Abstract
An elevated intraocular pressure (IOP) is one of the most important risk factors for the development of glaucoma, which causes progressive optic neuropathy. Lowering IOP is currently the only therapeutic approach to the treatment of glaucoma. Tafluprost, a novel prostaglandin analogue, was recently launched onto the market as an ocular hypotensive agent. Tafluprost is potent in its affinity for the prostanoid FP receptor and in its intraocular lowering efficacy. Moreover, it enhances the ocular hemodynamics and has neuroprotective effects. Clinical studies have demonstrated its efficacy at decreasing intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Keywords: open-angle glaucoma, normal tension glaucoma, receptor affinity, intraocular pressure
Introduction
To date, four prostaglandin (PG) derivatives (unoprostone, latanoprost, travoprost and bimatoprost) have been marketed across the world. In addition, in 2008 tafluprost was marketed in some European countries and in Japan. Prost-type PG derivatives (that is, PG derivatives other than unoprostone) are now the drugs of first choice for the treatment of glaucoma because of their potent intraocular pressure (IOP)-lowering activity, the low likelihood of systemic adverse reactions, the once-daily application, and good patient adherence. As of end July 2009, tafluprost has been approved in 21 countries. No preservative-free tafluprost preparation has yet been marketed in Japan, even though both a preservative-containing preparation (Tapros®) and a preservativefree preparation (Taflotan®) have been marketed in other countries. This review will describe the pharmacological profile of tafluprost (the latest PG derivative) and also the clinical studies of this drug conducted across the world, while focusing especially on those conducted in Japan.
Pharmacological profile of tafluprost
Tafluprost was developed by Santen Pharmaceutical Co. Ltd. (Osaka, Japan) and Asahi Glass Co. Ltd. (Tokyo, Japan), their original concept having been to develop a new product with an efficacy and safety comparable to or higher than those of latanoprost (a prostanoid FP receptor agonist).
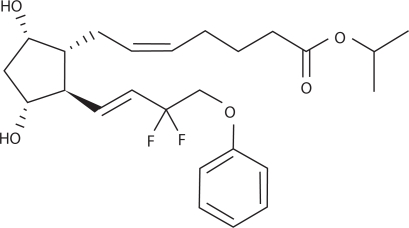
Prost-type PG derivatives (ie, those other than unoprostone), were discovered as compounds that retained the 15-position hydroxyl group of natural PGF2α, and had high pharmacological activity. The hydroxyl group at the 15-position was considered indispensable for the manifestation of the activity of PGs.1 However, tafluprost is a novel PGF2α derivative in which the 15-position hydroxyl group is substituted by 2 fluorine atoms (Figure 1). Because of this substitution, ketonization by 15-hydroxy-dehydrogenase (one of the major pathways involved in the metabolization of PGs) does not occur with this compound. As a result, it is metabolized only through beta-oxidation of the alpha-chain of PG skeleton.2 Because of this lack of one pathway for its metabolization, tafluprost may expected to have a prolonged action, but it has been shown to lack a tendency to accumulate.2 In PG derivatives other than bimatoprost, the alpha-chain ending of the PG skeleton has been isopropyl-esterified, and for this reason these derivatives are converted into an active form (carboxylic acid form) by corneal esterase, and penetrate rapidly into the anterior chamber of the eye. Tafluprost, too, is an isopropylester type of PG derivative.
Figure 1.
Structure of tafluprost.
