.9) | 179.3 (106.4) | .80 |
| Insulin IAUC, μU/min/mLb | 415.7 (304.2) | 354.7 (205.4) | .56 |
| Insulin sensitivity, (×10−4/min−1)/(μU/mL)b | 1.4 (0.8) | 2.1 (1.9) | .20 |
| Acute insulin response, μU/mL × 10 minb | 1415.8 (1079.0) | 1145.9 (658.0) | .59 |
| Disposition index, ×10−4 min−1b | 1501.5 (794.4) | 1573.1 (913.7) | .84 |
| Energy, kcal | 2032.6 (669.9) | 1747.2 (538.1) | .11 |
| Calories from fat, % | 31.9 (6.1) | 33.1 (6.0) | .52 |
| Calories from protein, % | 15.3 (3.2) | 16.6 (3.6) | .19 |
| Calories from carbohydrate, % | 53.9 (8.1) | 51.7 (6.6) | .32 |
| Calories from added sugar, % | 17.4 (6.7) | 12.2 (4.3) | .003 |
| Fiber, g per 1000 kcal | 7.6 (2.8) | 9.3 (3.5) | .07 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IAUC, incremental area under the curve.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; insulin to picomoles per liter, multiply by 6.945.
χ2 Tests were used for categorical variables and independent t tests, for continuous variables. Sample sizes for dual-energy x-ray absorptiometry were 23 in sugar intake decreasers and 22 in sugar intake increasers. Sample sizes for magnetic resonance imaging were 22 in sugar intake decreasers and 18 in sugar intake increasers.
Variables were not normally distributed so statistical tests were run with log-transformed data. For BMI percentile, a transformation involving ln(highest value + 1)−y was used.
Table 2.
Baseline Characteristics by Those Individuals Who Decreased or Increased Fiber Intake Relative to Caloric Intakea
| Mean (SD) |
|||
|---|---|---|---|
| Decreased Fiber Intake (n=20) |
Increased Fiber Intake (n=20) |
P Value | |
| Sex, M/F, % | 40/60 | 59/41 | .25 |
| Randomization group, control/nutrition/combo, % | 20/40/40 | 34/41/24 | .40 |
| Age, y | 15.3 (1.1) | 15.5 (1.0) | .44 |
| Height, cm | 163.6 (7.0) | 166.8 (8.4) | .17 |
| Weight, kgb | 89.4 (21.4) | 96.2 (23.8) | .31 |
| BMI | 33.3 (7.2) | 34.4 (7.1) | .60 |
| BMI percentileb | 96.3 (3.9) | 96.9 (4.1) | .45 |
| Total fat mass, kgb | 32.2 (11.8) | 33.6 (12.1) | .73 |
| Visceral fat, L | 1.7 (0.7) | 1.6 (0.8) | .72 |
| Subcutaneous fat, Lb | 8.4 (4.0) | 9.5 (4.2) | .47 |
| Total lean tissue mass, kgb | 51.5 (7.9) | 56.3 (9.7) | .10 |
| Fasting glucose level, mg/dL | 91.3 (7.8) | 92.9 (6.5) | .43 |
| 2-h Glucose level, mg/dLb | 134.0 (28.5) | 125.4 (23.0) | .27 |
| Glucose IAUC, mg/min/dLb | 107.4 (59.4) | 98.4 (47.5) | .85 |
| Fasting insulin level, μU/mLb | 29.5 (13.7) | 27.0 (16.7) | .39 |
| 2-h Insulin level, μU/mLb | 181.1 (107.0) | 188.4 (148.1) | .90 |
| Insulin IAUC, μU/min/mLb | 364.4 (211.5) | 404.8 (296.9) | .79 |
| Insulin sensitivity, (×10−4/min−1)/(μU/mL)b | 2.0 (1.9) | 1.6 (1.1) | .28 |
| Acute insulin response, μU/mL × 10 minb | 1351.7 (1017.7) | 1255.2 (854.7) | .77 |
| Disposition index, ×10−4 min−1b | 1497.7 (686.1) | 1558.4 (945.2) | .75 |
| Energy, kcal | 1928.6 (696.3) | 1887.8 (582.5) | .82 |
| Calories from fat, % | 31.0 (6.5) | 33.4 (5.5) | .17 |
| Calories from protein,% | 16.9 (3.3) | 15.2 (3.4) | .09 |
| Calories from carbohydrate, % | 53.6 (7.3) | 52.4 (7.7) | .57 |
| Calories from added sugar, % | 13.7 (5.8) | 16.0 (6.5) | .22 |
| Fiber, g per 1000 kcal | 10.3 (3.3) | 7.1 (2.4) | .001 |
χ2 Tests were used for categorical variables and independent t tests, for continuous variables. Sample sizes for dual-energy x-ray absorptiometry were 19 in fiber intake decreasers and 26 in fiber intake increasers. Sample sizes for magnetic resonance imaging were 14 in fiber intake decreasers and 26 in fiber intake increasers.
