Abstract
Background
Geographical differences in amyotrophic lateral sclerosis (ALS) incidence have been reported in the literature, but comparisons across previous studies are limited by different methods in case ascertainment and by the relatively small size of the studied populations. To address these issues, the authors undertook a pooled-analysis of European population-based ALS registries.
Methods
All new incident ALS cases in subjects 18 years old and older were identified prospectively in six population-based registries in three European countries (Ireland, United Kingdom, Italy) in the two year period 1998-1999 with a reference population of almost 24 million.
Results
Based on 1,028 identified incident cases, the crude annual incidence rate of ALS in the general European population was 2.16 per 100,000 person-years; 95% CI 2.0-2.3), with similar incidence rates across all registries. The incidence was higher among men (3.0 per 100,000 person-years; 95% CI = 2.8 to 3.3) than among women (2.4 per 100,000 person-years; 95% CI=2.2 to 2.6). Spinal onset ALS was more common among men compared to women, particularly in the 70-80 year age group. Disease occurrence decreases rapidly after 80 years of age.
Conclusions
ALS incidence is homogeneous across Europe. Sex differences in incidence may be explained by the higher incidence of spinal onset ALS among males and the age-related disease pattern suggests that ALS occurs within a susceptible group within the population rather than being a disease of aging.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease of unknown aetiology that is characterized by rapidly progressive paralysis leading ultimately to death within three to five years of symptom onset. The field of ALS epidemiology matured in the early 1990's when a number of population-based registers were established to prospectively determine the incidence of ALS in defined geographical regions. In contrast to previous retrospective and mortality studies, the incidence rates published by these registries were remarkably similar, ranging from 1.7 to 2.3 cases per 100,000 per year.1-8 This relative homogeneity is due to a confluence of factors inherent to registry design: first, each registry utilized prospective ascertainment of cases (with consequent minimization of loss to follow-up); second, all registries employed multiple sources of information to ensure complete case ascertainment within their catchment area; third, each subject was followed over the course of illness to confirm or refute the diagnosis of ALS and to facilitate collection of detailed clinical data concerning the natural history of the disease; finally, a consensus diagnostic criteria for ALS had been established in 1990, which allowed standardization of the diagnosis of ALS across the different registers.9
Despite the methodological standards employed by each of these prospective, population-based studies, each individual register is limited by the small size of its catchment population which restricts the number of newly diagnosed cases ascertained each year, and by the nature of its healthcare system. For example, the Irish population in 2000 was ∼4 million, meaning that the Irish ALS register only enrolls ∼70 incident cases per year. Because of these limitations of the individual registers, several important epidemiological questions remain unanswered: does the incidence of ALS decrease among the very elderly (suggesting a decrease in the pool of susceptible persons in the general population) or does the incidence continue to increase with age (supporting the hypothesis that motor neuron degeneration is part of the aging process of the CNS)?; is the incidence of ALS in women truly lower than in men, and if so, are gender differences uniform across ALS subtype and age group?10 A clearer understanding of ALS epidemiology would undoubtedly provide useful insights into genetic or environmental risk factors that may be relevant to disease pathogenesis.
To address these important issues and to overcome size limitations of the individual registers, we combined the epidemiological data collected by six European ALS registers for the two year period, 1998 – 1999. These registers included the Irish ALS Register (catchment population of 3.9 million Irish residents), the Scottish MND Register (5.1 million Scottish residents), the Lancashire register (1.6 million residents in NorthWest England), the Piemonte register (4.3 million residents in Northern Italy), the Lombardy register (4.9 million residents in Northern Italy) and the Puglia register (4.1 million residents in Southern Italy). In this paper we describe the epidemiology of ALS in this large dataset comprising almost 24 million people collected across Europe.
Methods
ALS Registries
This study was based on data from six prospective, population-based ALS registers (see Table 1 for details). Each registry utilized multiple sources of information to ensure complete case ascertainment of individuals diagnosed with ALS within their defined geographic area, and identified cases were prospectively followed over the course of their disease. The provision of free or heavily subsidized public medicine in Ireland, the United Kingdom and Italy ensured that the entire population at risk of ALS was likely to visit a neurologist at some stage during their illness and thus be ascertained by their respective register. Using the six ALS registers, we identified all residents in the catchment populations in whom ALS was diagnosed during the two year period from January 1, 1998, to December 31, 1999.
Table 1.
