Abstract
Background
Livestock has recently been identified as a new reservoir of methicillin-resistant Staphylococcus aureus (MRSA). Most isolates belong to ST398 and are non-typeable with PFGE using SmaI, making it difficult to study transmission and outbreaks. Therefore, a new PFGE using Cfr9I, a neoschizomer of SmaI was optimized and evaluated to investigate ST398 isolates.
Results
After optimizing and evaluating the Cfr9I PFGE, clear and reproducible banding patterns were obtained from all previously non-typeable MRSA (NTSmaI -MRSA) isolates. The PFGE patterns of ST398 isolates showed more diversity than with spa-typing and/or MLST. The PFGE results showed diversity within and between the two most prevalent spa-types of NTSmaI -MRSA (t011 and t108). No match was found, when comparing banding patterns of the NTSmaI -MRSA with 700 different PFGE types, obtained with SmaI digestion, in our database of more than 4000 strains. Furthermore, possible transmission among veterinarians and their family members was investigated and an outbreak of ST398 MRSA in a residential care facility was confirmed with the Cfr9I PFGE.
Conclusions
The adjusted PFGE can be used as a method for selecting important and distinct ST398 isolates for further research. The adjustments in the PFGE protocol using Cfr9I are easy to implement to study the ST398 clonal lineage in laboratories which already have a PFGE facility.
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of nosocomial and community-associated infections worldwide. Most cases of community-associated MRSA (CA-MRSA) have been associated with skin and soft-tissue infections in previously healthy individuals [1,2]. Since 2003, pigs [3-7] and other animals such as horses [8,9], poultry [10] and calves [11] have been identified as a new reservoir for CA-MRSA. Most of the livestock related MRSA strains share the same multi locus sequence typing (MLST) type, namely ST398. Throughout Europe [9,12-14], Canada [6] and in the United States [15] ST398 has been found in association with animal husbandry, indicating a worldwide clonal lineage. Although the clinical importance of ST398 is still controversial, there are reports indicating transmission and infections among humans [16-18]. Pulsed Field Gel Electrophoresis (PFGE) using SmaI is considered to be the gold standard for typing MRSA isolates [19]. When PFGE was performed on ST398 isolates, no banding patterns could be generated, due to methylation of the SmaI site [20]. Therefore, ST398 isolates are referred to as PFGE non-typeable (NTSmaI)-MRSA. Some years ago staphylococcal protein A (spa) typing was introduced as a highly discriminatory typing method to characterize S. aureus isolates [21,22]. However, spa-typing of the ST398 isolates revealed very limited variation within this group and 80% of our ST398 isolates had either spa-type t011, t108 or t034 [23]. Recently, a multiple-locus variable number of tandem repeat analysis (MLVA) has been presented [24]. Although MLVA is significantly more discriminatory than spa-typing, it was unable to yield a better discrimination of the isolates of the ST398 lineage. The lack of a typing method that can discriminate ST398 strains has hampered studies on the origin and transmission routes of this MRSA clade.
In the Netherlands all first MRSA isolates obtained from patients with staphylococcal disease and from patients that carry the pathogen are sent to the National MRSA reference centre for typing. In 2007, 30% of all forwarded MRSA isolates were NTSmaI -MRSA [23].
Recently, a neoschizomer of SmaI, designated as Cfr9I, was shown to be insensitive for the DNA-methylation leading to NTSmaI -MRSA isolates. In two studies this restriction enzyme was used for generating PFGE profiles of NTSmaI -MRSA isolates [18,25]. In the study presented here we optimized PFGE with restriction enzyme Cfr9I and evaluated its use to characterize NTSmaI -MRSA isolates.
The data will yield important information about the genetic diversity of the ST398 clonal lineage in the Netherlands and demonstrates that Cfr9I PFGE is a powerful tool to study possible transmission and outbreaks of MRSA isolates, previously not typeable by conventional PFGE approaches.