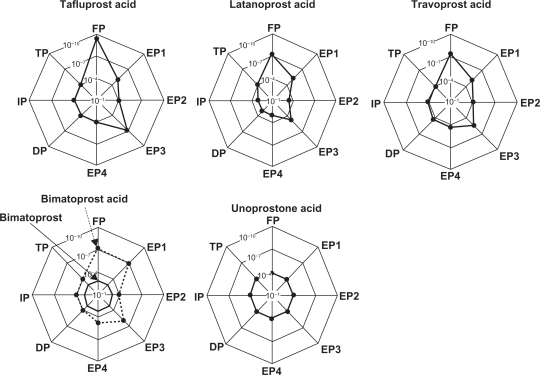
It has been reported that tafluprost not only has an affinity for the FP receptor that is about 12 times higher than that of latanoprost, but that it has almost no potential to bind to the other receptors.3 Among the other PG derivatives, the affinity of travoprost for the prostanoid FP receptor has been reported to be 2.8 times as high as that of latanoprost.4 For both unoprostone and bimatoprost, the receptors on which they act were uncertain at the time of launch onto the market. However, each had been reported to have a low affinity for the prostanoid FP receptor.5,6 Later, only nonmetabolized bimatoprost was found not to bind to the conventional FP receptor, but instead to the prostamide receptor, which is a complex receptor consisting of FP and splice-variant FP receptors.7 Figure 2 shows images indicating the receptorbinding affinities of PG derivatives for various prostanoid receptors. A simple summary of these reports on the affinities of the existing PG derivatives for the FP receptor would be that tafluprost has the highest affinity for the FP receptor among the PG derivatives currently available (notably, higher than that of latanoprost). We previously reported that the IOP-lowering activity of each of these PG derivatives was absent in prostanoid FP receptor-knockout mice, indicating that the FP receptor is involved to a greater or lesser extent in the IOP-lowering action of each PG derivative.8,9
Figure 2.
Receptor affinities of various prostaglandin derivatives (schematic representation).
Efficacy and safety of tafluprost – nonclinical trials
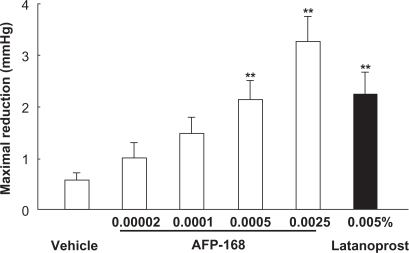
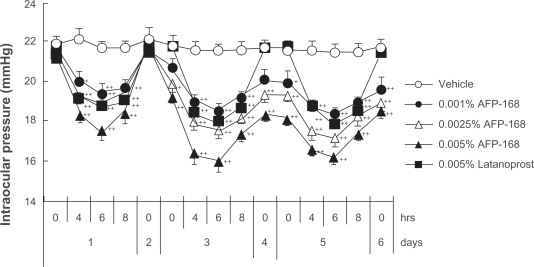
The IOP-lowering activity of tafluprost has been demonstrated both in ocular normotensive monkeys and in laser-induced ocular hypertensive monkeys.3 In the former the maximal IOP reductions achieved with a single dose of tafluprost (0.00002 to 0.0025%) were dose-dependent, with statistical significance being reached at doses of 0.0005 and 0·0025%, and the potency of tafluprost at 0·0005% was almost equal to that of latanoprost at 0·005% (Figure 3).3 Similarly, in laser-induced ocular hypertensive monkeys, single-dose applications of tafluprost (0.00002 to 0.0025%) induced a dose-dependent IOP reduction.3 When normotensive monkeys received repeated doses of tafluprost (0.0015%, 0.0025%, or 0.005%) once daily for 5 days, this drug exhibited IOP-lowering effects not only on the first day, but also on the third and fifth days of administration, without an attenuation of efficacy over time (Figure 4).3 When 0.005% of latanoprost was administered instead in the same study, the IOP measured at 24 hours after each dose was equal to the baseline IOP level. However, when any one of the above concentrations of tafluprost was administered, the IOP levels at the trough-points on the third to fifth days were significantly lower than those in the vehicle-treatment group (Figure 4).3 These results suggest that the duration of action of tafluprost may become longer upon repeated dosing.
Figure 3.
Intraocular pressure (IOP)-lowering effects of a single application of tafluprost in normotensive monkeys.
Reproduced with permission from Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78:768–776.3 Copyright © 2004 Elsevier.