Methodological details of the six ALS Registers used to estimate epidemiology of ALS in Europe.
| Sources of case information | Population size | Year case ascertainment commenced | Area (km2) | |
|---|---|---|---|---|
| Ireland4,8 | ALS specialist clinics, neurologists, neurophysiologists, neuropathologists, neurosurgeons, primary care physicians, and local charitable organizations | 3,917,203 (2002 census) | 1993 | 70,273 |
| Lancashire, UK3 | ALS specialist clinics, neurologists, neurophysiologists, hospital discharge databases | 1,623,667 (2001 census) | 1986 | 5,706 |
| Lombardy, Italy7 | ALS specialist clinics, neurologists, neurophysiologists, hospital discharge databases, and local charitable organizations | 4,947,554 (2001 census) | 1998 | 23,851 |
| Piemonte, Italy5 | ALS specialist clinics, neurologists, and hospital discharge databases | 4,341,733 (2006 est.) | 1995 | 28,662 |
| Puglia, Italy6 | ALS specialist clinics, neurologists, hospital discharge database, and local charitable organizations | 4,086,613 (2001 census) | 1998 | 19,357 |
| Scotland1,2 | ALS specialist clinics, neurologists, neurophysiologists, hospital discharge databases, mortality databases, and local charitable organizations | 5,062,011 (2001 census) | 1989 | 78,772 |
Diagnostic and residency criteria
All patients fulfilling the diagnostic criteria for suspected, possible, probable or definite ALS according to the original El Escorial criteria were included in the current study.9 Cases of progressive bulbar palsy, progressive muscular atrophy and primary lateral sclerosis were also included among the incident cases for this study. All patients underwent neurophysiological testing on at least one occasion. Patients for this study must have established residency in their respective catchment area for at least one year before diagnosis, thus excluding those who might have migrated into the area for diagnosis or treatment.
Data management
Demographic and clinical data were available for each individual diagnosed with ALS included in the current study. Site of symptom onset was recorded as either spinal-onset or bulbar-onset disease. Generalized-onset disease was classified as bulbar-onset disease by convention. Age at time of symptom onset and age at diagnosis were recorded as whole years. Delay in diagnosis was the period between symptom onset and establishment of the diagnosis of ALS. Anonymized data were submitted to a central coordinating office at Mario Negri Institute in Milan, Italy for data analysis. Statistical analyses were performed using SAS version 9.1 (Cary, NC). The collection of patient clinical data for each register was approved by the appropriate local Ethical Committee.
Data analysis
The entire population of each catchment area older than 18 years was considered to be at risk of developing ALS. The lower age limit was defined by the El Escorial criteria to avoid diagnostic confusion with juvenile onset motor neuron diseases.9 Age- and gender-specific incidence rates for the European population were calculated from the observed numbers of new cases for all six registers combined, divided by the combined age- and gender-specific person-years of observation estimated from census data for each geographical region. Rates were also calculated separately for individual catchment regions. To compare the incidence rates of ALS between these six geographical regions, differences in the demographic structure between populations were corrected by adjusting the incidence rates of each population to the 2000 European population using the direct method.11 Ninety-five percent confidence intervals were based on Poisson distribution or on normal approximation to the Poisson.
Results
During the two-year study period from January 1, 1998 to December 31, 1999, 1,028 individuals residing within the six catchment areas were diagnosed as having suspected, possible, probable or definite ALS. Of these, 154 patients were Irish residents, 231 patients were Scottish, 54 resided in Lancashire& South Cumbria (UK), and 589 were Italian patients residing in Piemonte (n = 265), Lombardy (n = 194) and Puglia (n = 130). The combined catchment population of the six ALS registers was 47.7 million person-years.
Incidence of ALS in Europe
Based on the 1,028 newly diagnosed cases, the average annual crude incidence rate of ALS in Europe was 2.16 per 100,000 person-years (95% CI=2.0 to 2.3). The average annual crude incidence rate for the European population 18 years and over was 2.7 per 100,000 person-years (95% CI=2.5 to 2.9, Table 2).
Table 2.