Methods
Bacterial isolates
The National Institute for Public Health and the Environment (RIVM) serves as the Dutch National MRSA reference center. All first MRSA isolates, one per patient, are sent to the RIVM for further typing. PFGE was carried out using restriction enzyme SmaI according to the Harmony protocol [26]. From this large MRSA collection a number of NTSmaI -MRSA was selected to optimize and validate the Cfr9I PFGE. To study the genetic diversity of the two most prevalent spa-types among NTSmaI -MRSA in the Netherlands, 60 NTSmaI -MRSA isolates (t011 (n = 30) and t108 (n = 30)) in 2008 from patients living in geographical dispersed regions in the Netherlands were used. In addition, 16 strains (8 pairs) from veterinarians and one of their family members, the latter whom did not have contact with animals and 40 pig and pig farmer isolates and 6 strains from an NTSmaI -MRSA outbreak in a residential care facility [18] were included in this study to assess the potential of the Cfr9I PFGE to identify transmissions. To validate the Cfr9I PFGE method, 10 typeable MRSA (T-MRSA) isolates and the reference strain NCTC 8325 were tested. Five non-typeable isolates were repeated 3 times with Cfr9I PFGE to ensure the reproducibility of the method.
Molecular typing
All isolates were characterized with spa typing [22]. Spa-types were assigned using Bionumerics software version 5.1 (Applied Maths, Sint-Martens-Latem, Belgium). SCCmec typing of the isolates was performed using the multiplex PCR described by Boye et al [27].
In order to obtain clear and reproducible PFGE banding patterns using Cfr9I as restriction enzyme, the Harmony PFGE protocol had to be adjusted. This resulted in the following protocol: From each isolate, 100 μl bacterial suspension of an overnight Trypton Soy Broth (TSB) culture, was embedded in a plug mold (Biorad) with 1.2% low-melting-point agarose (Seakem gold®, Biorad). Then, 500 μl lysostaphine (100 μg/ml, Sigma) was added and incubated for 6 h at 37°C. Subsequently, the plugs were incubated overnight at 55°C with 500 μl Proteinase K (50 μg/ml, Merck). The plugs were then washed, 6 to 10 times in a shaking incubator for 30 min. in 1 × Tris-EDTA buffer (Fluka, pH 7) at 50°C in order to remove cell debris. Finally, the plugs were equilibrated in 1 × Cfr9I buffer (Fermentas, Ontario, Canada) for 15 min. at room temperature prior to digestion and then submerged in 200 μl of 1 × Cfr9I reaction buffer containing 40 U of Cfr9I restriction enzyme (Fermentas, Ontario, Canada). The reaction tubes were incubated overnight at 37°C in a shaking incubator. Further steps were carried out according to the Harmony protocol [26]. Briefly, a 1% agarose gel was poured into a gel tray and positioned in a contour-clamped homogeneous electric field (CHEF) (Biorad) tank and submerged in 1,700 ml of 0.5 × Tris-Borate-EDTA (TBE). The total run time was 22 h at 14°C with an initial pulse time of 5 s, a final pulse time of 50 s and a voltage of 6 V/cm or 200 V. Gels were stained in ethidium bromide (1 μg/ml, Invitrogen) and viewed and photographed with UV transillumination. Digital images were analyzed using Bionumerics software, version 5.1. If a difference in PFGE pattern was observed, a new pulsed field type was assigned. The definition of a PFGE cluster was based on a similarity cutoff of 80% [28] (Dice coefficient, represented by UPGMA, 0.5% optimization and 1.0% tolerance). Different PFGE clusters were given in alphabetical order. Every band difference within a PFGE cluster resulted in adding a numerical order to the pulsed field cluster.
Results
Optimization and validation of the Cfr9I PFGE method
In the initial experiments the SmaI restriction enzyme was replaced by Cfr9I and exactly the same conditions were used as in the original PFGE protocol. This led to uninformative PFGE patterns consisting mainly of smears and faint bands obtained through partial digestion of the genomic DNA. A higher lysostaphine concentration (100 μg/ml), longer incubation steps for lysis (6 h), proteinase K and digestion overnight and hot washes at 50°C - instead of washes at room temperature - produced clear and reproducible banding profiles.