Variables were not normally distributed so statistical tests were run with log-transformed data. For BMI percentile, a transformation involving ln(highest value +1)−y was used.
Baseline characteristics by sugar and fiber intake categories are also shown in Table 1 and Table 2. The sugar intake decreasers had a mean (SD) decrease of 47 (42) g/d of added sugar intake and the fiber intake increasers had a mean (SD) increase of 5 (8) g/d of total fiber intake. There were no significant differences at baseline in sugar or fiber intake categories for age, sex, Tanner stage, height, measures of adiposity, or glucose/insulin indexes (P>.05). There was a trend toward significance for the sugar intake decreasers to have a higher BMI at baseline than the sugar intake increasers (35.6 vs 32.0; P=.08). Though there were no significant differences in macronutrient intake by sugar or fiber intake categories at baseline (P>.05), the sugar intake decreasers had a higher percentage of calories from added sugar intake at baseline (P=.003) and the fiber intake increasers had a lower baseline intake of fiber (in grams) per 1000 calories (P=.001).
Comparisons of raw change scores by sugar and fiber intake categories are shown in Table 3. For the added sugar intake category comparisons, the only significant difference was in insulin IAUC, where the group who decreased sugar intake showed a reduction of 121 μU/min/mL as compared with a decrease of 36 μU/min/mL in those who did not decrease sugar intake (P=.02). In the dietary fiber intake category comparisons, those who increased fiber intake had a significant decrease in BMI (−0.6 vs +0.5; P=.02) and in visceral fat (−0.2 vs +0.006; P=.04) as compared with those who did not, but there were no significant differences in other metabolic outcomes.
Table 3.
Unadjusted Changes in Metabolic Outcomes by Dietary Improvement Categoriesa
| Decreased Added Sugar Intake |
Increased Added Sugar Intake |
Decreased Fiber Intake |
Increased Fiber Intake |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change Score (SD) |
Sample Size |
Change Score (SD) |
Sample Size |
P Value |
Change Score (SD) |
Sample Size |
Change Score (SD) |
Sample Size |
P Value |
|
| BMI | −0.3 (1.9) | 25 | 0.1 (1.0) | 20 | .31 | 0.5 (1.1) | 20 | −0.6 (1.7) | 27 | .02 |
| Total fat mass, kgb | 0.1 (3.0) | 22 | −0.1 (1.7) | 20 | .93 | 0.4 (2.1) | 19 | −0.2 (2.5) | 25 | .38 |
| Visceral fat, L | −0.1 (0.3) | 20 | 0.0 (0.1) | 17 | .15 | 0.006 (0.1) | 14 | −0.2 (0.3) | 25 | .04 |
| Fasting glucose level, mg/dL | −0.5 (6.4) | 26 | −4.1 (6.9) | 21 | .08 | −1.4 (5.2) | 20 | −2.7 (7.6) | 29 | .48 |
| 2-h Glucose level, mg/dLb | −7.0 (24.8) | 26 | −12.8 (24.6) | 21 | .62 | −13.8 (28.2) | 20 | −4.1 (22.9) | 29 | .27 |