Age and gender-specific incidence of ALS per 100,000 person-years among those 18 years and over based on combined data from the six European ALS Registers for the two year period, 1998 - 1999
| Males | Females | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agea | Casesb | Person-yearc | Incidenced | 95% CI | Cases | Person-year | Incidence | 95% CI | Cases | Person-year | Incidence | 95% CI |
| 18-19 | 2 | 596,328 | 0.3 | 0.0-0.8 | 0 | 571,361 | - | - | 2 | 1,167,688 | 0.2 | 0.0-0.4 |
| 20-24 | 1 | 1,563,778 | 0.1 | 0.0-0.2 | 1 | 1,525,677 | 0.1 | 0.0-0.2 | 2 | 3,089,455 | 0.1 | 0.0-0.2 |
| 25-29 | 6 | 1,720,225 | 0.3 | 0.1-0.6 | 6 | 1,706,432 | 0.4 | 0.1-0.6 | 12 | 3,426,657 | 0.4 | 0.2-0.5 |
| 30-34 | 5 | 1,856,398 | 0.3 | 0.0-0.5 | 2 | 1,859,155 | 0.1 | 0.0-0.3 | 7 | 3,715,553 | 0.2 | 0.0-0.3 |
| 35-39 | 11 | 1,869,135 | 0.6 | 0.2-0.9 | 3 | 1,885,445 | 0.2 | 0.0-0.3 | 14 | 3,754,580 | 0.4 | 0.2-0.6 |
| 40-44 | 24 | 1,693,896 | 1.4 | 0.8-2.0 | 14 | 1,710,534 | 0.8 | 0.4-1.2 | 38 | 3,404,430 | 1.1 | 0.8-1.5 |
| 45-49 | 35 | 1,565,127 | 2.2 | 1.5-3.0 | 23 | 1,582,262 | 1.5 | 0.9-2.0 | 58 | 3,147,389 | 1.8 | 1.4-2.3 |
| 50-54 | 38 | 1,606,043 | 2.4 | 1.6-3.1 | 37 | 1,615,772 | 2.3 | 1.6-3.0 | 75 | 3,221,815 | 2.3 | 1.8-2.9 |
| 55-59 | 77 | 1,348,148 | 5.7 | 4.4-7.0 | 39 | 1,385,152 | 2.8 | 1.9-3.7 | 116 | 2,733,300 | 4.2 | 3.5-5.0 |
| 60-64 | 72 | 1,280,496 | 5.6 | 4.3-6.9 | 79 | 1,367,302 | 5.8 | 4.5-7.1 | 151 | 2,647,798 | 5.7 | 4.8-6.6 |
| 65-69 | 95 | 1,098,073 | 8.7 | 6.9-10.4 | 84 | 1,261,865 | 6.7 | 5.2-8.1 | 179 | 2,359,938 | 7.6 | 6.5-8.7 |
| 70-74 | 96 | 900,352 | 10.7 | 8.5-12.8 | 76 | 1,170,278 | 6.5 | 5.0-8.0 | 172 | 2,070,630 | 8.3 | 7.1-9.5 |
| 75-79 | 63 | 649,547 | 9.7 | 7.3-12.1 | 69 | 990,265 | 7.0 | 5.3-8.6 | 132 | 1,639,811 | 8.0 | 6.7-9.4 |
| 80-84 | 24 | 324,676 | 7.4 | 4.4-10.3 | 24 | 602,654 | 4.0 | 2.4-5.6 | 48 | 927,330 | 5.2 | 3.7-6.6 |
| >85 | 5 | 245,499 | 2.0 | 0.3-3.8 | 17 | 643,615 | 2.6 | 1.4-3.9 | 22 | 889,113 | 2.5 | 1.4-3.5 |
| Total | 554 | 18,317,722 | 3.0 | 2.8-3.3 | 474 | 19,877,768 | 2.4 | 2.2-2.6 | 1028 | 38,195,487 | 2.7 | 2.5-2.9 |
Age at diagnosis
Number of cases of ALS diagnosed in the six European ALS registers for the two year period, 1998-1999
combined person-years for the six European for the two year period, 1998-1999
per 100,000 person-years
Male and female incidence of ALS in Europe
There was a predominance of men among the ALS incident cases: 554 versus 474 women. The male and female annual crude incidence rates in the population 18 years and over were 3.0 (95% CI=2.8 to 3.3) and 2.4 per 100,000 person-years (95% CI=2.2 to 2.6; ratio of male: female incidence rates = 1.3, Table 2). To explore the possibility that the apparent male/female differences were due to gender-based variations in demographic structure, we directly adjusted the male and female incidence rates to the 2000 European population. The rate of ALS remained higher among men than among women after adjustment, (3.1 and 2.2 per 100,000 person-years among men and women after adjustment; male:female incidence rate ratio = 1.4).
