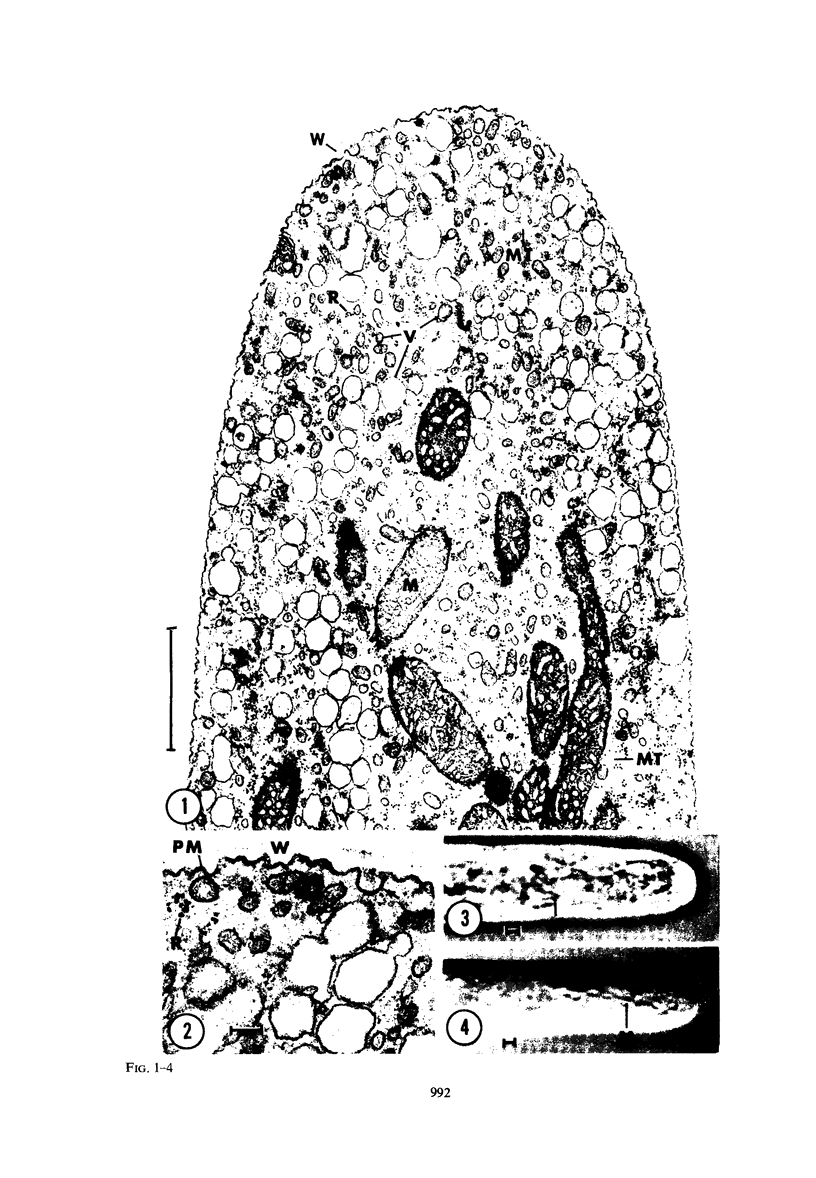
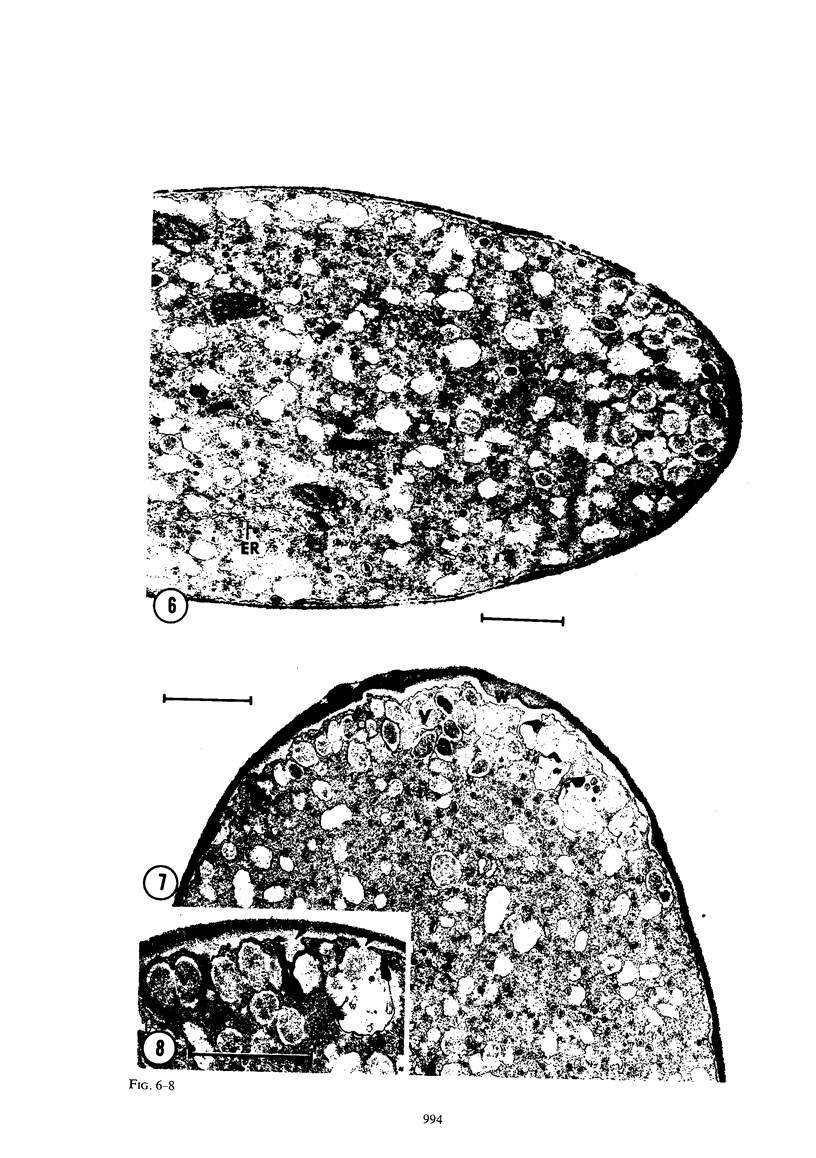
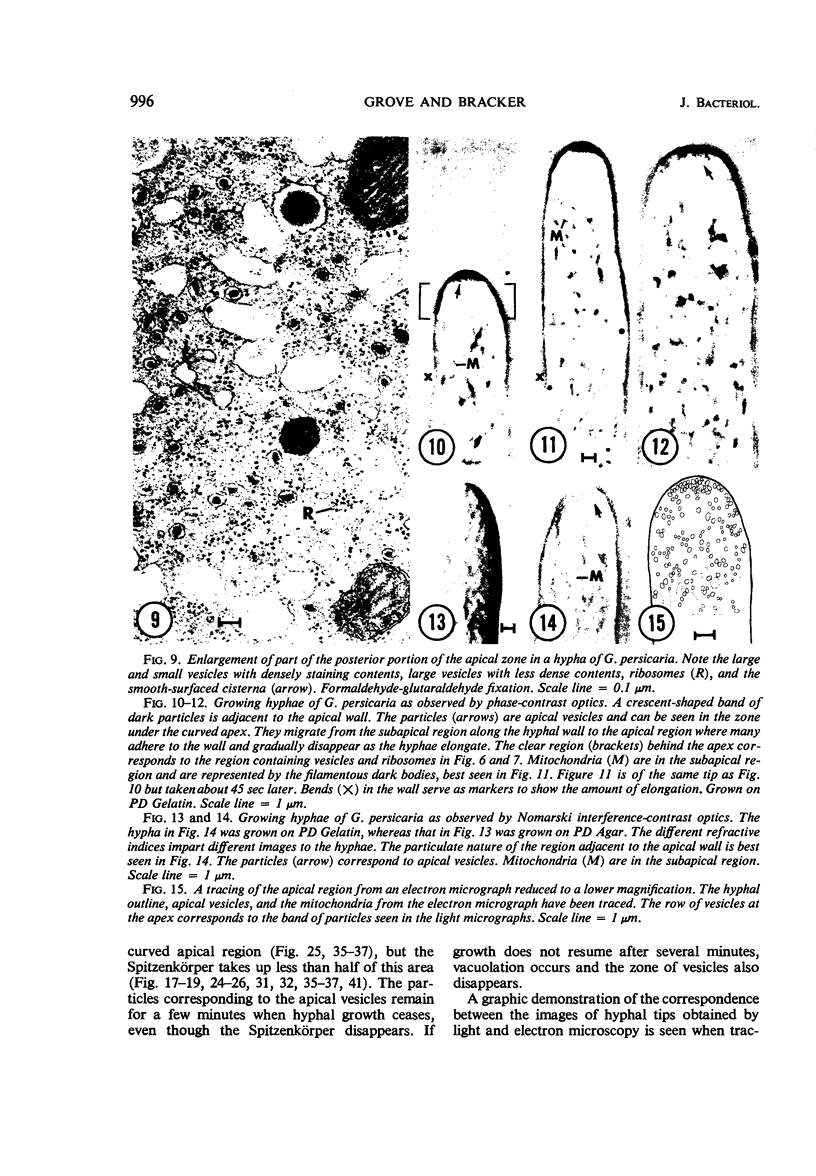
Abstract
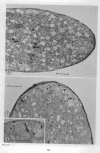
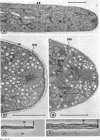
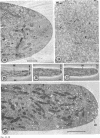
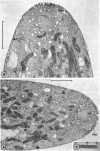
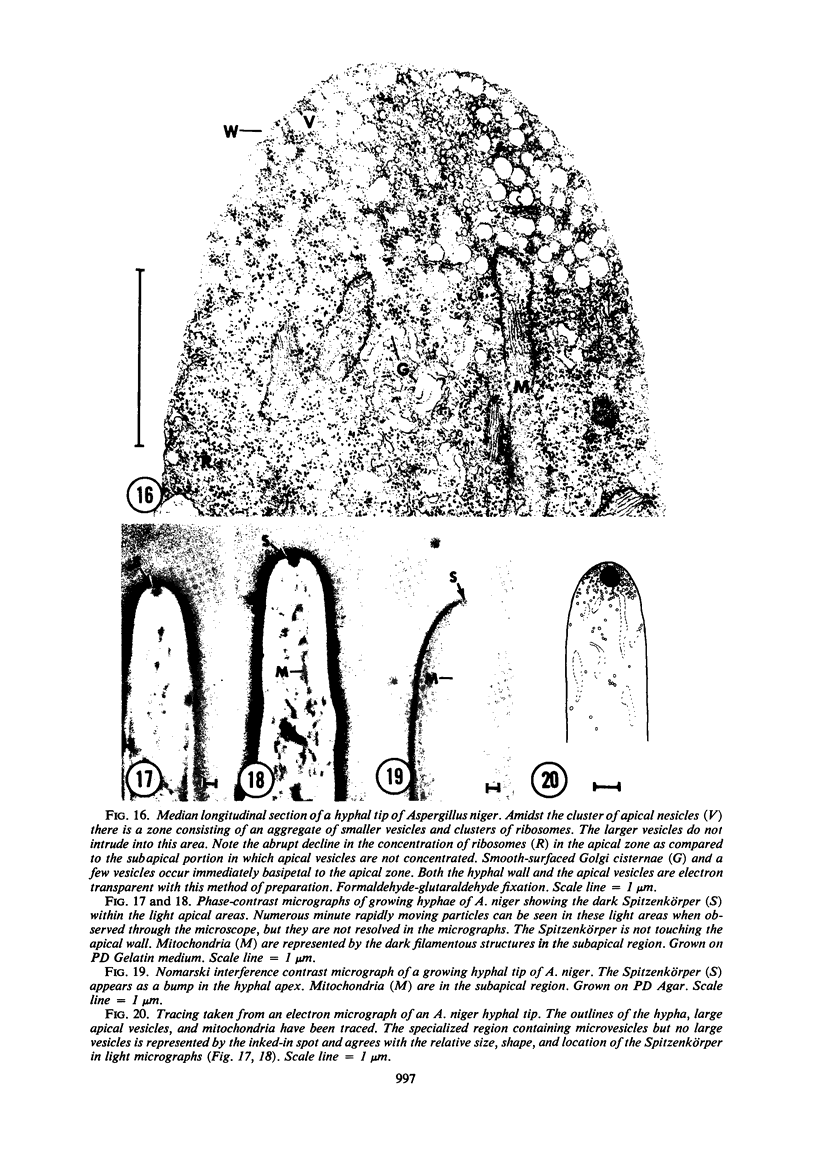
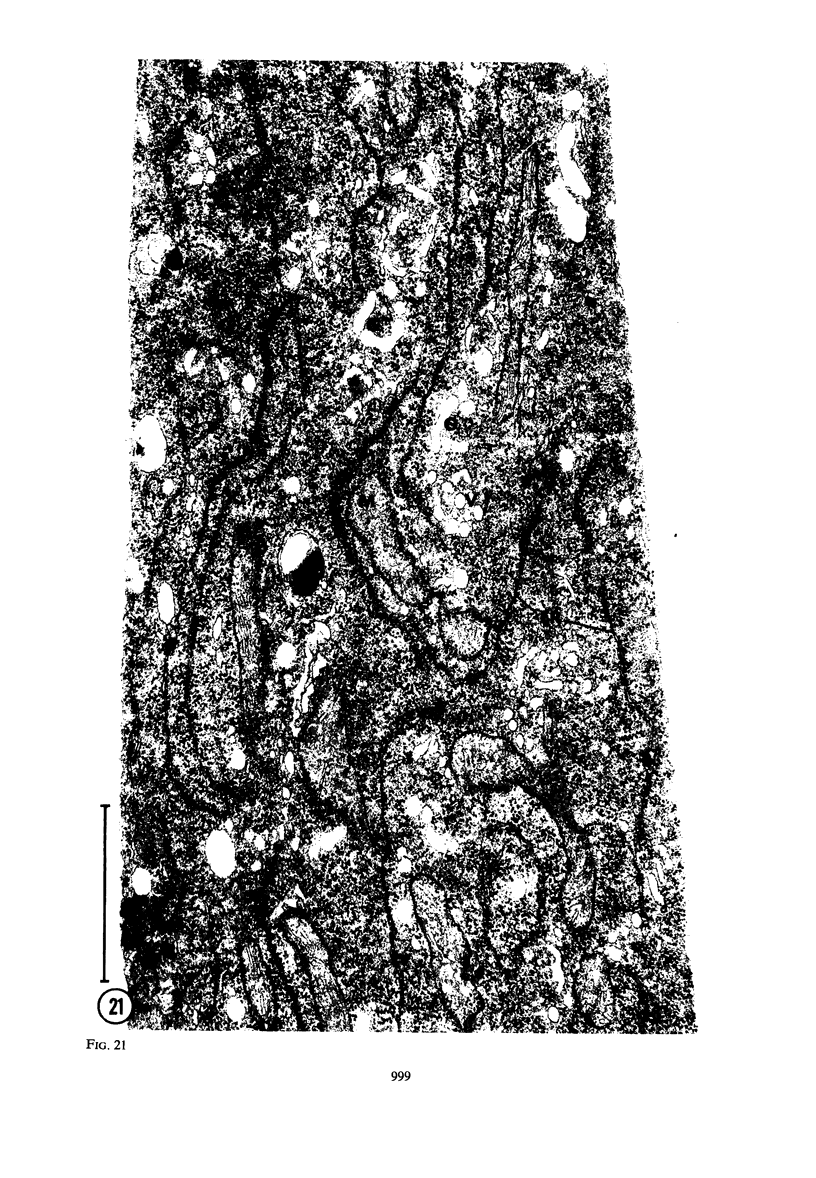
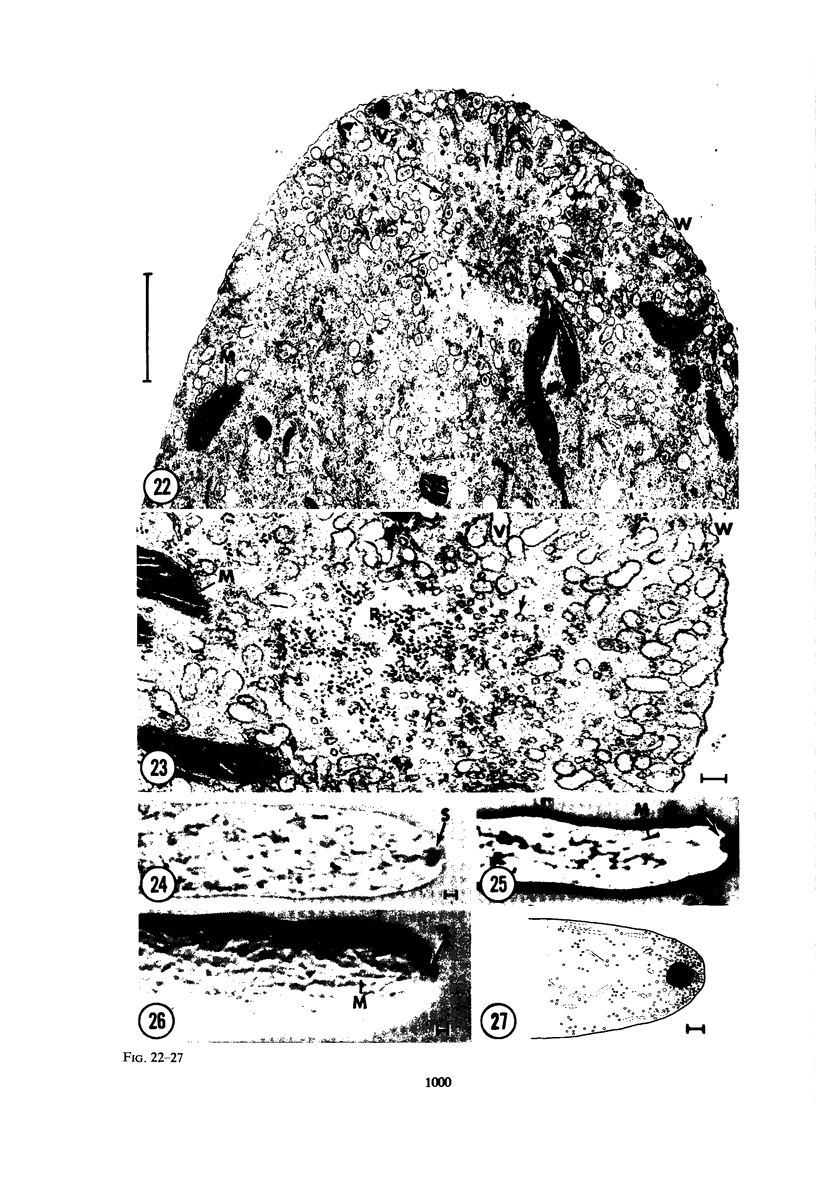
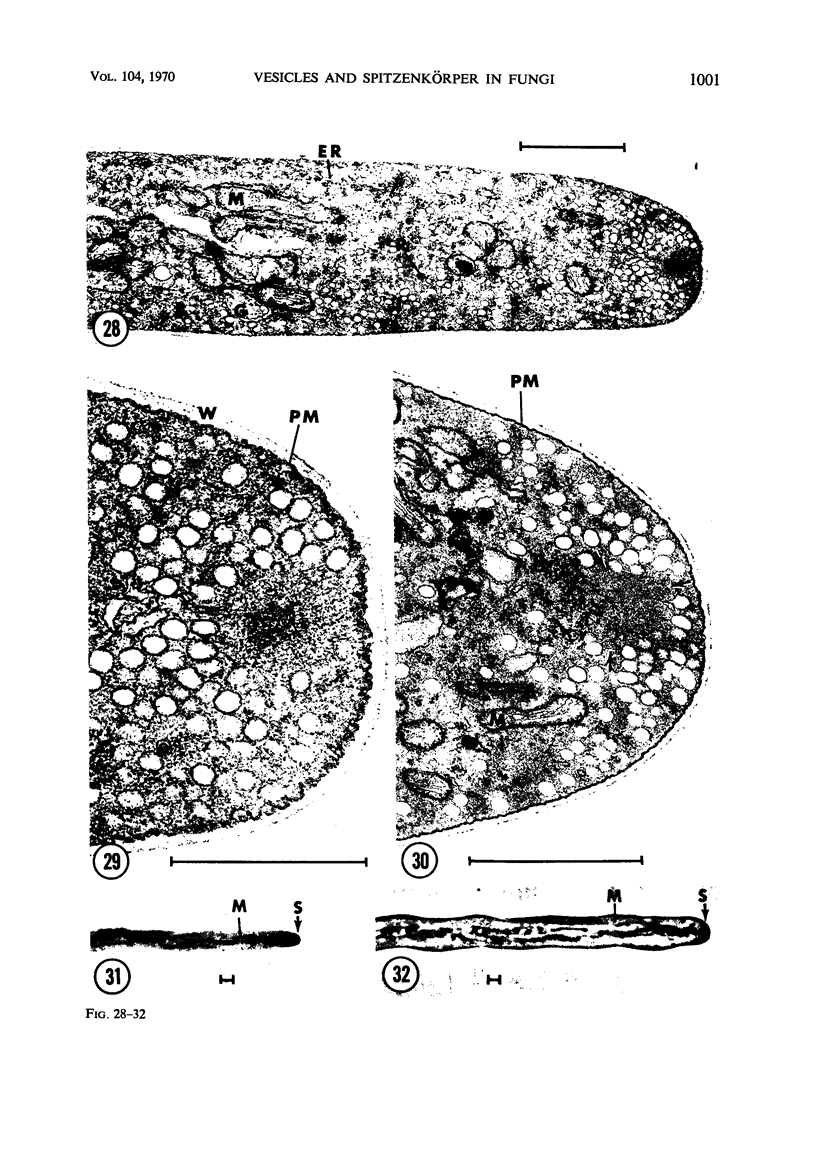