Notes: Tafluprost is indicated as AFP-168.
Data represent the mean ± SEM for 12 animals. **P < 0.01 vs vehicle (Tukey–Kramer test).
Figure 4.
Intraocular pressure (IOP)-lowering effects of once-daily multiple applications of tafluprost and latanoprost in normotensive monkeys.
Reproduced with permission from Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78:768–776.3 Copyright © 2004 Elsevier.
Notes: Tafluprost is indicated as AFP-168.
IOP change was calculated from time 0 on day 1. Drugs were instilled at 10:30 a.m. on each day from day 1 to day 5. Data represent the mean + SEM. for 10 animals. *P < 0.05;
**P < 0.01 vs vehicle (Dunnett’s multiple-range test).
Like latanoprost, tafluprost lowers IOP by stimulating the outflow of aqueous humor through the uveoscleral passway.3 Although latanoprost and tafluprost lower IOP through actions on the same receptor and through the same mechanism, the magnitude of the IOP reduction in a given individual may differ between these two drugs. In our recent study on monkeys that had a low susceptibility to latanoprost and displayed an inadequate reduction of IOP in response to latanoprost, the magnitude of the IOP reduction induced by tafluprost (0.0015%) was greater than that induced by latanoprost (0.005%) in all ten monkeys tested.10 This suggests that switching to tafluprost may be useful in cases in which the IOP reduction achieved with latanoprost is insufficient.
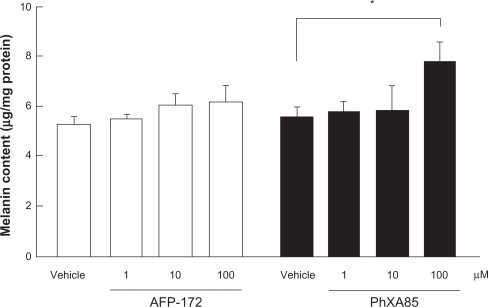
One of the known adverse reactions to PG derivatives is pigmentation of the iris and/or eyelids. Interesting data have been collected relating to the likelihood of tafluprost inducing such pigmentation. Melanoma cells that had been incubated for 4 days in a medium supplemented with a high concentration of tafluprost failed to exhibit the elevated melanin content seen in cultures containing the same concentration of latanoprost (Figure 5).3 Admittedly, a very high concentration of each drug was used in this study. Nevertheless, the lack of reaction in the tafluprost-treated cells, despite the presence of such a reaction in the latanoprost-treated ones, may indicate (a) that the incidence of iris or eyelid pigmentation will be low with tafluprost and (b) that if such pigmentation does develop following treatment with this drug, it will not be severe.
Figure 5.
Effects of tafluprost on melanin formation in B16 melanoma cells.
Reproduced with permission from Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78:768–776.3 Copyright © 2004 Elsevier.
Notes: The carbonic acid form of tafluprost is indicated as AFP-172, and that of latanoprost as PhXA85.
Drugs were added to the culture medium for 4 days. Data represent the mean ± SEM from four experiments. *P < 0.05, vs vehicle (Dunnett’s multiple-range test).
Other data have raised the possibility that tafluprost might improve blood flow as well as other PG derivatives. For instance, Izumi N et al11 noted that administration of tafluprost ophthalmic solution elevated feline retinal blood flow. Moreover, in a rabbit model of disturbed blood flow induced by administration of endothelin-1 (ET-1) into the vitreous, an improvement in blood flow was demonstrated with tafluprost.12 Indeed, when latanoprost, travoprost, or tafluprost ophthalmic solution was administered 2 hours before the intravitreous injection of ET-1, the disturbance of the retinal blood flow was significantly inhibited in all the groups. However, such a significant effect was observed only in the tafluprost-treated group when the same ophthalmic solutions were administered 4 hours before intravitreous injection of ET-1. This result suggests that the ocular blood flow-improving effect may be more prolonged with tafluprost than with latanoprost or travoprost. Regarding the mechanism responsible for the blood flow improvement, Dong Y et al13 noted that tafluprost relaxed rabbit ciliary artery smooth muscle, and that the relaxation was not dependent on endothelium-derived factors. Moreover, it apparently did not involve a reduction in agonist-induced Ca2+ influx or increased Ca2+ extrusion into the extracellular space. However, they did obtain evidence that the tafluprost-induced relaxation might be due, at least in part, to an inhibition of the capacitative entry of extracellular Ca2+. Since we really do not have sufficient means of measuring blood flow in animal models and human eyes, the clinical relevance of improvement of ocular blood flow by PG derivatives including tafluprost should be verified in the future study.