Table 2 shows the age- and gender-specific incidence rates for the combined European cohort. Incidence rates generally increased with advancing age in both men and women, increasing rapidly after the age of 40 years, reaching a peak at 70 to 74 years for men and 65-69 years for women and declining thereafter. Median age at diagnosis of ALS was 65.2 years (interquartile range, 56.0 to 72.2 years) for men and 67.0 years (interquartile range, 59.0 to 74.0 years) for women. The variability of the estimates (i.e. the 95% CI) increased among the elderly due to the smaller numbers of cases diagnosed among this demographic group: there were 68 patients over age 80 representing 6.6% of the total cohort and only 22 patients were older than 85 years of age (1.9% of total cohort).
Clinical features of ALS in Europe
Clinical features of the incident cases for those aged 18 years and over are shown in Table 3. 634 (61.2%) of the 1,028 ALS cases presented with purely limb symptoms, 310 (30.1%) had bulbar or generalized symptoms at onset and the site of symptom onset was unspecified among the remaining 84 (8.2%) patients. Age- and gender-specific incidence rates of generalized or bulbar ALS were similar among men and women with only a slightly higher incidence among women in the oldest age group (Figure 1). In contrast, the incidence of spinal onset ALS was significantly higher for men compared to women, particularly between the ages of 65 and 84 years when the incidence of ALS reached its peak (Figure 1).
Table 3.
Demographic and clinical details of ALS incident cases for the two year period, 1998 - 1999, classified by region.
| Country (Region) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Piemonte (N=265) | Lombardy (N=194) | Puglia (N=130) | Ireland (N=154) | Scotland (N=231) | Lancashire (N=54) | Total (N=1028) | ||
| Age (Mean ± SD) | 65.9±10.8 | 61.9±12.6 | 62.5±11.5 | 63.2±15.3 | 66.5±11.7 | 65.4±10.6 | 64.4±12.3 | |
| Median Time, days, to Diagnosis (IQR) | 240 (150-390) | 255 (159-372) | 271 (180-540) | 280 (180-495) | 270 (180-390) | 389 (267-584) | 270 (175-421) | |
| Sex (Males) | 144 (54.3%) | 104 (53.6%) | 81 (62.3%) | 74 (48.1%) | 125 (54.1%) | 26 (48.2%) | 554 (53.9%) | |
| Familial ALS (%) | 8(3.2%) | Na | 4 (2%) | 7 (4.6%) | Na | Na | Na | |
| EEC 98 | ||||||||
| Definite | 119 (44.9%) | 92 (47.4%) | 29 (22.3%) | 90 (58.4%) | 116 (50.2%) | 3 (5.6%) | 449 (43.7%) | |
| Probable | 79 (29.8%) | 54 (27.8%) | 44 (33.9%) | 37 (24.0%) | 0 (0%) | 17 (31.5%) | 231 (22.5%) | |
| Possible | 67 (25.3%) | 33 (17.0%) | 45 (34.6%) | 10 (6.5%) | 43 (18.6%) | 10 (18.5%) | 208 (20.2%) | |
| Suspected | 0 (0%) | 15 (7.7%) | 12 (9.2%) | 7 (4.6%) | 37 (16.0%) | 24 (44.4%) | 95 (9.2%) | |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 10 (6.5%) | 35 (15.2%) | 0 (0%) | 45 (4.4%) | |
| Site of onset | ||||||||
| Spinal | 182 (68.7%) | 132 (68.0%) | 96 (73.9%) | 76 (49.4%) | 116 (50.2%) | 32 (59.3%) | 634 (61.7%) | |
| Bulbar | 83 (31.3%) | 54 (27.8%) | 25 (19.2%) | 53 (34.4%) | 41 (17.8%) | 22 (40.7%) | 278 (27.0%) | |
| Generalized† | 0 (0%) | 4 (2.1%) | 9 (6.9%) | 19 (12.3%) | 0 (0%) | 0 (0%) | 32 (3.1%) | |
| Unspecified | 0 (0%) | 4 (2.1%) | 0 (0%) | 6 (3.9%) | 74 (32.0%) | 0 (0%) | 84 (8.2%) | |
EEC 98 = El Escorial Classification
Generalized-onset ALS is classified as bulbar-onset ALS by convention
Na: prevalence of familial cases was not available
Figure 1.