After optimizing the PFGE method with Cfr9I, high quality banding patterns from all selected (n = 124) previously non-typeable ST398 MRSA isolates were obtained. For validation, both PFGE protocols (SmaI and Cfr9I) were performed on 10 typeable MRSA isolates and the reference strain NCTC 8325. Side-by-side comparison of SmaI and Cfr9I PFGE profiles yielded identical banding patterns consistent with unequivocal comparability of both restriction patterns. Reproducibility of the method was confirmed with 5 NTSmaI -MRSA isolates which were re-analyzed 3 times and yielded identical banding patterns.
Genetic diversity of NTSmaI -MRSA
All PFGE patterns of the NTSmaI -MRSA were compared with a database consisting of more than 4000 isolates containing over 700 different PFGE types obtained with SmaI digestion. Surprisingly, newly-obtained banding patterns of NTSmaI -MRSA isolates did not match with any known PFGE cluster in the national database of MRSA isolates collected since 2002.
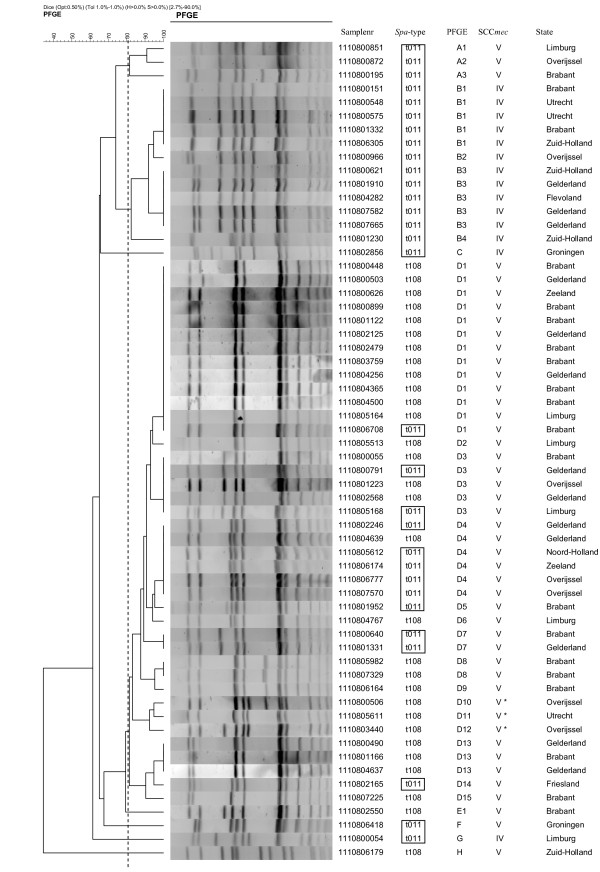
Thirty t011 isolates revealed 16 different PFGE patterns (figure 1). The largest PFGE cluster consisted of 5 isolates, and 5 patterns were found more than once (n = 19). No correlation was found between PFGE cluster and geographic location. The minimal similarity (Dice coefficient, represented by UPGMA, 0.5% optimization and 1.0% tolerance) between the different patterns was 64% (data not shown). Thirty t108 isolates revealed 14 different PFGE patterns (figure 1). The largest cluster contained 12 isolates and 4 patterns were found more than once (n = 20). The clusters showed no geographical correlation. The minimal similarity of the t108 isolates was 50% (data not shown). One t108 isolate yielded a very distinct PFGE pattern (figure 1, pattern H). Without this isolate the minimal similarity of the t108 isolates would be 80%. The minimal similarity of the 60 NTSmaI -MRSA isolates was 35%, but most isolates share 80% or more similarity (figure 1). SCCmec typing of the 60 NTSmaI -MRSA isolates showed SCCmec type IV (n = 14) and SCCmec type V (n= 43). Three isolates yielded a variant of SCCmec type V (indicated in figure 1 with V*) and no SCCmec types I, II or III were found (figure 1).
Figure 1.
Dendrogram of the Cfr9I PFGE results of NTSmaI-MRSA isolates with the 2 most prevalent spa-types in the Netherlands.