In a recent study, Kanamori et al14 reported neuroprotective effects of tafluprost. In a rat model in which retinal ganglion cell apoptosis was induced by serum-removal or by glutamate exposure, tafluprost suppressed the apoptosis in a concentration-dependent manner. They also noted that in a rat model of optic-nerve crush, treatment with tafluprost ophthalmic solution increased the survival rate of retinal ganglion cells.
Efficacy and safety of tafluprost – clinical trials
Efficacy – IOP-lowering effect
Clinical studies on tafluprost have been carried out both in Western countries14–19 and in Japan, in each case using preservativecontaining tafluprost preparations. Two phase I studies were conducted in England, one on healthy Japanese men and one on healthy non-Japanese men.15,16 The therapeutic concentration of tafluprost has been set at 0.0015% on the basis of results from phase II dose-response study performed in Japan. In Japan, three phase III clinical studies have been carried out using taflprost at that concentration (0.0015%). Incidentally, for the dosage and administration of this drug, it is indicated that it should be administered once daily (one drop/dose).
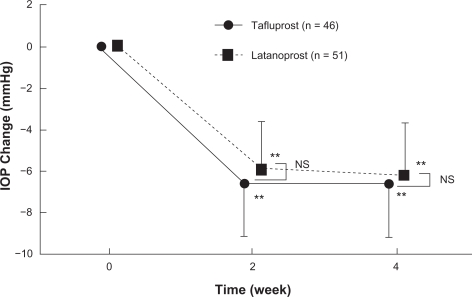
Of the three phase III clinical studies conducted in Japan, one was designed as a non-inferiority study aimed at comparing the efficacy of tafluprost with that of latanoprost. In that study – a randomized, single-blind comparative study involving 109 patients with primary open angle glaucoma or ocular hypertension (reference drug: latanoprost ophthalmic solution) – the magnitude of the IOP reduction in the tafluprost and latanoprost treatment group after 4 week administration was 6.6 ± 2.5 mmHg (27.6 ± 9.6%) and 6.2 ± 2.5 mmHg (25.9 ± 9.7%), respectively. Thus endorsing the noninferiority of this drug versus latanoprost (Figure 6).20 Moreover, the percentage of patients showing a reduction of 20% or more in IOP was 80.4% in the tafluprost treatmentgroup against 70.6% in the latanoprost-treatment group.
Figure 6.
Time course of intraocular pressure (IOP) change in phase II clinical study in patients with primary open angle glaucoma or ocular hypertension (noninferiority study between Tapros® and latanoprost).
Notes: Data represent mean ± SD **P < 0.01, vs pre-dosing value (paired-t test).
NS: not significant, comparison between Tapros® and latanoprost (Student t-test).
The second phase III clinical study of tafluprost was designed as a long-term dosing study primarily aimed at evaluating its safety in prolonged use. In that study – involving 351 patients with open angle glaucoma (including normotensive glaucoma) or ocular hypertension – the magnitude of the IOP reduction seen following treatment with tafluprost studied within the range 4.9 to 5.7 mmHg throughout the 52-week study period. Thus, this drug exerted its IOP-lowering effect in a stable manner during prolonged use (see Product Overview: http://www.santen.co.jp/medical/common/pdf/info_package/tenpu/tapros.pdf).