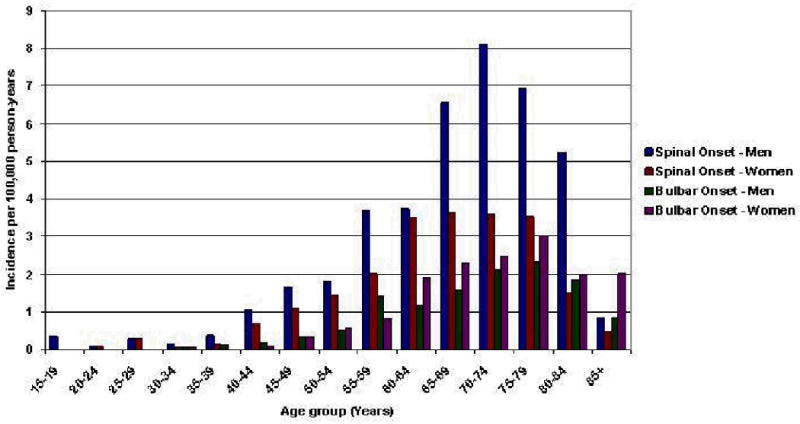
Age- and gender-specific incidence rates of ALS in Europe during the two-year period 1998-1999 classified according to site of onset†
† Generalized-onset ALS is classified as bulbar-onset ALS by convention
The overall (male and female) age-adjusted incidence rate for spinal ALS was 1.7 per 100,000 person-years (95% CI, 1.6 to 1.9). Stratified by gender, we observed an age-adjusted incidence rate for spinal ALS of 2.3 (95% CI, 2.0 to 2.5) for males and 1.3 per 100,000 person-years (95% CI, 1.2 to 1.5) for females. The overall (male and female) age-adjusted incidence rate for bulbar and generalized ALS was 0.8 per 100,000 person-years (95% CI, 0.7 to 0.9). Stratified by gender, we observed an age-adjusted incidence rate for bulbar and generalized ALS of 0.8 per 100,000 person-years (95% CI, 0.6 to 0.9) and 0.8 per 100,000 person-years (95% CI, 0.7 to 0.9).
Two thirds of European ALS patients met the El Escorial criteria for probable or definite ALS at the time of their diagnosis and thus would have been considered eligible for clinical trials (680 patients, 66.1%). However, the percentage of trial eligible patients varied considerably across Europe from 37.0% in Lancashire to 82.5% of patients in Ireland. The mean time from symptom onset to diagnosis across the six ALS registers was 370.9 days (±372.4), while the median time was 270 days (Interquartile range, IQR, 175-421). We did not observe a significant difference in median diagnostic delay between patients with bulbar or generalized onset (median (IQR) = 247 (170.8-388.2) days) compared with those with spinal onset (median (IQR) =278 (176-450) days, Wilcoxon rank sum p=0.22).
Variation in incidence and clinical characteristics of ALS across Europe
The incidence of ALS in each of the six catchment areas is shown in Table 4. After direct adjustment to the 2000 European population, the overall incidence rate of ALS was lowest in Lancashire (1.5 per 100,000 person-years) and highest in Ireland (2.7 per 100,000 person-years). The reference population for the Lancashire was much smaller than in the other five registries, which may have contributed to a less precise estimate. This is reflected by the large 95% confidence interval associated with the Lancashire estimate (95% CI, 1.1 to 1.9).
Table 4.