| Glucose IAUC, mg/min/dLb | −11.2 (37.3) | 26 | −0.6 (47.3) | 21 | .18 | −5.8 (42.6) | 20 | −4.3 (42.0) | 29 | .46 |
| Fasting insulin level, μU/mLb | −3.1 (11.7) | 26 | −3.7 (10.0) | 21 | .79 | −2.9 (8.2) | 20 | −1.8 (15.1) | 29 | .96 |
| 2-h Insulin level, μU/mLb | −46.1 (122.2) | 26 | −47.1 (112.9) | 21 | .37 | −22.7 (149.5) | 20 | −23.4 (175.3) | 29 | .73 |
| Insulin IAUC, μU/min/mLb | −121.1 (179.6) | 26 | −36.3 (164.4) | 21 | .02 | −19.3 (226.5) | 20 | −77.3 (225.8) | 29 | .12 |
| Insulin sensitivity, (×10 −4/min −1)/(μU/mL)b | 0.3 (0.8) | 25 | 0.02 (0.9) | 21 | .62 | −0.3 (2.0) | 20 | 0.2 (1.0) | 28 | .06 |
| Acute insulin response, μU/mL × 10 minb | −61.5 (339.7) | 25 | 201.7 (546.6) | 21 | .18 | 73.31 (536.9) | 20 | 18.7 (415.9) | 28 | .08 |
| Disposition index, ×10−4 min−1b | 228.9 (872.5) | 25 | 166.8 (910.3) | 21 | .79 | 101.0 (828.7) | 20 | 332.2 (915.9) | 28 | .26 |
A decrease in added sugar intake is defined as a decrease in the percentage of calories from added sugar (postintervention - preintervention). An increase in fiber intake is defined as an increase in total fiber intake of any magnitude relative to caloric intake. Differences in change scores by category were assessed by independent t tests and data are presented as mean (SD).
Variables were not normally distributed so statistical tests were run with log - transformed data but untransformed values are reported for ease of interpretation.
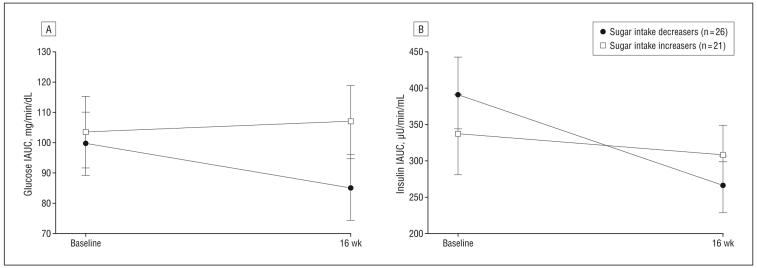
Significant results for the repeated-measures ANCOVA analyses are shown in Figure 2 and Figure 3. In the analyses with sugar intake category as the between-subjects factor, there were significant time × sugar intake category interactions for both glucose IAUC and insulin IAUC, controlling for sex, randomization group, and baseline added sugar consumption. Those who reduced added sugar intake had a significant reduction in glucose IAUC (−15% vs +3%; P=.049) (Figure 2A) and insulin IAUC (−33% vs −9%; P=.02) (Figure 2B) compared with those who increased added sugar intake. Body composition was evaluated as a covariate in both models and was not significant (P>.05) and therefore not included in the final models. Changes in adiposity or glucose/insulin index outcomes, including SI, AIR, and DI, were not significantly different in those who reduced added sugar intake vs those who did not.
Figure 2.
Adjusted changes in postchallenge glucose response (A) and insulin secretion (B) by sugar intake improvement categories. Sugar intake improvement was defined as a decrease of any magnitude in the percentage of calories from added sugar intake. Models are adjusted for sex, randomization group, and baseline added sugar intake. Body composition was evaluated in the model but removed because it was not significant. IAUC indicates incremental area under the curve.