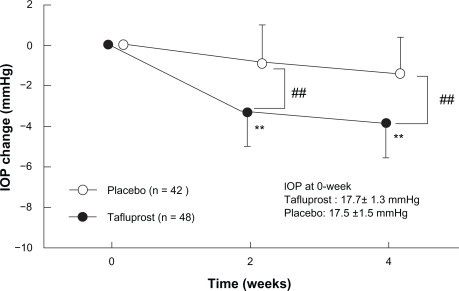
The third phase III clinical study was designed as a comparative study aimed at evaluating the efficacy of tafluprost in patients with normal tension glaucoma. According to the results of the epidemiological survey of glaucoma in Japan carried out in Tajimi City, Gifu Prefecture in 2000 and 2001 (often referred to as the “Tajimi Study”), the prevalence of glaucoma at ages over 40 is 5% in Japan, and normal tension glaucoma accounts for more than 70% of all glaucoma cases.21 To date, however, no IOP-lowering agent has been shown in clinical trials to be effective against normal tension glaucoma (the most frequent type of glaucoma in Japan). In unpublished randomized masked comparative study involving 94 patients with normotensive glaucoma (reference drug: placebo ophthalmic solution), the magnitude of the IOP reduction in the tafluprost-treatment group was 4.0 mmHg (95% confidence interval: 3.5 to 4.5 mmHg), and this was significantly greater than that in the placebo-treatment group (Figure 7).
Figure 7.
Time course of intraocular pressure (IOP) change in phase II clinical study in patients with normal tension glaucoma (source: Product Overview http://www.santen.co.jp/medical/common/pdf/info_package/tenpu/tapros.pdf).
Notes: Data represents mean ± SD. **P < 0.01, vs pre-dosing value (paired t-test).
##P < 0.01, comparison between placebo and Tapros® (Student t-test).
The following clinical studies were conducted not in Japan, but in Europe. For tafluprost versus latanoprost, noninferiority was demonstrated by ANOVA (and almost by ANCOVA) in a phase III, 24-month study in patients with open-angle glaucoma or ocular hypertension.17 In addition, the IOP-lowering efficacy of tafluprost in adjunctive use with timolol demonstrated by Egorov and Ropo.18 A clinical equivalence between preservativefree and preservative-containing prescriptions has been reported both by Uusitalo H et al19 in healthy volunteers, and by Hamacher T et al22 in patients with glaucoma or ocular hypertension. Thus the penetration of tafluprost into the anterior chamber is apparently not affected by preservative benzalkonium chloride. At present, there are insufficient clinical data comparing tafluprost and other PG derivatives because of its short marketing history. Further comparison studies should be conducted to verify the potential efficacy of tafluprost.
Safety
The phase III clinical trial of 4 weeks aimed at comparing to latanoprost revealed that the incidence of adverse reactions did not differ between those two treatment groups (40.0% and 48.1%, respectively, P = 0.443, Fisher’s exact test). The most common adverse reaction was conjunctival hyperemia (16.4%, n = 55), which was higher than that of latanoprost (13.0%, n = 54), but not significant. Of the total population studied in Japan (483 patients), 326 patients (67.5%) showed adverse reactions to tafluprost ophthalmic solution 0.0015% (including abnormal changes in laboratory parameters). The most common adverse reactions reported were conjunctival hyperemia (151 cases, 31.3%), eyelash abnormalities (93 cases, 19.3%), itching sensation (85 cases, 17.6%), ocular irritation (65 cases, 13.5%), and iris pigmentation (39 cases, 8.1%). No adverse reaction unknown for existing PG derivatives was noted. The adverse reactions were mild in more than 95% of all cases, and no severe adverse reaction was noted. Like the other PG derivatives, this drug requires particular care when used for aphakic eyes or intraocular lensimplanted eyes, in patients with bronchial asthma or a history of it, in patients with endophthalmitis (iritis, uveitis), or in pregnant, women, or lactating women, and so on. Patients with herpes infection are not included in the group requiring particularly careful administration. These adverse reactions by tafluprost were comparable to other new PG derivatives such as travoprost and bimatoprost.