Crude and adjusted incidence per 100,000 person-years of ALS by catchment area and site of onset
| Registry | Cases | Denominator (person years) | Incidence | 95% CI | Adjusted Incidence | 95% CI |
|---|---|---|---|---|---|---|
| Lancashire | ||||||
| Spinal | 32 | 3,246,334 | 0.9 | 0.6-1.2 | 0.9 | 0.6-1.2 |
| Generalized + Bulbar | 22 | 3,246,344 | 0.6 | 0.4-0.9 | 0.6 | 0.3-0.9 |
| Ireland | ||||||
| Spinal | 76 | 7,455,000 | 1.0 | 0.8-1.3 | 1.3 | 1.0-1.6 |
| Generalized + Bulbar | 72 | 7,455,000 | 1.0 | 0.7-1.2 | 1.3 | 1.0-1.6 |
| Lombardy | ||||||
| Spinal | 132 | 9,895,108 | 1.3 | 1.1-1.6 | 1.2 | 1.0-1.4 |
| Generalized + Bulbar | 58 | 9,895,108 | 0.6 | 0.4-0.7 | 0.5 | 0.4-0.7 |
| Piemonte | ||||||
| Spinal | 182 | 8,815,564 | 2.1 | 1.8-2.4 | 1.7 | 1.5-2.0 |
| Generalized + Bulbar | 83 | 8,815,564 | 0.9 | 0.7-1.1 | 0.8 | 0.6-0.9 |
| Puglia | ||||||
| Spinal | 96 | 7,959,576 | 1.2 | 1.0-1.4 | 1.2 | 1.0-1.4 |
| Generalized + Bulbar | 34 | 7,959,576 | 0.4 | 0.3-0.6 | 0.4 | 0.3-0.6 |
| Scotland | ||||||
| Spinal | 153 | 10,124,022 | 1.5 | 1.3-1.8 | 1.5 | 1.3-1.7 |
| Generalized + Bulbar | 41 | 10,124,022 | 0.4 | 0.3-0.5 | 0.4 | 0.3-0.5 |
The clinical features of ALS in each of the six catchment areas are reported in Table 3. After adjustment, the highest incidence rate of spinal ALS was observed in Piemonte (1.7 per 100,000 person-years, 95% CI=1.5 to 2.0), whereas the lowest was observed in England (0.9 per 100,000 person-years, 95% CI=0.6 to 1.2). The adjusted incidence rate of bulbar ALS was the highest in Ireland compared to its occurrence among those in both Puglia and Scotland. The median age at onset was highest in Scotland and Lancashire (68.7 years and 67.9 years, respectively). Lombardy and Ireland had the lowest median age at onset of all the registries (62.8 years and 64.7 years, respectively, Table 3). The number of familial cases was higher in Ireland compared to Piemonte and Puglia, though family history data was limited to these three regions (Table 3). The mean diagnostic delay was 499.7 days (±359.6) in Lancashire compared to Ireland, which had a mean diagnostic delay of 466.6 days (±566.0). The lowest mean diagnostic delay was observed in Lombardy and Piemonte (321.4 ± 290.3 days and 334.1 ± 290.3 days, respectively, Table 3). After adjustment, the lowest incidence for females was observed in Puglia (1.2 per 100,000 person-years, 95% CI=0.8 to 1.5). Among males, the lowest incidence rate was observed in Lancashire (1.5 per 100,000 person-years, 95% CI=0.9 to 2.1). An incidence of 2.8 per 100,000 person-years for males was observed in both Ireland (95% CI=2.1 to 3.5) and Scotland (95% CI=2.3 to 3.2). The highest incidence among females was observed in Ireland (2.6, 95% CI=2.0 to 3.2). After adjustment, the male:female incidence rate ratio was 1.1 in both Lancashire and Ireland. The highest male:female incidence rate ratio (1.8) was observed in Puglia.
Discussion
This study reports the incidence and clinical features of ALS in Europe based on nearly 50 million person-years of observation collected from six ALS registers that are actively maintained in Ireland, Scotland, England and Italy. The large number of ALS patients collected in this study (n = 1,028) allowed the epidemiology of this fatal neurodegenerative disease to be accurately quantified. Pooling of data was possible because the six European ALS registries utilized matching study designs including multiple sources of information to ensure complete case ascertainment within a large catchment area, longitudinal follow-up of patients to ensure accuracy of the diagnosis of ALS and the application of uniform standard diagnostic criteria.
The current study found the incidence of ALS in Europe to be 2.1 per 100,000 person-years. This figure is very similar to previous reports of ALS incidence at different time intervals from these registers1-8 and from other epidemiological studies based on the US population12, indicating that it is likely to be accurate and representative of the true rate of ALS among white Caucasian populations. It is worth noting that each register employed slightly different sources of information (Table 1) within disparate healthcare systems of different countries across Northern and Southern Europe. This suggests that there was no systematic bias arising from the manner in which registries collect case data within a population. Furthermore, the uniform frequency of ALS suggests that the same environmental and/or genetic factors may underlie disease within the white population.