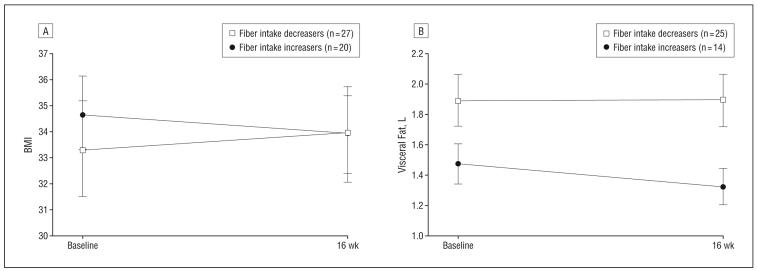
Figure 3.
Adjusted changes in body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) (A) and visceral fat (B) by fiber intake improvement categories. Fiber intake improvement is defined as an increase in fiber intake of any magnitude relative to caloric intake. Models are adjusted for sex, randomization group, and baseline fiber intake. Visceral fat model also included baseline and posttest subcutaneous fat.
In the repeated-measures ANCOVA analyses with fiber intake category as the between-subjects factor, there were significant time × fiber intake category interactions for both BMI and visceral adipose tissue, controlling for sex, randomization group, baseline fiber intake, and subcutaneous adipose tissue (in the visceral adipose tissue model). Those who increased fiber intake had a significant reduction in BMI (−2% vs +2%; P=.01) (Figure 3A) and visceral adipose tissue (−10% vs no change; P=.03) (Figure 3B) compared with those who decreased fiber intake. There were no other time × fiber intake category interactions for other measures of adiposity, such as fat mass or subcutaneous fat, or glucose/insulin index outcomes, including SI, AIR, and DI.
There was considerable overlap in sugar and fiber intake categories: 78% (21 of 27) of those who reduced sugar intake also increased fiber intake, and 72% (21 of 29) of those who increased fiber intake also decreased sugar intake (data not shown). However, when sugar and fiber intake categories were tested together as 2 factors in the same repeated-measures ANCOVA model, there were no significant interactions for any of the adiposity measures or glucose/insulin indexes (P>.05).
COMMENT
The main findings from the current analysis show that overweight Latino adolescents who decreased added sugar intake by an average of 47 g/d, equivalent to the sugar in 1 can of soda, had an average 33% decrease in insulin secretion as assessed by IAUC during an OGTT. Additionally, participants who increased fiber intake by an average of 5 g/d, equivalent to the amount in a ½ cup of beans, had an average 10% reduction in visceral adipose tissue volume. Moreover, these dietary changes were independent of intervention group assignment and children assigned to the control group were as likely to make dietary improvements and to show metabolic improvements as those assigned to a rigorous 16-week intervention.
In particular, 57% of the control participants decreased their sugar intake and 71% increased their fiber intake, in the absence of any nutrition intervention. This effect could be attributed to contamination effects because the control participants were not blinded to the purpose of the intervention. The recruitment materials and consent forms for the study explained that the purpose of the intervention was to focus on a decrease in sugar intake and an increase in fiber intake. Furthermore, the change in the control group could also be attributed to the Hawthorne effect: when participants enrolled in the study, some became motivated to make these dietary changes on their own, knowing that they would be observed. Further analyses are warranted to explore whether intrinsic motivation and other psychosocial variables at baseline predict changes in sugar and/or fiber intake. In addition, these results prompt the question of whether it is necessary to conduct elaborate interventions in people who might already be intrinsically motivated to change.
Regardless of intervention group, participants who were able to reduce sugar intake and/or increase fiber intake showed notable metabolic improvements related to risk reduction for type 2 diabetes. Although there was overlap in sugar and fiber intake improvement, we found that reductions in sugar intake were more related to glucose and insulin indexes whereas increases in fiber intake were more related to adiposity parameters. A reduction in visceral fat indicates a reduction in risk for type 2 diabetes, considering that to a greater degree than total body fat, visceral fat has been shown to be negatively associated with SI.15 In addition, a reduction in insulin response to oral glucose likely indicates a reduction in insulin secretion in response to an increase in SI. If SI increases, less insulin is required and insulin secretion decreases. Accordingly, insulin IAUC has been shown to be an indirect index of SI.16 Although not significant, we also saw an increase of 0.3 in SI, as measured by intravenous glucose tolerance test, in the participants who reduced their sugar intake as compared with an increase of only 0.02 in those who increased their sugar intake.