Transmission of ST398 isolates
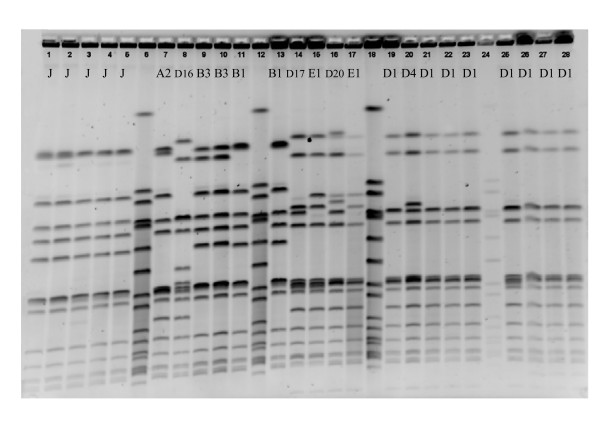
The results of Cfr9I PFGE of 8 pairs of veterinarians and one of their close family members showed that 5 pairs gave indistinguishable banding patterns suggesting possible transmission of ST398 (figure 2 shows 2 pairs of indistinguishable banding patterns). Two pairs that did not match also had different spa-types (figure 2). One pair which had the same spa-type differed in a single PFGE band (data not shown). Six isolates belonging to an outbreak in a residential care facility with spa-types t2383 and t011 all shared the same banding pattern (figure 2). Furthermore, the transmission between pigs, pig farmers and their family on 9 different pig farms (table 1, figure 2) was studied. Farms 1 to 5 shared the same spa-type whereas on farms 6 to 9, two or more different spa-types were present. The number of different PFGE patterns (B1-K) differed between farms, ranging from indistinguishable patterns (farm 4) to 5 different PFGE patterns (farm 8). PFGE patterns B1, D1, D3, D4 and E1 were found on several farms (table 1). The minimal similarity within the farms varied from 52% (farm 5) to 100% (farm 4) and the minimal similarity between the farms was 61% (data not shown). Figure 2 shows the PFGE results of farm 6 with 4 different PFGE patterns and from farm 9 which all had indistinguishable PFGE patterns.
Figure 2.
PFGE patterns of ST398 isolates digested with Cfr9I restriction enzyme using NCTC 8325 as the reference standard. Lanes 6, 12, 18, and 24, NCTC 8325; Lanes 1-5, isolates from an outbreak in a residential care facility, all PFGE pattern J; Lanes 7-8, and 14-15, two pairs of a veterinarian and a close family member with distinct PFGE patterns; Lanes 9-11, and 13, two pairs of a veterinarian and a close family member with identical banding patterns; Lanes 16-17, and 19-22, isolates of pig farm 6 with four different PFGE patterns; Lanes 23, and 25-28, isolates from pig farm 9 with identical banding patterns
Table 1.
Overview of transmission of ST398 MRSA on 9 farms (n = 40)
| Strain nr | Farm | spa-type | Origin | PFGE pattern | Coefficient* |
|---|---|---|---|---|---|
| 1110701181 | 1 | t011 | farmer | B3 | 70 |
| 1110700844 | 1 | t011 | pig | D7 | |
| 1110701184 | 2 | t011 | farmer | D4 | 86 |
| 1110700857 | 2 | t011 | pig | D4 | |
| 1110701182 | 2 | t011 | employee | E1 | |
| 1110701185 | 2 | t011 | relative | E1 | |
| 1110701429 | 3 | t011 | pig | B1 | 87 |
| 1110701595 | 3 | t011 | relative | B2 | |
| 1110701592 | 3 | t011 | farmer | D19 | |
| 1110701192 | 4 | t108 | farmer | D1 | 100 |
| 1110700908 | 4 | t108 | pig | D1 | |
| 1110701196 | 5 | t567 | farmer | D18 | 52 |
| 1110701197 | 5 | t567 | relative | D18 | |
| 1110700912 | 5 | t567 | pig | I | |
| 1110701611 | 6 | t108 | dust | D1 | 84 |
| 1110701614 | 6 | t108 | dust | D1 | |
| 1110701604 | 6 | t108 | pig | D1 | |
| 1110701200 | 6 | t011 | farmer | D20 | |
| 1110701612 | 6 | t011 | dust | D4 | |
| 1110701605 | 6 | t011 | pig | D4 | |
| 1110701201 | 6 | t011 | relative | E1 | |
| 1110701600 | 7 | t2741 | employee | D14 | 95 |
| 1110701596 | 7 | t011 | farmer | D14 | |
| 1110701580 | 7 | t011 | pig | D14 | |
| 1110701601 | 7 | t108 | employee | D21 | |
| 1110701576 | 7 | t011 | pig | D21 | |
| 1110701577 | 7 | t011 | pig | D21 | |
| 1110700882 | 8 | t011 | pig | B1 | 66 |
| 1110700884 | 8 | t108 | pig | D1 | |
| 1110700876 | 8 | t108 | pig | D3 | |
| 1110700889 | 8 | t2330 | dust | D4 | |
| 1110701188 | 8 | t2330 | relative | D4 | |
| 1110701191 | 8 | t2330 | relative | D4 | |