In previous studies the small number of ALS patients and general population over the age of 80 has hampered accurate measurement of incidence among this age group. Analysis of the 70 cases over the age of 80 reported in the current study clearly demonstrates that the frequency of motor neuron degeneration decreases among the very elderly, and suggests that the pool of susceptible individuals in the population is reduced after a certain age. A similar age pattern has been described in sporadic Alzheimer's disease13 where it is known that the frequency of ApoE4, the major genetic risk factor for the sporadic form of Alzheimer's disease, is lower among the elderly compared to the other age groups in the general population.14;15 Although compelling, the results of the current study should be interpreted cautiously, as case ascertainment among the elderly may be problematic for a number of reasons: exclusion of ALS mimic syndromes is more difficult among this age group; the shorter survival interval associated with disease in the elderly mean that such patients may die before reaching diagnostic neurological services; finally, physicians may avoid an unfavorable diagnosis such ALS in subjects with short life expectancy because of their age.16
The higher incidence of ALS observed among men in our study is consistent with past research, but the magnitude of the difference was smaller than previously reported. Our data indicates that these gender-based differences incidence were largely due to the higher occurrence of limb-onset ALS among men. Limb-onset ALS was two-fold more common among European men than among women at all ages, particularly in the 70 – 80 age group. In contrast, women had only a slighter higher incidence of limb-onset compared to bulbar generalized across age groups. Similar findings have been reported in the Italian and in Irish populations.7;8 Though a precise etiological interpretation is not possible, we speculate that gender-based differences in site of disease onset may be due to the involvement of a gender-related environmental risk factor. For example, repeated traumatic events and strenuous physical activity have been proposed to be risk factors for ALS and are more common in men because of occupational or sport activities.
Some limitations of our study should be considered. Individuals with ALS who did not seek medical attention or who died prior to establishing the diagnosis would not have been enrolled in the registers. This is particularly a concern among the elderly. In subjects with Parkinson disease, door to door surveys have shown that almost 40% of patients remain undiagnosed even in countries with public healthcare systems17, but this method of collecting epidemiological data is not feasible in ALS epidemiological research because of the relatively low incidence of the disease. However, the similarity of ALS incidence figures across Europe, despite the use of different sources of case ascertainment, suggests that the number of undiagnosed cases is low. Furthermore, capture-recapture studies, which is the most reliable assessment of case ascertainment, demonstrates a high ascertainment rate for ALS registers.2;18 Finally, the inclusion of patients diagnosed with progressive muscular atrophy (i.e. suspected ALS according to the El Escorial criteria9) and primary lateral sclerosis (i.e. possible ALS) may have falsely inflated incidence rates across the various registries, as there is debate as to whether these diagnostic entities truly represent ALS. This effect was likely to be minimal, as longitudinal studies indicate that the majority of such cases progress clinically and ultimately meet the criteria to be reclassified as probable or definite ALS.19
Minor differences in study design between the registers are unavoidable and are largely driven by local healthcare infrastructure. The Italian registers utilized a dense network of neurological departments, neurophysiology units and neurologists working in each health district. In Ireland, Scotland and England, the number of neurological facilities and neurologists were limited and the referral patterns were simpler. A family study of ALS is currently underway in Ireland which may explain the higher number of familial ALS cases observed in this region. Perhaps the most striking difference between the registers is the higher occurrence of bulbar-onset ALS in the Irish population compared to the Italian population – this is also reflected in the difference in gender incidence rate ratio between the two regions. It is possible that the rate of bulbar-onset is higher in Ireland than on continental Europe as a function of the slightly higher rates of familial ALS population, though there is no indication that bulbar-onset disease is commoner among familial cases. It is also possible that there are regional differences in how neurologists classify patients as bulbar- or limb-onset disease, and it is noteworthy that the El Escorial diagnostic criteria do not standardize reporting of site of symptom onset.
Finally, our current study reports a similar incidence of ALS among three distinct European sub-populations, namely from Celtic, Anglo-Saxon and Mediterranean regions. Although these distinct ethnic groups are representative of European heritage, it remains possible that the incidence of ALS is different in other European regions such as Central or Eastern Europe, Scandinavia, or Southwestern Europe. Furthermore, the analysis of ALS epidemiology reported in the current study may be relevant only to white Caucasians, whereas the pattern of ALS occurrence among other ethnic groups is largely unknown and requires further investigation.20,21
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging (project Z01 AG000949-02) and the National Institute on Neurological Disorders and Stroke (NINDS), Istituto Superiore di Sanità, Contract n° 526D/8, Rome, Italy, and the ALS Association.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, with the exception of Bryan Traynor who is a NIH, US federal employee, on a worldwide basis to the BMJ Publishing Group Ltd, and its Licensees to permit this article (if accepted) to be published in JNNP and any other BMJPGL products and to exploit all subsidiary rights, as set out in our license.