It is worthwhile to explore why we saw significant results in outcomes associated with the OGTT, namely glucose and insulin IAUC, but not in indexes from the intravenous glucose tolerance test, namely SI, AIR, and DI, though the directionality of the results was consistent. One explanation for the modest changes in the intravenous glucose tolerance test measures could be the relatively short intervention period of 16 weeks. The body is more responsive to oral delivery of glucose considering that it is a more natural condition, and the oral response includes mechanisms that are not triggered during intravenous delivery, such as the release of gastrointestinal hormones that facilitate insulin secretion from the beta cells after eating.17
Our results add to the literature in that we are the first, to our knowledge, to test an intensive randomized control intervention focused on quality of carbohydrates in overweight Latino adolescents and to find that reductions in sugar intake and increases in fiber intake have associated metabolic benefits. These findings are consistent with the adult literature, in which prospective studies have shown that added sugar intake is a risk factor for the development of type 2 diabetes while fiber intake is a protective factor. As far as we know, no other interventions besides our pilot study18 have tested a specific high-fiber intervention with youth, although it has been shown cross-sectionally in youth that whole-grain consumption is associated with lower BMI and increased insulin sensitivity.19 Other investigators have shown beneficial metabolic results from interventions targeting sweetened beverages with adolescents of other ethnicities. For example, in a school-based intervention with Zuni adolescents aimed to reduce consumption of soft drinks, Teufel and Ritenbaugh20 found a reduction in fasting and 30-minute insulin levels in students after a 2-year intervention. In a randomized controlled pilot study with an ethnically diverse group of adolescents, Ebbeling et al21 found that reductions in sugar-sweetened beverage consumption were associated with a reduction in BMI, specifically in adolescents who had the highest BMI values at baseline. This particular finding parallels what we show in the present analysis, considering that participants who reduced their added sugar intake had a marginally higher BMI at baseline and a significantly higher baseline consumption of added sugar. Perhaps youth with more room for improvement at the time of enrollment have better responses to dietary interventions. In comparison with the findings of Ebbeling et al, we only found a small, nonsignificant decrease in BMI in the participants who reduced their added sugar intake. However, our study was 16 weeks while the Ebbeling et al study was 25 weeks, focused entirely on decreasing sugar-sweetened drink intake, and included the weekly provision of alternative beverages. In the Ebbeling et al study, insulin and glucose indexes were not reported; therefore, we are unable to compare results for these parameters.
In conclusion, through this secondary analysis of response to a 16-week intervention, we found that overweight Latino youth who decreased added sugar intake or increased fiber intake showed stronger improvements in risk factors for type 2 diabetes, specifically in insulin response to an oral glucose challenge or in visceral fat. Modest changes in sugar and fiber consumption, equivalent to omitting 1 can of soda or adding 1 serving of beans daily, could lead to substantial improvements in adiposity and metabolic parameters. Furthermore, given that the control group demonstrated similar dietary changes as the intervention groups, our results suggest that intensive interventions may not be necessary to achieve modifications in sugar and fiber intake. Accordingly, nutritional guidance given in the primary care or community setting may be sufficient to promote the suggested dietary changes in some individuals. In addition, policies that promote reduced intake of added sugar and increased intake of fiber could be effective public health strategies for the prevention of type 2 diabetes in this high-risk population.
Acknowledgments
Funding/Support: This work was supported by National Cancer Institute Cancer Control and Epidemiology Research Training Grant T32 CA 09492, University of Southern California Center for Transdisciplinary Research on Energetics and Cancer grant U54 CA 116848, National Institute of Child Health and Human Development grant RO1 HD/HL 33064, the Dr Robert C. and Veronica Atkins Foundation, and National Center for Research Resources/National Institutes of Health grant M01 RR 00043.