| 1110700890 | 8 | t108 | dust | K | |
| 1110701791 | 9 | t108 | dust | D1 | 86 |
| 1110701783 | 9 | t108 | pig | D1 | |
| 1110701788 | 9 | t108 | pig | D1 | |
| 1110703030 | 9 | t108 | relative | D1 | |
| 1110703031 | 9 | t588 | relative | D1 | |
| 1110703032 | 9 | t108 | relative | D3 | |
* Dice similarity coefficient, using UPGMA. Optimization 0,5%, position tolerance
Discussion
MRSA isolates belonging to the ST398 clonal lineage are hard to discriminate based on spa-typing and/or MLST, hampering the assessment of transmission and outbreaks. Therefore, other techniques such as a modified PFGE could provide a new opportunity to differentiate ST398 isolates. The restriction enzyme SmaI does not cut the DNA of NTSmaI -MRSA isolates, due to methylation of the SmaI site. However, Cfr9I, a neoschizomer of SmaI, can be used for generating PFGE profiles of the NTSmaI -MRSA isolates. When the standard SmaI protocol was used for Cfr9I, banding patterns with smears and partial digests appeared. Other recently published articles seemed to have encountered similar problems with their Cfr9I PFGE [18,25]. The results indicated that lysis of ST398 isolates and digestion with restriction enzyme Cfr9I is more cumbersome than lysis of typeable MRSA and digestion with SmaI [29]. After modifying the protocol, banding patterns of similar quality as those of typeable MRSA isolates digested with SmaI were obtained. All previously non-typeable MRSA isolates can be typed with the optimized PFGE method providing a new opportunity to differentiate the ST398 clonal lineage.
From April 2002 until January 2008, all MRSA isolates sent to the RIVM have been typed with PFGE using SmaI as restriction enzyme creating a database with more than 4000 isolates with over 700 different PFGE types. Since Cfr9I recognizes the same restriction site as SmaI, Cfr9I enables analysis and comparison of the patterns with other profiles in our database. No comparison was found when comparing banding patterns of NTSmaI -MRSA with known PFGE patterns, suggesting that SmaI restriction modification is confined to a defined clonal lineage. Recently, ST398 isolates were typed using amplified fragment length polymorphism (AFLP). These data also suggested that ST398 is a distinct cluster recently introduced into the Dutch patient population [30].
The PFGE patterns of the two most prevalent spa-types (t011 and t108) within the NTSmaI -MRSA isolates showed more variation than spa-typing or MLST. The genetic diversity within the ST398 clonal lineage of MRSA sharing the same spa-type creates an opportunity for improved investigation of outbreak and potential transmission events. Spa-typing, which is currently used as a MRSA typing standard, cannot differentiate these isolates further. Using Cfr9I PFGE, spa-type t011 seemed to be more diverse than t108. Although the minimal similarity of the t108 isolates was 50%, this was mainly caused by a single isolate with a very distinct PFGE pattern (pattern H). Without this isolate the minimal similarity of the t108 isolates was 80%. The t011 isolates showed a minimal similarity of 64% (data not shown). SCCmec typing showed an almost equal distribution between SCCmec type IV (n = 14) and V (n = 16) for t011 isolates, whereas all t108 isolates carried SCCmec type V or a SCCmec type V variant. Huijsdens and colleagues performed SCCmec typing on 300 NTSmaI -MRSA isolates and they showed similar results [23]. This variation in SCCmec types may also indicates a higher diversity among t011 MRSA isolates compared to t108 isolates.