References
- 1.The Scottish Motor Neuron Disease Register: a prospective study of adult onset motor neuron disease in Scotland. Methodology, demography and clinical features of incident cases in 1989. J Neurol Neurosurg Psychiatry. 1992;55:536–41. doi: 10.1136/jnnp.55.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes RB, Colville S, Parratt J, Swingler RJ. The incidence of motor nueron disease in Scotland. J Neurol. 2007;254:866–9. doi: 10.1007/s00415-006-0454-y. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JD, Gatrell AC, Al Hamad A, Davies RB, Batterby G. Geographical epidemiology of residence of patients with motor neuron disease in Lancashire and south Cumbria. J Neurol Neurosurg Psychiatry. 1998;65:842–7. doi: 10.1136/jnnp.65.6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995-1997: a population-based study. Neurology. 1999;52:504–9. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 5.Incidence of ALS in Italy: evidence for a uniform frequency in Western countries. Neurology. 2001;56:239–44. doi: 10.1212/wnl.56.2.239. [DOI] [PubMed] [Google Scholar]
- 6.Logroscino G, Beghi E, Zoccolella S, Palagano R, Fraddosio A, Simone IL, Lamberti P, Lepore V, Serlenga L. Incidence of amyotrophic lateral sclerosis in southern Italy: a population based study. J Neurol Neurosurg Psychiatry. 2005;76:1094–8. doi: 10.1136/jnnp.2004.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beghi E, Millul A, Micheli A, Vitelli E, Logroscino G. Incidence of ALS in Lombardy, Italy. Neurology. 2007;68:141–5. doi: 10.1212/01.wnl.0000250339.14392.bb. [DOI] [PubMed] [Google Scholar]
- 8.O'Toole O, Traynor BJ, Brennan P, Sheehan C, Frost E, Corr B, Hardiman O. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry. 2008;79:30–2. doi: 10.1136/jnnp.2007.117788. [DOI] [PubMed] [Google Scholar]
- 9.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 10.Beghi E, Logroscino G, Chio A, Hardiman O, Mitchell D, Swingler R, Traynor BJ. The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta. 2006;1762:1150–7. doi: 10.1016/j.bbadis.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Fleiss JL. Statistical methods for rates and proportions. New York: Wiley & Sons; 1981. p. 244. [Google Scholar]
- 12.McGuire V, Longstreth WT, Jr, Koepsell TD, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology. 1996;47:571–3. doi: 10.1212/wnl.47.2.571. [DOI] [PubMed] [Google Scholar]
- 13.Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JC. Apolipoprotein E epsilon4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: The Cache County Study. Arch Gen Psychiatry. 2004;61:518–24. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- 14.Henderson AS, Easteal S, Jorm AF, Mackinnon AJ, Korten AE, Christensen H, Croft L, Jacomb PA. Apolipoprotein E allele epsilon 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387–90. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- 15.Schachter F, Faure-Delanef L, Guenot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 16.Chiò A, Borasio GD. Breaking the news in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:195–201. doi: 10.1080/14660820310017326. [DOI] [PubMed] [Google Scholar]
- 17.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–4. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 18.Chiò A, Ciccone G, Calvo A, Vercellino M, Di Vito N, Ghiglione P, Mutani R. Validity of hospital morbidity records for amyotrophic lateral sclerosis. A population-based study. J Clin Epidemiol. 2002;55:723–7. doi: 10.1016/s0895-4356(02)00409-2. [DOI] [PubMed] [Google Scholar]
- 19.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch Neurol. 2000;57:1171–6. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- 20.Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology. 2007;68:1002–7. doi: 10.1212/01.wnl.0000258551.96893.6f. [DOI] [PubMed] [Google Scholar]
- 21.Zaldivar T, Gutierrez J, Lara G, Carbonara M, Logroscino G, Hardiman O. Reduced frequency of ALS in an ethnically mixed population: a population-based mortality study. Neurology. 2009;72:1640–5. doi: 10.1212/WNL.0b013e3181a55f7b. [DOI] [PubMed] [Google Scholar]