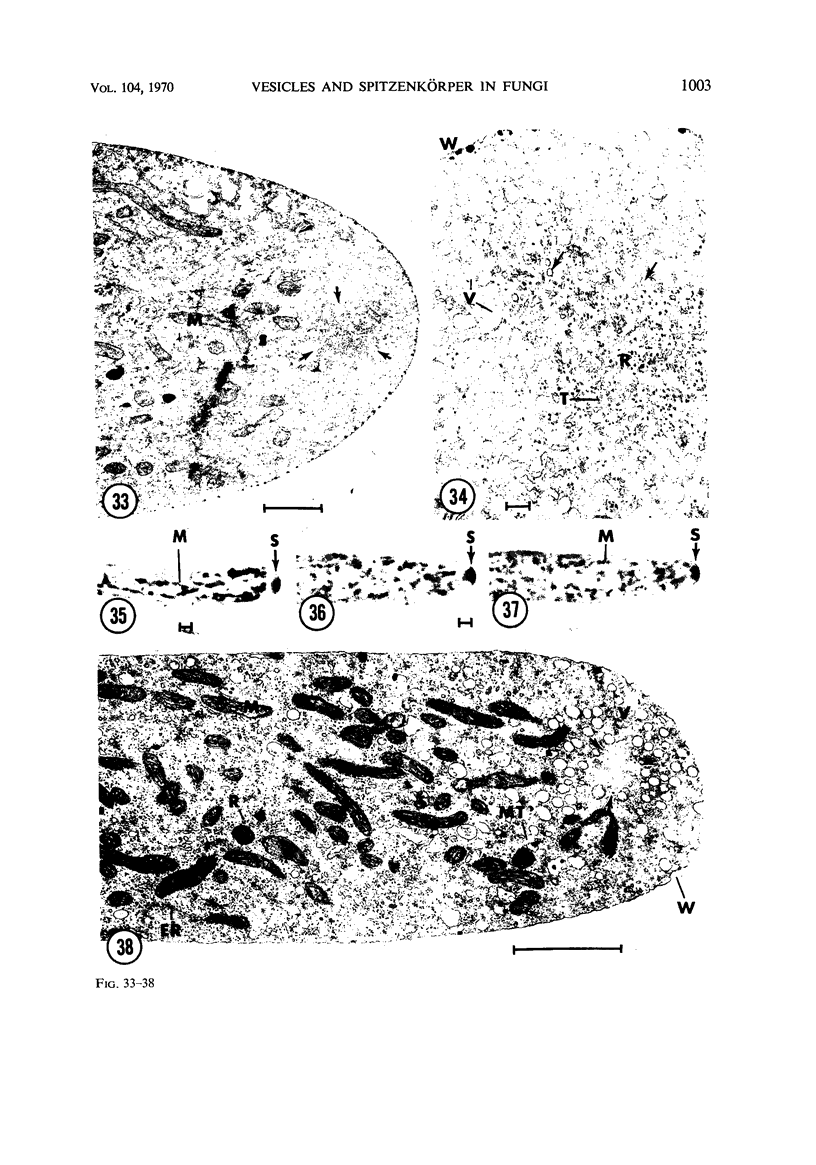
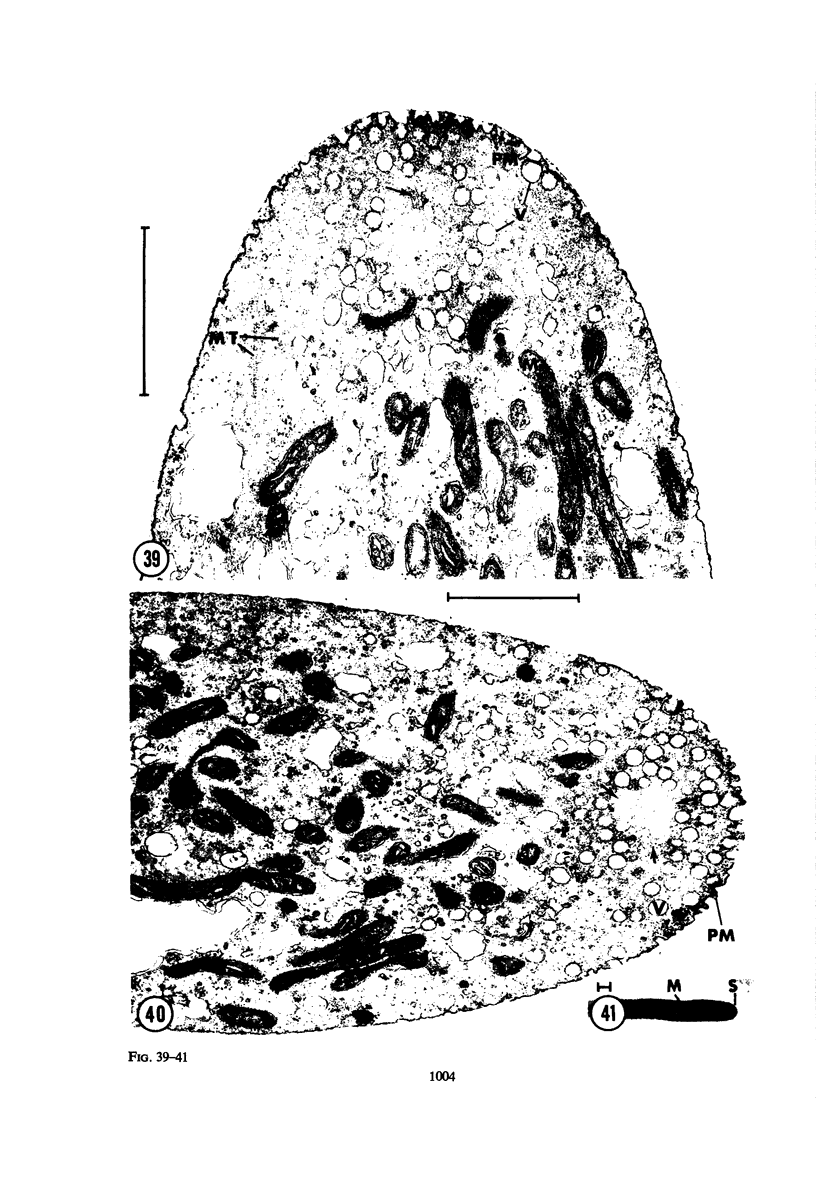
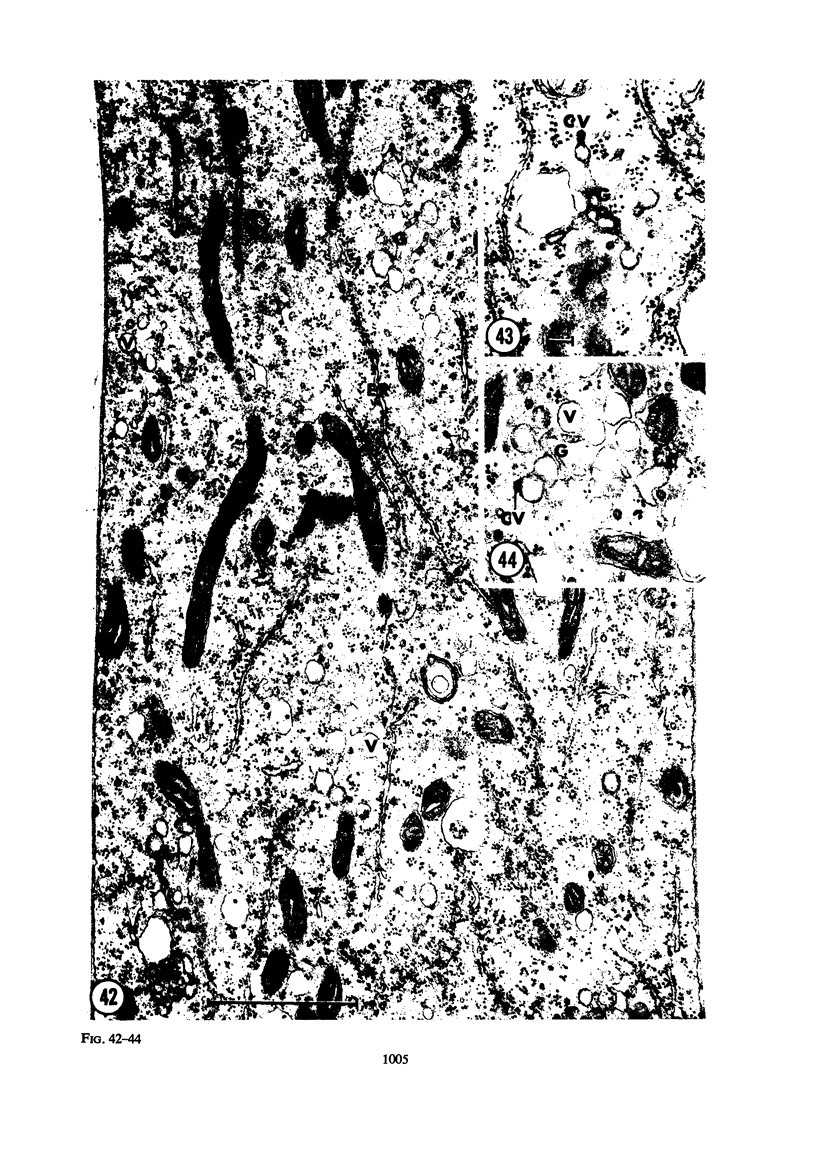
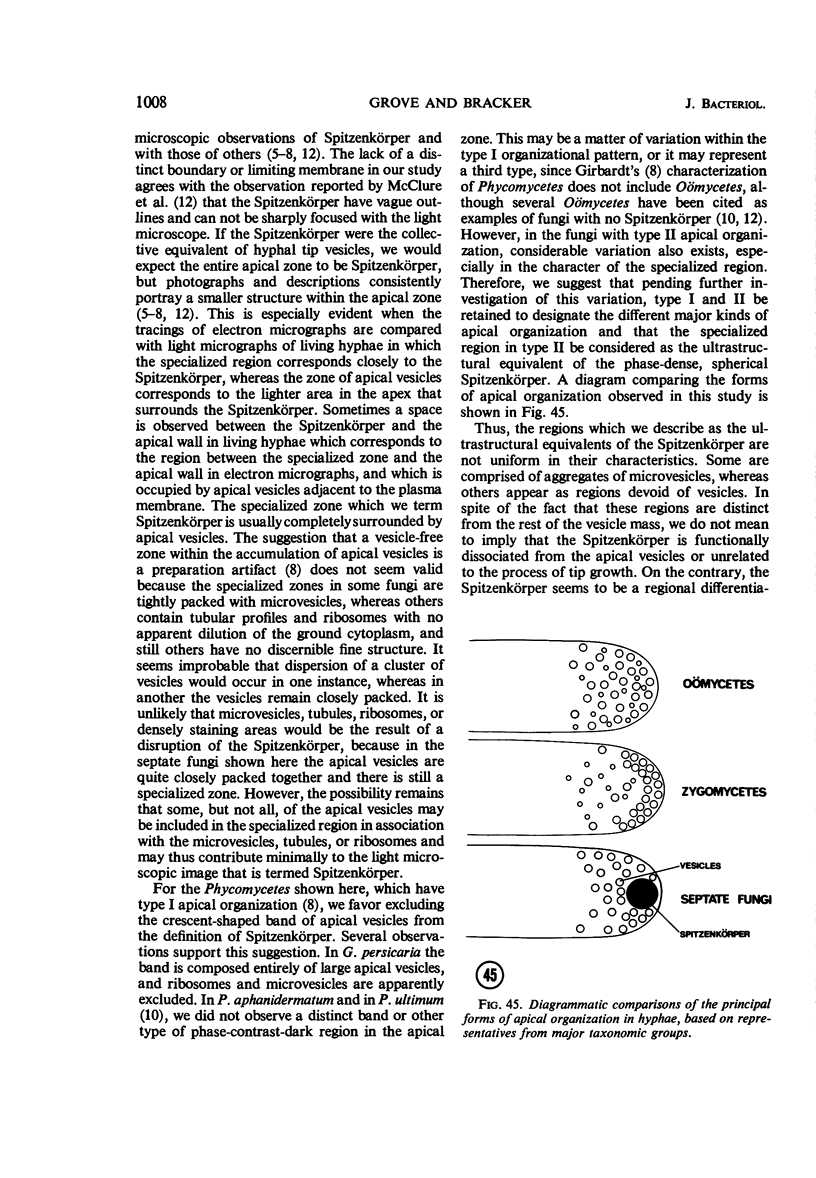
Hyphal tips of fungi representing Oömycetes, Zygomycetes, Ascomycetes, Basidiomycetes, and Deuteromycetes were examined by light and electron microscopy and compared with respect to their protoplasmic organization. In all fungi studied, there is a zone at the hyphal apex which is rich in cytoplasmic vesicles but nearly