The minimal similarity of the Cfr9I PFGE patterns among ST398 isolates was 35% and showed variation within spa-types, but the diversity within this lineage is still limited. Furthermore, one isolate with spa-type t108 yielded a very distinct PFGE pattern which causes the similarity to be 35% (figure 1). When excluding this isolate from the dendrogram the minimal similarity was 62%. Comparing the PFGE results using the criteria by Tenover et al. and when a similarity cut-off of 80% was applied, most NTSmaI -MRSA isolates should be classified as one PFGE cluster [31,32]. However, the Cfr9I PFGE is still better in discriminating possible differences between NTSmaI -MRSA isolates.
No geographical relation could be found in either spa-type. However, most NTSmaI -MRSA isolates are found in areas with the highest pig density. This could be explained by the frequent movement of pigs between farms in the Netherlands. This facilitates the dissemination of ST398 MRSA on a national scale. A similar situation took place during the foot- and -mouth epidemic in England of 2001 [33].
To provide additional resolution on the molecular evolution and dissemination of MRSA lineages, several typing techniques such as PFGE, SCCmec- and spa-typing have been developed. Since PFGE with SmaI does not digest the DNA of ST398 isolates, spa-typing has been the method of choice for characterizing NTSmaI -MRSA isolates. However, given the low diversity in spa-types it is hard to ascertain health care-associated transmission if two or more different spa-types are present in the same institution. Fanoy et al. described an outbreak in a residential care facility where two spa-types (t2383 and t011) were prevalent [18]. After re-examination of the same isolates the PFGE profiles using Cfr9I were indistinguishable, indicating isogenicity. Moreover, the discriminatory ability of spa-typing of NTSmaI -MRSA is compromised by the fact that more than 80% of the NTSmaI -MRSA in the Netherlands belong either to spa-type t011 or t108 [23]. With the modified Cfr9I PFGE a better tool for epidemiological investigation has become available.
The results obtained by Cfr9I PFGE of isolates from veterinarians and their close family members showed possible transmission of ST398. Five out of eight pairs had identical profiles. The family members had themselves no contact with animals and were presumably infected by the occupationally exposed veterinarian. Two pairs of PFGE patterns among family members were not identical. Their isolates also had different spa-types. Family members may have been colonized by one MRSA through the veterinarian and subsequently the veterinarian may have been re-colonized by another MRSA after occupational exposure. One pair differed only in a single PFGE band probably as a consequence of micro-evolution.
A study on nine different farms revealed that the PFGE patterns of isolates from seven farms were related, but PFGE patterns varied within and between the farms. For example, farm 7, yielded only 2 very closely related PFGE patterns (D14, D21; similarity 95%), while other farms, like farm 8, showed 5 different PFGE patterns (B1, D1, D3, D4 and K) and had a similarity of only 66%. Different batches of animals entering the farm, carrying different NTSmaI -MRSA, could have caused variation within farms. Further study is needed to confirm that farms with a fast turnover of pigs indeed show a higher diversity of PFGE patterns of NTSmaI -MRSA.
Conclusions
In conclusion, the modified PFGE protocol for Cfr9I provided highly informative banding patterns and showed good reproducibility. The PFGE results showed diversity within and between the two most prevalent spa-types among NTSmaI -MRSA. PFGE confirmed transmission of the ST398 clonal lineage within families and in a residential care facility. The modified PFGE approach can be used as a method for selecting important and distinct ST398 isolates for further research. The adjustments in the PFGE protocol using Cfr9I are easy to implement in laboratories which already have a PFGE facility, creating a powerful tool to study the ST398 clonal lineage.
Authors' contributions
TB carried out all molecular typing and drafted the manuscript. AJN participated in the design of the study and revised the manuscript critically for important intellectual content. LMS has made substantial contributions to conception and design of the study. KWZ was responsible for analysis and interpretation of the data and revised the manuscript critically. JAJWK has been involved in drafting the manuscript and revising it critically for important intellectual content. HG participated in the design of the study and has given final approval of the version to be published. XWH participated in the design of the study, has been involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Thijs Bosch, Email: thijs.bosch@rivm.nl.
Albert J de Neeling, Email: han.de.neeling@rivm.nl.