Tafluprost 0.0015% ophthalmic solution for the Japanese market
Table 1 shows the composition of the product to be marketed in Japan. It contains 15 μg tafluprost per mL, a concentration that is the lowest among the PG derivative preparations on the Japanese market. It is a clear, colorless aqueous ophthalmic solution, with a pH of 5.7 to 6.3 and an osmotic pressure ratio of 1.0 to 1.1.
Table 1.
Composition and properties of tafluprost 0.0015% ophthalmic solution for the Japanese market
| Brand name | Tapros® ophthalmic solution 0.0015% |
| Active ingredient | Tafluprost |
| Content | 15 μg/mL |
| Inactive ingredients | Polysorbate 80, glycerin, ethylenediaminetetraacetate, sodium dihydrogen phosphate, benzalkonium chloride, pH adjuster |
| pH | 5.7–6.3 |
| Osmotic pressure ratio | 1.0–1.1 |
| Appearance | Clear, colorless aqueous solution |
This product is intended to be stored at room temperature, and its expiration period is 3 years after manufacture. It is provided in a plastic bottle called “Dimple®” bottle. Hyodo et al24 reported that this container was highly praised in an evaluation of various indicators of the impressions of users, and that elderly patients (≥65 years old) could use it as easily as nonelderly patients. Thus, since this ophthalmic solution can be stored at room temperature and is supplied in a highly comfortable container, there is expected to be high adherence of patients to the dosing instructions.
Conclusion
Tafluprost 0.0015% ophthalmic solution contains a new PG analogue with an IOP-lowering activity and safety comparable to those of latanoprost. Since it can be stored at room temperature and is supplied in a container that patients find easy to handle, there is expected to be a high patient adherence to the dosing instructions. Tafluprost is thus a promising new alternative for glaucoma treatment.
Acknowledgments/disclosure
The author has no proprietary interest in any aspect of this review. The author thanks Yasutaka Takagi Santen Phamaceutical Co. Ltd., for excellent technical assistance and pharmacological advice.
References
- 1.Resul B, Stjernschantz J. Structure-activity relationships of prostaglandin analogues as ocular hypotensive agents. Curr Opin Ther. 1993;3:781–795. [Google Scholar]
- 2.Fukano Y, Kawazu K. Disposition and metabolism of a novel prostanoid antiglaucoma medication, tafluprost, following ocular administration to rats. Drug Metab Dispos. 2009;37:1622–1634. doi: 10.1124/dmd.108.024885. [DOI] [PubMed] [Google Scholar]
- 3.Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78:768–776. doi: 10.1016/j.exer.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Sharif NA, Kelly CR, Crider JY, et al. Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J Ocul Pharmacol Ther. 2003;19:501–515. doi: 10.1089/108076803322660422. [DOI] [PubMed] [Google Scholar]
- 5.Goh Y, Kishino J. Pharmacological characterization of prostaglandinrelated ocular hypotensive agents. Jpn J Ophthalmol. 1994;38:236–245. [Google Scholar]
- 6.Woodward DF, Krauss AH-P, Chen J, et al. The pharmacology of bimatoprost. Surv Ophthalmol. 2001;45(Suppl 4):S337–S345. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- 7.Liang Y, Woodward DF, Guzman VM, et al. Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. Br J Pharmacol. 2008;154:1079–1093. doi: 10.1038/bjp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota T, Aihara M, Narumiya S, et al. The effects of prostaglandin analogues on IOP in prostanoid FP-receptor–deficient mice. Invest Ophthalmol Vis Sci. 2005;46:4159–4163. doi: 10.1167/iovs.05-0494. [DOI] [PubMed] [Google Scholar]