devoid of other cell components. Some vesicle profiles are continuous with the plasma membrane at the apices of these tip-growing cells. The subapical zones of hyphae contain an endomembrane system which includes smooth-surfaced cisternae associated with small clusters of vesicles. The findings are consistent with the hypothesis that vesicles produced by the endomembrane system in the subapical region become concentrated in the apex where they are incorporated at the expanding surface. Septate fungi (Ascomycetes, Basidiomycetes, and Deuteromycetes) have an apical body (Spitzenkörper) which is associated with growing hyphal tips. In electron micrographs of these fungi, an additional specialized region within the accumulation of apical vesicles is shown for the first time. This region corresponds on the bases of distribution among fungi, location in hyphae, size, shape and boundary characteristics to the Spitzenkörper seen by light microscopy. This structure is not universally associated with tip growth, whereas apical vesicles are widespread among tip-growing systems.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S., Nelson N., Cota-Robles E. A novel apical corpuscle in hypahe of Mucor rouxii. J Bacteriol. 1968 Jun;95(6):2399–2402. doi: 10.1128/jb.95.6.2399-2402.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. M., Carroll G. C. Fine-structural correlates of growth in hyphae of Ascodesmis sphaerospora. J Bacteriol. 1968 Feb;95(2):658–671. doi: 10.1128/jb.95.2.658-671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr, Franke W. W., Kleinig H., Falk H., Sitte P. Scale formation in chrysophycean algae. I. Cellulosic and noncellulosic wall components made by the Golgi apparatus. J Cell Biol. 1970 May;45(2):246–271. doi: 10.1083/jcb.45.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E., Morré D. J. Cytomembrane differentiation in the endoplasmic reticulum-Golgi apparatus-vesicle complex. Science. 1968 Jul 12;161(3837):171–173. doi: 10.1126/science.161.3837.171. [DOI] [PubMed] [Google Scholar]
- McClure W. K., Park D., Robinson P. M. Apical organization in the somatic hyphae of fungi. J Gen Microbiol. 1968 Feb;50(2):177–182. doi: 10.1099/00221287-50-2-177. [DOI] [PubMed] [Google Scholar]
- Moor H. Endoplasmic reticulum as the initiator of bud formation in yeast (S. cerevisiae). Arch Mikrobiol. 1967 Jun 6;57(2):135–146. doi: 10.1007/BF00408697. [DOI] [PubMed] [Google Scholar]
- Northcote D. H. Structure and function of plant-cell membranes. Br Med Bull. 1968 May;24(2):107–112. doi: 10.1093/oxfordjournals.bmb.a070609. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps J. D. Further ultrastructural observations on polysaccharide localization in plant cells. J Cell Sci. 1968 Mar;3(1):55–64. doi: 10.1242/jcs.3.1.55. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]