Leo M Schouls, Email: leo.schouls@rivm.nl.
Kim W van der Zwaluw, Email: kim.van.der.zwaluw@rivm.nl.
Jan AJW Kluytmans, Email: jankluytmans@gmail.com.
Hajo Grundmann, Email: hajo.grundmann@rivm.nl.
Xander W Huijsdens, Email: xander.huijsdens@rivm.nl.
References
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- de Neeling AJ, Broek MJ van den, Spalburg EC, van Santen-Verheuvel MG, Dam-Deisz WD, Boshuizen HC, Giessen AW van de, van Duijkeren E, Huijsdens XW. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet Microbiol. 2007;122:366–372. doi: 10.1016/j.vetmic.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Huijsdens XW, van Dijke BJ, Spalburg E, van Santen-Verheuvel MG, Heck ME, Pluister GN, Voss A, Wannet WJ, de Neeling AJ. Community-acquired MRSA and pig-farming. Ann Clin Microbiol Antimicrob. 2006;5:26. doi: 10.1186/1476-0711-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek IV van den, van Cleef BA, Haenen A, Broens EM, Wolf PJ van der, Broek MJ van den, Huijsdens XW, Kluytmans JA, Giessen AW van der, Tiemersma EW. Methicillin-resistant Staphylococcus aureus in people living and working in pig farms. Epidemiol Infect. 2008. pp. 1–9. [DOI] [PubMed]
- Khanna T, Friendship R, Dewey C, Weese JS. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet Microbiol. 2008;128:298–303. doi: 10.1016/j.vetmic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 2005;11:1965–1966. doi: 10.3201/eid1112.050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Strommenger B, Witte W, Stanek C. Clusters of infections in horses with MRSA ST1, ST254, and ST398 in a veterinary hospital. Microb Drug Resist. 2008;14:307–310. doi: 10.1089/mdr.2008.0845. [DOI] [PubMed] [Google Scholar]
- Witte W, Strommenger B, Stanek C, Cuny C. Methicillin-resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg Infect Dis. 2007;13:255–258. doi: 10.3201/eid1302.060924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoons D, Van Hoorebeke S, Hermans K, Butaye P, de Kruif A, Haesebrouck F, Dewulf J. Methicillin-resistant Staphylococcus aureus in poultry. Emerg Infect Dis. 2009;15:452–453. doi: 10.3201/eid1503.080696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooij TA, Jenkins J, Thijssen S. MRSA in calves. Infectieziekten Bulletin. 2007;18:234–236. [Google Scholar]
- Armand-Lefevre L, Ruimy R, Andremont A. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg Infect Dis. 2005;11:711–714. doi: 10.3201/eid1105.040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardabassi L, Stegger M, Skov R. Retrospective detection of methicillin resistant and susceptible Staphylococcus aureus ST398 in Danish slaughter pigs. Vet Microbiol. 2007;122:384–386. doi: 10.1016/j.vetmic.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Meemken D, Cuny C, Witte W, Eichler U, Staudt R, Blaha T. Occurrence of MRSA in pigs and in humans involved in pig production--preliminary results of a study in the northwest of Germany. Dtsch Tierarztl Wochenschr. 2008;115:132–139. [PubMed] [Google Scholar]
- Smith TC, Male MJ, Harper AL, Kroeger JS, Tinkler GP, Moritz ED, Capuano AW, Herwaldt LA, Diekema DJ. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS ONE. 2008;4:e4258. doi: 10.1371/journal.pone.0004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekkelenkamp MB, Sekkat M, Carpaij N, Troelstra A, Bonten MJ. Endocarditis due to methicillin-resistant Staphylococcus aureus originating from pigs. Ned Tijdschr Geneeskd. 2006;150:2442–2447. [PubMed] [Google Scholar]
- Yu F, Chen Z, Liu C, Zhang X, Lin X, Chi S, Zhou T, Chen Z, Chen X. Prevalence of Staphylococcus aureus carrying Panton-Valentine leukocidin genes among isolates from hospitalised patients in China. Clin Microbiol Infect. 2008;14:381–384. doi: 10.1111/j.1469-0691.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- Fanoy E, Helmhout LC, Vaart WL van der, Weijdema K, van Santen-Verheuvel MG, Thijsen SF, de Neeling AJ, van Wamel WJ, Manaskova SH, Kingma-Thijssen JL. An outbreak of non-typeable MRSA within a residential care facility. Euro Surveill. 2009;14(1):19080. pii. [PubMed] [Google Scholar]