Role of the Sponsors: None of the funders participated in the design or conduct of the study or the collection, management, analysis, or interpretation of the data.
Additional Contributions: We would like to thank project manager Christina Ayala, MPH, and the SANO LA team as well as the nursing staff at the General Clinical Research Center. In addition, we are grateful to our study participants and their families for their involvement.
Trial Registration: clinicaltrials.gov Identifier: NCT00697580
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Goran MI, Bergman R, Cruz M, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25(12):2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 3.Cruz ML, Weigensberg MJ, Huang T, Ball GDC, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89(1):108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 4.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28(10):2519–2524. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 5.Ventura EEDJ, Alexander KE, Shaibi GQ, et al. Dietary intake and the metabolic syndrome in overweight Latino children. J Am Diet Assoc. 2008;108(8):1355–1359. doi: 10.1016/j.jada.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JN, Ventura E, Weigensberg M, et al. The relation of sugar intake to beta-cell function in overweight Latino children. Am J Clin Nutr. 2005;82(5):1004–1010. doi: 10.1093/ajcn/82.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JN, Ventura EE, Shaibi GQ, et al. Reduction in added sugar intake and improvement in insulin secretion in overweight Latina adolescents. Metab Syndr Relat Disord. 2007;5(2):183–193. doi: 10.1089/met.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbeling CB, Leidig MM, Sinclair KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am J Clin Nutr. 2005;81(5):976–982. doi: 10.1093/ajcn/81.5.976. [DOI] [PubMed] [Google Scholar]
- 9.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reducedglycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157(8):773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 10.Davis JN, Kelly LE, Lane CJ, et al. Randomized control trial of a nutrition education and strength training program to prevent obesity related diseases in overweight Latino adolescents. Obesity. In press. [Google Scholar]
- 11.Centers for Disease Control and Prevention . 2000 CDC Growth Charts. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; Atlanta, GA: 2000. US Publication 314. [Google Scholar]
- 12.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ADA Type 2 diabetes in children and adolescents. Pediatrics. 2000;105(3 pt 1):671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 15.Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25(9):1631–1636. doi: 10.2337/diacare.25.9.1631. [DOI] [PubMed] [Google Scholar]
- 16.Weigensberg MJ, Cruz ML, Goran MI. Association between insulin sensitivity and post-glucose challenge plasma insulin values in overweight Latino youth. Diabetes Care. 2003;26(7):2094–2099. doi: 10.2337/diacare.26.7.2094. [DOI] [PubMed] [Google Scholar]
- 17.Campioni M, Toffolo G, Shuster LT, Service FJ, Rizza RA, Cobelli C. Incretin effect potentiates beta-cell responsivity to glucose as well as to its rate of change: OGTT and matched intravenous study. Am J Physiol Endocrinol Metab. 2007;292(1):E54–E60. doi: 10.1152/ajpendo.00033.2006. [DOI] [PubMed] [Google Scholar]
- 18.Davis JN, Ventura EE, Alexander KE, et al. Feasibility of a home-based versus classroom-based nutrition intervention to reduce obesity and type 2 diabetes in Latino youth. Int J Pediatr Obes. 2007;2(1):22–30. doi: 10.1080/17477160601133077. [DOI] [PubMed] [Google Scholar]
- 19.Steffen LM, Jacobs DR, Jr, Murtaugh MA, et al. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am J Epidemiol. 2003;158(3):243–250. doi: 10.1093/aje/kwg146. [DOI] [PubMed] [Google Scholar]
- 20.Teufel NI, Ritenbaugh C. Development of a primary prevention program: insight gained in the Zuni Diabetes Prevention Program. Clin Pediatr (Phila) 1998;37(2):131–141. doi: 10.1177/000992289803700211. [DOI] [PubMed] [Google Scholar]
- 21.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117(3):673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]