- 9.Ota T, Aihara M, Saeki T, et al. The IOP-lowering effects and mechanism of action of tafluprost in prostanoid receptor-deficient mice. Br J Ophthalmol. 2007;91:673–676. doi: 10.1136/bjo.2006.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aihara M, Kurashima H, Ishida N, et al. Effect of tafluprost on the intraocular pressure in latanoprost low-responder monkeys. World Glaucoma Congress; Boston, USA. 2009. [Google Scholar]
- 11.Izumi N, Nagaoka T, Sato E, et al. Short-term effects of topical tafluprost on retinal blood flow in cats. J Ocul Pharmacol Ther. 2008;24:521–526. doi: 10.1089/jop.2007.0065. [DOI] [PubMed] [Google Scholar]
- 12.Kurashima H, Akaishi T, Odani-Kawabata N, et al. The effect of prostaglandins on endothelin-1-induced impairment of ocular blood flow in rabbits. Annual Meeting of The Association for Research in Vision and Ophthalmology; Fort Lauderdale, USA. 2008. [Google Scholar]
- 13.Dong Y, Watabe H, Su G, et al. Relaxing effect and mechanism of tafluprost on isolated rabbit ciliary arteries. Exp Eye Res. 2008;87:251–256. doi: 10.1016/j.exer.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Kanamori A, Naka M, Fukuda M, et al. Tafluprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2009;247:1353–1360. doi: 10.1007/s00417-009-1122-6. [DOI] [PubMed] [Google Scholar]
- 15.Sutton A, Gilvarry A, Ropo A. A comparative, placebo-controlled study of prostanoid fluoroprostaglandin-receptor agonists tafluprost and latanoprost in healthy males. J Ocul Pharm Ther. 2007;23:359–365. doi: 10.1089/jop.2006.0061. [DOI] [PubMed] [Google Scholar]
- 16.Sutton A, Gouws P, Ropo A. Tafluprost, a new potent prostanoid receptor agonist: A dose-response study on pharmacodynamics and tolerability in healthy volunteers. Int J Clin Pharm Ther. 2008;46:400–406. doi: 10.5414/cpp46400. [DOI] [PubMed] [Google Scholar]
- 17.Uusitalo HMT, Pillunat LE, Baudouin C, et al. Phase III, 24-month study investigating the efficacy and safety of tafluprost vs latanoprost in patients with open-angle glaucoma or ocular hypertension. Acta Ophthalmologica. 2008;86(S243) [Google Scholar]
- 18.Egorov E, Ropo A. Adjunctive use of tafluprost with timolol provides additive effects for reduction of intraocular pressure in patients with glaucoma. Eur J Ophthalmol. 2009;19:214–222. doi: 10.1177/112067210901900207. [DOI] [PubMed] [Google Scholar]
- 19.Uusitalo H, Kaarniranta K, Ropo A. Pharmacokinetics, efficacy and safety profiles of preserved and preservative-free tafluprost in healthy volunteers. Acta Ophthalmol Suppl. 2008;242:7–13. doi: 10.1111/j.1755-3768.2008.01380.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuwayama Y, Komemusi S. Phase III confirmatory study of 0.0015% DE-085 (Tafluprost) ophthalmic solution as compared to 0.005% Latanoprost ophthalmic solution in patients with open-angle glaucoma or ocular hypertension. Atarashii Ganka. 2008;25:1595–1602. [Google Scholar]
- 21.Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology. 2004;111:1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Hamacher T, Airaksinen J, Saarela V, et al. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: Results from a pharmacodynamics analysis. Acta Ophthalmol Suppl. 2008;242:14–19. doi: 10.1111/j.1755-3768.2008.01381.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Iwase A, Araie M, et al. The Tajimi Study Report 2: Prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112:1661–1669. doi: 10.1016/j.ophtha.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Hyodo R, Mizoue S, Kawasaki S, et al. Investigation of glaucoma eyedrop bottle usability by elderly patients. Journal of the Eye. 2007;24:371–376. [Google Scholar]