- Kaufmann ME. Pulsed-field gel electrophoresis. Totowa N.J.: Humana press; 1998. [Google Scholar]
- Bens CC, Voss A, Klaassen CH. Presence of a novel DNA methylation enzyme in methicillin-resistant Staphylococcus aureus isolates associated with pig farming leads to uninterpretable results in standard pulsed-field gel electrophoresis analysis. J Clin Microbiol. 2006;44:1875–1876. doi: 10.1128/JCM.44.5.1875-1876.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenay HM, Bunschoten AE, Schouls LM, van Leeuwen WJ, Vandenbroucke-Grauls CM, Verhoef J, Mooi FR. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur J Clin Microbiol Infect Dis. 1996;15:60–64. doi: 10.1007/BF01586186. [DOI] [PubMed] [Google Scholar]
- Harmsen D, Claus H, Witte W, Rothganger J, Claus H, Turnwald D, Vogel U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijsdens XW, Bosch T, van Santen-Verheuvel MG, Spalburg E, Pluister GN, van Luit M, Heck MEOC, Haenen A, de Neeling AJ. Molecular characterization of PFGE non-typeable methicillin-resistant Staphylococcus aureus in the Netherlands, 2007. Eurosurveillance. 2009;14(38) doi: 10.2807/ese.14.38.19335-en. [DOI] [PubMed] [Google Scholar]
- Schouls LM, Spalburg EC, van Luit M, Huijsdens XW, Pluister GN, van Santen-Verheuvel MG, Heide HG van der, Grundmann H, Heck ME, de Neeling AJ. Multiple-locus variable number tandem repeat analysis of Staphylococcus aureus: comparison with pulsed-field gel electrophoresis and spa-typing. PLoS ONE. 2009;4:e5082. doi: 10.1371/journal.pone.0005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M, Dumortier C, Taylor BS, Miller M, Vasquez G, Yunen J, Brudney K, Sanchez EJ, Rodriguez-Taveras C, Rojas R. Staphylococcus aureus ST398, New York City and Dominican Republic. Emerg Infect Dis. 2009;15:285–287. doi: 10.3201/eid1502.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchan S, Kaufmann ME, Deplano A, de Ryck R, Struelens M, Zinn CE, Fussing V, Salmenlinna S, Vuopio-Varkila J, El Solh N. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J Clin Microbiol. 2003;41:1574–1585. doi: 10.1128/JCM.41.4.1574-1585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye K, Bartels MD, Andersen IS, Moller JA, Westh H. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin Microbiol Infect. 2007;13:725–727. doi: 10.1111/j.1469-0691.2007.01720.x. [DOI] [PubMed] [Google Scholar]
- McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksnys V, Pleckaityte M. Catalytic and binding properties of restriction endonuclease Cfr9I. Eur J Biochem. 1993;217:411–419. doi: 10.1111/j.1432-1033.1993.tb18260.x. [DOI] [PubMed] [Google Scholar]
- van Belkum A, Melles DC, Peeters JK, van Leeuwen WB, van Duijkeren E, Huijsdens XW, Spalburg E, de Neeling AJ, Verbrugh HA. Dutch Working Party on Surveillance and Research of M-S. Methicillin-resistant and -susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg Infect Dis. 2008;14:479–483. doi: 10.3201/eid1403.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struelens MJ, Deplano A, Godard C, Maes N, Serruys E. Epidemiologic typing and delineation of genetic relatedness of methicillin-resistant Staphylococcus aureus by macrorestriction analysis of genomic DNA by using pulsed-field gel electrophoresis. J Clin Microbiol. 1992;30:2599–2605. doi: 10.1128/jcm.30.10.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S, Zhang Z, Donaldson AI, Garland AJ. The pathogenesis and diagnosis of foot-and-mouth disease. J CompPathol. 2003;129:1–36. doi: 10.1016/s0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]